Abstract
The enigmatic coexistence of O2-sensitive nitrogenase and O2-evolving photosynthesis in diazotrophic cyanobacteria has fascinated researchers for over two decades. Research efforts in the past 10 years have revealed a range of O2 sensitivity of nitrogenase in different strains of cyanobacteria and a variety of adaptations for the protection of nitrogenase from damage by both atmospheric and photosynthetic sources of O2. The most complex and apparently most efficient mechanisms for the protection of nitrogenase are incorporated in the heterocysts, the N2-fixing cells of cyanobacteria. Genetic studies indicate that the controls of heterocyst development and nitrogenase synthesis are closely interrelated and that the expression of N2 fixation (nif) genes is regulated by pO2.
Full text
PDF




























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich A., Shilo M. Photooxidative death in blue-green algae. J Bacteriol. 1972 Sep;111(3):682–689. doi: 10.1128/jb.111.3.682-689.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. G., Carr N. G. The developmental biology of heterocyst and akinete formation in cyanobacteria. Crit Rev Microbiol. 1981;9(1):45–100. doi: 10.3109/10408418109104486. [DOI] [PubMed] [Google Scholar]
- Almon H., Böhme H. Components and activity of the photosynthetic electron transport system of intact heterocysts isolated from the blue-green alga Nostoc muscorum. Biochim Biophys Acta. 1980 Aug 5;592(1):113–120. doi: 10.1016/0005-2728(80)90118-8. [DOI] [PubMed] [Google Scholar]
- BULEN W. A., BURNS R. C., LECOMTE J. R. NITROGEN FIXATION: HYDROSULFITE AS ELECTRON DONOR WITH CELL-FREE PREPARATIONS OF AZOTOBACTER VINELANDII AND RHODOSPIRILLUM RUBRUM. Proc Natl Acad Sci U S A. 1965 Mar;53:532–539. doi: 10.1073/pnas.53.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebout B. M., Paerl H. W., Crocker K. M., Prufert L. E. Diel interactions of oxygenic photosynthesis and n(2) fixation (acetylene reduction) in a marine microbial mat community. Appl Environ Microbiol. 1987 Oct;53(10):2353–2362. doi: 10.1128/aem.53.10.2353-2362.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benemann J. R., Weare N. M. Hydrogen Evolution by Nitrogen-Fixing Anabaena cylindrica Cultures. Science. 1974 Apr 12;184(4133):174–175. doi: 10.1126/science.184.4133.174. [DOI] [PubMed] [Google Scholar]
- Biggins D. R., Postgate J. R. Nitrogen fixation by cultures and cell-free extracts of Mycobacterium flavum 301. J Gen Microbiol. 1969 May;56(2):181–193. doi: 10.1099/00221287-56-2-181. [DOI] [PubMed] [Google Scholar]
- Bone D. H. Kinetics of synthesis of nitrogenase in batch and continuous culture of Anabaena flos-aquae. Arch Mikrobiol. 1971;80(3):242–251. doi: 10.1007/BF00410125. [DOI] [PubMed] [Google Scholar]
- Bothe H., Tennigkeit J., Eisbrenner G. The utilization of molecular hydrogen by the blue-green alga Anabaena cylindrica. Arch Microbiol. 1977 Jul 26;114(1):43–49. doi: 10.1007/BF00429628. [DOI] [PubMed] [Google Scholar]
- Broda E., Peschek G. A. Nitrogen fixation as evidence for the reducing nature of the early biosphere. Biosystems. 1983;16(1):1–8. doi: 10.1016/0303-2647(83)90021-7. [DOI] [PubMed] [Google Scholar]
- Brusca J. S., Hale M. A., Carrasco C. D., Golden J. W. Excision of an 11-kilobase-pair DNA element from within the nifD gene in anabaena variabilis heterocysts. J Bacteriol. 1989 Aug;171(8):4138–4145. doi: 10.1128/jb.171.8.4138-4145.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buikema W. J., Haselkorn R. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 1991 Feb;5(2):321–330. doi: 10.1101/gad.5.2.321. [DOI] [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARNAHAN J. E., MORTENSON L. E., MOWER H. F., CASTLE J. E. Nitrogen fixation in cell-free extracts of Clostridium pasteurianum. Biochim Biophys Acta. 1960 Nov 18;44:520–535. doi: 10.1016/0006-3002(60)91606-1. [DOI] [PubMed] [Google Scholar]
- Capone D. G., O'neil J. M., Zehr J., Carpenter E. J. Basis for Diel Variation in Nitrogenase Activity in the Marine Planktonic Cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1990 Nov;56(11):3532–3536. doi: 10.1128/aem.56.11.3532-3536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardemil L., Wolk C. P. The polysaccharides from heterocyst and spore envelopes of a blue-green alga. Structure of the basic repeating unit. J Biol Chem. 1979 Feb 10;254(3):736–741. [PubMed] [Google Scholar]
- Carpenter E. J., Price C. C. Marine oscillatoria (Trichodesmium): explanation for aerobic nitrogen fixation without heterocysts. Science. 1976 Mar 26;191(4233):1278–1280. doi: 10.1126/science.1257749. [DOI] [PubMed] [Google Scholar]
- Codd G. A. Carboxysomes and ribulose bisphosphate carboxylase/oxygenase. Adv Microb Physiol. 1988;29:115–164. doi: 10.1016/s0065-2911(08)60347-1. [DOI] [PubMed] [Google Scholar]
- Cohen Y., Padan E., Shilo M. Facultative anoxygenic photosynthesis in the cyanobacterium Oscillatoria limnetica. J Bacteriol. 1975 Sep;123(3):855–861. doi: 10.1128/jb.123.3.855-861.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. M., Fay P. Special aspects of nitrogen fixation by blue-green algae. Proc R Soc Lond B Biol Sci. 1969 Apr 1;172(1029):357–366. doi: 10.1098/rspb.1969.0026. [DOI] [PubMed] [Google Scholar]
- Cox R. M. Physiological studies on nitrogen fixation in the blue-green alga Anabaena cylindrica. Arch Mikrobiol. 1966 Mar 31;53(3):263–276. doi: 10.1007/BF00446673. [DOI] [PubMed] [Google Scholar]
- Daday A., Platz R. A., Smith G. D. Anaerobic and aerobic hydrogen gas formation by the blue-green alga Anabaena cylindrica. Appl Environ Microbiol. 1977 Nov;34(5):478–483. doi: 10.1128/aem.34.5.478-483.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton H., Postgate J. R. Effect of oxygen on growth of Azotobacter chroococcum in batch and continuous cultures. J Gen Microbiol. 1968 Dec;54(3):463–473. doi: 10.1099/00221287-54-3-463. [DOI] [PubMed] [Google Scholar]
- Ditta G., Virts E., Palomares A., Kim C. H. The nifA gene of Rhizobium meliloti is oxygen regulated. J Bacteriol. 1987 Jul;169(7):3217–3223. doi: 10.1128/jb.169.7.3217-3223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Donze M., Haveman J., Schiereck P. Absence of photosystem 2 in heterocysts of the blue-green alga Anabaena. Biochim Biophys Acta. 1972 Jan 21;256(1):157–161. doi: 10.1016/0005-2728(72)90170-3. [DOI] [PubMed] [Google Scholar]
- Dunn J. H., Wolk C. P. Composition of the cellular envelopes of Anabaena cylindrica. J Bacteriol. 1970 Jul;103(1):153–158. doi: 10.1128/jb.103.1.153-158.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Issack R., Kennedy C., Postgate J. R., Ratcliffe H. D. Nitrogenase synthesis in Klebsiella pneumoniae: comparison of ammonium and oxygen regulation. J Gen Microbiol. 1978 Feb;104(2):277–285. doi: 10.1099/00221287-104-2-277. [DOI] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 1990 Oct;9(10):3379–3388. doi: 10.1002/j.1460-2075.1990.tb07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich D. W., Burris R. H. Complementary functioning of the component proteins of nitrogenase from several bacteria. J Bacteriol. 1978 Jun;134(3):936–943. doi: 10.1128/jb.134.3.936-943.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A., Reich S., Böger P. Modification of dinitrogenase reductase in the cyanobacterium Anabaena variabilis due to C starvation and ammonia. J Bacteriol. 1990 Feb;172(2):748–755. doi: 10.1128/jb.172.2.748-755.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAY P. HETEROTROPHY AND NITROGEN FIXATION IN CHLOROGLOEA FRITSCHII. J Gen Microbiol. 1965 Apr;39:11–20. doi: 10.1099/00221287-39-1-11. [DOI] [PubMed] [Google Scholar]
- Fay P. Cell differentiation and pigment composition in Anabaena cylindrica. Arch Mikrobiol. 1969;67(1):62–70. doi: 10.1007/BF00413682. [DOI] [PubMed] [Google Scholar]
- Fay P., Cox R. M. Oxygen inhibition of nitrogen fixation in cell-free preparations of blue-green algae. Biochim Biophys Acta. 1967;143(3):562–569. doi: 10.1016/0005-2728(67)90061-8. [DOI] [PubMed] [Google Scholar]
- Fay P. Factors influencing dark nitrogen fixation in a blue-green alga. Appl Environ Microbiol. 1976 Mar;31(3):376–379. doi: 10.1128/aem.31.3.376-379.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P., Kulasooriya S. A. Tetrazolium reduction and nitrogenase activity in heterocystous blue-green algae. Arch Mikrobiol. 1972;87(4):341–352. doi: 10.1007/BF00409133. [DOI] [PubMed] [Google Scholar]
- Fay P. Photostimulation of nitrogen fixation in Anabaena cylindrica. Biochim Biophys Acta. 1970 Sep 1;216(2):353–356. doi: 10.1016/0005-2728(70)90226-4. [DOI] [PubMed] [Google Scholar]
- Fay P., Stewart W. D., Walsby A. E., Fogg G. E. Is the heterocyst the site of nitrogen fixation in blue-green algae? Nature. 1968 Nov 23;220(5169):810–812. doi: 10.1038/220810b0. [DOI] [PubMed] [Google Scholar]
- Fay P., Walsby A. E. Metabolic activities of isolated heterocysts of the blue-green alga Anabaena cylindrica. Nature. 1966 Jan 1;209(5018):94–95. doi: 10.1038/209094a0. [DOI] [PubMed] [Google Scholar]
- Fleming H., Haselkorn R. Differentiation in Nostoc muscorum: nitrogenase is synthesized in heterocysts. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2727–2731. doi: 10.1073/pnas.70.10.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming H., Haselkorn R. The program of protein synthesis during heterocyst differentiation in nitrogen-fixing blue-green algae. Cell. 1974 Oct;3(2):169–170. doi: 10.1016/0092-8674(74)90121-4. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986 May 15;247(1):1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Gallon J. R., LaRue T. A., Kurz W. G. Photosynthesis and nitrogenase activity in the blue-green alga Gloeocapsa. Can J Microbiol. 1974 Dec;20(12):1633–1637. doi: 10.1139/m74-254. [DOI] [PubMed] [Google Scholar]
- Garcia-González M., Mateo P., Bonilla I. Boron Protection for O(2) Diffusion in Heterocysts of Anabaena sp. PCC 7119. Plant Physiol. 1988 Jul;87(3):785–789. doi: 10.1104/pp.87.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick S., Oren A., Padan E. Occurrence of facultative anoxygenic photosynthesis among filamentous and unicellular cyanobacteria. J Bacteriol. 1977 Feb;129(2):623–629. doi: 10.1128/jb.129.2.623-629.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings T. H., Jr, Staehelin L. A. Changes in thylakoid structure associated with the differentiation of heterocysts in the cyanobacterium, Anabaena cylindrica. Biochim Biophys Acta. 1979 Jun 5;546(3):373–382. doi: 10.1016/0005-2728(79)90074-4. [DOI] [PubMed] [Google Scholar]
- Giovannoni S. J., Turner S., Olsen G. J., Barns S., Lane D. J., Pace N. R. Evolutionary relationships among cyanobacteria and green chloroplasts. J Bacteriol. 1988 Aug;170(8):3584–3592. doi: 10.1128/jb.170.8.3584-3592.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I., Nadler V., Hochman A. Mechanism of nitrogenase switch-off by oxygen. J Bacteriol. 1987 Feb;169(2):874–879. doi: 10.1128/jb.169.2.874-879.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Carrasco C. D., Mulligan M. E., Schneider G. J., Haselkorn R. Deletion of a 55-kilobase-pair DNA element from the chromosome during heterocyst differentiation of Anabaena sp. strain PCC 7120. J Bacteriol. 1988 Nov;170(11):5034–5041. doi: 10.1128/jb.170.11.5034-5041.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Wiest D. R. Genome rearrangement and nitrogen fixation in Anabaena blocked by inactivation of xisA gene. Science. 1988 Dec 9;242(4884):1421–1423. doi: 10.1126/science.3144039. [DOI] [PubMed] [Google Scholar]
- Gotto J. W., Yoch D. C. Regulation of Rhodospirillum rubrum nitrogenase activity. Properties and interconversion of active and inactive Fe protein. J Biol Chem. 1982 Mar 25;257(6):2868–2873. [PubMed] [Google Scholar]
- Grillo J. F., Bottomley P. J., Van Baalen C., Tabita F. R. A mutant of Anabaena sp. CA with oxygen-sensitive nitrogenase activity. Biochem Biophys Res Commun. 1979 Jul 27;89(2):685–693. doi: 10.1016/0006-291x(79)90684-3. [DOI] [PubMed] [Google Scholar]
- Gussin G. N., Ronson C. W., Ausubel F. M. Regulation of nitrogen fixation genes. Annu Rev Genet. 1986;20:567–591. doi: 10.1146/annurev.ge.20.120186.003031. [DOI] [PubMed] [Google Scholar]
- HINO S., WILSON P. W. Nitrogen fixation by a facultative bacillus. J Bacteriol. 1958 Apr;75(4):403–408. doi: 10.1128/jb.75.4.403-408.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker H., Veeger C. Involvement of the cytoplasmic membrane in nitrogen fixation by Azotobacter vinelandii. Eur J Biochem. 1977 Jul 1;77(1):1–10. doi: 10.1111/j.1432-1033.1977.tb11634.x. [DOI] [PubMed] [Google Scholar]
- Haaker H., Veeger C. Regulation of respiration and nitrogen fixation in different types of Azotobacter vinelandii. Eur J Biochem. 1976 Apr 1;63(2):499–507. doi: 10.1111/j.1432-1033.1976.tb10253.x. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. C., Kostel P. J., Benemann Purification and properties of nitrogenase from the cyanobacterium, Anabaena cylindrica. Eur J Biochem. 1979 Jul;98(1):275–284. doi: 10.1111/j.1432-1033.1979.tb13186.x. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. C. Molecular aspects of nitrogen fixation by photosynthetic prokaryotes. Crit Rev Microbiol. 1987;14(1):1–48. doi: 10.3109/10408418709104434. [DOI] [PubMed] [Google Scholar]
- Hartmann A., Burris R. H. Regulation of nitrogenase activity by oxygen in Azospirillum brasilense and Azospirillum lipoferum. J Bacteriol. 1987 Mar;169(3):944–948. doi: 10.1128/jb.169.3.944-948.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselkorn R. Organization of the genes for nitrogen fixation in photosynthetic bacteria and cyanobacteria. Annu Rev Microbiol. 1986;40:525–547. doi: 10.1146/annurev.mi.40.100186.002521. [DOI] [PubMed] [Google Scholar]
- Haury J. F., Wolk C. P. Classes of Anabaena variabilis mutants with oxygen-sensitive nitrogenase activity. J Bacteriol. 1978 Nov;136(2):688–692. doi: 10.1128/jb.136.2.688-692.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead A., Robinson R., Stewart W. D. Nitrogenase activity in extracts of heterocystous and non-heterocystous blue-green algae. Arch Mikrobiol. 1970;74(3):235–243. doi: 10.1007/BF00408884. [DOI] [PubMed] [Google Scholar]
- Helber J. T., Johnson T. R., Yarbrough L. R., Hirschberg R. Regulation of nitrogenase gene expression in anaerobic cultures of Anabaena variabilis. J Bacteriol. 1988 Feb;170(2):552–557. doi: 10.1128/jb.170.2.552-557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry L. E., Gogotov I. N., Hall D. O. Superoxide dismutase and catalase in the protection of the proton-donating systems of nitrogen fixation in the blue-green alga Anabaena cylindrica. Biochem J. 1978 Aug 15;174(2):373–377. doi: 10.1042/bj1740373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman A., Burris R. H. Effect of oxygen on acetylene reduction by photosynthetic bacteria. J Bacteriol. 1981 Aug;147(2):492–499. doi: 10.1128/jb.147.2.492-499.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchins J. P., Burris R. H. Light and dark reactions of the uptake hydrogenase in anabaena 7120. Plant Physiol. 1981 Sep;68(3):712–716. doi: 10.1104/pp.68.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchins J. P., Burris R. H. Occurrence and localization of two distinct hydrogenases in the heterocystous cyanobacterium Anabaena sp. strain 7120. J Bacteriol. 1981 Apr;146(1):209–214. doi: 10.1128/jb.146.1.209-214.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchins J. P., Burris R. H. Physiological reactions of the reversible hydrogenase from anabaena 7120. Plant Physiol. 1981 Sep;68(3):717–721. doi: 10.1104/pp.68.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchins J. P., Hind G. Flash spectroscopic characterization of photosynthetic electron transport in isolated heterocysts. Arch Biochem Biophys. 1983 Jul 1;224(1):272–282. doi: 10.1016/0003-9861(83)90210-2. [DOI] [PubMed] [Google Scholar]
- Isogai Y., Iida A., Mochizuki K., Okabe H., Yokose T. [Therapeutic and pathophysiologic consideration on diabetic microangiopathy from the view point of the blood rheology (author's transl)]. Horumon To Rinsho. 1980 Feb;28(2):149–154. [PubMed] [Google Scholar]
- Jones C. W., Brice J. M., Wright V., Ackrell B. A. Respiratory protection of nitrogenase in Azotobacter vinelandii. FEBS Lett. 1973 Jan 15;29(2):77–81. doi: 10.1016/0014-5793(73)80530-7. [DOI] [PubMed] [Google Scholar]
- Jones L. W., Bishop N. I. Simultaneous measurement of oxygen and hydrogen exchange from the blue-green alga anabaena. Plant Physiol. 1976 Apr;57(4):659–665. doi: 10.1104/pp.57.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallas T., Rebière M. C., Rippka R., Tandeau de Marsac N. The structural nif genes of the cyanobacteria Gloeothece sp. and Calothrix sp. share homology with those of Anabaena sp., but the Gloeothece genes have a different arrangement. J Bacteriol. 1983 Jul;155(1):427–431. doi: 10.1128/jb.155.1.427-431.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellar P. E., Paerl H. W. Physiological adaptations in response to environmental stress during an n(2)-fixing anabaena bloom. Appl Environ Microbiol. 1980 Sep;40(3):587–595. doi: 10.1128/aem.40.3.587-595.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucas R. Nitrogen fixation by Klebsiella grown in the presence of oxygen. Can J Microbiol. 1972 Dec;18(12):1845–1850. doi: 10.1139/m72-288. [DOI] [PubMed] [Google Scholar]
- Kranz R. G., Foster-Hartnett D. Transcriptional regulatory cascade of nitrogen-fixation genes in anoxygenic photosynthetic bacteria: oxygen- and nitrogen-responsive factors. Mol Microbiol. 1990 Nov;4(11):1793–1800. doi: 10.1111/j.1365-2958.1990.tb02027.x. [DOI] [PubMed] [Google Scholar]
- Kranz R. G., Haselkorn R. Anaerobic regulation of nitrogen-fixation genes in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6805–6809. doi: 10.1073/pnas.83.18.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz R. G., Haselkorn R. Characterization of nif regulatory genes in Rhodopseudomonas capsulata using lac gene fusions. Gene. 1985;40(2-3):203–215. doi: 10.1016/0378-1119(85)90043-5. [DOI] [PubMed] [Google Scholar]
- Krieg N. R., Hoffman P. S. Microaerophily and oxygen toxicity. Annu Rev Microbiol. 1986;40:107–130. doi: 10.1146/annurev.mi.40.100186.000543. [DOI] [PubMed] [Google Scholar]
- Kuhla J., Oelze J. Dependence of nitrogenase switch-off upon oxygen stress on the nitrogenase activity in Azotobacter vinelandii. J Bacteriol. 1988 Nov;170(11):5325–5329. doi: 10.1128/jb.170.11.5325-5329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasooriya S. A., Lang N. J., Fay P. The heterocysts of blue-green algae. 3. Differentiation and nitrogenase activity. Proc R Soc Lond B Biol Sci. 1972 Jun 6;181(1063):199–209. doi: 10.1098/rspb.1972.0046. [DOI] [PubMed] [Google Scholar]
- Kumar A., Tabita F. R., Van Baalen C. High endogenous nitrogenase activity in isolated heterocysts of Anabaena sp. strain CA after nitrogen starvation. J Bacteriol. 1983 Aug;155(2):493–497. doi: 10.1128/jb.155.2.493-497.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambein F., Wolk C. P. Structural studies on the glycolipids from the envelope of the heterocyst of Anabaena cylindrica. Biochemistry. 1973 Feb 27;12(5):791–798. doi: 10.1021/bi00729a002. [DOI] [PubMed] [Google Scholar]
- Layzell D. B., Hunt S., Palmer G. R. Mechanism of Nitrogenase Inhibition in Soybean Nodules : Pulse-Modulated Spectroscopy Indicates that Nitrogenase Activity Is Limited by O(2). Plant Physiol. 1990 Apr;92(4):1101–1107. doi: 10.1104/pp.92.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex M., Carr N. G. The metabolism of glucose by heterocysts and vegetative cells of Anabaena cylindrica. Arch Microbiol. 1974;101(2):161–167. doi: 10.1007/BF00455936. [DOI] [PubMed] [Google Scholar]
- Lex M., Silvester W. B., Stewart W. D. Photorespiration and nitrogenase activity in the blue-green alga, Anabaena cylindrica. Proc R Soc Lond B Biol Sci. 1972 Jan 18;180(1058):87–102. doi: 10.1098/rspb.1972.0007. [DOI] [PubMed] [Google Scholar]
- Lex M., Stewart W. D. Algal nitrogenase, reductant pools and photosystem I activity. Biochim Biophys Acta. 1973 Feb 22;292(2):436–443. doi: 10.1016/0005-2728(73)90049-2. [DOI] [PubMed] [Google Scholar]
- Lockau W., Peterson R. B., Wolk C. P., Burris R. H. Modes of reduction of nitrogen in heterocysts isolated from Anabaena species. Biochim Biophys Acta. 1978 May 10;502(2):298–308. doi: 10.1016/0005-2728(78)90051-8. [DOI] [PubMed] [Google Scholar]
- Ludatscher R. M., Friedman-Birnbaum R., Bergman R., Lichtig C. Structural alterations of collagen fibrils in the reactive type of elastosis perforans serpiginosa. Arch Dermatol Res. 1988;280(5):319–322. doi: 10.1007/BF00440606. [DOI] [PubMed] [Google Scholar]
- Meyer J., Kelley B. C., Vignais P. M. Aerobic nitrogen fixation by Rhodopseudomonas capsulata. FEBS Lett. 1978 Jan 15;85(2):224–228. doi: 10.1016/0014-5793(78)80460-8. [DOI] [PubMed] [Google Scholar]
- Mortenson L. E., Mower H. F., Carnahan J. E. III. NITROGEN FIXATION BY ENZYME PREPARATIONS. Bacteriol Rev. 1962 Mar;26(1):42–50. doi: 10.1128/br.26.1.42-50.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E. The role of dihydrogen and hydrogenase in nitrogen fixation. Biochimie. 1978;60(3):219–223. doi: 10.1016/s0300-9084(78)80817-7. [DOI] [PubMed] [Google Scholar]
- Mortenson L. E., Thorneley R. N. Structure and function of nitrogenase. Annu Rev Biochem. 1979;48:387–418. doi: 10.1146/annurev.bi.48.070179.002131. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Haselkorn R. Nitrogen fixation (nif) genes of the cyanobacterium Anabaena species strain PCC 7120. The nifB-fdxN-nifS-nifU operon. J Biol Chem. 1989 Nov 15;264(32):19200–19207. [PubMed] [Google Scholar]
- Murry M. A., Horne A. J., Benemann J. R. Physiological Studies of Oxygen Protection Mechanisms in the Heterocysts of Anabaena cylindrica. Appl Environ Microbiol. 1984 Mar;47(3):449–454. doi: 10.1128/aem.47.3.449-454.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A., Rippka R., Kunisawa R. Heterocyst formation and nitrogenase synthesis in Anabaena sp. A kinetic study. Arch Mikrobiol. 1971;76(2):139–150. doi: 10.1007/BF00411788. [DOI] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Balkwill D. L., Stevens S. E., Jr Heterocyst differentiation in the cyanobacterium Mastigocladus laminosus. J Bacteriol. 1984 Feb;157(2):514–525. doi: 10.1128/jb.157.2.514-525.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A., Padan E. Induction of anaerobic, photoautotrophic growth in the cyanobacterium Oscillatoria limnetica. J Bacteriol. 1978 Feb;133(2):558–563. doi: 10.1128/jb.133.2.558-563.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER C. A., SCUTT P. B. The effect of oxygen on nitrogen fixation by Azotobacter. Biochim Biophys Acta. 1960 Feb 26;38:230–238. doi: 10.1016/0006-3002(60)91236-1. [DOI] [PubMed] [Google Scholar]
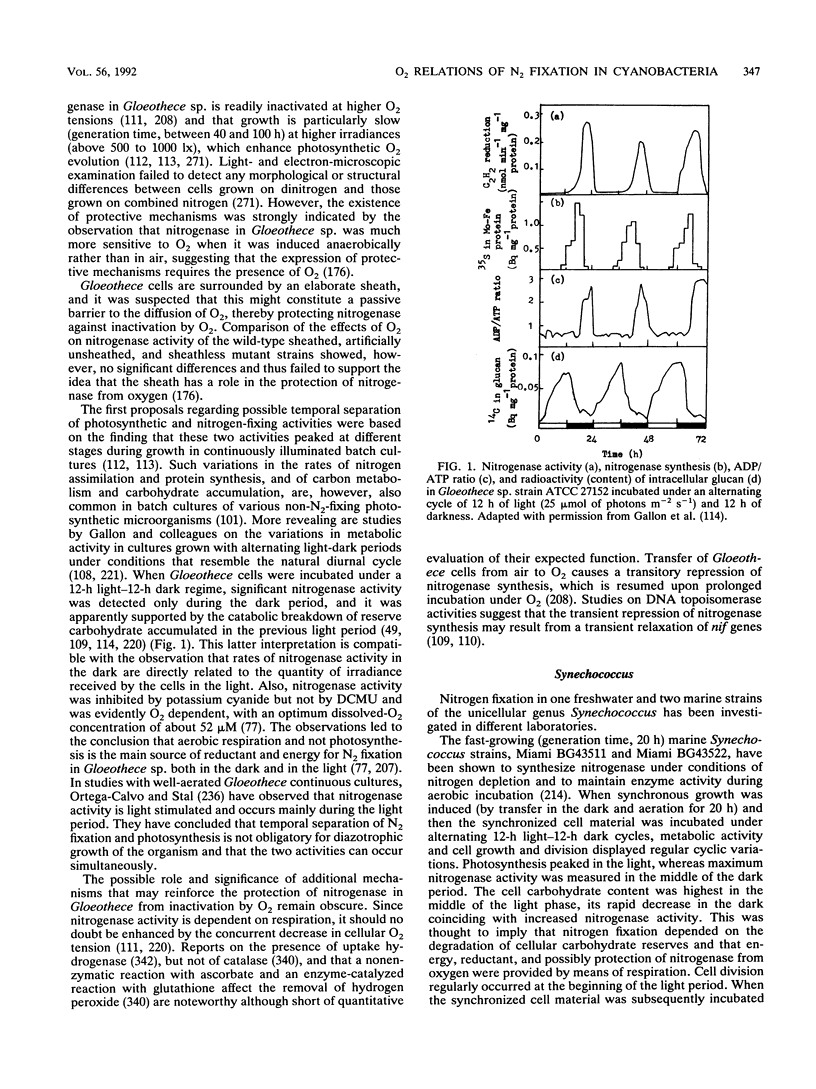
- PENGRA R. M., WILSON P. W. Physiology of nitrogen fixation by Aerobacter aerogenes. J Bacteriol. 1958 Jan;75(1):21–25. doi: 10.1128/jb.75.1.21-25.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H. W., Bebout B. M. Direct measurement of o2-depleted microzones in marine oscillatoria: relation to n2 fixation. Science. 1988 Jul 22;241(4864):442–445. doi: 10.1126/science.241.4864.442. [DOI] [PubMed] [Google Scholar]
- Paerl H. W., Bland P. T. Localized Tetrazolium Reduction in Relation to N(2) Fixation, CO(2) Fixation, and H(2) Uptake in Aquatic Filamentous Cyanobacteria. Appl Environ Microbiol. 1982 Jan;43(1):218–226. doi: 10.1128/aem.43.1.218-226.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H. W., Kellar P. E. Nitrogen-fixing anabaena: physiological adaptations instrumental in maintaining surface blooms. Science. 1979 May 11;204(4393):620–622. doi: 10.1126/science.204.4393.620. [DOI] [PubMed] [Google Scholar]
- Paerl H. W., Priscu J. C., Brawner D. L. Immunochemical localization of nitrogenase in marine trichodesmium aggregates: relationship to n(2) fixation potential. Appl Environ Microbiol. 1989 Nov;55(11):2965–2975. doi: 10.1128/aem.55.11.2965-2975.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelroy R. A., Rippka R., Stanier R. Y. Metabolism of glucose by unicellular blue-green algae. Arch Mikrobiol. 1972;87(4):303–322. doi: 10.1007/BF00409131. [DOI] [PubMed] [Google Scholar]
- Peterson R. B., Burris R. H. Properties of heterocysts isolated with colloidal silica. Arch Microbiol. 1976 May 3;108(1):35–40. doi: 10.1007/BF00425090. [DOI] [PubMed] [Google Scholar]
- Peterson R. B., Dolan E., Calvert H. E., Ke B. Energy transfer from phycobiliproteins to photosystem I in vegetative cells and heterocysts of Anabaena variabilis. Biochim Biophys Acta. 1981 Feb 12;634(2):237–248. doi: 10.1016/0005-2728(81)90142-0. [DOI] [PubMed] [Google Scholar]
- Peterson R. B., Friberg E. E., Burris R. H. Diurnal variation in n(2) fixation and photosynthesis by aquatic blue-green algae. Plant Physiol. 1977 Jan;59(1):74–80. doi: 10.1104/pp.59.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B., Wolk C. P. Localization of an uptake hydrogenase in anabaena. Plant Physiol. 1978 Apr;61(4):688–691. doi: 10.1104/pp.61.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkos P. T., Bodmer S., Tabita F. R. Oxygen inactivation and recovery of nitrogenase activity in cyanobacteria. J Bacteriol. 1983 Jan;153(1):182–190. doi: 10.1128/jb.153.1.182-190.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post E., Kleiner D., Oelze J. Whole cell respiration and nitrogenase activities in Azotobacter vinelandii growing in oxygen controlled continuous culture. Arch Microbiol. 1983 Jan;134(1):68–72. doi: 10.1007/BF00429410. [DOI] [PubMed] [Google Scholar]
- Price G. D., Badger M. R. Expression of Human Carbonic Anhydrase in the Cyanobacterium Synechococcus PCC7942 Creates a High CO(2)-Requiring Phenotype : Evidence for a Central Role for Carboxysomes in the CO(2) Concentrating Mechanism. Plant Physiol. 1989 Oct;91(2):505–513. doi: 10.1104/pp.91.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalle L. S., Burris R. H. D-erythrose supports nitrogenase activity in isolated Anabaena sp. strain 7120 heterocysts. J Bacteriol. 1984 Feb;157(2):350–356. doi: 10.1128/jb.157.2.350-356.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Rippka R., Neilson A., Kunisawa R., Cohen-Bazire G. Nitrogen fixation by unicellular blue-green algae. Arch Mikrobiol. 1971;76(4):341–348. doi: 10.1007/BF00408530. [DOI] [PubMed] [Google Scholar]
- Roberts G. P., Brill W. J. Genetics and regulation of nitrogen fixation. Annu Rev Microbiol. 1981;35:207–235. doi: 10.1146/annurev.mi.35.100181.001231. [DOI] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Rodríguez H., Rivas J., Guerrero M. G., Losada M. Ca requirement for aerobic nitrogen fixation by heterocystous blue-green algae. Plant Physiol. 1990 Apr;92(4):886–890. doi: 10.1104/pp.92.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saville B., Straus N., Coleman J. R. Contiguous organization of nitrogenase genes in a heterocystous cyanobacterium. Plant Physiol. 1987 Sep;85(1):26–29. doi: 10.1104/pp.85.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherings G., Haaker H., Wassink H., Veeger C. On the formation of an oxygen-tolerant three-component nitrogenase complex from Azotobacter vinelandii. Eur J Biochem. 1983 Oct 3;135(3):591–599. doi: 10.1111/j.1432-1033.1983.tb07693.x. [DOI] [PubMed] [Google Scholar]
- Schopf J. W., Packer B. M. Early Archean (3.3-billion to 3.5-billion-year-old) microfossils from Warrawoona Group, Australia. Science. 1987 Jul 3;237:70–73. doi: 10.1126/science.11539686. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver W. S., Postgate J. R. Evolution of asymbiotic nitrogen fixation. J Theor Biol. 1973 Jul;40(1):1–10. doi: 10.1016/0022-5193(73)90160-4. [DOI] [PubMed] [Google Scholar]
- Simpson F. B., Burris R. H. A nitrogen pressure of 50 atmospheres does not prevent evolution of hydrogen by nitrogenase. Science. 1984 Jun 8;224(4653):1095–1097. doi: 10.1126/science.6585956. [DOI] [PubMed] [Google Scholar]
- Smith B. E., Lang G. Mössbauer spectroscopy of the nitrogenase proteins from Klebsiella pneumoniae. Structural assignments and mechanistic conclusions. Biochem J. 1974 Feb;137(2):169–180. doi: 10.1042/bj1370169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Van Baalen C., Tabita F. R. Alteration of the Fe protein of nitrogenase by oxygen in the cyanobacterium Anabaena sp. strain CA. J Bacteriol. 1987 Jun;169(6):2537–2542. doi: 10.1128/jb.169.6.2537-2542.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Van Baalen C., Tabita F. R. Control of nitrogenase recovery from oxygen inactivation by ammonia in the cyanobacterium Anabaena sp. strain CA (ATCC 33047). J Bacteriol. 1990 May;172(5):2788–2790. doi: 10.1128/jb.172.5.2788-2790.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. V., Evans M. C. Soluble nitrogenase from vegetative cells of the blue-green alga Anabaena cylindrica. Nature. 1970 Mar 28;225(5239):1253–1254. doi: 10.1038/2251253a0. [DOI] [PubMed] [Google Scholar]
- Spiller H., Shanmugam K. T. Physiological conditions for nitrogen fixation in a unicellular marine cyanobacterium, Synechococcus sp. strain SF1. J Bacteriol. 1987 Dec;169(12):5379–5384. doi: 10.1128/jb.169.12.5379-5384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John R. T., Shah V. K., Brill W. J. Regulation of nitrogenase synthesis by oxygen in Klebsiella pneumoniae. J Bacteriol. 1974 Jul;119(1):266–269. doi: 10.1128/jb.119.1.266-269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. D., Haystead A., Pearson H. W. Nitrogenase activity in heterocysts of blue-green algae. Nature. 1969 Oct 18;224(5216):226–228. doi: 10.1038/224226a0. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Lex M. Nitrogenase activity in the blue-green alga Plectonema boryanum strain 594. Arch Mikrobiol. 1970;73(3):250–260. doi: 10.1007/BF00410626. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Rowell P., Rai A. N. Cyanobacteria-eukaryotic plant symbioses. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):205–228. doi: 10.1016/s0769-2609(83)80106-9. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Rowell P., Tel-or E. Nitrogen fixation and the heterocyst in blue-green algae. Biochem Soc Trans. 1975;3(3):357–361. doi: 10.1042/bst0030357. [DOI] [PubMed] [Google Scholar]
- Storch T. A., Saunders G. W., Ostrofsky M. L. Diel nitrogen fixation by cyanobacterial surface blooms in sanctuary lake, pennsylvania. Appl Environ Microbiol. 1990 Feb;56(2):466–471. doi: 10.1128/aem.56.2.466-471.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tel-Or E., Huflejt M. E., Packer L. Hydroperoxide metabolism in cyanobacteria. Arch Biochem Biophys. 1986 Apr;246(1):396–402. doi: 10.1016/0003-9861(86)90485-6. [DOI] [PubMed] [Google Scholar]
- Tel-Or E., Luijk L. W., Packer L. An inducible hydrogenase in cyanobacteria enhances n2 fixation. FEBS Lett. 1977;78(1):49–52. doi: 10.1016/0014-5793(77)80270-6. [DOI] [PubMed] [Google Scholar]
- Tel-Or E., Luijk L. W., Packer L. Hydrogenase in N2-fixing cyanobacteria. Arch Biochem Biophys. 1978 Jan 15;185(1):185–194. doi: 10.1016/0003-9861(78)90158-3. [DOI] [PubMed] [Google Scholar]
- Tel-Or E., Stewart W. D. Photosynthetic electron transport, ATP synthesis and nitrogenase activity in isolated heterocysts of Anabaena cylindrica. Biochim Biophys Acta. 1976 Feb 16;423(2):189–195. doi: 10.1016/0005-2728(76)90177-8. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Synthesis of glutamate in Aerobacter aerogenes by a hitherto unknown route. Biochem J. 1970 Apr;117(2):405–407. doi: 10.1042/bj1170405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. Absence of the pigments of photosystem II of photosynthesis in heterocysts of a blue-green alga. Nature. 1970 Oct 10;228(5267):181–183. doi: 10.1038/228181b0. [DOI] [PubMed] [Google Scholar]
- Thomas J. Relationship between age of culture and occurrence of the pigments of photosystem II of photosynthesis in heterocysts of a blue-green alga. J Bacteriol. 1972 Apr;110(1):92–95. doi: 10.1128/jb.110.1.92-95.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B. Regulation of the assimilation of nitrogen compounds. Annu Rev Biochem. 1978;47:1127–1162. doi: 10.1146/annurev.bi.47.070178.005403. [DOI] [PubMed] [Google Scholar]
- Walker C. C., Yates M. G. The hydrogen cycle in nitrogen-fixing Azotobacter chroococcum. Biochimie. 1978;60(3):225–231. doi: 10.1016/s0300-9084(78)80818-9. [DOI] [PubMed] [Google Scholar]
- Wang Z. C., Burns A., Watt G. D. Complex formation and O2 sensitivity of Azotobacter vinelandii nitrogenase and its component proteins. Biochemistry. 1985 Jan 1;24(1):214–221. doi: 10.1021/bi00322a031. [DOI] [PubMed] [Google Scholar]
- Weare N. M., Benemann J. R. Nitrogen fixation by Anabaena cylindrica. II. Nitrogenase activity during induction and aging of batch cultures. Arch Mikrobiol. 1973 Oct 19;93(2):101–112. [PubMed] [Google Scholar]
- Weare N. M., Benemann J. R. Nitrogenase activity and photosynthesis in Plectonema boryanum. J Bacteriol. 1974 Jul;119(1):258–265. doi: 10.1128/jb.119.1.258-265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. W., Shilo M. Heterotrophic growth of the filamentous blue-green alga Plectonema boryanum. Arch Microbiol. 1975;102(2):123–127. doi: 10.1007/BF00428356. [DOI] [PubMed] [Google Scholar]
- Willison J. C., Jouanneau Y., Colbeau A., Vignais P. M. H2 metabolism in photosynthetic bacteria and relationship to N2 fixation. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):115–135. doi: 10.1016/s0769-2609(83)80100-8. [DOI] [PubMed] [Google Scholar]
- Winkenbach F., Wolk C. P. Activities of enzymes of the oxidative and the reductive pentose phosphate pathways in heterocysts of a blue-green alga. Plant Physiol. 1973 Nov;52(5):480–483. doi: 10.1104/pp.52.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P., Cai Y., Cardemil L., Flores E., Hohn B., Murry M., Schmetterer G., Schrautemeier B., Wilson R. Isolation and complementation of mutants of Anabaena sp. strain PCC 7120 unable to grow aerobically on dinitrogen. J Bacteriol. 1988 Mar;170(3):1239–1244. doi: 10.1128/jb.170.3.1239-1244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P. Movement of carbon from vegetative cells to heterocysts in Anabaena cylindrica. J Bacteriol. 1968 Dec;96(6):2138–2143. doi: 10.1128/jb.96.6.2138-2143.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J. T., Silvey J. K. Nitrogen fixation by gloeocapsa. Science. 1969 Aug 29;165(3896):908–909. doi: 10.1126/science.165.3896.908. [DOI] [PubMed] [Google Scholar]
- Zehr J. P., McReynolds L. A. Use of degenerate oligonucleotides for amplification of the nifH gene from the marine cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1989 Oct;55(10):2522–2526. doi: 10.1128/aem.55.10.2522-2526.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr J. P., Ohki K., Fujita Y. Arrangement of nitrogenase structural genes in an aerobic filamentous nonheterocystous cyanobacterium. J Bacteriol. 1991 Nov;173(21):7055–7058. doi: 10.1128/jb.173.21.7055-7058.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcelos L., Fay P. Nitrogen metabolism and ultrastructure in Anabaena cylindrica. I. The effect of nitrogen starvation. Arch Mikrobiol. 1974 Mar 28;96(4):271–279. [PubMed] [Google Scholar]
- van Gorkom H. J., Donze M. Localization of nitrogen fixation in Anabaena. Nature. 1971 Nov 26;234(5326):231–232. doi: 10.1038/234231b0. [DOI] [PubMed] [Google Scholar]