Abstract
Advances in functional neurosurgery have expanded the treatment of Parkinson’s disease (PD), from early lesional procedures to targeted electrical stimulation of specific nodes in the basal ganglia circuitry. Deep brain stimulation (DBS), applied to selected patients with Parkinson’s disease (PD) and difficult-to-manage motor fluctuations, yields substantial reductions in off time and dyskinesia. Outcomes for DBS targeting the two major studied targets in PD, the subthalamic nucleus (STN) and the internal segment of the globus pallidus (GPi), appear to be broadly similar and the choice is best made based on individual patient factors and surgeon preference. Emerging concepts in DBS include examination of new targets, such as the potential efficacy of pedunculopontine nucleus (PPN) stimulation for treatment of freezing and falls, the utilization of pathologic oscillations in the beta band to construct an adaptive “closed-loop” DBS, and new technologies, including segmented electrodes to steer current toward specific neural populations.
Keywords: Parkinson disease, deep brain stimulation, patient selection, beta band oscillations
INTRODUCTION
The surgical treatment of Parkinson’s disease (PD) has evolved sporadically over the last century. Early attempts at relieving parkinsonian symptoms included bilateral posterior cervical rhizotomy by Leriche in 19121 and later by others in an attempt to improve rigidity and tremor. Later surgeries focused on interruption of the pyramidal tracts (motor cortex,2 cervical spinal cord,3 and cerebral peduncle).4 Surgery on the basal ganglia for PD, particularly the pallidofugal fibers, was initially explored by Meyers in 1951,5 and given support by the favorable outcome on a parkinsonian patient whose anterior choroidal artery was serendipitously ligated by Cooper when performing a pedunculotomy.6 With the advent of stereotactic surgery, reports by Spiegel and Wycis7 and others on the benefits of pallidotomy began to surface, and eventually thalamotomy was found to reduce parkinsonian tremor. In the 1960s, levodopa was found to dramatically improve the symptoms of PD8 and there was a temporary hiatus in the surgical treatment of PD. Despite the profound efficacy of levodopa, complications such as dyskinesias and motor fluctuations prompted a renewed interest in the surgical treatment of PD in the early 1990s, primarily through the previously utilized lesional surgeries. Although stimulation of the deep structures of the brain had been performed previously,9 the modern era of chronic DBS was led by the reports of Benabid and colleagues, first of the ventral intermediate nucleus of the thalamus (Vim),10 then later the subthalamic nucleus (STN)11 and the internal segment of the globus pallidus (GPi).12 Thalamic DBS was approved by the US FDA in 1997 for the treatment of tremor associated with essential tremor and PD, and STN and pallidal DBS in 2002 for the treatment of PD. In a short time, DBS became an established treatment for advanced Parkinson’s disease. Evidence has accumulated supporting the long term efficacy of DBS, and at the same time new technology has continued to refine the procedure.
CASE STUDY
A 66 year old man presents to the Movement Disorders Center for evaluation and treatment of worsening symptoms from his PD. He had the onset of right-hand resting tremor at the age of 58 years, followed by micrographia, decreased dexterity when typing, then later slowness and shuffling of gait. He ultimately had good improvement with levodopa for several years. Over time, however, the duration of benefit from his medication doses shortened, and he required increasing doses of medication to obtain the same benefit. Adjunctive medications including a COMT inhibitor, dopamine agonist, and a MAO-B inhibitor yielded modest improvement, but motor fluctuations became progressively more difficult to control, alternating between “on” periods with good function and “off” periods marked by freezing of gait and near-immobility. Sleep was disrupted by painful off period symptoms returning in the middle of the night. Moderate to severe dyskinesia complicated his functioning in the “on” periods, partially attenuated by the addition of amantadine. Depression was present but adequately treated with an antidepressant.
PATIENT SELECTION
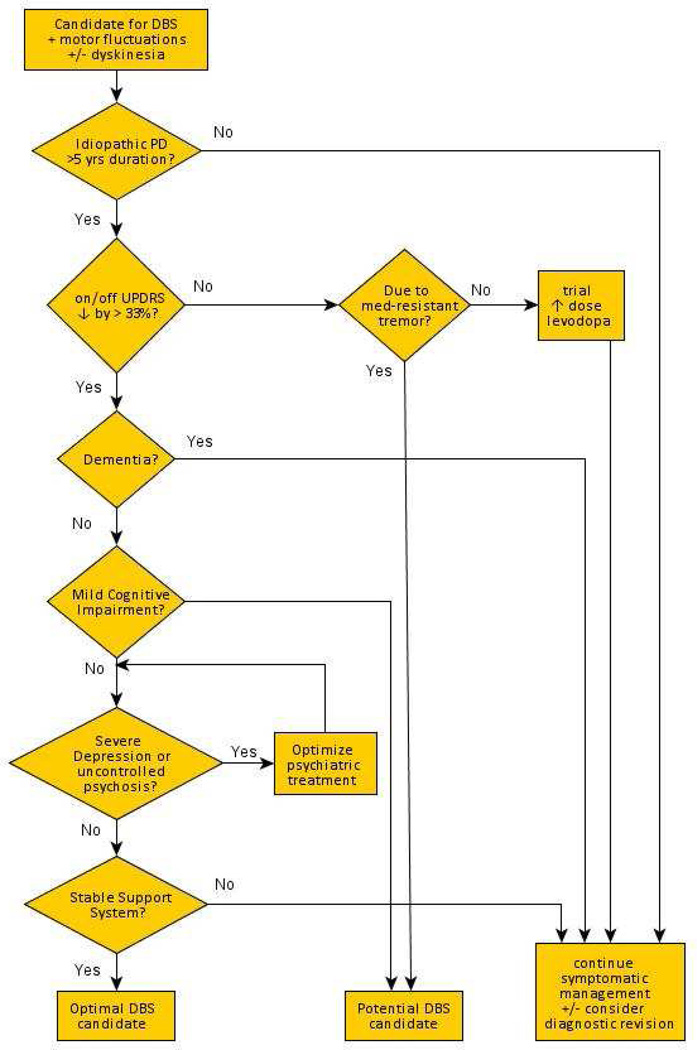
The Core Assessment Program for Intracerebral Transplantations (CAPIT) was developed and published in 1992,13 in an attempt to standardize patient selection, inclusionary criteria, and reporting of outcomes in studies of intracerebral transplantation (originally, fetal dopamine neurons). This protocol was later updated and adapted to both ablative and neurostimulation procedures in the Core Assessment Program for Surgical Interventional Therapies (CAPSIT) protocol.14 From this protocol and with experience over time, a set of standardized evaluations have evolved, identifying candidates who will derive lasting benefits from the procedure, while being cognitively, emotionally, and socially prepared for DBS.15 It is important for centers to evaluate patients in a standardized fashion in order to accurately identify those patients in whom the benefits of the surgery will outweigh the potential risks. A decision-making algorithm may be helpful (figure 1).
Figure 1.
Decision-making algorithm for patient selection for DBS surgery in Parkinson’s disease.
DBS performed in a patient with an atypical parkinsonism may not benefit and, in fact, may accelerate the symptomatic decline of these conditions.16 Therefore, a diagnosis of idiopathic PD is a prerequisite for consideration of functional neurosurgery. Disability that requires consideration for DBS after a disease duration of five years or less suggests the presence of a PD mimic, most often an atypical parkinsonism, such as the parkinsonian variant of multiple system atrophy. A pre-operative evaluation of levodopa responsiveness is mandatory, with a 33% decrease in the Unified Parkinson’s Disease Rating Scale (UPDRS) part III motor score often suggested as the threshold beyond which surgical benefits materialize. DBS mostly benefits symptoms that are improved with levodopa, with the possible exception of medication-resistant tremor (where benefit from DBS may exceed that of medication). The presurgical evaluation helps to establish a patient’s realistic expectations from surgery.
Preoperative cognitive assessments should be performed to exclude patients with dementia. In addition, behavioral assessments are necessary to identify significant depression, given the small but serious risk of attempted suicide reported in some cases.17 Finally, a sufficient social support network is essential in order to help care for the patient during the post-operative and stimulation titration process, which can take several months.
An “appropriate DBS candidate” may come from three major categories. The above case, a cognitively intact patient with idiopathic PD, good response to levodopa but with motor fluctuations including shortened duration of benefit from their medication and bothersome dyskinesia despite optimal medical management, should be considered for DBS. Alternatively, a patient with well-controlled PD except for medication-resistant tremor may also benefit from the procedure. Finally, patients with poor symptom control due to inability to tolerate adequate doses of levodopa may also benefit from surgical intervention. Except in the latter, less common scenario, response to levodopa remains the best predictor for surgical response, as magnitude of benefit to DBS matches but does not outperform that of levodopa response.
SURGICAL TARGET SELECTION
Thalamic stimulation is effective at reducing tremor in PD, and while the benefit is sustained, Vim DBS does not address bradykinesia or other PD symptoms, which invariably progress over time.18,19 Preferred targets include the STN and GPi, and the choice between them depends on individual patient factors and the experience of the surgeon. STN stimulation has consistently across studies been shown to allow for greater reduction in dopaminergic medication post-operatively. GPi stimulation is often more effective at reducing dyskinesia directly, unaccounted for by a reduction in dopaminergic medications. Although there is some suggestion that GPi DBS is “safer” from a neurocognitive standpoint,20 leading some to suggest this target for cognitively impaired patients who are otherwise good candidates,21 controlled clinical trials have not demonstrated significant cognitive differences in outcomes between the two targets.
OPERATIVE PROCEDURE
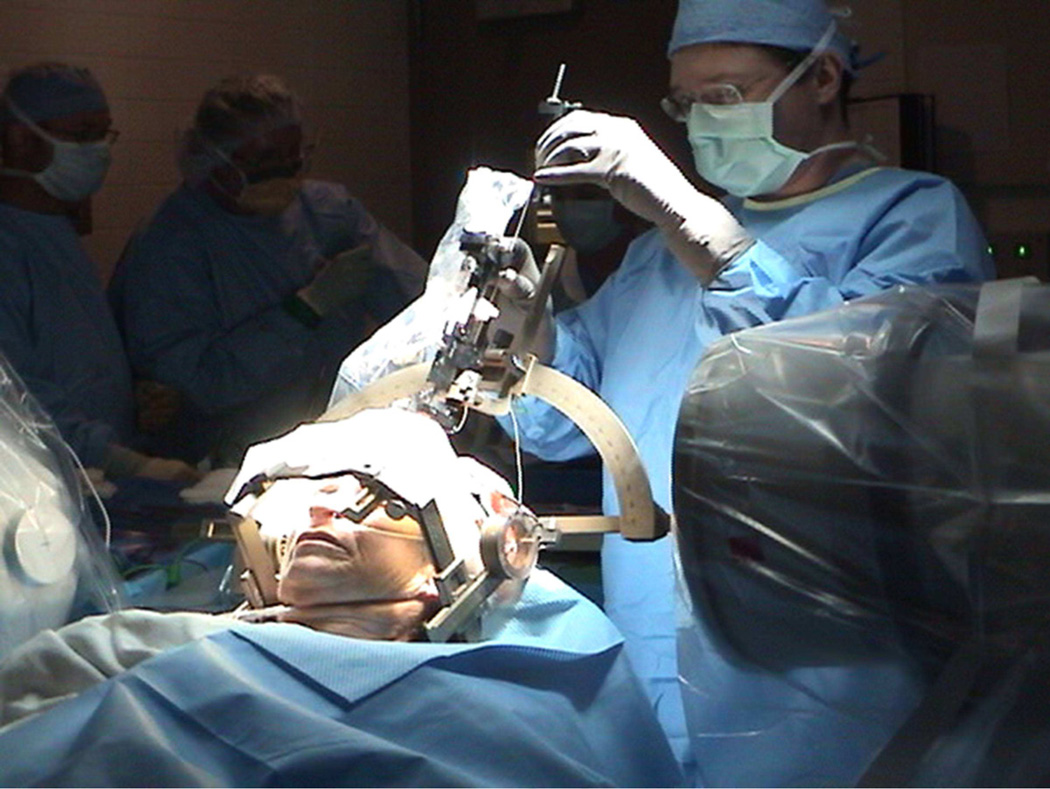
The location of the surgical target is typically chosen preoperatively on MRI by the neurosurgeon, based on visual landmarks. Co-registration with a standardized brain atlas can also be used. Brain mapping software determines the three dimensional coordinates of the target which can then be entered into a frame secured to the patient’s skull. If a frameless system is utilized, the angle and depth of the target is calculated with respect to skull fiducial markers. After burr hole placement, a microelectrode is slowly passed along the trajectory, and the depth of the target is identified based on microelectrode recording. The stimulating macroelectrode is then placed (figure 2) and tested intraoperatively to verify the threshold for side effects (depending on the target these can include paresthesias, muscle contraction, conjugate eye deviation, visual phosphenes). The macroelectrode is secured into position and the contralateral target is approached in a similar manner if a bilateral procedure is being performed. The electrodes are then connected to an implantable pulse generator (IPG), often in a separate procedure.
Figure 2.
Intraoperative placement of the DBS electrode.
POST-OPERATIVE PROGRAMMING
Initial programming of the DBS stimulators often takes place several weeks after implantation, to permit the “lesion effect” of improved function following lead implantation to dissipate, allowing for easier identification of symptoms and improvements from stimulation. The quadripolar electrode has contacts spaced 1.5 mm or 0.5 mm apart depending on the lead used, and stimulation can be delivered in a monopolar (one active electrode contact as cathode with the IPG as anode) or bipolar (electrode contacts used for both cathode and anode) fashion. The most effective electrode contact configuration gives the greatest benefit with the least amount of side effects due to current spread to surrounding structures. Stimulation amplitude is started at a low setting and increased gradually over time, which can allow for a reduction in medication. Programming optimization can take up to 4–6 months and may require multiple adjustment sessions.
OUTCOMES
STN and GPi DBS have been shown in several randomized controlled clinical trials to be superior to medical management alone.22–24 DBS patients gained on average 4.4 to 4.6 hours of “on” time without troublesome dyskinesia, had 1–2.6 fewer hours per day of “on” time with troublesome dyskinesia, and 2.4–4.2 fewer hours per day of “off” time. Quality of life, off-medication UPDRS motor scores, and sleep have also been reported to improve. Recent randomized trials comparing STN to GPi DBS showed no differences in motor function between groups, in both unilateral25 and bilateral26 stimulation. Given the similar outcome, it has been recommended that individual patient factors (e.g. STN if greater medication reduction is desired, GPi if greater antidyskinetic effect is pursued) and surgeon preference dictate target selection. Another randomized trial did find a significant difference in motor scores as a secondary outcome favoring STN compared to GPi DBS, but no difference in the primary outcomes of functional health (disability score) or cognitive, mood, or behavioral effects.27
DBS COMPLICATIONS
The incidence of serious adverse events related to DBS varies depending on the study. In three large multicenter trials,24,26,27 intracranial or intracerebral hemorrhage occurred in 2% of patients, ischemic stroke in 0–1%, implantation site infection in 3–8%, and seizures in 0–3%. A post-operative confusional state was also be seen in up to 21% in one of the trials,26 although this required additional hospitalization in only 2%. Lead fracture and device malfunction are also possible complications, which can cause loss of clinical benefit.28
The prevalence of suicidal ideation may increase after DBS, with a multicenter retrospective survey finding a rate of 0.90% suicide attempts and 0.45% suicide completions.29 Declines in cognition in longitudinal follow-up have been documented30,31 however it remains unclear whether DBS hastens this decline. Smaller series initially suggested that DBS may have a negative impact on frontal executive functioning, in some patients leading to a “mental state comparable to progressive supranuclear palsy.”32 However, larger studies comparing DBS patients to best medical management found overall cognitive scores (e.g. Mattis dementia rating scale) to be similar, although found declines in verbal fluency (both semantic and phonemic), working memory, and processing speed.23,33 Verbal fluency has been most consistently decreased following STN DBS across many studies and meta-analyses.34
EMERGING CONCEPTS
Axial features of PD
Although DBS is very helpful in reducing motor fluctuations and dyskinesia, it may be less effective in treating certain axial symptoms such as freezing of gait and falls, and indeed these symptoms are often less responsive to dopaminergic therapy as well. DBS of the pendunculopontine nucleus (PPN), known from animal research to be an important center for locomotion, has been studied as one treatment approach for these resistant symptoms. Thus far, data from only small open-label trials are available,35–38 and while all of the trials reported improvement in duration of freezing and falls, blinded assessments in one trial37 did not show improvements. Limiting factors in these studies were the small sample size (4–6 patients in each study) and the challenge of targeting a location that can be difficult to visualize on MRI sequences and does not as of yet have a clearly defined microelectrode recording signature. Further research is needed to determine if the PPN will fulfill the promise of a target to improve postural instability and other axial symptoms.
Early versus late DBS
While DBS is currently recommended for PD patients with at least five years of disease duration and motor disability that does not improve with optimization of medication therapy, there has been growing interest in examining whether earlier implantation of DBS may be associated with larger and longer lasting improvements in quality of life, before social and occupational activities are threatened.39 A recent clinical trial of 251 patients with PD and “early” motor complications (after a mean disease duration of 7.5 years) randomized to STN DBS plus medical therapy or medical therapy alone, demonstrated greater quality of life, as measured by the Parkinson's Disease Questionnaire (PDQ-39), in the DBS plus medical therapy group after 2 years, compared to the medical therapy alone group.40 However the definition of “early” has been subject to debate and the long-term efficacy (and potential disease modification) of earlier implantation remains unknown.
Intermediate-frequency stimulation
High-frequency stimulation has long been held as the necessary mechanism for DBS-based improvement since it was first investigated.10 Later research confirmed that stimulation frequencies above 130 Hz were the most effective in the STN.41 Frequencies less than 20 Hz appear to worsen bradykinesia,41,42 but there is some evidence that frequencies in the 60–80 Hz range could improve freezing and gait,43 although the duration of this benefit may be transient.44
Rate versus pattern of neuronal firing
Updated models of basal ganglia physiology suggest that motor benefit from DBS result from changes in the pattern of neuronal firing, particularly related to the synchronicity of intrinsic neuronal oscillations in the basal ganglia, rather than simple changes to the firing rate.45 This synchronicity can manifest as an increase in the power of specific frequencies of local field potentials (LFPs) when recording from particular nuclei within the basal ganglia. In the STN and GPi, LFP oscillations in the beta band frequency (11–30 Hz) are associated with worsened motor function in the off medication state,46 and the power of these oscillations is attenuated by voluntary movement, and dopamine,47,48 suggesting that the beta band may be “pathological” in PD. It has been demonstrated that DBS also attenuates the beta band synchrony,49 leading to research into “closed-loop” or adaptive DBS.50 Suppression of oscillatory activity through a closed loop paradigm was more effective than standard open loop stimulation in controlling parkinsonian motor symptoms in the MPTP-treated African green monkey.51 Other advances in DBS technology such as segmented electrodes may provide the ability to “steer” the direction of stimulation and shape the electrical field to better encompass desired structures but exclude spread to fibers contributing to side effects of stimulation.52,53
SUMMARY
DBS surgery is a very effective treatment for the appropriately selected PD patient and can afford decreased motor fluctuations, reduced “off” time, and improvement in dyskinesia. Outcomes for STN and GPi targets appear to be largely similar and the choice of target is best made based on individual patient factors and surgeon preference. The inner workings of the basal ganglia, or the “dark basement” of the mind as Wilson referred to them,54 are slowly yielding their secrets, led in large part by research based on surgically implanted patients. Adaptive, “closed-loop” DBS and new technologies including segmented electrodes may help to further refine and improve this procedure.
Key points.
The surgical treatment of Parkinson’s disease (PD) has progressed from destructive and lesional procedures to focused and targeted brain stimulation.
The effects of deep brain stimulation (DBS) typically mirror the benefits of levodopa, however with the benefit of reducing motor fluctuations and off time, as well as decreasing dyskinesia.
The two main targets for DBS in PD, the subthalamic nucleus (STN) and internal segment of the globus pallidus (GPi) appear to give similar outcomes although individual patient factors and surgeon expertise may be important.
Developing concepts in DBS including closed loop paradigms and segmented electrodes promise to further refine this therapy.
Acknowledgments
Dr. Espay is supported by the K23 career development award (NIMH, 1K23MH092735); has received grant support from CleveMed/Great Lakes Neurotechnologies and Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Solvay (Abbot/Abbvie), Chelsea Therapeutics, TEVA, Impax, Merz, Solstice Neurosciences, Eli Lilly, and USWorldMeds; and honoraria from Novartis, UCB, TEVA, the American Academy of Neurology, and the Movement Disorders Society. He serves as Associate Editor of Movement Disorders and Frontiers in Movement Disorders and on the editorial board of The European Neurological Journal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Duker has nothing to disclose.
References
- 1.Leriche Ueber Chirurgischen Eingriff bei Parkinson’s scher Krankheit. Neurologische Zeitblaetter. 1912;13:1093–1096. [Google Scholar]
- 2.Klemme RM. Surgical treatment of dystonia, paralysis agitans and athetosis. Arch Neurol Pysch. 1940;44:926. [Google Scholar]
- 3.Putnam TJ. Relief from unilateral paralysis agitans by section of the pyramidal tract. Arch Neurol. 1938;40:1049–1050. [Google Scholar]
- 4.Walker AE. Cerebral pedunculotomy for the relief of involuntary movements. II. Parkinsonian tremor. J Nerv Ment Dis. 1952 Dec;116(6):766–775. doi: 10.1097/00005053-195212000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Meyers R. Surgical experiments in the therapy of certain “extrapyramidal” diseases: a current evaluation. Acta Psychiatr Neurol Suppl. 1951;67:1–42. [PubMed] [Google Scholar]
- 6.Cooper IS. Ligation of the anterior choroidal artery for involuntary movements; parkinsonism. Psychiatr Q. 1953;27(2):317–319. doi: 10.1007/BF01562492. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel EA, Wycis HT, Marks M, Lee AJ. Stereotaxic Apparatus for Operations on the Human Brain. Science. 1947 Oct 10;106(2754):349–350. doi: 10.1126/science.106.2754.349. [DOI] [PubMed] [Google Scholar]
- 8.Cotzias GC, Van Woert MH, Schiffer LM. Aromatic amino acids and modification of parkinsonism. N Engl J Med. 1967 Feb 16;276(7):374–379. doi: 10.1056/NEJM196702162760703. [DOI] [PubMed] [Google Scholar]
- 9.Hariz MI, Blomstedt P, Zrinzo L. Deep brain stimulation between 1947 and 1987: the untold story. Neurosurg Focus. 2010 Aug;29(2):E1. doi: 10.3171/2010.4.FOCUS10106. [DOI] [PubMed] [Google Scholar]
- 10.Benabid AL, Pollak P, Louveau A, Henry S, De Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987 Jan;50ca(1–6):344–346. doi: 10.1159/000100803. [DOI] [PubMed] [Google Scholar]
- 11.Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995 Jan 14;345(8942):91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 12.Siegfried J, Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery. 1994 Dec;35(6):1126–1129. doi: 10.1227/00006123-199412000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992 Jan;7(1):2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 14.Defer GL, Widner H, Marié RM, Rémy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD) Mov Disord. 1999 Jul;14(4):572–584. doi: 10.1002/1531-8257(199907)14:4<572::aid-mds1005>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Lang A, Widner H. Deep brain stimulation for Parkinson’s disease: patient selection and evaluation. Mov Disord. 2002;17(Suppl 3):s94–s101. doi: 10.1002/mds.10149. [DOI] [PubMed] [Google Scholar]
- 16.Shih LC, Tarsy D. Deep brain stimulation for the treatment of atypical parkinsonism. Mov Disord. 2007 Nov 15;22(15):2149–2155. doi: 10.1002/mds.21648. [DOI] [PubMed] [Google Scholar]
- 17.Doshi PK, Chhaya N, Bhatt MH. Depression leading to attempted suicide after bilateral subthalamic nucleus stimulation for Parkinson’s disease. Mov Disord. 2002 Sep;17(5):1084–1085. doi: 10.1002/mds.10198. [DOI] [PubMed] [Google Scholar]
- 18.Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996 Feb;84(2):203–214. doi: 10.3171/jns.1996.84.2.0203. [DOI] [PubMed] [Google Scholar]
- 19.Rehncrona S, Johnels B, Widner H, Törnqvist A-L, Hariz M, Sydow O. Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov Disord. 2003 Feb;18(2):163–170. doi: 10.1002/mds.10309. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto J-L, Pollak P, Rehncrona S, et al. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain. 2005 Oct;128(Pt 10):2240–2249. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- 21.Rouaud T, Dondaine T, Drapier S, Haegelen C, Lallement F, Péron J, et al. Pallidal stimulation in advanced Parkinson’s patients with contraindications for subthalamic stimulation. Mov Disord. 2010 Sep;25(12):1839–1846. doi: 10.1002/mds.23171. [DOI] [PubMed] [Google Scholar]
- 22.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006 Aug 31;355(9):896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 23.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009 Jan;301(1):63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurology. 2010 Jun;9(6):581–591. doi: 10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009 May;65(5):586–595. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Follett Ka, Weaver FM, Stern M, Hur K, Harris CL, Luo P, et al. Pallidal versus subthalamic deepbrain stimulation for Parkinson’s disease. N Engl J Med. 2010 Jun;362(22):2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 27.Odekerken VJ, Van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013 Nov 15;12(1):37–44. doi: 10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- 28.Guridi J, Rodriguez-Oroz MC, Alegre M, Obeso J a. Hardware complications in deep brain stimulation: electrode impedance and loss of clinical benefit. Parkinsonism Relat Disord. 2012 Jul;18(6):765–769. doi: 10.1016/j.parkreldis.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Voon V, Krack P, Lang AE, Lozano AM, Dujardin K, Schüpbach M, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson’s disease. Brain. 2008 Oct;131(Pt 10):2720–2728. doi: 10.1093/brain/awn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schüpbach WMM, Chastan N, Welter ML, Houeto JL, Mesnage V, Bonnet aM, et al. Stimulation of the subthalamic nucleus in Parkinson’s disease: a 5 year follow up. J Neurol Neurosurg Psychiatry. 2005 Dec;76(12):1640–1644. doi: 10.1136/jnnp.2005.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver FM, Follett Ka, Stern M, Luo P, Harris CL, Hur K, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty-six-month outcomes. Neurology. 2012 Jul 3;79(1):55–65. doi: 10.1212/WNL.0b013e31825dcdc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saint-Cyr JA, Trépanier LL, Kumar R, Lozano AM, Lang AE. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson’s disease. Brain. 2000 Oct;123(Pt 10):2091–2108. doi: 10.1093/brain/123.10.2091. [DOI] [PubMed] [Google Scholar]
- 33.Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol. 2008 Jul;7(7):605–614. doi: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]
- 34.Parsons TD, Rogers Sa, Braaten AJ, Woods SP, Tröster AI. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: a meta-analysis. Lancet Neurol. 2006 Jul;5(7):578–588. doi: 10.1016/S1474-4422(06)70475-6. [DOI] [PubMed] [Google Scholar]
- 35.Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007 Jun;130(Pt 6):1596–1607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- 36.Moro E, Hamani C, Poon Y-Y, Al-Khairallah T, Dostrovsky JO, Hutchison WD, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain. 2010 Jan;133(Pt 1):215–224. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- 37.Ferraye MU, Debû B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain. 2010 Jan;133(Pt 1):205–214. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- 38.Khan S, Gill SS, Mooney L, White P, Whone a, Brooks DJ, et al. Combined pedunculopontinesubthalamic stimulation in Parkinson disease. Neurology. 2012 Apr 3;78(14):1090–1095. doi: 10.1212/WNL.0b013e31824e8e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espay AJ, Vaughan JE, Marras C, Fowler R, Eckman MH. Early versus delayed bilateral subthalamic deep brain stimulation for parkinson’s disease: a decision analysis. Mov Disord. 2010 Jul;25(10):1456–1463. doi: 10.1002/mds.23111. [DOI] [PubMed] [Google Scholar]
- 40.Schuepbach WMM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, et al. Neurostimulation for Parkinson’s Disease with Early Motor Complications. N Engl J Med. 2013 Feb 14;368(7):610–622. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- 41.Moro E, Esselink RJA, Xie J, Hommel M, Benabid AL, Pollak P. The impact on Parkinson’s disease of electrical parameter settings in STN stimulation. Neurology. 2002 Sep 10;59(5):706–713. doi: 10.1212/wnl.59.5.706. [DOI] [PubMed] [Google Scholar]
- 42.Eusebio A, Chen CC, Lu CS, Lee ST, Tsai CH, Limousin P, et al. Effects of low-frequency stimulation of the subthalamic nucleus on movement in Parkinson’s disease. Exp Neurol. 2008 Jan;209(1):125–130. doi: 10.1016/j.expneurol.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreau C, Defebvre L, Destée A, Bleuse S, Clement F, Blatt JL, et al. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology. 2008 Jul 8;71(2):80–84. doi: 10.1212/01.wnl.0000303972.16279.46. [DOI] [PubMed] [Google Scholar]
- 44.Ricchi V, Zibetti M, Angrisano S, Merola A, Arduino N, Artusi CA, et al. Transient effects of 80 Hz stimulation on gait in STN DBS treated PD patients: a 15 months follow-up study. Brain Stimul. 2012 Jul;5(3):388–392. doi: 10.1016/j.brs.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Brown P, Marsden CD. What do the basal ganglia do? Lancet. 1998 Jun;351(9118):1801–1804. doi: 10.1016/s0140-6736(97)11225-9. [DOI] [PubMed] [Google Scholar]
- 46.Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord. 2003 Apr;18(4):357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- 47.Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson’s disease. Brain. 2002 Jun;125(Pt 6):1196–1209. doi: 10.1093/brain/awf128. [DOI] [PubMed] [Google Scholar]
- 48.Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, et al. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002 Jun;125(Pt 6):1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- 49.Bronte-Stewart H, Barberini C, Koop MM, Hill BC, Henderson JM, Wingeier B. The STN beta-band profile in Parkinson’s disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp Neurol. 2009 Jan;215(1):20–28. doi: 10.1016/j.expneurol.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Marceglia S, Rossi L, Foffani G, Bianchi A, Cerutti S, Priori A. Basal ganglia local field potentials: applications in the development of new deep brain stimulation devices for movement disorders. Expert Rev Med Devices. 2007;4(5):605–614. doi: 10.1586/17434440.4.5.605. [DOI] [PubMed] [Google Scholar]
- 51.Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011 Oct 20;72(2):370–384. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 52.Martens HCF, Toader E, Decré MMJ, Anderson DJ, Vetter R, Kipke DR, et al. Spatial steering of deep brain stimulation volumes using a novel lead design. Clin Neurophysiol. 2011 Mar;122(3):558–566. doi: 10.1016/j.clinph.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 53.Chaturvedi A, Foutz TJ, McIntyre CC. Current steering to activate targeted neural pathways during deep brain stimulation of the subthalamic region. Brain Stimul. 2012 Jul;5(3):369–377. doi: 10.1016/j.brs.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson S. Modern Problems in Neurology. London: Arnold & Company; 1928. [Google Scholar]