Abstract
Nitric oxide (NO) is an endogenous vasodilator as well as natural inhibitor of platelet adhesion and activation that can be released from a NO donor species, such as diazeniumdiolated dibutylhexanediamine (DBHD/N2O2) within a polymer coating. In this study, various Food and Drug Administration approved poly(lactic-co-glycolic acid) (PLGA) species were evaluated as additives to promote a prolonged NO release from DBHD/N2O2 within a plasticized poly(vinyl chloride) (PVC) matrix. When using an ester-capped PLGA additive with a slow hydrolysis time, the resulting coatings continuously release between 7–18×10-10 mol cm-2 min-1 NO for 14 d at 37°C in PBS buffer. The corresponding pH changes within the polymer films were visualized using pH sensitive indicators and are shown to correlate with the extended NO release pattern. The optimal combined diazeniumdiolate/PLGA-doped NO release (NOrel) PVC coating was evaluated in vitro and its effect on the hemodynamics was also studied within a 4 h in vivo extracorporeal circulation (ECC) rabbit model of thrombogenicity. Four out of 7 control circuits clotted within 3 h, whereas all the NOrel coated circuits were patent after 4 h. Platelet counts on the NOrel ECC were preserved (79 ± 11% compared to 54 ± 6% controls). The NOrel coatings showed a significant decrease in the thrombus area as compared to the controls. Results suggest that by using ester-capped PLGAs as additives to a conventional plasticized PVC material containing a lipophilic diazeniumdiolates, the NO release can be prolonged for up to 2 weeks by controlling the pH within the organic phase of the coating.
Keywords: Diazeniumdiolate, Extracorporeal Circulation, Nitric Oxide, Platelets, PLGA
1. Introduction
Blood/material interaction is critical to the success of implantable medical devices, ranging from simple catheters, stents and grafts, to complex extracorporeal artificial organs that are used in thousands of patients every day.1 Thrombosis is one of the primary problems associated with clinical application of blood contacting materials. Despite a thorough understanding of the mechanisms of blood–surface interactions and decades of bioengineering research effort, the ideal non-thrombogenic prosthetic surface remains an unsolved problem.2 Over the last 50 years much has been learned about foreign surface-induced thrombosis and attempts to prevent it with systemic anticoagulation and surface modifications. Surface modifications have included using pure, very smooth silicone rubber3 or polyurethane,4 pre-exposure of the surfaces to albumin5 and other coating proteins,6 and surface binding of heparin in an ionic7 as well as a covalent fashion.8 Despite extensive research to develop a non-thrombogenic surface that mimics the endothelium, none of these modifications have been successful.
In 1993 Radomski and Moncada9 described nitric oxide (NO) as one of two potent vasodilators secreted by normal endothelium that has the ability to inhibit platelet adhesion and aggregation to the blood vessel wall. The amount of NO released from the surface of a normal and stimulated endothelium has been estimated to be in the range of 0.5 – 4×10-10 mol cm-2 min-1.10 Nitric oxide has been extensively studied for its inhibitory effects on circulating platelet and monocyte activation that leads to aggregation and ultimately initiation of thrombosis.11–14 A wide range of NO donors such as S-nitrosothiols,15, 16 N-hydroxy-N-nitrosoamines,17 N-diazeniumdiolates18, 19 and nitrosyl metal complexes20 have been studied over the past decade, as a means to release NO either by systemic infusion21 or locally released from a polymer surface.22 Despite the promising potential of NO releasing materials, their development has been hindered due to challenges in prolonging the NO release beyond a few days.
Diazeniumdiolates have been one of the most widely studied NO donors, which release NO through proton23 or thermal24 driven mechanisms. Prior work has shown that NO can be released from the NO donor compound, diazeniumdiolated dibutylhexanediamine (DBHD/N2O2), when this species is incorporated into hydrophobic polymer films.22 While DBHD/N2O2 is an excellent donor for incorporation into polymers to create NO release coatings, the loss of NO from this molecule creates free lipophilic amine species within the polymer that react with water, thereby increasing the pH within the organic polymer phase which effectively turns off the NO production before a significant fraction of the total NO payload has been released.25 In earlier work, to overcome this complication, tetrakis-(p-chlorophenyl)-borate was employed as an additive to maintain a low enough pH within the organic polymer phase and to promote a sustained NO flux.22, 25 However, in a recent cell proliferation study it was shown that tetrakis-(p-chlorophenyl)-borate was not successful in prolonging the NO release beyond few days and, further, this compound was found to be cytotoxic towards endothelial and smooth muscle cells.26
The work presented herein focuses on a completely different approach to address this pH control problem and hence to greatly prolong the NO release, one of the key challenges with NO delivery materials. The method involves the use of poly(lactide-co-glycolide) (PLGA) species as additives to help stabilize the pH within the organic phase polymeric coatings. The addition of PLGA can be used to control the flux of NO emitted from polymers containing diazeniumdiolate species by helping to control the pH within the polymer phase.27, 28 PLGA is a biodegradable and biocompatible polymer29–31 that can be used as a small additive to sustain the NO flux for a prolonged period by the slow formation of lactic and glycolic acid within the base polymer layer of the coating. Ester linkages of the PLGA are slowly hydrolyzed as small amounts of water penetrate the polymer from the surrounding aqueous environment to generate lactic and glycolic acid within the polymer matrix.32, 33 The presence of this continuous acid production reaction compensates for the increase in pH from generation of organo-ammonium hydroxide (reaction of liberated free amines from DBHD with water in the polymer film) from the NO release reaction, thereby maintaining a greater rate of NO release for longer periods of time.
In the literature there are reports of two main strategies that utilize PLGA to deliver NO from diazeniumdiolate species. The first strategy is dispersing the NO donor compound within the PLGA matrix, creating a completely biodegradable NO release material. Yoo et al. employed PLGA microparticles with an NO donor (diethylenetriamine diazeniumdiolate (DETA NONOate), a low molecular weight diazeniumdiolate) within to deliver NO for a very short 6 h period.34 In another study, Cai et al. studied in vitro effects of NO releasing PLGA films on biofilm formation.35 In this study the authors dispersed the NO donor compound in a completely hydrolysable PLGA matrix, however due to the toxicity concerns of the product amine, leaching is a potential limitation of these films. In the second strategy, Zhou and Meyerhoff have shown that PLGA has the potential to act as a proton donor to enhance the release of NO from a polymer material that had covalently linked diazeniumdiolate groups for a relatively short 20 h period.27 Although these approaches have had some limited success, prolonging the NO release still remains one of the great challenges preventing use of NO releasing materials in clinical application.
Herein, we report the use of PLGA as an additive in a poly(vinyl chloride) (PVC)/dioctyl sebacate (DOS) matrix to control NO release from a lipophilic diazeniumdiolate (DBHD/N2O2) species added to the organic phase of the coating to address the need to prolong the NO release beyond a few days. A comparison was made between PLGAs that have different end group chemistries; either a free carboxylic acid or an ester end group. The hydrolysis rate of the PLGA is primarily determined by the copolymer ratio, the nature of the end group (e.g., free acid or ester), and molecular weight. It is shown that the hydrolysis rate and acid content of the given PLGA species used greatly influences the NO release profile. Optimized film compositions prepared with ester end-capped PLGA additives can sustain high fluxes of NO for up to 14 d. Further, incorporation of pH indicators in the coatings provides a means to correlate the NO release with the observed pH changes within the PVC/DOS matrix. The newly formulated PLGA/diaziumdiolate-doped PVC/DOS coatings were further tested in an in vivo rabbit extracorporeal circulation (ECC) model to assess platelet count and function preservation, in addition to reduction in the thrombus coverage area. The new PLGA-doped NOrel PVC coatings could provide a breakthrough for achieving sustained preservation of circulating platelets, an important goal for longer-term ECC situations, such as ECMO, or other blood-contacting devices (e.g. catheters, vascular grafts, etc.).
2. Materials and Methods
2.1. Materials
Tygon™ poly(vinyl chloride) (PVC) tubing was purchased from Fisher Healthcare (Houston, TX). High molecular weight poly(vinyl chloride) (PVC), dioctyl sebacate (DOS), anhydrous tetrahydrofuran (THF), anhydrous acetonitrile, bromothymol blue, bromocresol green, sodium chloride, potassium chloride, sodium phosphate dibasic, and potassium phosphate monobasic were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Various poly(D,L-lactide-co-glycolide) materials, including 5050DLG1A and 5050DLG7E, were obtained from SurModics Pharmaceuticals Inc. (Birmingham, AL). N,N’-Dibutyl-1,6-hexanediamine (DBHD) was purchased from Alfa Aesar (Ward Hill, MA). DBHD/N2O2 was synthesized by treating DBHD with 80 psi NO gas purchased from Cryogenic Gases (Detroit, MI) at room temperature for 48 h, as previously described.25
2.2. Preparation of NOrel films for NO release and pH studies
As mentioned earlier, the main focus of this work is to use PLGA as an additive in a PVC/DOS polymer matrix to prolong the NO release from a lipophilic DBHD/N2O2 species (Fig. 1). In this study, 5050DLG1A (1–2 wk hydrolysis rate) and 5050DLG7E (1–2 mo hydrolysis rate) PLGAs were used. These product names identify polymer mole ratio, polymer type, inherent viscosity and the end group designation (ester or acid). For example, 5050DLG7E stands for: 50 mole% DL-lactide, 50 mole% glycolide, 0.7dL/g and ‘E’ for an ester end group (Table 1). The hydrolysis rate of the PLGA is primarily determined by the copolymer ratio, end group, and molecular weight (as determined from the inherent viscosity). A variety of NO releasing film formulations were prepared using the solvent evaporation method in order to optimize the NO release duration. NO releasing films consisting of 10 wt% PLGA (5050DLG1A or 5050DLG7E) + 25 wt% DBHD/N2O2 in 2:1 PVC/DOS were prepared with 80 mg PLGA, 200 mg DBHD/N2O2, 173 mg DOS and 347 mg PVC in 5 mL THF. The NO releasing films were cast in Teflon rings (d = 2.5 cm) and cured for 2 d under nitrogen. Disks (d = 0.9 cm) were cut from the parent NO releasing film and dip coated 8 times using the top coating solution (375 mg DOS, 750 mg PVC in 15 mL THF) in 10 min intervals. Top coated films were dried under nitrogen conditions for 1 d to minimize the loss of NO due to ambient moisture. Similarly, control films were also prepared using solvent evaporation followed by top coating as described above. The 10 wt% PLGA control film solution consisted of 80 mg PLGA, 240 mg DOS and 480 mg PVC in 5 mL THF. The 25 wt% DBHD/N2O2 control film (without PLGA) consisted of 200 mg DBHD/N2O2, 200 mg DOS, and 400 mg PVC in 5 mL THF. The final films had a total thickness of ~1000 µm including a topcoat of ~200 µm, which were measured using a Mitutoyo digital micrometer (Metron Precision Inc.).
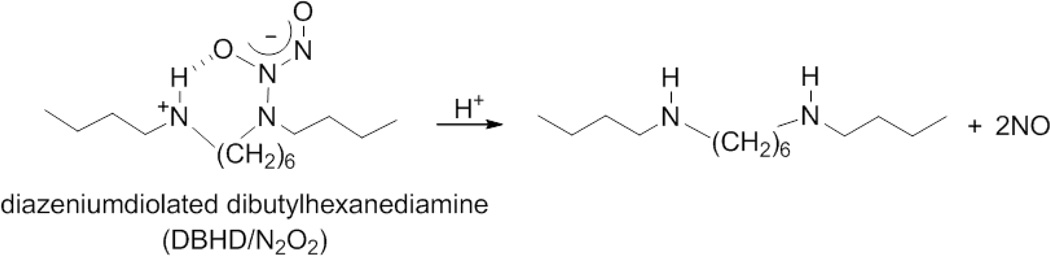
Fig. 1.
Reaction mechanism of diazeniumdiolated dibutylhexanediamine (DBHD/N2O2) and poly(lactic-co-glycolic acid) (PLGA).
Table 1.
Analytical information for the 5050DLG1A and 5050DLG7E poly(lacide-co-glycolide) additives.
| PLGA | Copolymer Ratio (1H NMR) (Lactide:Glycolide)* |
Acid number (mg KOH/g PLGA) |
Inherent viscosity* (dl/g) |
Molecular weight(GPC)* |
|
|---|---|---|---|---|---|
| Mw (kDa) |
PDI | ||||
| 5050DLG7E | 52:48 | 60.4 ± 2.5 | 0.08 | 4.1 | 2.1 |
| 5050DLG7E | 51:49 | 2.4 ± 0.8 | 0.65 | 106 | 1.6 |
Information provided in Analytical Report from Lakeshore Biomaterials.
For the pH studies, the films were prepared as described above with the exception of adding given pH indicators to the active layer solution. The pH indicators, bromocresol green or bromothymol blue, were present in the active layer casting solution at 0.025 wt%. Photos of the pH films were taken each day with Nikon L24 digital camera to monitor the change in pH as indicated by the color of the incorporated pH indicators.
2.3 Acid content of PLGA
The acid number, which is a measure of the initial acid content of the PLGA and is directly related to the free carboxylic acid functionalities, was determined by a previously reported titration method.36 Briefly, approximately 50 mg of PLGA was dissolved in 10 mL of a 1:1 mixture of acetone and THF. This solution was immediately titrated with 0.01 N KOH in methanol to a stable pink endpoint. Phenolphthalein in methanol (0.1 wt%) was used as the indicator for the titration. Titrations were performed in triplicate.
2.4. Preparation of NOrel coated ECC loops
A plasticized PVC coating containing 25 wt% DBHD/N2O2, an NO donor, was prepared using a method previously reported.22 Briefly, triple layers of polymeric coatings that included a base layer, NO releasing layer and top layer were individually coated onto the inner surface of the Tygon™ tubing that formed the ECC (Fig. 2). Top and base coats were employed for three main reasons: 1) to prevent leaching of DBHD/N2O2; 2) to neutralize the surface charge; and 3) to yield a smoother finish to the surface. The base coat of 100% PVC was used to reduce the NO diffusion into the tubing wall, and maximize the NO release towards the blood-contacting surface. The base layer was prepared by dissolving 600 mg PVC in 10 mL THF (Solution A). The NO releasing layer was prepared by dissolving 770 mg PVC, 385 mg DOS, 180 mg PLGA and 450 mg DBHD/N2O2 in 10 mL THF to obtain a slightly cloudy dispersion of the diazeniumdiolate in the solution (Solution B). The top coat solution was prepared using 181 mg PVC, 362 mg DOS plasticizer and 10 mL THF (Solution C). The control ECC solution was prepared by dissolving 1070 mg PVC, 535 mg DOS, and 180 mg PLGA in 10 mL THF (Solution D).
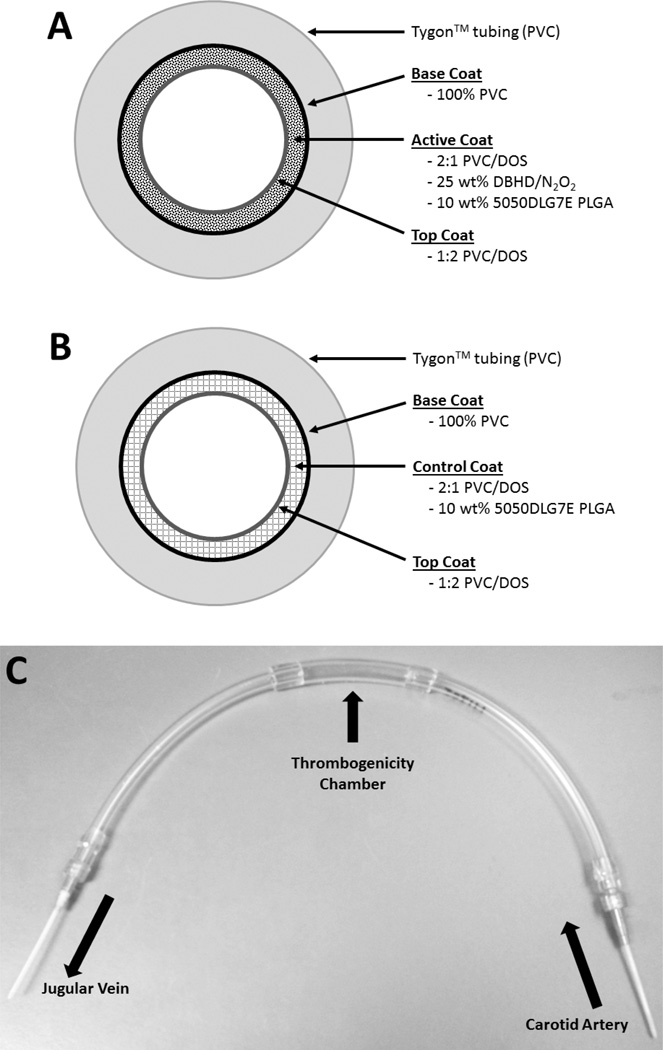
Fig. 2.
Schematic of the NO releasing coating (A), control coating (B) and assembled ECC loop (C). The base coat of 100% PVC limits NO diffusion into the Tygon™ tubing. The NO releasing ECC consists of an active coat of plasticized PVC doped with a lipophilic DBHD/N2O2 and the PLGA additive. The control ECC consists of plasticized PVC doped with the PLGA additive. The top coat of plasticized PVC over the active/control coats provides a smooth surface and neutralizes the charge on the surface of the assembled ECC.
The ECC configuration used in this study (with rabbits) was described previously.22 Briefly, the ECC consisted of a 16-gauge and 14-gauge IV polyurethane angiocatheters (Kendall Monoject Tyco Healthcare Mansfield, MA), two 16 cm in length ¼ inch inner diameter (ID) Tygon™ tubing and a 8 cm length of 3/8 inch ID Tygon™ tubing which created a thrombogenicity chamber where thrombus could form more easily due to more turbulent blood flow. Each component of the ECC was coated first with a base coating of Solution A, followed by two coats of the NO releasing layer (Solution B) or control layer (Solution D) and finally the top coat (Solution C) as shown in Figs. 2A and 2B. The circuitry was filled with each solution, which was then removed. Each coat was allowed to dry for at least 1 h. The ECC was pieced together, starting at the left carotid artery side, with the 16-gauge angiocatheter, one 15 cm length ¼ inch ID tubing, the 8 cm length thrombogenicity chamber, the second 15 cm length ¼ inch ID tubing and finally the 14-gauge angiocatheter (Fig. 2C). The angiocatheters were interfaced with tubing using 2 luer-lock PVC connectors. The 3/8 inch ID tubing and the ¼ inch tubing were welded together using THF. The assembled ECCs were allowed to cure under nitrogen for 20 min and then dried under vacuum for 2 d, to minimize the negative effects of ambient moisture on the coating. The trilayer configuration for both NO releasing and control ECCs had a total thickness of approximately 150–200 µm., much thinner than the free standing multilayer films described above.
2.5. NO release measurements
Nitric oxide released from the films was measured using a Sievers chemiluminescence Nitric Oxide Analyzer (NOA), model 280 (Boulder, CO). The chemiluminiscence nitric oxide analyzer (NOA) is considered the gold standard for detecting NO and is widely used to measure NO release from materials.22, 25, 35, 37, 38 The major advantage of using the NOA is that the most troublesome interferents such as nitrites and nitrates are not transferred from the sample vessel to the reaction cell; thus, NO selectivity is enhanced.39, 40 A sample of the film was placed in 4 mL PBS buffer at 37° C. Nitric oxide liberated from the film was continuously swept from the headspace of the sample cell and purged from the buffer with a nitrogen sweep gas and bubbler into the chemiluminescence detection chamber. The flow rate was set to 200 mL/min with a chamber pressure of 5.4 Torr and an oxygen pressure of 6.0 psi. Films were incubated in 4 mL of PBS buffer at 37°C for a 2 week period and tested for NO release at various time points. Buffer was replaced every day to prevent saturation of NO. In addition, a uniform segment was cut from the coated ECC loop and was tested for 4 h in vitro for NO release in PBS buffer prior to blood exposure. After the surgery, a section of the ECC loop was tested for NO release in PBS buffer post blood exposure.
2.6. The rabbit thrombogenicity model
The animal handling and surgical procedures were approved by the University Committee on the Use and Care of Animals in accordance with university and federal regulations. A total of 14 New Zealand white rabbits (Myrtle’s Rabbitry, Thompson’s Station, TN) were used in this study. All rabbits (2.5–3.5 kg) were initially anesthetized with intramuscular injections of 5 mg/kg xylazine injectable (AnaSed® Lloyd Laboratories Shenandoah, Iowa) and 30 mg/kg ketamine hydrochloride (Hospira, Inc. Lake Forest, IL). Maintenance anesthesia was administered via isoflurane gas inhalation at a rate of 1.5–3% via mechanical ventilation which was done via a tracheotomy and using an A.D.S. 2000 Ventilator (Engler Engineering Corp. Hialeah, FL). Peek inspiratory pressure was set to 15 cm of H2O and the ventilator flow rate set to 8 L/min. In order to aid in maintenance of blood pressure stability, IV fluids of Lactated Ringer’s were given at a rate of 10 mL/kg/h. For monitoring blood pressure and collecting blood samples, the rabbits’ right carotid artery was cannulated using a 16-gauge IV angiocatheter (Jelco®, Johnson & Johnson, Cincinnati, OH). Blood pressure and derived heart rate were monitored with a Series 7000 Monitor (Marquette Electronics Milwaukee, WI). Body temperature was monitored with a rectal probe and maintained at 37°C using a water-jacketed heating blanket. Prior to placement of the arteriovenous (AV) custom-built extracorporeal circuit (ECC), the rabbit left carotid artery and right external jugular vein were isolated and baseline hemodynamics as well as arterial blood pH, pCO2, pO2, and total hemoglobin were measured using an ABL 825 blood-gas analyzer. In addition, baseline blood samples were collected for platelet and total white blood cell (WBC) counts which were measured on a Coulter Counter Z1 (Coulter Electronics Hialeah, FL). Plasma fibrinogen levels were determined using a Dade Behring BCS Coagulation Analyzer (Siemans Deerfield, IL), activated clotting times (ACT) were monitored using a Hemochron Blood Coagulation System Model 801 (International Technidyne Corp. Edison, NJ), platelet function was assessed using a Chrono-Log optical aggregometer model 490 (Havertown, PA).
After baseline blood measurements, the custom-built ECC was placed into position by cannulating the left carotid artery for ECC inflow and the right external jugular vein for ECC outflow. The flow through the ECC was initiated by unclamping the arterial and venous sides of ECC and blood flow in circuit was monitored with an ultrasonic flow probe and flow meter (Transonic HT207 Ithaca, NY). Animals were not systemically anticoagulated during the experiments.
After 4 h on ECC, the circuits were clamped, removed from animal, rinsed with 60 mL of saline and drained. Any residual thrombus in the larger tubing of ECC (i.e., thrombogenicity chamber) was photographed and the degree of thrombus image was quantitated using Image J imaging software from National Institutes of Health (Bethesda, MD). Prior to euthanasia, all animals were given a dose of 400 U/kg sodium heparin to prevent necrotic thrombosis. The animals were euthanized using a dose of Fatal Plus (130 mg/kg sodium pentobarbital) (Vortech Pharmaceuticals Dearborn, MI). All animals underwent gross necropsy after being euthanized, including examination of the lungs, heart, liver and spleen for any signs of thromboembolic events.
2.7. Blood sampling
Rabbit whole blood samples were collected in non-anticoagulated 1 cc syringes for ACT, 3.2% sodium citrate vacutainers (Becton, Dickinson. Franklin Lakes, NJ) in 3 cc volumes for cell counts, aggregometry, and 1 cc syringes containing 40 U/mL of sodium heparin (APP Pharmaceuticals, LLC Schaumburg, IL) for blood-gas analysis. Following the initiation of ECC blood flow, blood samples were collected every hour for 4 h for ex vivo measurements. Samples were used within 2 h of collection to avoid any activation of platelets, monocytes or plasma fibrinogen.
2.8. Platelet aggregometry
Rabbit platelet aggregation was assayed based on the Born’s turbidimetric method using a Chrono-Log optical aggregometer. Briefly, citrated blood (1:10 blood to ACD) was collected (6 mL) and platelet-rich plasma (PRP) was obtained by centrifugation at 110×g for 15 min. Platelet-poor plasma (PPP) was obtained by another centrifugation of the PRP-removed blood sample at 2730×g for 15 min and was used to normalize the PRP for aggregation. Normalized PRP was incubated for 10 min at 37°C and then 25 µg/mL collagen (Chrono-PAR #385 Havertown, PA) was added. As shown by Major et al., the percentage of aggregation was determined 3 min after the addition of collagen using Chrono-Log Aggrolink software.22, 41
Statistical Analysis
Data are expressed as mean ± SEM (standard error of the mean). Comparison of ECC results between the various NOrel and control polymer groups were analyzed by a one-way ANOVA with a multiple comparison of means using Student’s t-test. All statistical analyses were performed using the statistical program SAS JMP (SAS Institute Cary, NC). Values of p<0.05 were considered statistically significant for all tests.
3. Results and Discussions
3.1 In vitro NO release from films containing DBHD/N2O2 in PVC/DOS with various PLGA additives
The diazeniumdiolate species investigated here, DBHD/N2O2, decomposes to generate NO primarily by a proton-driven mechanism.25 A tetrakis-(p-chlorophenyl)-borate derivative was used previously as a lipophilic additive counteranion to stabilize the pH within NO releasing polymers prepared with DBHD/N2O2.25 However, the borate derivative is not an ideal additive because of its toxicity.26 In this study, PLGA additives with varying hydrolysis rates were used as a replacement to the borate derivative to act as a proton donor source to control the NO release from DBHD/N2O2-doped PVC coatings. It is well known that, in the presence of water, the ester bonds in PLGA hydrolyze to yield lactic and glycolic acids, and that PLGA is a widely used biodegradable/biocompatible polymer that has been approved by FDA for numerous products.42
The films used in this study had a three layer configuration: base coat, active coat and top coat. The base and top-coat consisted of PVC/DOS in 2:1 ratio and the active coat consisted of PVC/DOS with 25 wt% DBHD/N2O2 and 10 wt% PLGA additive. As demonstrated by Reynolds et al.,25 PVC films containing DBHD/N2O2 with a 2:1 ratio of PVC/DOS had a more prolonged NO release when compared to 1:1 or 1:2 ratio of PVC/DOS. Increasing the DOS content of the polymer increases the water uptake, resulting in a higher initial burst of NO release. Therefore, in this study, a 2:1 ratio of PVC/DOS was used. Top and base coats were employed for three main reasons indicated above in the Experimental Section. In this study, 5050DLG1A (1–2 week hydrolysis rate) and 5050DLG7E (1–2 month hydrolysis rate) PLGAs were compared.
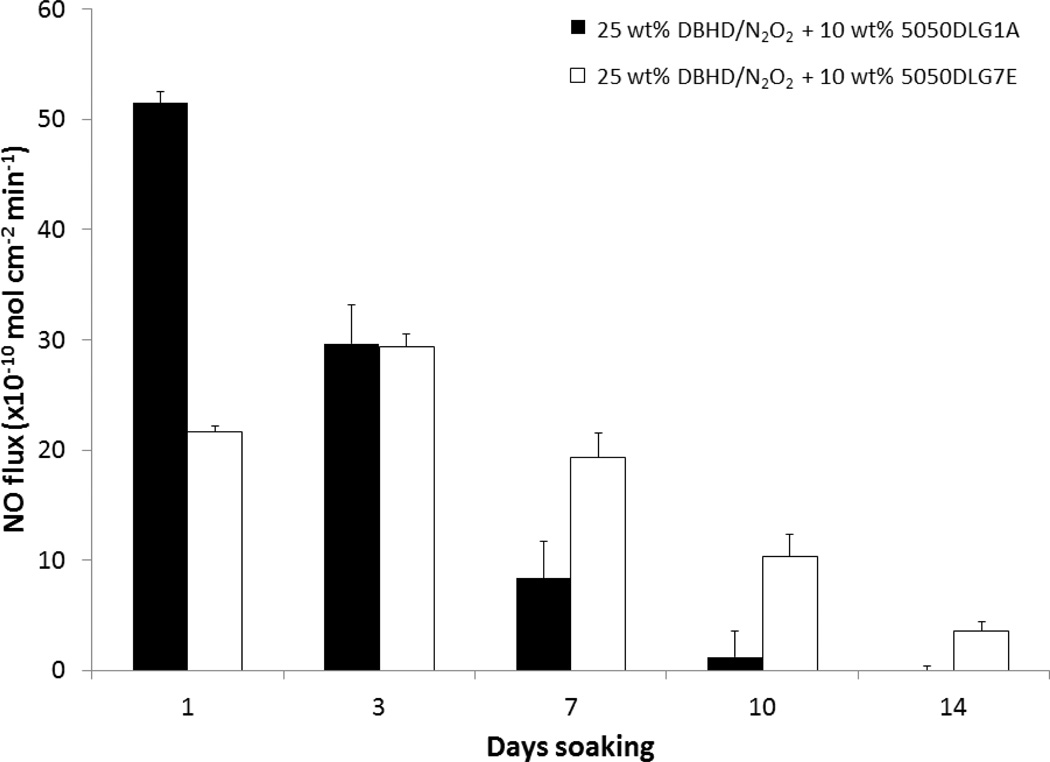
It has been previously reported that DBHD/N2O2 within PVC films without an additive releases NO, producing the corresponding diamine, DBHD, that raises the pH within the polymer film slowing and eventually stopping the NO release in 1–2 d.25 Use of a PLGA additive promotes a more sustained NO release. As shown in Fig. 3, DBHD/N2O2 in plasticized PVC with 5050DLG1A as the additive had an initial burst of NO due to high proton activity, but the NO release quickly diminished over a 10 d period. In contrast, the PVC films prepared with the 5050DLG7E additive had a more consistent NO flux with no initial burst of NO, and this enabled the NO release to be prolonged for a 14 d period. Not only does the 5050DLG1A hydrolyze and produce acid monomers more quickly than the 5050DLG7E, but it has a higher initial acid content (compared to the ester capped PLGA) (Table 1). The higher acid content and faster hydrolysis rate of the 5050DLG1A directly correlates to the high initial burst and greater initial NO fluxes which quickly depletes the DBHD/N2O2 reservoir.
Fig. 3.
NO release profiles of 25 wt% DBHD/N2O2 films containing 10 wt% 5050DLG1A (1–2 wk hydrolysis rate) and 5050DLG7E (1–2 mo hydrolysis rate) PLGA additives in PVC/DOS polymer matrix. The data are means ± SEM.
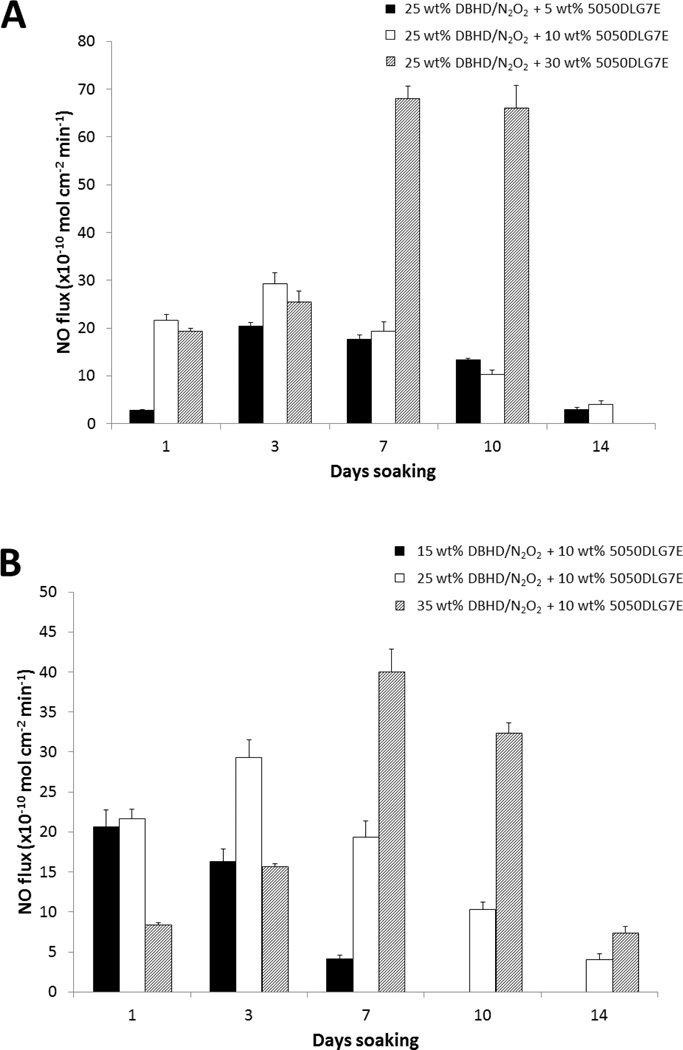
From Fig. 3 it is apparent that the 5050DLG7E additive films exhibit little or no initial burst of NO and release the NO for a prolonged time period. In order to optimize the film formulation containing 5050DLG7E, the amounts of PLGA (5–30 wt%) and DBHD/N2O2 (15–35 wt%) were varied. In Fig. 4A, the amount of DBHD/N2O2 is kept constant at 25 wt%, while the 5050DLG7E PLGA amount is varied from 5–30 wt%. The 5 wt% 5050DLG7E films are shown to release NO for 14 d; however, the fluxes were quite low for the first few days of soaking. These low fluxes indicate that the 5 wt% PLGA is not adequate to compensate for the pH increase due to production of free DBHD diamine within the film. Increasing the 5050DLG7E to 30 wt% yields films that exhibit high fluxes from days 7–10 due to the increased amount of acid monomers being produced, resulting in complete depletion of the NO reservoir before day 14. The ideal NO release coating has a consistent NO release, with little variation in the NO flux from day-to-day under physiological conditions. The films with 10 wt% 5050DLG7E had little variation in the NO flux until days 10–14, while the 5% and 30% films gave fluxes that were either low or very high. From this data one can conclude that using 10 wt% 5050DLG7E PLGA gives the more constant and sustained NO release profile. Based on our calculations, approximately 85% of the theoretical NO is recovered from plasticized PVC doped with 10 wt% 5050DLG7E and 25 wt% DBHD/N2O2 coatings. The 15% of the theoretical NO is lost during coating preparation and curing, which is likely due to the residual acid monomers present in the PLGA and thermal NO release at room temperature. This loss of NO is difficult to avoid during the coating and curing process.
Fig. 4.
NO release profiles of plasticized PVC doped with 25 wt% DBHD/N2O2 and 5, 10, or 30 wt% 5050DLG7E (A). NO release profiles of plasticized PVC doped with 30 wt% 5050DLG7E and 15, 25, or 35 wt% DBHD/N2O2 (B). The data are means ± SEM.
In Fig. 4B the amount of 5050DLG7E is kept constant at 10 wt%, while the amount of DBHD/N2O2 is varied from 15–35 wt%. Decreasing the amount of DBHD/N2O2 to 15 wt% limits the available NO load and only releases NO for 7 days. Increasing the DBHD/N2O2 content of the films to 35 wt% yields a lower initial NO flux, but overall did not show any gain in the number of days of NO release when compared to the 25 wt% DBHD/N2O2 films (Fig. 4B). Based on the data shown in Fig. 4B,25 wt% DBHD/N2O2 with 10 wt% 5050DLG7E appears to be optimal film composition in terms of sustained NO release for 14 d. Hence, this formulation was used for subsequent antithrombosis evaluation in an ECC rabbit model.
3.2. Correlating NO release and pH change in the films
At 37°C, incubation of DBHD/N2O2 films in PBS enables NO to be released through a proton driven mechanism and the diamine DBHD product formed increases the pH within the PVC film. The pH increase causes the NO release rate to decrease and eventually cease completely, without delivering the entire NO payload. In contrast, using PLGA as an additive in appropriate proportion helps ensure that the DBHD/N2O2 is the limiting reagent and the entire NO payload is eventually released. PLGA continues to hydrolyze creating a more acidic environment essential for NO release. Hydrolysis of PLGA takes place simultaneously with NO release, balancing the pH of the film in a pH range that favors NO release. However, the key to optimizing the NO release from these formulations is balancing the rates of PLGA hydrolysis with the rate of DBHD amine production.
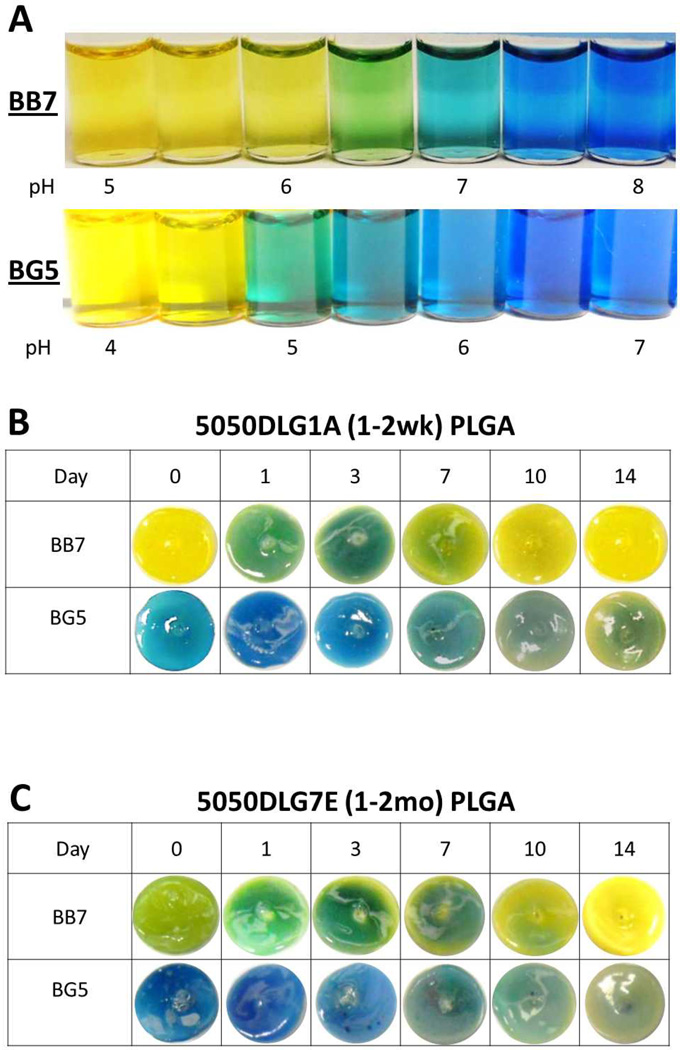
It is challenging to measure the rate constants of the diazeniumdiolate decomposition and PLGA hydrolysis, since one reaction directly effects the other. That is, the formation of the diamine causes the coating to become basic, which will further catalyze (increase rate of) the PLGA hydrolysis. Therefore, the rate constants for the two reactions will both change over time, and be somewhat dependent on one another. Hence, it is not possible to assess the kinetics of each reaction independently. Studying the pH changes within the polymer matrix via the addition of pH indicator dyes, however, allows for qualitative correlation between the observed pH changes and NO release profile. Studying the pH changes within the polymer matrix as a function of time provides a means to further support the hypothesis that the addition of PLGA to the PVC films derives its benefit via control of the polymer phase pH. In previous work, Chromoionophore II (9-dimethylamino-5[4-(16-butyl–2,14-dioxo-3,15-dioxaeicosyl)phenylimino]benzo[a]phenoxazine) was doped into a PVC/DOS film with DBHD/N2O2. However, this pH indicator only demonstrated the mechanism whereby this matrix becomes more basic over time without any detailed correlation to the observed NO release rate.25 The pH within pure PLGA matrices has been studied previously using confocal microscopy with an acidic pH sensitive probe Lysosensor yellow/blue.32 In the present study, doping the films with simple pH indicator dyes allows a convenient and inexpensive way to visualize the pH changes that occur throughout the 14 d incubation period. The amount of dye added to the films is crucial, as too little dye will prevent visual interpretation, while too much dye will compete with the DBHD/N2O2 reaction. As shown in Fig. 5A, bromothymol blue (BB7) has a pH transition range of 6–7 and bromocresol green (BG5) of 4–5, where yellow is acidic and blue indicates basic conditions.
Fig. 5.
Comparison of color changes of bromocresol green (BG5) and bromothymol blue (BB7) in PBS buffer at various pH values (A). Comparison of color changes of BG5 and BB7 doped with 25 wt% DBHD/N2O2 and 10 wt% of 5050DLG1A PLGA in plasticized PVC polymer matrix (B). Comparison of color changes of BG5 and BB7 doped with 25 wt% DBHD/N2O2 and 10 wt% of 5050DLG7E PLGA in plasticized PVC polymer matrix (C). All films were incubated at 37°C for 14 d in PBS buffer.
The DBHD/N2O2 films containing DBHD/N2O2 (without PLGA additive) and the pH indicator dyes initially have a basic environment (see Fig. S1 in ESI). This basic environment is maintained throughout the incubation time period. This demonstrates that without an additive, the pH in the DBHD/N2O2 only films remains basic (from free DBHD within the DBHD/N2O2 preparation) and prevents any further NO release. The dyes were also added to the PLGA + PVC/DOS films (without any DBHD/N2O2), and all showed an acidic environment (see Fig. S1 in ESI).
In contrast, the films doped with 10 wt% 5050DLG1A and 25 wt% DBHD/N2O2 release NO for 10 d, but exhibit a burst of NO on the first day of soaking. As shown in Fig. 5B, the pH indicators in these 5050DLG1A-doped films indicate an initial acidic environment (pH ~5–6). This initial acidic environment correlates to the observed large NO burst on day 1, caused by the high free acid content in the 5050DLG1A (Table 1). After 1 d of soaking, the 5050DLG1A film with the BB7 dye indicate an increase in pH (color change from yellow to green). This increase of pH is due to the high flux of NO that occurs (Fig. 3) thereby producing significant amounts of the free DBHD amine within the film during a short period of time. By days 7–10, the NO flux diminishes significantly, during which time both dyes gradually indicate a decrease in film pH (films turned green and then yellow), an indication that the DBHD/N2O2 reservoir has been depleted as the PLGA continues to hydrolyze, recreating an acidic environment (pH ~5).
Additionally, as reported above, NO release profiles of the 5050DLG7E-doped films yield the best balance between the hydrolysis rate of PLGA and NO release from DBHD/N2O2, providing a prolonged NO release profile. The pH indicators show (Fig. 5C) that these films also are initially acidic (pH ~6–7), but less acidic than the 5050DLG1A films. In fact, the 5050DLG7E polymer possesses a much lower acid content and therefore slower hydrolysis rate in comparison to the films containing the 5050DLG1A polymer additive (Table 1); therefore, no initial burst of NO is observed. This lower initial acid content is crucial to prolonging the NO release from these films. The films containing 5050DLG7E PLGA exhibit little color change until days 10–14, when they begin to become more acidic. This demonstrates that the acid production rate (from the PLGA hydrolysis) and DBHD amine production rate is closely balanced within these films, explaining the consistency of the pH and NO release from day-to-day. These films also turned yellow by day 14, indicating the depletion of the NO reservoir. In short, the use of pH indicators within the films provides further evidence that the 5050DLG7E PLGA hydrolysis rate balances the decomposition rate of the DBHD/N2O2, producing the optimum pH and concomitant prolonged NO release/flux profile.
3.3. PLGA-doped NOrel films in ECC and effects on rabbit hemodynamics
ECC circuits coated (Fig. 2) (on inner walls) with PVC containing the a 5050DLG7E PLGA/DBHD/N2O2 NO release formulation were tested for NO release flux, pre- and post-4 h rabbit blood exposure. The optimized PVC coating material continuously releases NO under physiological conditions at levels that exceeds the physiological NO release from endothelial cells (0.5–4 × 10-10 mol cm-2 min-1).10 The NO release as measured using chemiluminescence NO analyzer shows a sustained NO flux of approximately 11 × 10-10 mol cm-2 min-1 for 4 h when measuring the pre-blood exposure NO release profile. The NO release from the ECC circuit does not decrease after exposure to the flowing blood. Indeed, after 4 h of blood flow, the NO flux was found to be 10 × 10-10 mol cm-2 min-1. The NO release profiles of the NOrel coatings were very similar when tested in PBS or plasma (see Fig. S2 ESI). The fact that the blood environment does not alter the kinetics of the NO release from the coating agrees well with the previously reported data for various NO release circuits.22 Further, given the prolonged NO release capability of the new coatings being tested, applications in much longer-term extracorporeal or other biomedical applications would be possible. Our goal here, however, is to demonstrate that the presence of PLGA does not alter the physiological effectiveness of the NO release, and this is most easily examined using the 4 h ECC test model.
The ECC blood flow was maintained at approximately 105 mL/min for the NOrel circuits over the 4 h animal test period. However, the blood flow typically drops from the initial 105 mL/min to approximately 80 mL/min in the first one hour for the control circuits, and then remains at 80 mL/min for the rest of the 4 h period. This maintenance of blood flow in the control circuits is due to the addition of intravascular fluids to the animal over the test period. The NOrel and control ECC loops have the same inner diameter (which remains static due to the rigidity of the tubing) and therefore maintains the integrity of the coating, hence having no effect on the blood flow rates. Monitoring the flow rate is a means to measure the time at which the ECC circuits has completely clotted. Since the control ECC loops often clotted, this blocked the blood flow through the tubing, thus the flow rates decreased. No significant difference in the mean arterial pressure of the animals on the NOrel vs. control circuits was noted, with pressures averaging 46±4 mmHg for both types of circuits. The activation clotting time for blood obtained from the test animals increases over the 4 h period for both NOrel and control coated circuits. As noted in previous studies,22 this behavior can be attributed to the increase in intravascular fluids and concomitant hemodilution effect.
3.4. Effects of PLGA-doped NOrel PVC polymer coatings on rabbit platelet function and thrombus formation
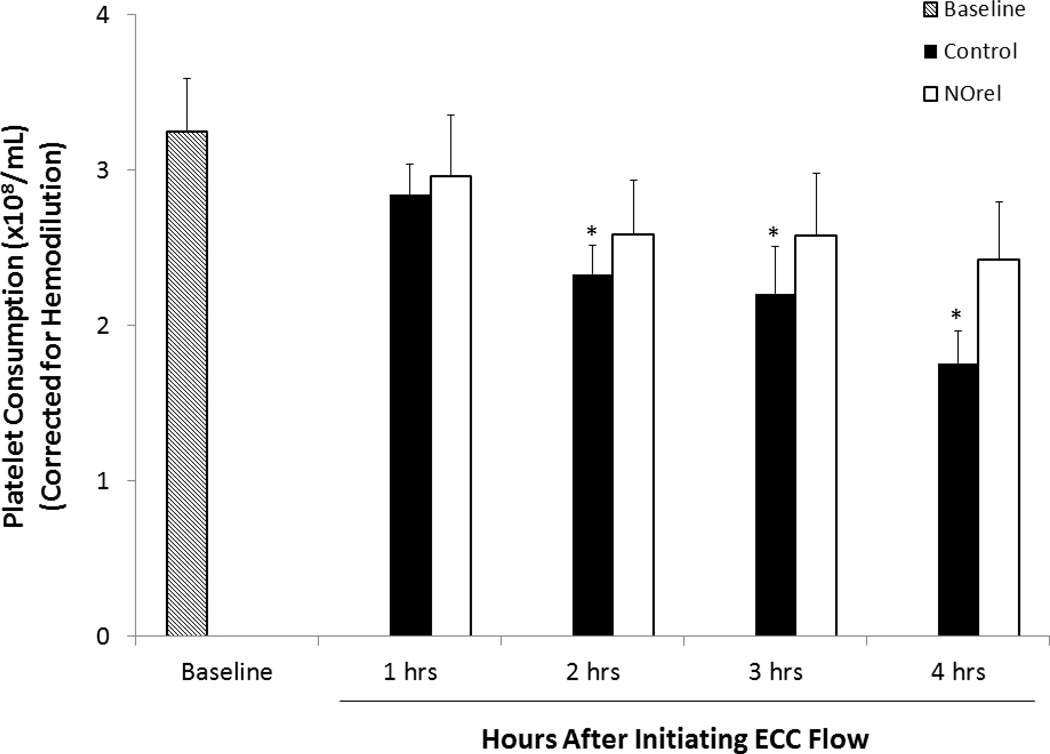
Platelet function during exposure to the NOrel and control polymer-coated ECCs was assessed by observing platelet count (Fig. 6) and percent of platelet aggregation, as determined by ex-vivo collagen (25 µg/mL) stimulation of PRP. Platelet count was corrected for any hemodilution due to added IV fluids into the rabbits. Four out of 7 control circuits clotted within 3 h, whereas all the 7 NOrel coated circuits remained patent after 4 h. The animals tested with the NOrel polymer coated ECCs showed 79 ± 11% preservation of the platelet count over the course of the 4 h blood contact period, whereas animals tested with the control polymer ECCs exhibited a time-dependent loss in platelet count (54 ± 6 %). The blood from animals subjected to the NOrel and control ECCs exhibited similar response to collagen-stimulated platelet aggregation over the course of 4 h blood exposure. The percent of platelet aggregation was determined by ex-vivo collagen stimulation of PRP, measured by optical turbidity.22 NOrel and control coated circuits were able to maintain 88% and 91% aggregation, respectively, compared to their baseline values. This indicates that NO did not adversely affect the platelets ability to aggregate and the platelets maintained their functionality even after 4 h of exposure to the NO releasing surface. The level of plasma fibrinogen to which the activated platelets bind during the 4 h ECC blood exposure was also assessed. The plasma fibrinogen levels were corrected for any hemodilution due to added IV fluids into the rabbits. The plasma fibrinogen levels decreased by approximately 10% for the control ECCs, whereas the fibrinogen levels for NOrel ECC experiments dropped by 18% in 4 h (see Fig. S3 in ESI). This was found to be consistent with the previously reported results where the decrease in plasma fibrinogen levels was attributed to the binding of fibrinogen to the ECC surfaces.22, 37
Fig.6.
Time dependent effects of NOrel ECC (25 wt% DBHD/N2O2 +10 wt% 5050DLG7E PLGA) as compared to control ECC on rabbit platelet count (i.e. consumption) as measured via Coulter counter. The data are means ± SEM. * = p < 0.05, baseline vs. control ECC circuits.
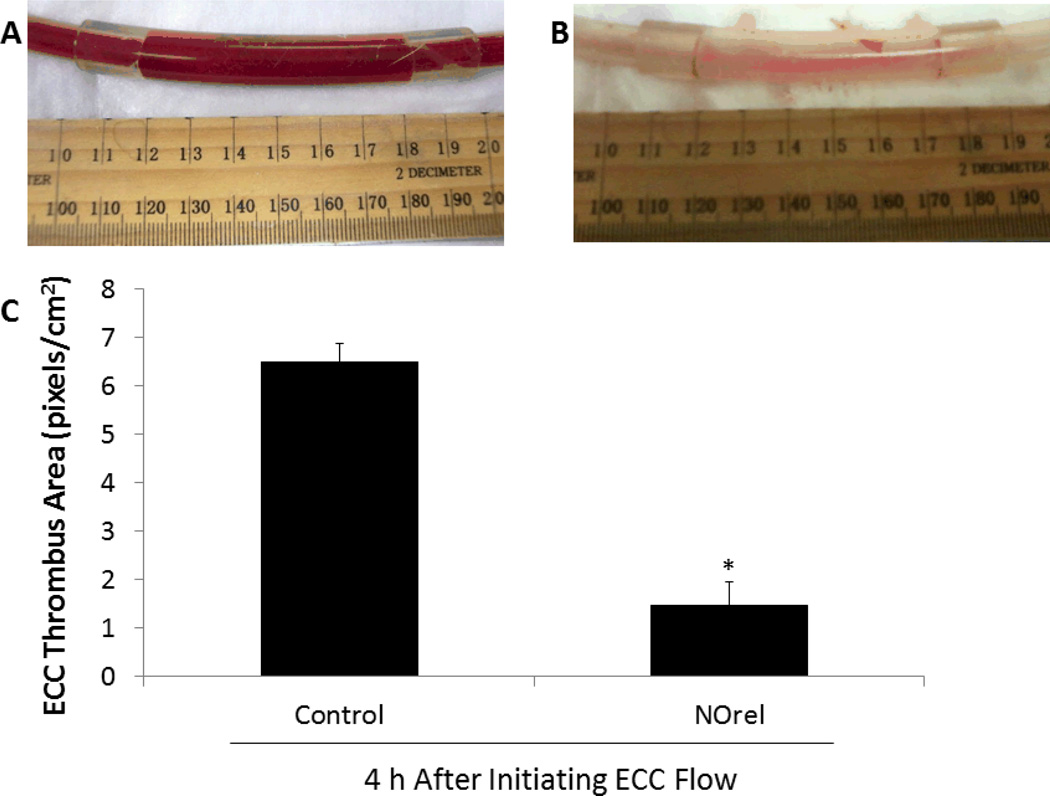
To ascertain the differential formation of thrombus in the thrombogenicity chamber (i.e., the 3/8 inch ID Tygon™ tubing 8 cm in length within the ECC loop) of the NOrel vs. control polymer-coated ECCs, 2-dimensional (2D) image analysis was performed after 4 h of blood exposure. Figures 7A and B, show representative images of the control and NOrel circuits, respectively, after being run for 4 h in the rabbit ECC model. Image J imaging software was used to calculate the representative 2D thrombus area (pixels/cm2) in each tubing chamber. These thrombi area measurements were quantitated and, as shown in Fig. 7C, the thrombus area of the NOrel polymer ECC was significantly reduced compared to the control polymer ECCs, 1.5 ± 0.5 and 6.5 ± 0.4 pixels/cm2, respectively. For an A-V shunt procedure, the lungs are the first main filter for the blood. After the 4 h experimental period, the lungs were evaluated for accumulated thromboemboli. The control ECCs rabbit lungs appeared to have more emboli accumulated in the lower lobes of both lungs then any of the NOrel ECC rabbit lungs.
Fig.7.
Evaluation of thrombus formation on NOrel and control polymer ECC after 4h blood exposure in rabbit thrombogenicity model. (A) Image of thrombus area in 3/8 inch I.D. tubing in the control ECC. (B) Image of thrombus area in 3/8 inch I.D. NOrel polymer ECC. (C) Quantitation of thrombus area as calculated with NIH Image J software using a 2D representation of thrombus. The data are means ± SEM. * = p<0.05, control ECC vs. NOrel ECC after 4h ECC flow.
5. Conclusions
This study demonstrates, for the first time, that an ester-capped PLGA material can be used as an additive within plasticized PVC films containing lipophilic diazeniumdiolate species, and the presence of the PLGA species sustains the NO release for much longer time periods than possible without the additive. By using various pH indicators it was shown that the hydrolysis rates of specific PLGA species employed can control the NO release properties by influencing the steady-state pH within the polymer films. Nitric oxide release from optimal PLGA-doped coatings used on the inner walls of ECC circuits was able to attenuate the activation of the platelets while maintaining their functionality. A significant reduction in the clot area was also seen with the new coatings vs. that observed on control ECC circuits. The new PLGA-doped NOrel PVC coatings are now under investigation in a 2–3 week rabbit model and could provide a breakthrough for achieving long-term preservation of circulating platelets, an important goal for longer-term ECC situations, such as ECMO.43
Supplementary Material
Acknowledgment
The authors declare this work is supported by the National Institutes of Health, Grants HL015434, EB000783 and K25HL111213.
References
- 1.Ratner BD. Biomaterials. 2007;28:5144–5147. doi: 10.1016/j.biomaterials.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratner BD. J. Biomater. Sci. Polym. Ed. 2000;11:1107–1119. doi: 10.1163/156856200744219. [DOI] [PubMed] [Google Scholar]
- 3.Kolobow T, Stool EW, Weathersby PK, Pierce J, Hayano F, Suaudeau J. Trans. Am. Soc. Artif. Intern. Organs. 1974;20A:269–276. [PubMed] [Google Scholar]
- 4.Szycher M. J. Biomater. Appl. 1988;3:297–402. doi: 10.1177/088532828800300207. [DOI] [PubMed] [Google Scholar]
- 5.Edmunds LH., Jr ASAIO J. 1995;41:824–830. [PubMed] [Google Scholar]
- 6.Didisheim P. ASAIO J. 1994;40:230–237. [PubMed] [Google Scholar]
- 7.Kim WS, Jacobs H. Blood Purificat. 1996;14:357–372. doi: 10.1159/000170288. [DOI] [PubMed] [Google Scholar]
- 8.Larm O, Larsson R, Olsson P. Biomater. Med. Devices Artif. Organs. 1983;11:161–173. doi: 10.3109/10731198309118804. [DOI] [PubMed] [Google Scholar]
- 9.Radomski MW, Moncada S. Adv. Exp. Med. Biol. 1993;344:251–264. doi: 10.1007/978-1-4615-2994-1_20. [DOI] [PubMed] [Google Scholar]
- 10.Vaughn MW, Kuo L, Liao JC. Am. J. Physiol. - Heart C. 1998;274:H2163–H2176. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 11.Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD. Blood. 2008;111:613–623. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen BL, Saitoh M, Ware JA. Am. J. Physiol. - Heart C. 1991;261:H1043–H1052. doi: 10.1152/ajpheart.1991.261.4.H1043. [DOI] [PubMed] [Google Scholar]
- 13.Sato Y, Hiramatsu Y, Homma S, Sato M, Sato S, Endo S, Sohara Y. J. Thorac. Cardiovasc. Surg. 2005;130:346–350. doi: 10.1016/j.jtcvs.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann AK, Aebert H, Reiz A, Freitag M, Husseini M, Ziemer G, Wendel HP. ASAIO J. 2004;50:193–199. doi: 10.1097/01.mat.0000123638.41808.59. [DOI] [PubMed] [Google Scholar]
- 15.de Souza GFP, Yokoyama-Yasunaka JKU, Seabra AB, Miguel DC, de Oliveira MG, Uliana SRB. Nitric Oxide. 2006;15:209–216. doi: 10.1016/j.niox.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Seabra AB, de Souza GFP, da Rocha LL, Eberlin MN, de Oliveira MG. Nitric Oxide. 2004;11:263–272. doi: 10.1016/j.niox.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Alston TA, Porter DJ, Bright HJ. J. Biol. Chem. 1985;260:4069–4074. [PubMed] [Google Scholar]
- 18.Annich GM, Meinhardt JP, Mowery KA, Ashton BA, Merz SI, Hirschl RB, Meyerhoff ME, Bartlett RH. Crit. Care Med. 2000;28:915–920. doi: 10.1097/00003246-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Schoenfisch MH, Mowery KA, Rader MV, Baliga N, Wahr JA, Meyerhoff ME. Anal. Chem. 2000;72:1119–1126. doi: 10.1021/ac991370c. [DOI] [PubMed] [Google Scholar]
- 20.Richter-Addo GB, Legzdins P. New York: Oxford, University Press; 1992. [Google Scholar]
- 21.Paulus WJ, Vantrimpont PJ, Shah AM. Circulation. 1994;89:2070–2078. doi: 10.1161/01.cir.89.5.2070. [DOI] [PubMed] [Google Scholar]
- 22.Major TC, Brant DO, Reynolds MM, Bartlett RH, Meyerhoff ME, Handa H, Annich GM. Biomaterials. 2010;31:2736–2745. doi: 10.1016/j.biomaterials.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies KM, Wink DA, Saavedra JE, Keefer LK. Journal of the American Chemical Society. 2001;123:5473–5481. doi: 10.1021/ja002899q. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Annich GM, Miskulin J, Osterholzer K, Merz SI, Bartlett RH, Meyerhoff ME. Biomaterials. 2002;23:1485–1494. doi: 10.1016/s0142-9612(01)00274-5. [DOI] [PubMed] [Google Scholar]
- 25.Batchelor MM, Reoma SL, Fleser PS, Nuthakki VK, Callahan RE, Shanley CJ, Politis JK, Elmore J, Merz SI, Meyerhoff ME. J. Med. Chem. 2003;46:5153–5161. doi: 10.1021/jm030286t. [DOI] [PubMed] [Google Scholar]
- 26.Wu B. PhD Dissertation. Ann Arbor: University of Michigan; 2009. [Google Scholar]
- 27.Zhou Z, Meyerhoff ME. Biomacromolecules. 2005;6:780–789. doi: 10.1021/bm049462l. [DOI] [PubMed] [Google Scholar]
- 28.Kaul S, Cercek B, Rengstrom J, Xu XP, Molloy MD, Dimayuga P, Parikh AK, Fishbein MC, Nilsson J, Rajavashisth TB, Shah PK. J. Am. Coll. Cardiol. 2000;35:493–501. doi: 10.1016/s0735-1097(99)00543-4. [DOI] [PubMed] [Google Scholar]
- 29.Holy CE, Dang SM, Davies JE, Shoichet MS. Biomaterials. 1999;20:1177–1185. doi: 10.1016/s0142-9612(98)00256-7. [DOI] [PubMed] [Google Scholar]
- 30.Lu L, Peter SJ, Lyman MD, Lai HL, Leite SM, Tamada JA, Uyama S, Vacanti JP, Langer R, Mikos AG. Biomaterials. 2000;21:1837–1845. doi: 10.1016/s0142-9612(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 31.Siegel SJ, Kahn JB, Metzger K, Winey KI, Werner K, Dan N. Eur. J. Pharm. Biopharm. 2006;64:287–293. doi: 10.1016/j.ejpb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Ding A, Schwendeman S. Pharm. Res. 2008;25:2041–2052. doi: 10.1007/s11095-008-9594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shenderova A, Ding AG, Schwendeman SP. Macromolecules. 2004;37:10052–10058. [Google Scholar]
- 34.Yoo JW, Lee JS, Lee CH. J. Biomed. Mater. Res. A. 2010;92A:1233–1243. doi: 10.1002/jbm.a.32434. [DOI] [PubMed] [Google Scholar]
- 35.Cai W, Wu J, Xi C, Meyerhoff ME. Biomaterials. 2012 doi: 10.1016/j.biomaterials.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Na DH, DeLuca PP. Pharm. Res. 2005;22:736–742. doi: 10.1007/s11095-005-2589-4. [DOI] [PubMed] [Google Scholar]
- 37.Lantvit SM, Barrett BJ, Reynolds MM. J Biomed Mater Res A. 2013 doi: 10.1002/jbm.a.34627. [DOI] [PubMed] [Google Scholar]
- 38.Miranda KM, Katori T, Torres de Holding CL, Thomas L, Ridnour LA, McLendon WJ, Cologna SM, Dutton AS, Champion HC, Mancardi D, Tocchetti CG, Saavedra JE, Keefer LK, Houk KN, Fukuto JM, Kass DA, Paolocci N, Wink DA. Journal of Medicinal Chemistry. 2005;48:8220–8228. doi: 10.1021/jm050151i. [DOI] [PubMed] [Google Scholar]
- 39.Hetrick EM, Schoenfisch MH. Annual Review of Analytical Chemistry. 2009:409–433. doi: 10.1146/annurev-anchem-060908-155146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coneski PN, Schoenfisch MH. Chem. Soc. Rev. 2012;41:3753–3758. doi: 10.1039/c2cs15271a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Major TC, Brant DO, Burney CP, Amoako KA, Annich GM, Meyerhoff ME, Handa H, Bartlett RH. Biomaterials. 2011;32:5957–5969. doi: 10.1016/j.biomaterials.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwendeman SP. Crit. Rev. Ther. Drug. 2002;19:73–98. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- 43.Oliver WC. Semin. Cardiothorac. Vasc. Anesth. 2009;13:154–175. doi: 10.1177/1089253209347384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.