Abstract
Arginine-vasopressin (AVP) facilitates water reabsorption by renal collecting duct principal cells and thereby fine-tunes body water homeostasis. AVP binds to vasopressin V2 receptors (V2R) on the surface of the cells and thereby induces synthesis of cAMP. This stimulates cellular signaling processes leading to changes in the phosphorylation of the water channel aquaporin-2 (AQP2). Protein kinase A phoshorylates AQP2 and thereby triggers the translocation of AQP2 from intracellular vesicles into the plasma membrane facilitating water reabsorption from primary urine. Aberrations of AVP release from the pituitary or AVP-activated signaling in principal cells can cause central or nephrogenic diabetes insipidus, respectively; an elevated blood plasma AVP level is associated with cardiovascular diseases such as chronic heart failure and the syndrome of inappropriate antidiuretic hormone secretion.
Here, we present a protocol for cultivation of primary rat inner medullary collecting duct (IMCD) cells, which express V2R and AQP2 endogenously. The cells are suitable for elucidating molecular mechanisms underlying the control of AQP2 and thus to discover novel drug targets for the treatment of diseases associated with dysregulation of AVP-mediated water reabsorption. IMCD cells are obtained from rat renal inner medullae and are used for experiments six to eight days after seeding. IMCD cells can be cultured in regular cell culture dishes, flasks and micro-titer plates of different formats, the procedure only requires a few hours, and is appropriate for standard cell culture laboratories.
Keywords: Cellular Biology, Issue 76, Bioengineering, Genetics, Molecular Biology, Biochemistry, Biomedical Engineering, Medicine, Pharmacology, Intercellular Signaling Peptides and Proteins, Exocytosis, Signal Transduction, Second Messenger Systems, Calcium Signaling, Cardiovascular Diseases, Hormones, Hormone Substitutes, and Hormone Antagonists, Life Sciences (General), water reabsorption, kidney, principal cells, vasopressin, cyclic AMP, aquaporin, animal model, cell culture
Introduction
In renal collecting duct principal cells, arginine-vasopressin (AVP) controls water reabsorption by stimulating the insertion of the water channel aquaporin-2 (AQP2) into the plasma membrane. AVP binds to the G protein-coupled vasopressin type-2 receptor (V2R) stimulating adenylyl cyclase and thereby cAMP formation. Initiation of this signaling cascade leads to activation of protein kinase A (PKA). PKA phosphorylates AQP2 at serine 256 (S256), which is the key trigger for its redistribution from intracellular vesicles into the plasma membrane. The membrane insertion facilitates water reabsorption along an osmotic gradient and fine-tunes body water homeostasis.
Dysregulation of AVP-mediated water reabsorption, due to aberrations of AVP secretion or AVP-activated signaling causes or is associated with severe diseases. Decreased water reabsorption caused by mutations of V2R or AQP2 leads to nephrogenic diabetes insipidus, whereas an elevated blood plasma AVP level is associated with excessive water reabsorption in cardiovascular diseases such as chronic heart failure and the syndrome of inappropriate antidiuretic hormone secretion (SIADH).
The significance of AVP-mediated water reabsorption stems not only from its implication in disease. The AVP-induced translocation of AQP2-bearing vesicles to and fusion with the plasma membrane represents a strictly cAMP-dependent exocytic process, which is currently not well understood. Other examples for a cAMP-dependent exocytosis are renin secretion in the kidney and H+ secretion in the stomach. Therefore, elucidation of molecular mechanisms governing the AQP2-translocation in renal principal cells not only helps to understand similar molecular processes in other cell types but may also pave the way to novel therapies for the treatment of diseases associated with disturbances of AVP-mediated water reabsorption.
Elucidating mechanisms controlling AQP2 requires collecting duct principal cell models. For this a number of different mammalian kidney cell lines are available. However, these models have several drawbacks. In many cell systems the protein level of AQP2 is low (Table 1; M1, HEK293, COS-7, MDCK, LLC-PK1)1-10, others ectopically overexpress human (MCD4, WT10)11,12 or rat (CD8)13 AQP2. In MCD4 and COS-7 cells the V2 receptor is not expressed. To our knowledge there is no permanent cell line as a model available, which is derived from the renal inner medullary collecting duct, that part of the kidney where AQP2 expression is most abundant, but from the cortical collecting duct1,11,13-16. Such cell models are for example the widely used immortalized mpkCCD (e.g.17) or the recently established mTERT-CCD14 cell lines. Both cell lines endogenously express AQP2 and V2R but since they are derived from the cortical collecting duct most likely contain a different proteome compared to inner medullary collecting duct (IMCD) cells. They must for example express different Na+ transport systems.
Here, we present a protocol to culture primary IMCD cells expressing V2R and AQP2 endogenously. This model, therefore, represents most closely the physiological situation in the renal collecting duct. As establishing the culture requires only standard laboratory equipment other laboratories can easily adopt this approach.
Protocol
1. Preparation
- Preparing the culture dishes
- Thaw Collagen Type IV O/N at 4 °C and dissolve it in 0.1% sterile acetic acid. The volume to use depends on the type and number of dishes in which the cells are to be seeded. Use 2 μg/cm2.
- Incubate dishes at least for 1 hr at RT and wash twice with distilled water (A. tridest).
- Allow the dishes to dry properly.
- Supplementation of medium
- Increase the glucose level up to 4.5 g/L by adding 1.75 g glucose to 500 ml medium. To adjust the medium to 600 mosmol, add 100 mM NaCl and 100 mM urea, to increase the osmolarity by 200 mosmol and 100 mosmol, respectively.
- Add 1% non-essential amino acids, 1% ultroser, 500 μM DBcAMP, 20 U/ml nystatin and 0.25 μg/ml gentamicin (1:200 dilution of stock solution with a concentration of 50 μg/ml) to 600 mosmol DMEM medium freshly on the day of preparation.
- Enzyme solution
- Add 1 mg/ml hyaluronidase and 2.2 mg/ml collagenase to DPBS-containing 0.25 μg/ml gentamicin and nystatin. Prepare 1 ml of this enzyme solution for the digestion of 2 kidney inner medullae. The solution must be sterilized by filtration and is stored in 50 ml Falcon tubes.
2. Cultivation of Primary Rat Inner Medullary Collecting Duct (IMCD) Cells
All animal handling procedures are to be carried out in compliance with the local and federal legislation.
Anaesthetize rats in CO2 (in a box saturated with CO2) or isoflurane (U-400 Anaesthesia unit, Univentor Ltd., Malta; air flow 350-400 ml/min with 2-2.5% isoflurane). Decapitate the animals for bleeding which permits exact detection of the border between the whitish inner and the darker outer medulla. In addition, the bleeding minimizes the number of isolated erythrocytes. Remove kidneys and wash them in sterile DPBS supplemented with gentamicin and nystatin (see above) on ice. All further steps are to be carried out under sterile clean bench conditions.
Transfer kidneys from the DPBS solution onto a sterile compress to remove excessive liquid. Remove the fatty kidney capsule with sterile forceps and scissors.
Having the kidney still fixed in the compress, cut slightly next to the longitudinal axis of the outer (cortical) side. Make sure not to cut through completely, but to leave it in one piece, connected in the renal pelvis.
With curved scissors carefully dissect the whitish inner medullae, which are surrounded by the rosé shimmering outer medulla. Collect them in DPBS, containing gentamicin and nystatin in a 60 mm culture dish on ice.
Once all medullae are isolated and collected on ice, reduce the volume of DPBS to a level where they are just covered with buffer. Use a sterile razorblade to cut the medullae into cubes of 5 mm3.
Melt and round the sharp end of a sterile Pasteur pipette by holding it briefly into a flame. Make sure the pipette is cooled before continuing.
Transfer the tissue suspension to the 50 ml Falcon tubes containing freshly prepared sterile enzyme solution with hyaluronidase and collagenase (see 1.3.1) by using the rounded Pasteur pipette. Close the lid tightly and incubate at 37 °C under continuous agitation (220 rpm) in a regular tabletop water bath for 1.5 - 2 hr.
For trituration of the suspension, pipette the mixture up and down several times with the round Pasteur pipette (see above) until the suspension becomes homogeneously turbid.
Centrifuge the suspension for 5 min at 300 x g and 16 °C. Discard the supernatant, resuspend the pellet thoroughly in DPBS, containing gentamicin and nystatin and centrifuge again. Repeat this washing step once.
Resuspend the cells in fully supplemented medium and seed them into culture dishes and/or micro-titer well plates. The preparation of 2 medullae (1 animal) will yield sufficient cells for one 60 mm culture dish.
After 24 hr, wash cells twice with 600 mosmol DMEM and add fresh medium. During the next week the cells should be washed 2-3 times (Figure 1). 6-8 days after seeding, the IMCD cells can be used for experiments. Remember to incubate them in medium without DBcAMP and nystatin for 24 hr before starting the experiment. Otherwise AQP2 will be located exclusively in the plasma membrane.
Representative Results
The successful cultivation of primary rat IMCD cells will result in a confluent monolayer 6-8 days after seeding (Figure 2). Per 60 mm culture dish there are approximately 6 x 106 cells. The cells tightly adhere to the culture dishes, as these were coated with collagen type IV, a basement membrane component18. Therefore, IMCD cells will not detach even during several thorough washing procedures. Up to 80% of the cultured cells express endogenously V2R and AQP2. These are the principal cells, whereas the cells lacking AQP2 are considered intercalated cells, non-IMCD cells derived from thin limbs of the loop of Henle, vasa recta or inner medullary interstitial cells. As we have previously shown, the medium osmolarity of 600 mosmol prevents down-regulation of AQP219. In addition, AQP2 expression is maintained by supplementing the medium with the membrane-permeable cAMP analogue DBcAMP. To reduce the AQP2 plasma membrane expression and thus to favor its intracellular localization the IMCD cells should be kept without DBcAMP approximately 24 hr before experiments. If most of the AQP2 is localized in the plasma membrane under control conditions, the time of DBcAMP-free cultivation should be prolonged. Lysis of cells grown in 60 mm culture dishes in standard buffers yields 1.5 mg protein10.
Upon short-term stimulation (30 min) with AVP or forskolin, a direct activator of adenylyl cyclase, AQP2 translocates from intracellular vesicles to the plasma membrane (Figure 2). In addition, AQP2 expression in IMCD cells increases upon a 30 min challenge with AVP or forskolin (Figure 3)10. The increase in AQP2 abundance is independent of enhanced transcription and translation. Forskolin or AVP stimulation leads to inhibition of p38-mitogen-activated-protein-kinase (p38-MAPK), which is associated with a decrease in the level of phosphorylation at serine 261 of AQP2 and a reduction in its poly-ubiquitination. Thus, the increase of AQP2 abundance upon stimulation is ascribed to decreased proteasomal degradation10. Taken together, IMCD cells allow investigating the mechanisms controlling AQP2.
| Cell line | Organism | ATCC Number or Reference |
| CAKI | Homo sapiens | HTB-46 |
| CD8 | Homo sapiens | Valenti et al., 199613 |
| COS-7 | Cercopithecus aethiops | CRL-1651 |
| HEK293 | Homo sapiens | CRL-1573 |
| LLC-PKI | Sus scrofa | CL-101 |
| M1 | Mus musculus | CRL-2038 |
| MCD4 | Mus musculus | Iolascon et al., 200711 |
| MDCK | Canis familiaris | CRL-2935 |
| mpkCCD | Mus musculus | Bens et al., 199915, Hasler et al. 200216 |
| mTERT-CCD | Mus musculus | Steele et al., 201014 |
| WT10 | Canis familiaris | Deen et al., 199712 |
Table 1. Mammalian kidney cell lines for studies of AQP2 control mechanisms. CAKI, mpkCCD and mTERT-CCD cells express AQP2 endogenously. In COS-7, HEK293, LLC-PK1, M1 and MDCK cell lines, AQP2 is hardly detectable. CD8, MCD4 and WT10 cells overexpress AQP2 ectopically.
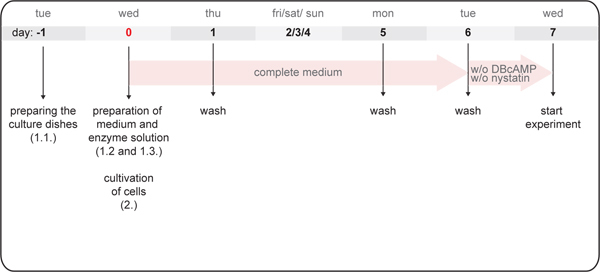 Figure 1. Schematic representation of the procedure for establishing IMCD cell cultures. Indicated are the order of experimental steps and the weekdays preferred for the experiments. Primary rat IMCD cells are seeded on day 0 and 1 week later are used for experiments. Numbers in brackets refer to the respective experimental steps described under "Protocol".
Figure 1. Schematic representation of the procedure for establishing IMCD cell cultures. Indicated are the order of experimental steps and the weekdays preferred for the experiments. Primary rat IMCD cells are seeded on day 0 and 1 week later are used for experiments. Numbers in brackets refer to the respective experimental steps described under "Protocol".
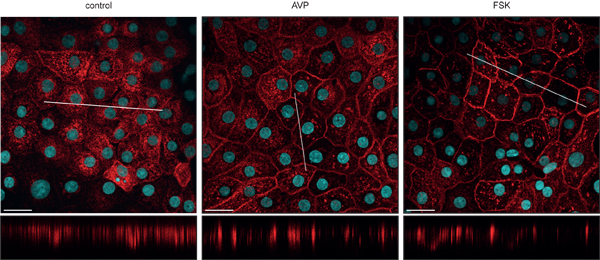 Figure 2. AQP2 inserts into the plasma membrane upon stimulation with AVP or forskolin. IMCD cells were left under control conditions or either stimulated with 100 nM AVP or 10 μM forskolin for 30 min at 37 °C. Cells were analyzed by immunofluorescence microscopy as described before (LSM 710; Carl Zeiss MicroImaging, Jena, Germany)10. Nuclei were visualized with DAPI, AQP2 was detected with a custom-made antibody (red; antibody H27)20,21. Upper panel, x-y scans; lower panel, x-z scans. Scale bars, 20 μm.
Figure 2. AQP2 inserts into the plasma membrane upon stimulation with AVP or forskolin. IMCD cells were left under control conditions or either stimulated with 100 nM AVP or 10 μM forskolin for 30 min at 37 °C. Cells were analyzed by immunofluorescence microscopy as described before (LSM 710; Carl Zeiss MicroImaging, Jena, Germany)10. Nuclei were visualized with DAPI, AQP2 was detected with a custom-made antibody (red; antibody H27)20,21. Upper panel, x-y scans; lower panel, x-z scans. Scale bars, 20 μm.
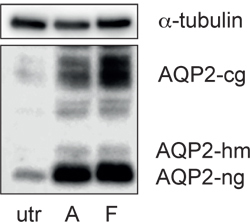 Figure 3. AQP2 protein abundance increases upon stimulation with vasopressin (AVP) and forskolin. IMCD cells were left untreated (utr) or either stimulated with 100 nM AVP (A) or 10 μM forskolin (F) for 30 min at 37 °C. Cells were lysed and complex glycosylated (AQP2 cg), high mannose (AQP2 hm) and non glycosylated AQP2 (AQP2 ng) was detected by immunoblot using specific antibodies against AQP2 (sc-9882, SantaCruz) as previously described10. As a loading control α-tubulin (CP06, Calbiochem) was detected. Shown is a representative result from > 3 experiments. Molecular weights are indicated (kDa).
Figure 3. AQP2 protein abundance increases upon stimulation with vasopressin (AVP) and forskolin. IMCD cells were left untreated (utr) or either stimulated with 100 nM AVP (A) or 10 μM forskolin (F) for 30 min at 37 °C. Cells were lysed and complex glycosylated (AQP2 cg), high mannose (AQP2 hm) and non glycosylated AQP2 (AQP2 ng) was detected by immunoblot using specific antibodies against AQP2 (sc-9882, SantaCruz) as previously described10. As a loading control α-tubulin (CP06, Calbiochem) was detected. Shown is a representative result from > 3 experiments. Molecular weights are indicated (kDa).
Discussion
We present a detailed protocol for the preparation and culturing of primary rat IMCD cells. The approach yields up to 21 cm2 of cells from one rat20. The experiment requires standard cell culture equipment and can be carried out by a single person within approximately 6 hr. Therefore, this approach is suitable as a standard laboratory method.
Primary rat IMCD cells can be seeded in culture dishes of different size, ranging from 96 well plates to 60 mm dishes. However, for growth in 384 well format the procedure requires optimization. We avoid using dishes larger than 60 mm as growth decelerated. The cells are suitable for a variety of experiments such as Western blotting, immunoprecipitation, DNA/RNA isolation, immunofluorescence microscopy, Ca2+ imaging and electrophysiology10,22,23.
Approximately 6 to 8 days after seeding, primary IMCD cells are grown to a confluent monolayer (Figure 2). The cell height is 3 - 5 μm on average24, and the cells are tightly attached and maintain a high level of endogenous AQP2 expression. Immunofluorescence microscopic detection of AQP2 indicates that up to 80% of the cells represent AQP2-positive principal cells (Figure 3;10,20 ). Morphologically the cells are characterized by long, straight cell borders, a nucleus embedded into cytoplasm with one to three nucleoli, and several prominent cytoplasmic granules20. The identity of the remaining 20% AQP2-negative cells in the culture has not unequivocally been defined. Maric et al. carried out phase-contrast microscopy and immunofluorescence microscopic analyses of tight junctions to visualize cell boundaries20. In AQP2-negative cells they observed curved cell borders with indentations and invaginations to their neighboring cells and a protruding nucleus with one nucleolus. Based on this morphology that is distinct from that of AQP2-positive cells, Maric et al. suggested that the majority of AQP2-negative cells are intercalated cells. In addition, the culture apparently contains few fibroblasts20. They only become apparent if the cultures are grown for more than 10 days when these cells start overgrowing the principal cells. Under our culture condition fibroblast do not display a typical fibroblast appearance. In line, Gonzalzez et al. found interstitial cells in similar primary IMCD cell cultures 5-8 days after seeding25.
Primary IMCD cell cultures prepared in slightly different ways but which possess similar properties as the ones described above are used in other laboratories. For example, Chou et al. seed primary IMCD cells on plastic surfaces that are not coated with collagen26. The primary IMCD cells prepared according to either protocol seem of similar high quality as for instance both are suitable for intracellular Ca2+ measurements22,23,26. Comprehensive lists of mRNAs and proteins expressed in primary IMCD cells were obtained from transcriptional profiling using Affimetrix technology27 and mass spectrometric proteome analysis28 (see also http://dir.nhlbi.nih.gov/papers/lkem/imcd).
Limitations of primary IMCD cells, as for many primary cells, include the low transfection rate (≈ 1%29). Thus overexpression of proteins is almost only possible if the encoding plasmids or the proteins themselves are microinjected (e.g.30). A further limitation is the lack of proper cellular polarity of primary IMCD cells. In response to AVP, AQP2 translocates predominantly into the basolateral plasma membrane (Figure 2). Our trials to induce proper polarity, i.e. to induce translocation of AQP2 to the apical plasma membrane, by growing the cells on various filters have not been successful, yet.
Taken together, primary IMCD cells, established as described above, possess the machinery for controlling AQP2 and are therefore a suitable model for studying AQP2 regulation. However, their use requires animals and should thus be limited to experiments that cannot be carried out in other established model systems (see above) and for validation of results that were obtained in other model systems.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG; KL 1415/3-2 and KL 1415/4-2).
References
- Stoos BA, Naray-Fejes-Toth A, Carretero OA, Ito S, Fejes-Toth G. Characterization of a mouse cortical collecting duct cell line. Kidney International. 1991;39:1168–1175. doi: 10.1038/ki.1991.148. [DOI] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. The Journal of general virology. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Ala Y, et al. Functional studies of twelve mutant V2 vasopressin receptors related to nephrogenic diabetes insipidus: molecular basis of a mild clinical phenotype. Journal of the American Society of Nephrology: JASN. 1998;9:1861–1872. doi: 10.1681/ASN.V9101861. [DOI] [PubMed] [Google Scholar]
- Richardson JC, Scalera V, Simmons NL. Identification of two strains of MDCK cells which resemble separate nephron tubule segments. Biochimica et Biophysica Acta. 1981;673:26–36. [PubMed] [Google Scholar]
- Chen Y, et al. Aquaporin 2 Promotes Cell Migration and Epithelial Morphogenesis. Journal of the American Society of Nephrology: JASN. 2012. [DOI] [PMC free article] [PubMed]
- Perantoni A, Berman JJ. Properties of Wilms' tumor line (TuWi) and pig kidney line (LLC-PK1) typical of normal kidney tubular epithelium. In vitro. 1979;15:446–454. doi: 10.1007/BF02618414. [DOI] [PubMed] [Google Scholar]
- Katsura T, et al. Constitutive and regulated membrane expression of aquaporin 1 and aquaporin 2 water channels in stably transfected LLC-PK1 epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7212–7216. doi: 10.1073/pnas.92.16.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull RN, Cherry WR, Weaver GW. The origin and characteristics of a pig kidney cell strain, LLC-PK. In vitro. 1976;12:670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- Nedvetsky PI, et al. Reciprocal regulation of aquaporin-2 abundance and degradation by protein kinase A and p38-MAP kinase. J. Am. Soc. Nephrol. 2010;21:1645–1656. doi: 10.1681/ASN.2009111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iolascon A, et al. Characterization of Two Novel Missense Mutations in the AQP2 Gene Causing Nephrogenic Diabetes Insipidus. Nephron Physiology. 2007;105:p33–p41. doi: 10.1159/000098136. [DOI] [PubMed] [Google Scholar]
- Deen PM, et al. Aquaporin-2 transfection of Madin-Darby canine kidney cells reconstitutes vasopressin-regulated transcellular osmotic water transport. Journal of the American Society of Nephrology: JASN. 1997;8:1493–1501. doi: 10.1681/ASN.V8101493. [DOI] [PubMed] [Google Scholar]
- Valenti G, Frigeri A, Ronco PM, D'Ettorre C, Svelto M. Expression and functional analysis of water channels in a stably AQP2-transfected human collecting duct cell line. The Journal of Biological Chemistry. 1996;271:24365–24370. doi: 10.1074/jbc.271.40.24365. [DOI] [PubMed] [Google Scholar]
- Steele SL, et al. Telomerase immortalization of principal cells from mouse collecting duct. American Journal of Physiology. Renal Physiology. 2010;299:F1507–F1514. doi: 10.1152/ajprenal.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bens M, et al. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. Journal of the American Society of Nephrology: JASN. 1999;10:923–934. doi: 10.1681/ASN.V105923. [DOI] [PubMed] [Google Scholar]
- Hasler U, et al. Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. The Journal of Biological Chemistry. 1074;277:10379–10386. doi: 10.1074/jbc.M111880200. [DOI] [PubMed] [Google Scholar]
- Miller RL, Sandoval PC, Pisitkun T, Knepper MA, Hoffert JD. Vasopressin inhibits apoptosis in renal collecting duct cells. American Journal of Physiology. Renal Physiology. 2012. [DOI] [PMC free article] [PubMed]
- Kleinman HK, et al. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Storm R, Klussmann E, Geelhaar A, Rosenthal W, Maric K. Osmolality and solute composition are strong regulators of AQP2 expression in renal principal cells. American Journal of Physiology. Renal Physiology. 2003;284:189–198. doi: 10.1152/ajprenal.00245.2002. [DOI] [PubMed] [Google Scholar]
- Maric K, Oksche A, Rosenthal W. Aquaporin-2 expression in primary cultured rat inner medullary collecting duct cells. Am. J. Physiol. 1998;275:796–801. doi: 10.1152/ajprenal.1998.275.5.F796. [DOI] [PubMed] [Google Scholar]
- Liebenhoff U, Rosenthal W. Identification of Rab3-, Rab5a- and synaptobrevin II-like proteins in a preparation of rat kidney vesicles containing the vasopressin-regulated water channel. FEBS Lett. 1995;365:209–213. doi: 10.1016/0014-5793(95)00476-p. [DOI] [PubMed] [Google Scholar]
- Lorenz D, et al. Cyclic AMP is sufficient for triggering the exocytic recruitment of aquaporin-2 in renal epithelial cells. EMBO Rep. 2003;4:88–93. doi: 10.1038/sj.embor.embor711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamma G, et al. The prostaglandin E2 analogue sulprostone antagonizes vasopressin-induced antidiuresis through activation of Rho. J. Cell Sci. 2003;116:3285–3294. doi: 10.1242/jcs.00640. [DOI] [PubMed] [Google Scholar]
- Maric K, et al. Cell volume kinetics of adherent epithelial cells measured by laser scanning reflection microscopy: determination of water permeability changes of renal principal cells. Biophys. J. 2001;80:1783–1790. doi: 10.1016/S0006-3495(01)76148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AA, et al. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension. 2011;57:594–599. doi: 10.1161/HYPERTENSIONAHA.110.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CL, et al. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. The Journal of Biological Chemistry. 2000;275:36839–36846. doi: 10.1074/jbc.M005552200. [DOI] [PubMed] [Google Scholar]
- Uawithya P, Pisitkun T, Ruttenberg BE, Knepper MA. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiological Genomics. 2008;32:229–253. doi: 10.1152/physiolgenomics.00201.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchapyjnikov D. Proteomic profiling of nuclei from native renal inner medullary collecting duct cells using LC-MS/MS. Physiological Genomics. 2010;40:167–183. doi: 10.1152/physiolgenomics.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan E, et al. Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J. Am. Soc. Nephrol. 2007;18:199–212. doi: 10.1681/ASN.2006020132. [DOI] [PubMed] [Google Scholar]
- Klussmann E, et al. An inhibitory role of Rho in the vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J. Biol. Chem. 2001;276:20451–20457. doi: 10.1074/jbc.M010270200. [DOI] [PubMed] [Google Scholar]


