Abstract
BACKGROUND
Sufficient evidence suggests that preoperative biliary stenting is associated with increased complication rates after pancreaticoduodenectomy.
METHODS
Surveillance, Epidemiology, and End Results (SEER) and linked Medicare claims data (1992–2007) were used to identify patients with pancreatic cancer who underwent pancreaticoduodenectomy. We evaluated trends in the use of preoperative biliary stenting, timing of physician visits relative to stenting, and time to surgical resection and symptoms in stented and unstented patients.
RESULTS
Pancreaticoduodenectomy was performed in 2,573 patients. 52.6% of patients underwent preoperative biliary stenting (N=1,354). Of these, 75.3% underwent endoscopic stenting only, 18.9% received a percutaneous stent, and 5.8% underwent both procedures. The overall stenting rate increased from 29.6% of patients in 1992–95 to 59.1% in 2004–07 (p<0.0001). Preoperative stenting was more common in patients with jaundice, cholangitis, pruritus, or coagulopathy (p<0.05 for all). 77.7% of stented patients had a stent placed prior to seeing a surgeon. Stenting prior to surgical consultation was associated with longer indwelling stent time compared to stenting after surgical consultation (37.3 vs. 27.0 days, p<0.0001). In addition, stented patients had longer times from surgeon visit to pancreatectomy than those who were not stented (24.2 days vs. 17.2 days, p< 0.0001).
CONCLUSION
Use of preoperative biliary stenting doubled from 1992–2007 despite evidence that stenting is associated with increased perioperative infectious complications. The majority of stenting occurred prior to surgical consultation and is associated with significant delay in time to operation. Surgeons should be involved early in order to prevent unnecessary stenting and improve outcomes.
Keywords: Pancreatic cancer, biliary stenting, endostent
INTRODUCTION
Preoperative biliary stenting was introduced in the 1960s and 1970s in an effort to improve surgical outcomes in pancreatic cancer patients undergoing curative-intent resection. This was done to correct physiologic disturbances induced by hyperbilirubinemia secondary to malignant obstruction, theoretically optimizing patients’ condition prior to operation and improving perioperative morbidity and mortality. Early retrospective studies1–4 and small prospective randomized trials 5–8 yielded mixed results, some finding benefit1–3, 5 with stenting and others finding no benefit.4, 6–8
The theoretical benefits of preoperative biliary stenting have not been consistently demonstrated in practice. Large retrospective analyses comparing stented to unstented patients either report no significant differences in surgical outcome9–12 or increased rates of infectious complications with preoperative biliary stenting.13–17 Several meta-analyses in the past decade corroborated these findings and recommend against the routine use of preoperative biliary stenting in patients undergoing pancreaticoduodenectomy.18–21 A recent prospective randomized trial reported a significantly increased overall complication rate in stented patients compared to those who proceeded directly to surgery, and many of the reported complications were related to the stenting procedure itself.22
In the setting of this evolving literature, we used the Surveillance, Epidemiology, and End Results (SEER) tumor registry and linked Medicare claims data to examine trends in use of preoperative biliary stenting from 1992–2007. In addition, we evaluated factors that were associated with the receipt of preoperative biliary stenting. Based on clinical experience, we hypothesized that preoperative biliary stenting had not decreased over this time period and that the majority of stenting occurred prior to surgical consultation/evaluation.
METHODS
This study was approved by the Institutional Review Board at the University of Texas Medical Branch.
Data Source
We used data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) tumor registry and linked Medicare claims data collected by the Center for Medicare and Medicaid Services. Developed by the National Cancer Institute, the SEER program collects information on cancer incidence and survival from population-based cancer registries currently covering approximately 28% of the U.S. population.23 SEER provides information on patient demographics, primary tumor site, histology, stage of disease, first course of treatment, and survival status.
The Medicare data include all claims for covered health care services, including inpatient and outpatient care, for all Medicare patients. The study included patients aged 66 years and older who were diagnosed from 1992 through 2007 and their Medicare claims through 2009.24
Cohort Selection (Figure 1)
Figure 1.
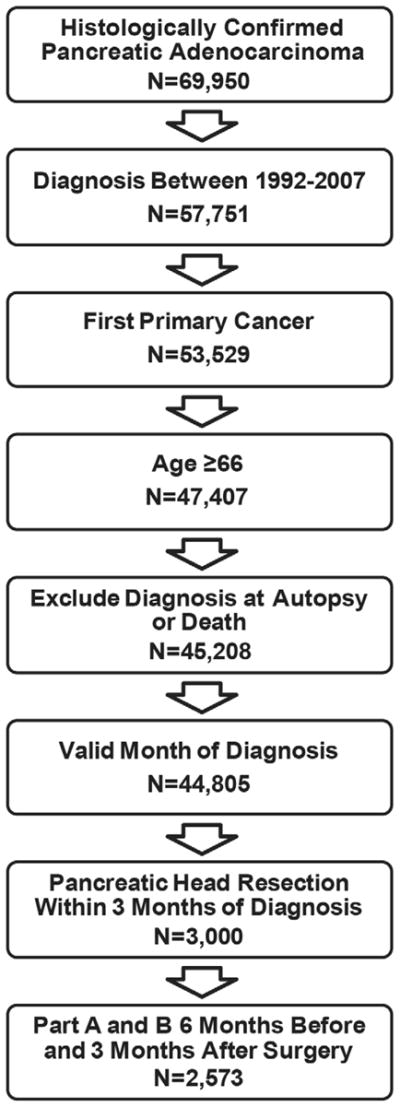
Cohort Selection. We included all patients with a first primary diagnosis of pancreatic adenocarcinoma between 1992–2007. Only patients age 66 and older with histologically confirmed and surgically resected adenocarcinoma within 3 months of diagnosis were included. Patients were excluded if they did not have Medicare Part A and B without HMO for six months before and three months after surgery.
Our cohort selection is summarized in Figure 1. Our cohort included patients with a primary diagnosis of adenocarcinoma of the pancreas between 1992–2007. International Classification of Disease for Oncology 3rd edition (ICD-O-3) morphology codes were used for adenocarcinoma (8000/3, 8010/3, 8020/3, 8021/3, 8022/3, 8140/3 8141/3, 8211/3, 8230/3, 8500/3, 8521/3, 8050/3, 8260/3, 8441/3, 8450/3, 8453/3, 8470/3, 8471/3, 8472/3, 8473/3, 8480/3, 8481/3, 8503/3). All International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM) and American Medical Association Common Procedure Terminology (CPT) codes used for the analysis are shown in Table 1. We included beneficiaries aged 66 and older who underwent pancreatic head resection within three months of diagnosis (Figure 1) and were enrolled in Medicare Part A and Part B without HMO for 6 months before and 3 months after surgery or until death. We excluded patients in whom pancreatic cancer was not the first primary and in whom the diagnosis was made at autopsy or by death certificate only.
Table 1.
ICD-9-CM and CPT Codes for Preoperative Procedures, Surgeon Visits, and Symptoms
| ICD-9 Codes | CPT/HCPCS codes | |
|---|---|---|
| Procedures | ||
| Pancreatic head resection | 52.6, 52.7, 52.51 | 48150, 48152, 48153, 48154, 48155 |
| ERCP | 51.10, 51.11, 51.84–51.87, 51.99, 52.13, 52.93 | 74328–74330, 43260–43269, 43271, 43272 |
| With endostent | 51.86, 51.87, 51.99 | 43267–43269 |
| Without endostent | 51.10, 51.11, 51.84, 51.85, 52.13, 52.93 | 74328–74330, 43260–43266, 43271, 43272 |
| PTC/ drain | 51.98, 87.51 | 74320 |
| Chemotherapy* | ICD-9 diagnosis codes: V58.1, V66.2, V67.2; ICD-9 procedure codes: 99.25; DRG code: 410 |
HCPCS/CPT codes: 96400- 96549, Q0083, Q0084, Q0085, J7150, J2353, J2354, J9000- J9999; Revenue center codes: 0331, 0332, 0335 |
| CT Abdomen | 88.01 | 74150, 74160, 74170, 74175, 76376, 76377 |
| MRI Abdomen | 88.97 | 74181, 74182, 74183, 74185 |
| EUS | NA | 76975, 43231, 43232, 43259 |
| Surgeon Visits | ||
| Outpatient Evaluation and Management | NA | 99201–99205, 99211–99215, 99241–99245 |
| Inpatient Evaluation and Management | NA | 99221–99223, 99231–99236, 99238, 99251–99255 |
| Symptoms | ||
| Jaundice | 782.4 | NA |
| Cholangitis | 576.1 | NA |
| Pruritis | 698.9 | NA |
| Coagulopathy | 286.7 | NA |
Neoadjuvant therapy defined as chemotherapy in the 6 months prior to surgical resection.
ERCP=endoscopic retrograde cholangiopancreatography, PTC= percutaneous transhepatic cholangiogram,CT= computed tomography, MRI= magnetic resonance imaging, EUS= endoscopic ultrasound, NA= not applicable
Measures
Endoscopic and percutaneous stenting were identified using both ICD-9 procedure codes and CPT codes in the carrier, Medicare Part A inpatient billing claims (MEDPAR), and outpatient standard analytic file (SAF) (Table 1). These codes included both insertion and replacement of stents or percutaneous biliary drains and excluded isolated pancreatic duct stenting. Date of the stenting procedure was obtained from Medicare claims, with the date of the first procedure used if multiple stenting procedures were performed. ICD-9 and CPT codes for endoscopic retrograde cholangiopancreatography (ERCP) without stenting (Table 1) were not included but were used to determine presumed failed endostenting if they occurred prior to or on the same day as percutaneous stenting. We also identified newer technologies such as CT, MRI, and endoscopic ultrasound (EUS) in carrier and outpatient SAF files with the following codes (CT abdomen: ICD-9: 88.01, CPT: 74150, 74160, 74170, 74175, 76376, 76377; MRI abdomen: ICD 9: 88.97, CPT: 74181, 74182, 74183, 74185, EUS: CPT: 76975, 43231, 43232, 43259).
Patients were classified as having: 1) localized disease (AJCC 0, IA, IB), 2) regional disease (AJCC IIA, IIB, III), or 3) distant disease (AJCC IV) using the SEER historic stage.25 The SEER historic stage is based on the best available data using a combination of pathologic observations, intraoperative observations, and clinical observations, in this priority order. Neoadjuvant therapy was defined as receipt of chemotherapy (Table 1) in the 6 months prior to surgical resection.
The date of surgery was determined from the associated Medicare claim. To identify the first surgeon visit, we used the carrier files to identify evaluation and management codes for office or other outpatient services and consultations and hospital inpatient services and consultations (Table 1). The Medicare Health Care Financing Administration specialty claims code associated with the claim was identified. Surgeon specialty was defined by Medicare specialty codes 02 (general surgery) and 91 (surgical oncology). The date of the first surgeon visit was defined as the date of the first visit to a surgeon before the date of surgical resection or the date of surgical resection if there was no prior documented surgeon visit. We identified the first gastroenterologist visit using evaluation and management claims for gastroenterologist (Medicare specialty code 10). The first visit was considered the date of first evaluation and management code or first endostent/ERCP code, whichever was earlier.
Demographic variables included age, gender, race, marital status, SEER region, quartiles of education, and median income. SEER-Medicare does not provide patient-level information for education and income; thus patients were placed into education and income quartiles based on zip code-level data. Zip code-level data were from the 2000 census and included median income and the percentage of residents with less than 12 years of education. Quartile 1 is the least educated (highest percentage without high school education) or lowest income quartile and quartile 4, the highest. Symptoms in the three months prior to surgery were identified using ICD-9 codes of inpatient or outpatient claims including jaundice (782.4), cholangitis (576.1), pruritus (698.9), and coagulopathy (286.7).
Statistical Analysis
Summary statistics were calculated for the entire cohort. We calculated the overall proportion of patients undergoing preoperative endoscopic or percutaneous stenting procedures. These were not mutually exclusive. Therefore, stented patients were classified as having undergone endoscopic, percutaneous, or both. In patients who underwent both endostenting and percutaneous stenting, we evaluated the relative timing of the two procedures. We also evaluated the timing of stent placement relative to surgical evaluation. Among stented patients, the proportion stented prior to seeing a surgeon was examined as were time trends in the rate of stenting prior to seeing a surgeon.
Time trends in the use of stenting procedures (overall, endoscopic, percutaneous) during the study period were examined using the Cochran-Armitage test for trend. Time trend analyses were performed using all SEER regions and then repeated excluding New Jersey, greater California, Kentucky, and Los Angeles, which were added in 2001 (first full year of data, 2000). As the results were identical, we report the results for the entire cohort.
We also evaluated the timing of stent placement relative to surgical evaluation. Among stented patients, the proportion stented prior to seeing a surgeon was examined as were time trends in the rate of stenting prior to seeing a surgeon.
Student’s t-test was used to compare time from surgical visit to surgical resection in stented and unstented patients and time from stenting to surgical resection in patients stented before and after seeing a surgeon. The proportion of patients who were stented within 1 week and 2 weeks of surgery was calculated.
Frequency of symptoms in the 3 months prior to surgery (jaundice, cholangitis, pruritus, coagulopathy) was calculated for the overall cohort. Bivariate analysis (chi-square) was used to determine the unadjusted association of patient, tumor, and operative characteristics with receipt of preoperative biliary stenting. Chi-square tests were also used to compare the rates of each individual symptom between stented and unstented patients as well as patients stented before and after seeing a surgeon. Multivariate logistic regression analysis was used to determine factors independently associated with preoperative biliary stenting.
Significance was accepted at the p<0.05 level. Statistical analysis was carried out using SAS Version 9.2 (Cary, N.C.).
RESULTS
Patient Demographics and Tumor Characteristics (Table 2)
Table 2.
Patient Demographics and Tumor Characteristics
| Number (%) N=2,573 |
|
|---|---|
| Patient Demographics | |
| Age (mean ±SD, years) | 73.8 ± 5.3 |
| Female | 1,400 (54.4) |
| Race | |
| White | 2,222 (86.4) |
| Black | 164 (6.4) |
| Hispanic | 30 (1.2) |
| Other | 157 (6.1) |
| Married | 1,578 (62.9) |
| Charlson comorbidity score | |
| 0 | 1136 (44.1) |
| 1 | 826 (32.1) |
| 2 | 360 (14.0) |
| 3+ | 251 (9.8) |
| SEER Region | |
| Atlanta | 88 (3.4) |
| Connecticut | 257 (10.0) |
| Detroit | 304 (11.8) |
| Greater California | 331 (12.9) |
| Hawaii | 57 (2.2) |
| Iowa | 181 (7.0) |
| Kentucky | 150 (5.8) |
| Los Angeles | 229 (8.9) |
| Louisiana | 125 (4.9) |
| New Jersey | 371 (14.4) |
| New Mexico | 62 (2.4) |
| San Francisco | 101 (3.9) |
| San Jose | 95 (3.7) |
| Seattle | 120 (4.7) |
| Utah | 99 (3.9) |
| Preoperative Signs/Symptoms | |
| Jaundice | 1650 (64.3) |
| Cholangitis | 265 (10.3) |
| Pruritis | 168 (6.5) |
| Coagulopathy | 17 (0.7) |
| Tumor Characteristics and Treatment | |
| Local/regional stage | 2,318 (90.1) |
| Tumor size in cm (N=2,269) | 3.3 ± 1.7 |
| Well/moderately differentiated | 1,414 (55.0) |
| Positive lymph nodes (N=2,304) | 1,402 (60.9) |
| Neoadjuvant therapy | 147 (5.7) |
2,573 patients underwent curative-intent surgical resection for adenocarcinoma of the head of the pancreas. The mean age at presentation was 73.8 ± 5.4 years. The majority of patients were female, white, and married. 2,318 patients (90.1%) had locoregional disease. One hundred forty-seven patients (5.7%) received neoadjuvant therapy in the 6 months prior to surgical resection. The distribution by SEER region is shown in Table 2. Potential indications for preoperative biliary drainage including jaundice, cholangitis, pruritus, and coagulopathy were seen preoperatively in 64.1%, 10.3%, 6.5%, and 0.7% of patients, respectively. Tumor characteristics are shown in Table 2.
Preoperative Biliary Drainage
Preoperative biliary drainage was performed in 1,354 patients (52.6%). Of these, 81.1% of patients underwent ERCP with endostenting and 24.7% underwent percutaneous biliary drainage. 75.3 % of patients who underwent preoperative biliary drainage underwent endostenting only, 18.9% percutaneous stenting only, and 5.8% underwent both procedures. In the 5.8% of patients (N=78) who received both endostenting and percutaneous drainage, 38.5% received the procedures on the same day (N=30), 24.4% had the endostent first (N=19), and 37.2% had the percutaneous biliary drain first (N=29). In patients who had a percutaneous transhepatic cholangiographic (PTC) stent only, 82.0% had an ERCP without endostent placement prior to PTC.
Time Trends in Preoperative Biliary Drainage (Figure 2)
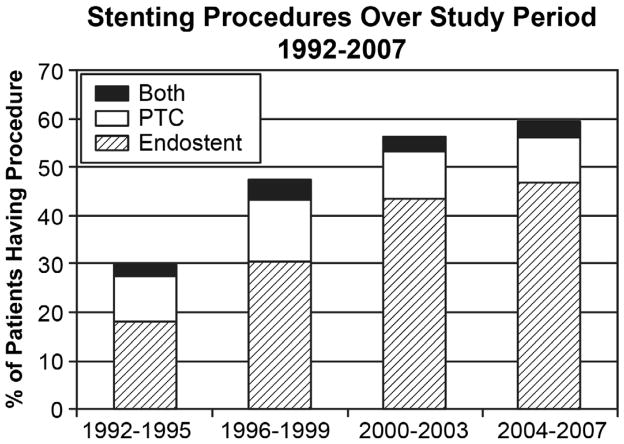
Figure 2.
Trends in Stenting Over Study Period, 1992–2007. Preoperative biliary stenting increased steadily over the study period, from 29.6% of patients in 1992–1995 to 59.1% of patients in 2004–7, p<0.0001. This increase is accounted for primarily by an increase in the rate of endostenting, from 20.2% of patients in 1992–1995 to 49.8% in 2004–2007 (p<0.0001).
Preoperative biliary stenting increased from 29.6% of patients in 1992–1995 to 59.1% in 2004–2007 (Figure 2, p<0.0001); this trend was largely attributed to an increase in the use of endostenting. The rate of endostenting increased significantly over time, from 20.2% in 1992–1995 to 49.8% in 2004–2007 (Figure 2, p<0.0001). The use of percutaneous stenting started at 11.5% in 1992–1995, peaked in the 1996–1999 time period at 16.7%, then returned to approximately 12% for the later two time periods (Figure 2, p=0.16). All time trends were identical when SEER regions that joined in 2001 were excluded (NJ, KY, greater CA, LA).
Concurrent Increase in Use of Other Tests
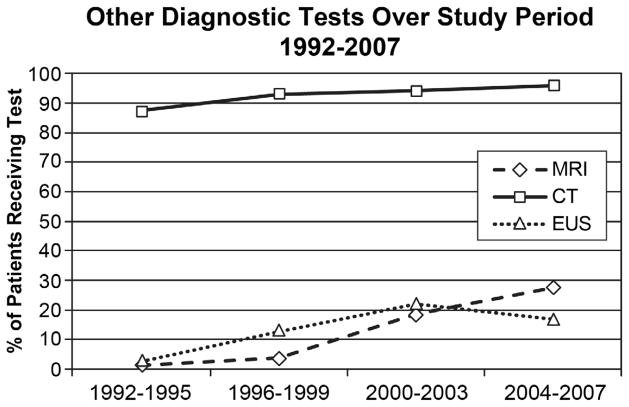
During the same time period, the use of several other diagnostic tests increased as well. Between 1992–1995 and 2004–2007, use of magnetic resonance imaging (MRI) increased from 1.5% to 21.6% (p<0.0001), CT use increased from 87.3% to 96.4%, and endoscopic ultrasound use increased from 2.7% in 1992–1995 to 16.8% in 2004–2007 (p<0.0001). Trends for all tests are shown in Figure 3.
Figure 3.
Trends in Use of Other Diagnostic Tests, 1992–2007. Preoperative diagnostic test use increased significantly during the study period. Computed tomography (CT) use increased from 87.3% to 96.4%, MRI from 1.5% to 21.6%, and EUS from 2.7% to 16.8% (p<0.0001 for all).
Of the 1,354 patients who underwent preoperative biliary stenting, 97.1% underwent CT, 20.5% underwent MRI, and 19.9% underwent EUS. Of these, 83.7% of CT scans, 53.1% of MRIs, and 12.2% of EUSs were performed prior to stenting, suggesting that staging before stenting occurred in most patients.
Timing of Preoperative Biliary Stenting and Surgeon/Gastroenterologist Visits
For the overall cohort we were unable to identify a surgeon visit in 443 patients (17.2%). In the 1,354 patients who underwent preoperative biliary stenting, we identified preoperative surgeon visits in 1,157 (85.4%). The date of surgery was the first surgeon-related claim in 14.6%. The preoperative biliary stent was placed prior to surgical evaluation in 77.7% of stented patients (N=1,052). This decreased to 73.9% when we included only those with documented preoperative surgeon visits. 1509 (58.7%) patients saw a gastroenterologist prior to seeing a surgeon. This pattern was consistent throughout the study period for all stented patients (p=0.55), those that saw a surgeon before surgery (p=0.25), and when the SEER regions joining in 2000 were excluded (N=800, p=0.31).
Timing of Preoperative Biliary Stenting and Surgical Resection
There was a longer time from diagnosis to surgery in stented compared to unstented patients (mean, 41.0 ± 42.7 days vs. 32.4 ± 45.8 days; median, 30 days vs. 21 days, p<0.0001). In patients who underwent preoperative biliary drainage, 69.6% had an interval of longer than two weeks between stent placement and surgery. The mean time from stenting to surgical resection was 35.0 ± 35.9 days (median, 23.0 days; Interquartile range: 12–42 days). Additional stenting procedures and/or stent changes were required in 234 (17.3%) of stented patients prior to surgical intervention, and 4.7% (N = 11) of these patients had cholangitis prior to the initial stent placement. Overall, 174 patients (12.9%) had documented cholangitis after initial stent placement. Of those patients with cholangitis after stenting, none had cholangitis documented prior to initial stent placement.
In the group of patients who underwent preoperative biliary stenting, those who were stented prior to seeing a surgeon had a longer time from stenting to surgical resection compared to those who were stented after seeing a surgeon (mean, 38.6 ± 37.3 days vs. 25.3 ± 29.7 days; median, 26 days vs. 14 days, p<0.0001). When we included only those with a documented surgeon evaluation prior to resection, the time from surgical consultation to surgical resection remained longer for stented compared to unstented patients (mean, 24.2 ± 28.34 days vs. 17.2 ± 26.21 days; median: 15 days vs. 8days, p<0.0001).
Factors Predicting Biliary Stenting
In an unadjusted analysis (Table 3), stenting increased with year of diagnosis. Men were more likely to undergo preoperative biliary stenting than women (p=0.05). Compared to black patients, white or Hispanic patients had higher rates of stenting (p=0.04). Patients in the lowest income (p=0.0006) and education quartiles (p=0.02) were less likely to be stented. The use of stenting was similar across Charlson comorbidity scores. Preoperative biliary stenting rates varied across SEER regions, with rates over 60% in Kentucky, Rural Georgia, Seattle, and Utah and less than 45% in Detroit, Hawaii, and Louisiana (p<0.0001). There was no difference in patient demographics, preoperative symptoms, and tumor characteristics across SEER regions with enough patients to compare. Age was similar in the stented and unstented groups (73.6 ± 5.2 years vs. 73.9 ± 5.4 years, p=0.19).
Table 3.
Bivariate Analysis: Factors Associated with Receipt of Preoperative Biliary Stenting
| % Undergoing Preoperative Biliary Stenting | p-value | |
|---|---|---|
| Year of Diagnosis | <0.0001 | |
| 1992–1995 | 29.6% | |
| 1996–1999 | 47.1% | |
| 2000–2003 | 55.9% | |
| 2004–2007 | 59.1% | |
| Patient Demographics | ||
| Sex | 0.05 | |
| Male | 54.7% | |
| Female | 50.9% | |
| Race | 0.04 | |
| White | 53.7% | |
| Black | 45.7% | |
| Hispanic | 56.7% | |
| Other | 44.6% | |
| Marital Status | 0.21 | |
| Married | 53.6% | |
| Unmarried | 51.1% | |
| Charlson comorbidity score | 0.36 | |
| 0 | 52.2% | |
| 1 | 51.0% | |
| 2 | 55.6% | |
| 3+ | 55.8% | |
| Income Quartiles | 0.0006 | |
| Quartile 1 (lowest) | 45.6% | |
| Quartile 2 | 55.6% | |
| Quartile 3 | 55.2% | |
| Quartile 4 (highest) | 54.4% | |
| Education Quartiles | 0.02 | |
| Quartile 1 (lowest) | 47.5% | |
| Quartile 2 | 55.6% | |
| Quartile 3 | 54.6% | |
| Quartile 4 (highest) | 53.2% | |
| SEER Region | <0.0001 | |
| Atlanta | 53.4% | |
| Connecticut | 51.0% | |
| Detroit | 44.7% | |
| Greater California | 56.5% | |
| Hawaii | 40.4% | |
| Iowa | 49.2% | |
| Kentucky | 62.0% | |
| Los Angeles | 47.2% | |
| Louisiana | 43.2% | |
| New Jersey | 59.3% | |
| New Mexico | 45.2% | |
| Rural Georgia | 66.7% | |
| San Francisco | 46.5% | |
| San Jose | 51.6% | |
| Seattle | 63.3% | |
| Utah | 64.6% | |
| Preoperative Signs/Symptoms | ||
| Jaundice | <0.0001 | |
| Yes | 66.2% | |
| No | 28.3% | |
| Cholangitis | <0.0001 | |
| Yes | 87.6% | |
| No | 48.6% | |
| Pruritus | <0.0001 | |
| Yes | 74.4% | |
| No | 51.1% | |
| Coagulopathy | 0.05 | |
| Yes | 76.5% | |
| No | 52.5% | |
| Tumor Characteristics and Treatment | ||
| Stage (N=2,496) | 0.0002 | |
| Localized | 44.1% | |
| Regional | 55.0% | |
| Distant | 47.8% | |
| Differentiation | 0.49 | |
| Poor | 53.7% | |
| Nodal status (regional disease only) (N=1,979) | 0.22 | |
| Positive | 55.7% | |
| Negative | 52.7% | |
| Neoadjuvant therapy | 0.008 | |
| Yes | 63.3% | |
| No | 52.0% | |
| Gastroenterologist visit before surgeon visit | <0.0001 | |
| Yes | 60.7% | |
| No | 41.2% |
Preoperative biliary stenting was more common in patients with preoperative jaundice, cholangitis, pruritus, or coagulopathy. 66.2% of jaundiced patients underwent stenting compared to 28.3% of patients without jaundice (p<0.0001). Similarly, patients with preoperative cholangitis (87.5% vs. 48.6%, p<0.0001), pruritus (74.4% vs. 51.1%, p<0.0001), and coagulopathy (76.5% vs. 52.5%, p=0.05) were more likely to be stented compared to patients without the sign/symptom. However, stenting was also common in patients without these documented symptoms. We also evaluated time trends in symptoms coded in the Medicare claims to see if there was a change in presentation of patients or documentation of their presentation over time. There was no change in the number of patients with jaundice, ranging from 39% in 1992–1995 to 37% in 2004–2007 (p=0.48). Only 168 patients had documented pruritus, and this increased slightly over time from 3% to 9% (p=0.005). Likewise, only 265 patients had cholangitis but the percentage of patients with cholangitis increased from 5% to 13% (p=0.003). Coagulopathy was stable over time around 1% (p=0.47).
In patients undergoing preoperative biliary stenting, tumor size was larger than in patients who were not stented (3.5 ± 1.8 cm vs. 3.1 ± 1.6 cm, p<0.0001). Stenting was more common in patients with regional disease.
Preoperative biliary stenting was more common in the 147 patients undergoing neoadjuvant therapy than in patients who did not (63.3% vs. 52.0%, p=0.008). Finally, patients who saw a gastroenterologist prior to seeing a surgeon were more likely to undergo preoperative biliary stenting (60.7% vs. 41.2%, p<0.0001).
There was no significant difference in the frequency of jaundice, cholangitis, or pruritus between the groups of patients stented before and after seeing a surgeon. Patients who were stented after seeing a surgeon were more likely to be coagulopathic compared to patients who were stented before seeing a surgeon (p=0.0006).
On multivariate logistic regression analysis (Table 4), the following factors were independently associated with receiving a preoperative biliary stent: later year of diagnosis, preoperative jaundice and cholangitis, larger tumor size, neoadjuvant therapy, and gastroenterologist visit prior to surgeon visit. The likelihood of undergoing preoperative biliary stenting varied significantly with SEER region. There was no difference in patient/tumor characteristics across SEER regions.
Table 4.
Multivariate Analysis: Factors Associated with Preoperative Biliary Stenting
| Factor | Odds Ratio | 95% CI |
|---|---|---|
| Patient Demographics | ||
| Age (per year) | 0.99 | 0.97–1.01 |
| Year of Diagnosis | 1.09 | 1.06–1.12 |
| Male (Female) | 1.14 | 0.92–1.41 |
| Race | ||
| White | 0.95 | 0.61–1.47 |
| Hispanic | 1.27 | 0.45–3.59 |
| Black | Reference | Reference |
| Other | 0.88 | 0.47–1.66 |
| Married (Unmarried) | 1.13 | 0.91–1.41 |
| Income quartiles | ||
| Quartile 1 | Reference | Reference |
| Quartile 2 | 1.29 | 0.93–1.80 |
| Quartile 3 | 1.15 | 0.78–1.71 |
| Quartile 4 | 1.05 | 0.67–1.67 |
| Education | ||
| Quartile 1 | Reference | Reference |
| Quartile 2 | 1.24 | 0.89–1.72 |
| Quartile 3 | 1.20 | 0.82–1.76 |
| Quartile 4 | 1.01 | 0.66–1.57 |
| SEER Region* | ||
| Atlanta | 1.72 | 0.83–3.55 |
| Connecticut | 2.02 | 1.10–3.71 |
| Detroit | 1.66 | 0.93–2.97 |
| Greater California | 1.90 | 1.09–3.31 |
| Hawaii | 1.11 | 0.44–2.80 |
| Iowa | 1.48 | 0.80–2.74 |
| Kentucky | 2.62 | 1.36–2.05 |
| Louisiana | Reference | Reference |
| Los Angeles | 2.02 | 1.10–3.71 |
| New Jersey | 1.79 | 1.01–3.15 |
| New Mexico | 0.84 | 0.33–2.12 |
| San Francisco | 1.53 | 0.75–3.12 |
| San Jose | 1.94 | 0.92–4.06 |
| Seattle | 3.89 | 1.98–7.65 |
| Utah | 3.37 | 1.66–6.88 |
| Preoperative Signs/Symptoms | ||
| Jaundice (no jaundice) | 5.53 | 4.40–6.95 |
| Cholangitis (no cholangitis) | 6.33 | 3.98–10.08 |
| Pruritus (no pruritus) | 1.58 | 1.02–2.45 |
| Coagulopathy (no coagulopathy) | 2.119 | 0.53–8.44 |
| Tumor Characteristics and Treatment | ||
| Tumor size (per 10 mm) | 0.99 | 0.98–0.99 |
| Stage | ||
| Localized | Reference | Reference |
| Regional | 1.26 | 0.90–1.78 |
| Distant | 1.10 | 0.64–1.90 |
| Differentiation (N=2,229) | ||
| Poor (vs. No) | 0.87 | 0.70–1.08 |
| Nodal status (N=2,304) | ||
| Positive | 1.10 | 0.87–1.40 |
| Negative | Reference | Reference |
| Neoadjuvant therapy | ||
| Yes (vs. No) | 2.71 | 1.58–4.66 |
| Gastroenterologist visit before surgeon visit | ||
| Yes (vs. No) | 1.80 | 1.46–2.22 |
Louisiana was chosen as reference because of low usage of preoperative biliary stenting amidst a sizable patient population. Rural Georgia was excluded because of few patients (N=3).
DISCUSSION
Despite evidence of increased complication rates and recommendations to avoid routine preoperative biliary stenting, the use of preoperative biliary stenting doubled between 1992 and 2007. The increase in preoperative biliary stenting was driven by a rise in the use of endostenting, while percutaneous stenting was used at a constant rate throughout the study period. Our population-based study found that 77% of patients are referred to a surgeon with a stent already in place, consistent with previous single institution studies reporting rates of 42%–79%. 26–29 Moreover, evaluation by a gastroenterologist prior to surgeon evaluation was associated with increased stenting.
The literature surrounding the issue of preoperative biliary stenting has long generated controversy amidst evolving stent technology and techniques, and advances in surgical technique and perioperative care. Several large retrospective analyses comparing stented to unstented patients demonstrated either no difference in outcome9–11 or increased rates of mortality,14 overall complications,13, 14, 27 overall infectious complications,14, 30 wound infection,13–17, 19, 27, 30 intraabdominal abscess,13, 14, 30 or pancreatic fistula formation15, 27, 30 with preoperative biliary stenting. Two meta-analyses in 200218, 21 showed no difference in outcome between stented and unstented patients and later meta-analyses in 2010 and 2011 demonstrated increased infectious complications.19, 20 A multicenter randomized controlled trial by van der Gaag et al. in 2010 assigned patients to either an early surgery group (resection within 1 week of diagnosis) or a drainage group (endostenting with 4–6 week drainage period prior to surgery).22 The severe complication rates in the early surgery group and the biliary drainage group were 39% and 74%, respectively (relative risk = 0.54; 95% CI: 0.41–0.71; p < 0.0001). A significant proportion of complications were related to the stenting procedure itself and not postoperative complications.
To our knowledge, our study is the first to evaluate trends in preoperative biliary stenting at the population level. In our multivariate analysis, we observed that patient symptoms and signs (jaundice, cholangitis, pruritus) were most strongly associated with biliary stenting, consistent with prior observations. 9, 14, 15, 31 However, stenting was also performed in 28% of patients without documented jaundice and approximately 50% of patients without cholangitis, pruritus, or coagulopathy. In addition, we observed significant geographic variation in the use of preoperative biliary stenting, with no evidence of differences in patient characteristics across SEER regions. This suggests that stenting was done based on provider preference and not patient or tumor characteristics.
Preoperative biliary stenting is indicated in patients who will undergo neoadjuvant therapy prior to surgical intervention. Previous studies have documented the safety and efficacy of preoperative stenting in this setting.32–34 In our study, only 147 patients underwent neoadjuvant therapy. Of these patients, 93 (63.3%) underwent preoperative stenting. In the setting of neoadjuvant therapy, stenting is appropriate to palliate jaundice while awaiting completion of therapy and restaging. However, of the 2,426 patients who did not undergo neoadjuvant therapy, 1,261 (52.0%) underwent preoperative biliary stenting. The observed increase in biliary stenting is not explained by an increase in receipt of neoadjuvant therapy in our cohort, as the use of neoadjuvant therapy did not change over time.
The median time from stenting to surgical resection was 35 days, and 70% of patients had delays of greater than two weeks from stenting to resection. Consistent with the recent randomized controlled trial22, we also observed significant complication rates related to the stenting procedure. Our study demonstrated that 13% of stented patients developed documented cholangitis and 17% required additional stenting procedures or stent changes prior to resection. A 2002 meta-analysis of randomized controlled trials reported drainage procedure-related complications in 27.4% and stent dysfunction in 33.8% of patients.18 Previous studies have documented hepatotoxicity, stent migration, and cholangitis as factors that may delay resection and may account for the long delay between stenting and surgical resection.10, 33, 35, 36 Conversely, the need for stent changes and development of cholangitis may be secondary to the delay and not vice versa.
The delay from diagnosis to resection was longer if the stent was placed prior to surgical evaluation, consistent with previous studies suggesting longer times to surgery for stented patients. 9–11, 27 It is well documented that preoperative biliary stenting colonizes the biliary tree with enteric flora.12, 19, 27, 28, 35, 37, 38 In addition, colonization and infection increase with increased duration of stenting and the presence of biliary contamination/infection is associated with increased rates of postoperative infectious complications and mortality10, 19, 27, 28, 35
Our study has several limitations. First, laboratory results such as bilirubin levels and liver function tests are not available in the SEER-Medicare data. Second, symptoms such as jaundice, cholangitis, coagulopathy, or pruritis are likely undercoded in claims data. Thus, we may overestimate stenting in asymptomatic patients. For example, it is possible that patients in the “non-jaundiced” group (ie. no claim for jaundice) actually had mildly elevated bilirubin or alkaline phosphatase levels, meaning the denominator would be lower.
In 14.6% of stented patients, we were unable to identify a surgeon visit prior to resection. We suspect that many of these patients were seen by surgeons as inpatients, and the surgeon visit was not documented in the hospital claim. It is also possible that there are errors in the Medicare physician specialty codes. For this reason, we evaluated stenting prior to surgical evaluation both in the overall cohort (using the date of surgery as the date of surgeon visit when no preoperative visit was identified) and in the subgroup with identified preoperative surgeon consultations. Finally, as our study goes through 2007, several of the studies recommending against its use occurred after the time period of our study and recent trends may have changed.
There are many situations, even in resected patients, where stenting is warranted. For example, stenting is appropriate in patients who need neoadjuvant chemoradiation and patients with severe symptoms related to their jaundice. Stenting may also be indicated in patients with significant comorbid medical illness who need further evaluation and optimization/stabilization of their medical conditions prior to surgery. In addition, stenting may be unavoidable in settings where operating room, surgeon, or patient availability is limited. There was no concomitant increase in jaundice over time to suggest the increase in stenting is clinically justified. Pruritus and cholangitis increased slightly over time. While it is impossible to tell if this increase represents a true change in symptoms or better coding, the small number of patients involved does not explain the doubling in stent rates. While we cannot determine the appropriateness of stenting in any specific instance, the increasing trend is concerning in light of consistent recommendations against the routine use of biliary stenting. Furthermore, geographic variation in use of stenting, coupled with an increase in the use of many other preoperative tests (EUS, MRI, CT), suggests a more widespread problem of overuse.39–43 In stented patients, the majority of diagnostic and staging tests were performed prior to stenting, suggesting careful staging. This provides additional evidence that referral patterns, and not the clinical picture, are driving preoperative biliary stenting; patients with staged, resectable disease are often being referred to gastroenterology before surgical evaluation without communication between the two specialties.
In conclusion, we demonstrated that preoperative biliary stenting has increased at an alarming rate despite consistent recommendations against its use. The majority of stenting occurred prior to surgical evaluation and was more likely if patients had symptoms or were seen by a gastroenterologist first. Biliary stenting was associated with a delay to surgery, which may have been due to stenting-related complications. These findings highlight the need for early communication between gastroenterologists and surgeons in the care of patients with pancreatic and other periampullary cancers. Multidisciplinary tumor boards are potential means to reduce unnecessary stenting and improve patient outcomes. Early surgeon involvement and multidisciplinary care offer opportunities for streamlining the evaluation of patients with early stage pancreatic cancer to avoid unnecessary procedures and improve processes of care for this patient population.
Acknowledgments
Funding: Supported by grants from the National Institutes of Health (1K07CA130983-01A1, UL1TR000071 and T32 DK007639), and the Cancer Prevention Research Institute of Texas Grant # #RP101207-P03
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should it be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Financial Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Denning DA, Ellison EC, Carey LC. Preoperative percutaneous transhepatic biliary decompression lowers operative morbidity in patients with obstructive jaundice. Am J Surg. 1981;141(1):61–5. doi: 10.1016/0002-9610(81)90013-1. [DOI] [PubMed] [Google Scholar]
- 2.Gundry SR, Strodel WE, Knol JA, et al. Efficacy of preoperative biliary tract decompression in patients with obstructive jaundice. Arch Surg. 1984;119(6):703–8. doi: 10.1001/archsurg.1984.01390180065011. [DOI] [PubMed] [Google Scholar]
- 3.Lygidakis NJ, van der Heyde MN, Lubbers MJ. Evaluation of preoperative biliary drainage in the surgical management of pancreatic head carcinoma. Acta Chir Scand. 1987;153(11–12):665–8. [PubMed] [Google Scholar]
- 4.Trede M, Schwall G. The complications of pancreatectomy. Ann Surg. 1988;207(1):39–47. doi: 10.1097/00000658-198801000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith RC, Pooley M, George CR, et al. Preoperative percutaneous transhepatic internal drainage in obstructive jaundice: a randomized, controlled trial examining renal function. Surgery. 1985;97(6):641–8. [PubMed] [Google Scholar]
- 6.Hatfield AR, Tobias R, Terblanche J, et al. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet. 1982;2(8304):896–9. doi: 10.1016/s0140-6736(82)90866-2. [DOI] [PubMed] [Google Scholar]
- 7.McPherson GA, Benjamin IS, Hodgson HJ, et al. Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg. 1984;71(5):371–5. doi: 10.1002/bjs.1800710522. [DOI] [PubMed] [Google Scholar]
- 8.Pitt HA, Gomes AS, Lois JF, et al. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985;201(5):545–53. doi: 10.1097/00000658-198505000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martignoni ME, Wagner M, Krähenbühl L, et al. Effect of preoperative biliary drainage on surgical outcome after pancreatoduodenectomy. Am J Surg. 2001;181(1):52–9. doi: 10.1016/s0002-9610(00)00528-6. discussion 87. [DOI] [PubMed] [Google Scholar]
- 10.Jagannath P, Dhir V, Shrikhande S, et al. Effect of preoperative biliary stenting on immediate outcome after pancreaticoduodenectomy. Br J Surg. 2005;92(3):356–61. doi: 10.1002/bjs.4864. [DOI] [PubMed] [Google Scholar]
- 11.Sewnath ME, Birjmohun RS, Rauws EA, et al. The effect of preoperative biliary drainage on postoperative complications after pancreaticoduodenectomy. J Am Coll Surg. 2001;192(6):726–34. doi: 10.1016/s1072-7515(01)00819-5. [DOI] [PubMed] [Google Scholar]
- 12.Karsten TM, Allema JH, Reinders M, et al. Preoperative biliary drainage, colonisation of bile and postoperative complications in patients with tumours of the pancreatic head: a retrospective analysis of 241 consecutive patients. Eur J Surg. 1996;162(11):881–8. [PubMed] [Google Scholar]
- 13.Heslin MJ, Brooks AD, Hochwald SN, et al. A preoperative biliary stent is associated with increased complications after pancreatoduodenectomy. Arch Surg. 1998;133(2):149–54. doi: 10.1001/archsurg.133.2.149. [DOI] [PubMed] [Google Scholar]
- 14.Povoski SP, Karpeh MS, Conlon KC, et al. Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann Surg. 1999;230(2):131–42. doi: 10.1097/00000658-199908000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohn TA, Yeo CJ, Cameron JL, et al. Do preoperative biliary stents increase postpancreaticoduodenectomy complications? J Gastrointest Surg. 2000;4(3):258–67. doi: 10.1016/s1091-255x(00)80074-8. discussion 267–8. [DOI] [PubMed] [Google Scholar]
- 16.Pisters PW, Hudec WA, Hess KR, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg. 2001;234(1):47–55. doi: 10.1097/00000658-200107000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodul P, Creech S, Pickleman J, et al. The effect of preoperative biliary stenting on postoperative complications after. Am J Surg. 2003;186(5):420–5. doi: 10.1016/j.amjsurg.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Sewnath ME, Karsten TM, Prins MH, et al. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236(1):17–27. doi: 10.1097/00000658-200207000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcea G, Chee W, Ong SL, et al. Preoperative biliary drainage for distal obstruction: the case against revisited. Pancreas. 2010;39(2):119–26. doi: 10.1097/MPA.0b013e3181bd65de. [DOI] [PubMed] [Google Scholar]
- 20.Qiu YD, Bai JL, Xu FG, et al. Effect of preoperative biliary drainage on malignant obstructive jaundice: a meta-analysis. World J Gastroenterol. 2011;17(3):391–6. doi: 10.3748/wjg.v17.i3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saleh MM, Nørregaard P, Jørgensen HL, et al. Preoperative endoscopic stent placement before pancreaticoduodenectomy: a meta-analysis of the effect on morbidity and mortality. Gastrointest Endosc. 2002;56(4):529–34. doi: 10.1067/mge.2002.128161. [DOI] [PubMed] [Google Scholar]
- 22.van der Gaag NA, Rauws EA, van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362(2):129–37. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 23. [Accessed December, 2012];SEER-Medicare: Brief Description of the SEER-Medicare Database. 2009 Nov; Available at: http://healthservices.cancer.gov/seermedicare/overview/
- 24.Research, Statistics, Data & Systems. [Accessed December, 2012]; Available at: http://www.cms.gov/Research-Statistics-Data-and-Systems/Research-Statistics-Data-and-Systems.html.
- 25.Young JLRS, Ries LAG, Fritz AG, Hurlbut AA, editors. NIH Pub No 01-4969. National Cancer Institute; Bethesda, MD: 2001. SEER Summary Staging Manual – 2000: Codes and Coding Instructions. [Google Scholar]
- 26.Isla AM, Griniatsos J, Riaz A, et al. Pancreaticoduodenectomy for periampullary malignancies: the effect of bile colonization on the postoperative outcome. Langenbecks Arch Surg. 2007;392(1):67–73. doi: 10.1007/s00423-006-0102-0. [DOI] [PubMed] [Google Scholar]
- 27.Morris-Stiff G, Tamijmarane A, Tan YM, et al. Pre-operative stenting is associated with a higher prevalence of post-operative complications following pancreatoduodenectomy. Int J Surg. 2011;9(2):145–9. doi: 10.1016/j.ijsu.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Cortes A, Sauvanet A, Bert F, et al. Effect of bile contamination on immediate outcomes after pancreaticoduodenectomy for tumor. J Am Coll Surg. 2006;202(1):93–9. doi: 10.1016/j.jamcollsurg.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Coates JM, Beal SH, Russo JE, et al. Negligible effect of selective preoperative biliary drainage on perioperative resuscitation, morbidity, and mortality in patients undergoing pancreaticoduodenectomy. Arch Surg. 2009;144(9):841–7. doi: 10.1001/archsurg.2009.152. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava S, Sikora SS, Kumar A, et al. Outcome following pancreaticoduodenectomy in patients undergoing preoperative biliary drainage. Dig Surg. 2001;18(5):381–7. doi: 10.1159/000050178. [DOI] [PubMed] [Google Scholar]
- 31.Herzog T, Belyaev O, Hessam S, et al. Bacteribilia with resistant microorganisms after preoperative biliary drainage--the influence of bacteria on postoperative outcome. Scand J Gastroenterol. 2012;47(7):827–35. doi: 10.3109/00365521.2012.679684. [DOI] [PubMed] [Google Scholar]
- 32.Gerke H, White R, Byrne MF, et al. Complications of pancreaticoduodenectomy after neoadjuvant chemoradiation in patients with and without preoperative biliary drainage. Dig Liver Dis. 2004;36(6):412–8. doi: 10.1016/s1590-8658(04)00096-9. [DOI] [PubMed] [Google Scholar]
- 33.Pisters PW, Hudec WA, Lee JE, et al. Preoperative chemoradiation for patients with pancreatic cancer: toxicity of endobiliary stents. J Clin Oncol. 2000;18(4):860–7. doi: 10.1200/JCO.2000.18.4.860. [DOI] [PubMed] [Google Scholar]
- 34.Eshuis WJ, van der Gaag NA, Rauws EA, et al. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Ann Surg. 2010;252(5):840–9. doi: 10.1097/SLA.0b013e3181fd36a2. [DOI] [PubMed] [Google Scholar]
- 35.Povoski SP, Karpeh MS, Conlon KC, et al. Preoperative biliary drainage: impact on intraoperative bile cultures and infectious morbidity and mortality after pancreaticoduodenectomy. J Gastrointest Surg. 1999;3(5):496–505. doi: 10.1016/s1091-255x(99)80103-6. [DOI] [PubMed] [Google Scholar]
- 36.Lai EC, Mok FP, Fan ST, et al. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81(8):1195–8. doi: 10.1002/bjs.1800810839. [DOI] [PubMed] [Google Scholar]
- 37.Herzog T, Belyaev O, Muller CA, et al. Bacteribilia after preoperative bile duct stenting: a prospective study. J Clin Gastroenterol. 2009;43(5):457–62. doi: 10.1097/MCG.0b013e318186b19b. [DOI] [PubMed] [Google Scholar]
- 38.Groen AK, Out T, Huibregtse K, et al. Characterization of the content of occluded biliary endoprostheses. Endoscopy. 1987;19(2):57–9. doi: 10.1055/s-2007-1018235. [DOI] [PubMed] [Google Scholar]
- 39.Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256(3):518–28. doi: 10.1097/SLA.0b013e318265bcdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung F, Yuan H, Yin L, et al. Elimination of preoperative testing in ambulatory surgery. Anesth Analg. 2009;108(2):467–75. doi: 10.1213/ane.0b013e318176bc19. [DOI] [PubMed] [Google Scholar]
- 41.Schein OD, Katz J, Bass EB, et al. The value of routine preoperative medical testing before cataract surgery. Study of Medical Testing for Cataract Surgery. N Engl J Med. 2000;342(3):168–75. doi: 10.1056/NEJM200001203420304. [DOI] [PubMed] [Google Scholar]
- 42.Vogt AW, Henson LC. Unindicated preoperative testing: ASA physical status and financial implications. J Clin Anesth. 1997;9(6):437–41. doi: 10.1016/s0952-8180(97)00060-3. [DOI] [PubMed] [Google Scholar]
- 43.Bryson GL, Wyand A, Bragg PR. Preoperative testing is inconsistent with published guidelines and rarely changes management. Can J Anaesth. 2006;53(3):236–41. doi: 10.1007/BF03022208. [DOI] [PubMed] [Google Scholar]