Abstract
Cardiac arrest followed by resuscitation often results in dramatic brain damage caused by ischemia and subsequent reperfusion of the brain. Global brain ischemia produces damage to specific brain regions shown to be highly sensitive to ischemia 1. Hippocampal neurons have higher sensitivity to ischemic insults compared to other cell populations, and specifically, the CA1 region of the hippocampus is particularly vulnerable to ischemia/reperfusion 2.
The design of therapeutic interventions, or study of mechanisms involved in cerebral damage, requires a model that produces damage similar to the clinical condition and in a reproducible manner. Bilateral carotid vessel occlusion with hypotension (2VOH) is a model that produces reversible forebrain ischemia, emulating the cerebral events that can occur during cardiac arrest and resuscitation. We describe a model modified from Smith et al. (1984) 2, as first presented in its current form in Sanderson, et al. (2008) 3, which produces reproducible injury to selectively vulnerable brain regions 3-6. The reliability of this model is dictated by precise control of systemic blood pressure during applied hypotension, the duration of ischemia, close temperature control, a specific anesthesia regimen, and diligent post-operative care. An 8-minute ischemic insult produces cell death of CA1 hippocampal neurons that progresses over the course of 6 to 24 hr of reperfusion, while less vulnerable brain regions are spared. This progressive cell death is easily quantified after 7-14 days of reperfusion, as a near complete loss of CA1 neurons is evident at this time.
In addition to this brain injury model, we present a method for CA1 damage quantification using a simple, yet thorough, methodology. Importantly, quantification can be accomplished using a simple camera-mounted microscope, and a free ImageJ (NIH) software plugin, obviating the need for cost-prohibitive stereology software programs and a motorized microscopic stage for damage assessment.
Keywords: Medicine, Issue 76, Biomedical Engineering, Neurobiology, Neuroscience, Immunology, Anatomy, Physiology, Cardiology, Brain Ischemia, ischemia, reperfusion, cardiac arrest, resuscitation, 2VOH, brain injury model, CA1 hippocampal neurons, brain, neuron, blood vessel, occlusion, hypotension, animal model
Introduction
Brain damage as a consequence of cardiac arrest and stroke is a leading cause of death and long-term disability. While cardiopulmonary resuscitation for victims of cardiac arrest succeeds in restoring spontaneous circulation in about 70,000 patients per year in the US 7,8 at least 60% of these patients subsequently die in the hospital as a result of extensive brain damage and only 3-10% of resuscitated patients can resume their former lifestyles 9,10 . Clearly, understanding the mechanisms that lead to brain damage following global brain ischemia and designing therapeutic interventions to minimize neurologic trauma is of critical importance.
Brain ischemia can be modeled utilizing multiple methods. Most commonly, brain ischemia is produced in the rodent by occluding a major blood vessel in the brain, the middle cerebral artery, thus producing a focal ischemic stroke 11,12 . While clinically important, focal brain ischemia is not an accurate method to study brain damage produced by cardiac arrest/resuscitation. To model this clinical paradigm the entire brain must be made ischemic followed by reintroduction of blood flow. To closely mimic this clinical presentation, investigators experimentally induce cardiac arrest followed by resuscitation with CPR and defibrillation 13,14 . This model is clinically relevant, however unpredictable resuscitation times can increase variability and may make data analysis difficult to interpret. Additionally, this model is associated with a high mortality rate, further increasing animal number necessary to test a hypothesis. Investigating the cerebral response to global ischemia and/or reperfusion in a more reproducible, consistent, and survivable insult may be preferred.
Global ischemia can be induced in the brain while preserving some blood flow systemically. This reduces mortality, while allowing investigation of the mechanisms of tissue damage in the brain 2. To produce global brain ischemia, it is necessary to interrupt or greatly limit flow in all four vessels that supply the brain, the internal carotid arteries and vertebral arteries. These vessels supply the brain with blood flow through a vascular structure called the Circle of Willis, which forms an anastomotic loop. This vascular architecture allows the brain to retain perfusion in the event of proximal vascular occlusion. Therefore, to induce complete ischemia of the brain, blood flow through all contributory vessels must occur. Carotid artery occlusion can be accomplished using a minimally invasive ventral neck cut-down and application of aneurism clips for a desired period. Interruption of blood flow via the vertebral arteries can be difficult, as they are incased in the transverse foramina of the vertebral column. Investigators have addressed this by electrocauterizing the vertebral arteries 24-48 hr prior to carotid occlusion and brain ischemia (4VO model) 15. In contrast to this approach, Smith, et al. developed a method of inducing global brain ischemia by reducing mean arterial blood pressure (MAP) systemically to 40 mmHg to reduce perfusion through the vertebral arteries to a point where blood flow is lost or greatly reduced 2. When coupled with carotid occlusion, this method produces ischemia throughout the forebrain, resulting in a pattern of brain damage that closely mimics that of cardiac arrest survivors. In a further refinement of this method, the model we present here requires tight MAP regulation at 30 mmHg ± 1mHg during the entire 8 min of ischemia. We found this alteration improves the reproducibility of brain damage induced by this model while preserving the low mortality rate of the original technique designed by Smith et al.
The precise phenotype of cell death and overall extent of tissue damage caused by the model presented here are directly dependent on ischemic duration 16. Following 8 min of ischemia, CA1 neurons exhibit delayed cell death, suggesting that there is a temporal window for therapeutic intervention during the reperfusion phase 15,17 . At the onset of reperfusion, neurons quickly regain function and no immediate cell death is detectable 18. However this insult causes induction of cell death cascades (apoptosis) that culminate in release of apoptogenic proteins from mitochondria, including cytochrome c, between 4-6 hr of reperfusion 3,19. Between 6 and 24 hr of reperfusion, neurons of the CA1 hippocampus have committed to cell demise, and the apoptotic cell death program is executed 19. It should be noted that cell death phenotype responsible for ischemic injury is highly controversial. Early studies have suggested necrosis is the primary cell death phenotype 20,21 , while other others report apoptosis as the principal mechanism 22,23 . In total, current evidence suggest that cells die of a spectrum of cell death phenotypes ranging from classic apoptosis to necrosis. The specific mode of cell death is dependent on many factors, with the degree of contribution of each phenotype depending on the severity of the insult, amongst other factors 24,25. By 24 hr of reperfusion, dying cells possess pyknotic nuclei, condensed cytosol with clear evidence of aggregated cellular contents, and loss of functional mitochondrial morphology. Dead cells are further broken down, engulfed by immune cells such as macrophages and/or microglia, and cleared from the CA1 hippocampal region. By 4-7 days of reperfusion, dead cells are removed, and all that remains are inflammatory cells and activated glial cells 17,26 . Therefore, 7 days of reperfusion represents an optimal time where CA1 hippocampal neuronal death can be quantified using simple, non-specific cell stains including Cresyl violet or hemotoxylin-eosin and counted based on morphologic inclusion criteria. Cells remaining at this late reperfusion interval can be counted as surviving cells, thus providing an index of brain damage.
If this model is to be utilized to test therapeutic interventions, it is suggested that the experimental design follow STAIR criteria (Stroke Therapy Academic Industry Roundtable) 27. These guidelines should be followed when designing and conducting a study, however are not discussed here.
Protocol
1. Preparation
All animal experiments must conform to institutional guidelines and receive approval by a respective animal care committee prior to initiation. All procedures presented here have been approved by the Wayne State University Institutional Animal Care and Use Committee and follow the guidelines on the ethical treatment of animals as put forth in the Guide for the Care and Use of Laboratory Animals and to the US Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training. Before beginning surgery, prepare necessary surgical material and a surgery recovery cage. This procedure is a survival surgery and it is therefore necessary to practice sterile technique.
To make a vascular catheter, cut an 8 inch length of polyethylene 50 (PE50) tubing and insert a blunted 23 gauge needle into one end. Catheters can be purchased individually pre-sterilized or purchased in bulk and then sterilized with ethylene glycol as needed.
Secure this needle onto one port of a three way stopcock and place another three way stopcock on the opposite port. Heparinize the stopcocks and catheter by running heparin saline solution through. Connect a 10 ml saline syringe onto the stopcock perpendicular and distal to the catheter. Connect the stopcock distal to the catheter to the pressure transducer tube, fill the system with saline and remove any air bubbles.
Setup the recovery cage in accordance with animal use regulations. The recovery room should be quiet and have low traffic. Important: Turn on the downdraft table or similar gas scavenging system before beginning surgery to filter vaporized isoflurane.
2. Surgical Preparation
Sprague-Dawley rats (300-350 g) are used in the 2VOH model of global forebrain ischemia. Anesthesia is induced and maintained with isoflurane and supplemented with a sub-dissociative dose of ketamine. We have observed that anesthesia maintained by isoflurane alone causes an increased occurrence of seizure during post-op recovery. Seizures can contribute to neural damage and mortality, which will impact the reproducibility of this model. Supplementing anesthesia with ketamine allows a much lower dose of isoflurane to reach a surgical grade of anesthesia. Using a homeostatic thermal blanket allows strict regulation of core temperature. The surgical procedure entails carotid artery cut-down and isolation, and femoral artery cut-down and cannulation.
Note: It is important to keep detailed records during each individual surgical procedure. Changes in core temperature, blood pressure, or other physiologic variables that occur prior to or during surgery may drastically alter results, and records can be analyzed to ensure there is procedural consistency between individuals and groups. Consistency in the physiologic parameters of the animal can be improved by fasting the animals prior to surgery. Experimenters are encouraged to determine the method that results in the most consistent pre-operated hemodynamics and serum glucose concentration for their studies.
Induce anesthesia by placing the rat into an induction chamber and fill with a 30% oxygen/70% nitrous oxide mixture at 5% isoflurane. Intubate with a 12 gauge catheter, using a rodent laryngoscope and ventilate with 30% oxygen/70% nitrous oxide mixture with 2.5% isoflurane. Ventilation rate should be 80 breaths per minute at 2.5 ml per breath.
Give an intra-peritoneal injection of ketamine (20 mg/kg) in saline and reduce isoflurane to 1.5%. Important: It is imperative to monitor the level of anesthesia throughout the procedure to ensure adequate surgical plane of anesthesia. To check depth of anesthesia, pinch between hind-limb digits, while monitoring pedal reflex. If the reflex is absent, anesthesia is adequate. After cannulation of the femoral artery, blood pressure can be used as an indicator of anesthesia level. Keep the dose of isoflurane as low as possible while maintaining a surgical plane of anesthesia to minimize the potential of post-ischemic seizures.
Incisions will be made on the neck and in the pelvic area. Shave the neck and the right pelvis, where the thigh meets the abdomen. Orient the rat in a supine position on the homeostatic thermo-blanket and insert the rectal thermometer using surgical lubricant. Apply protective eye lubricant. A 60 watt light bulb can help regulate temperature. The bulb should never be closer than 8 inches from the animal. Important: Temperatures above or below normal physiologic temperature (37 °C) can greatly influence final neurologic damage. Maintain core temperature at 37 °C ± 0.5 °C.
Scrub the incision areas with betadine and rinse with 70% ethanol. Repeat this two more times. Place a surgical field over the rat, and cut holes to expose the incision areas. Important: Carotid and femoral arteries can be isolated without causing any trauma to the surrounding musculature, which will improve recovery and minimized subsequent need for post-operative analgesia.
Pending adequate anesthesia, use a No. 10 scalpel to make a midline incision along the neck, then, using hemostats bluntly dissect between the salivary glands until reaching the sternohyoid muscle, the prominent midline muscle group covering the trachea. Again using careful blunt dissection technique, separate the major muscle groups of the sternohyoid from the sternomastoid, bilaterally. These two muscle groups form a triangular indentation which can be used as a landmark to locate the neurovascular bundle that contains the carotid artery. The common carotid branches into the internal and external carotid arteries. Isolate the common carotid artery proximal to the bifurcation.
Gently separate these two muscles and locate the carotid artery. To help identify this vessel look for a pulse. Isolate the carotid arteries on both sides by passing a ~5 inch length of 3-0 silk suture beneath the vessel. Clear away fascia from the vessels. Caution: The vagus nerve and sympathetic chains are included in the cervical neurovascular bundle and care should be taken to avoid causing damage to it when isolating the carotid artery.
Use scissors to make a second incision at the groin, along the indentation where hind-limb thigh muscles meets the abdomen. Dissect beneath the abdominal muscle, along the thigh muscles until you reach the inguinal ligament. This will expose the femoral neurovascular bundle. Carefully isolate the femoral artery by passing a ~5 inch length of 3-0 silk suture beneath the vessel. Clear away fascia leaving 5-7 mm of exposed vessel.
Tie a permanent knot at the distal end of the exposed artery. Note: Permanent knots should begin with a friction knot, followed by a square knot (total of two knots). This rule includes suture knots. Place another 3-0 silk suture around the proximal end of the artery and tie a loose knot.
Apply traction on the suture at the proximal end of the artery to occlude blood flow, by pulling the sutures taught. Make a small incision across the top of the vessel with ophthalmic scissors. Inadequate traction on the vessel will result in bleeding, which can be stopped by applying heavier traction. Close the catheter at the stopcock.
Using a vascular introducer, insert the catheter tubing into the vessel, 7-9 mm past the vessel incision and toward the midline. Once the catheter is placed at the desired distance, tie the loose knot around the vessel and catheter to secure it in place. Remove traction from the vessel and allow it to lie naturally.
Administer 0.3 ml heparin (100U/ml in saline), intravenously. Flush any blood out of the catheter with a small amount of saline to prevent clotting. Turn on the pressure monitor and calibrate the equipment. Open the stopcocks to allow the transducer to detect blood pressure. To obtain precise measurements of blood pressure, positioning of the system should not be changed after calibration.
Isoflurane dosage should be adjusted to yield a mean arterial blood pressure (MAP) of 110 mmHg to 130 mmHg. This MAP should be indicative of adequate surgical plane of anesthesia.
3. Ischemia Protocol
As mentioned previously, four vessels supply perfusion to the brain. Proximal occlusion of the two carotid arteries will not result in brain ischemia because the vertebral arteries will compensate through the Circle of Willis. It has been shown that carotid occlusion, coupled with induced systemic hypotension, will limit perfusion through the vertebral arteries resulting in brain ischemia. Here we describe the protocol for withdrawing blood to produce hypotension and clamping the carotid arteries to produce controlled, reversible ischemia. See Figure 1.
Blood oxygen and carbon dioxide, glucose concentration and pH can vary among individuals in the sample group and cause variation in the injury. Monitoring these physiological variables can reduce variability of the infarction. As mentioned, temperature is another parameter which is important to regulate, however brain temperature may not correspond to core temperature, as perfusion is greatly restricted during experimental ischemia28. Our specific surgical setup results in temperature changes in the brain that mirror changes in core temperature during ischemia. However, it is important for the experimenter to determine this empirically by monitoring brain temperature with thermocouples or by other means to standardizing the procedure. If core temperature does not mirror brain temperature, it is important to maintain the brain temperature normothermic during the entire procedure, irrespective of core temperature.
Randomization dictates that the surgeon randomizes the animal to ischemia or sham-operated control group at this point. Sham surgery will follow all procedures exactly the same as ischemic animals excluding any reduction in blood pressure and carotid artery occlusion. It is important that the sham-operated controls be under the same dose of anesthesia for a duration that matches that of the ischemic animals. If there is a treatment group included, which requires anesthesia before or after ischemia, sham and untreated ischemia groups should match the anesthesia dose and duration of the treatment group.
Ready hemostatic clips and clip applicator. Clips should completely occlude the vessel without causing any trauma. Ischemia can be induced when hemodynamics have become consistent. Connect a 10 ml heparinized syringe onto the stopcock, perpendicular and proximal to the catheter. There should be 0.3 ml (30 units) of heparinized saline inside the syringe to prevent coagulation of the withdrawn blood.
Set a timer for 1 min and open the stopcock to the heparinized syringe, so blood can be withdrawn.
Withdraw blood by pulling on the syringe plunger. If too much suction is applied, the vessel will collapse or seal against the catheter opening and no blood will be drawn. It should be possible to remove 7-9 ml of blood in 1 min. If this is not the case, advanced the catheter further into the artery and retry. We find that 7-9 ml of blood must be removed to reduce the MAP near 30 mmHg, the target blood pressure to achieve ischemia. Important: Ensure the withdrawn blood is kept at 37 °C to avoid cooling during blood reinfusion.
After the one minute blood draw (7-9 ml of blood should be withdrawn), apply hemostatic clips to the carotid arteries and begin a timer for 8 min. Immediately check blood pressure. If the MAP is not at 30 mmHg, infuse or withdraw blood slowly to achieve 30 mmHg ± 1 mmHg. Continue this practice throughout ischemia to maintain MAP of 30 mmHg. Important: Record blood pressure throughout the entire ischemia protocol. Failure to reduce blood pressure to 30 mmHg may limit ischemia. Monitor core and/or brain temperature closely during reinfusion as increases or decreases in temperature may influence brain damage.
At the end of 8 min, begin reperfusion by removing the hemostatic clips and reinfusing the blood slowly, 2 ml per minute.
When the blood has been reinfused the cannula can be removed from the femoral artery. To avoid any bleeding during post-op, secure two separate knots proximal to the incision on the artery.
Suture the incisions with a discontinuous pattern using an inverse cutting needle, with 5-0 VICRYL suture. This suture will dissolve, so it will not require removal. Note: During the procedure, if sufficient anesthesia cannot be reached with lower levels of isoflurane, administer an additional dose of ketamine.
4. Analgesia and Recovery
Animal welfare is the priority during recovery as well as the surgical procedure. Post-operative care should follow the specific guidelines of the institution. Animals that undergo global brain ischemia are susceptible to seizures. To minimize seizure occurrence it is important that the recovery room have minimal stimulation, i.e. noises and visual disturbances. Under our animal use protocol, we house post-operative animals for 72 hr in a secluded room for this purpose.
After the incisions have been sutured the rat can be weaned off the ventilator. Turn off the isoflurane and continue ventilation at 30% oxygen/70%nitrous oxide until the rat begins to breathe against the ventilator.
Administer 0.5 mg/kg butorphanol in 5 ml saline, subcutaneously between the shoulder blades.
Rapidly reinfusing blood may increase right ventricular preload. This increases pulmonary circulation pressure and could cause pulmonary edema. Signs of this include labored respiration and crackling sounds during respiration. Applying positive end expiratory pressure will help improve pulmonary edema.
When voluntary control of respiration is regained, extubate, remove the thermometer and turn off the heating blanket. At this point the animal can be placed into a recovery cage. The recovery cage should be positioned so half of the cage is on top of a water circulating blanket (34 °C) for the first 24 hr of reperfusion. Place the animal on the part of the cage floor over the heating blanket to aid in temperature maintenance. Once the animal begins to recover from anesthesia and analgesia, it will move around the cage to thermoregulate itself. Animals will be housed separately during post-op.
The animal should have unlimited access to water. For two days post-op, this water should contain 4 mg/kg liquid Tylenol solution. In addition to rodent chow, sweetened breakfast cereal is provided in the cage to stimulate eating and prevent weight loss.
Cover half of the cage with a drape and closely monitor post-operative recovery.
As the animal recovers from anesthesia it is important to monitor for signs of distress. Labored or inconsistent respiration can be a sign of distress, possibly caused by pulmonary edema or airway irritation during intubation. Butorphenol will sedate the animal, reducing activity, however mild activity should be restored within 1 hr. We find animals will begin eating treats and drinking within 4 hr of extubation. By 12 hr, normal behavior and feeding activity should be resumed. Another sign of distress is weight loss; an animal should lose no more than 20% of original body weight in any 48 hr span, if excessive weight loss is observed the animal should be euthanized.
Humane intervention is necessary if seizures occur. This type of seizure activity is generally seen 24 to 48 hr after reperfusion. Seizures can cause increased brain damage that is independent of the ischemic insult per se, thus seizure activity should be considered an exclusion criteria set in place prior to initiation of the study 29. Seizures can occur when no one is present, so it is important to recognize the signs. Bedding will be disturbed and possibly expelled from the cage. A postictal rat will have a languid disposition with rapid, labored breathing and/or have a bruised or bleeding nose.
Note: Any signs of pain or distress are immediately alleviated with analgesics. If sympotoms are not alleviated with one dose of analgesics or persist after the fourth consecutive dose of analgesia, the animal will be euthanized and excluded from the study. Surgical wounds should be inspected for proper healing for eight days post-op.
5. Tissue Collection and Processing
The brain is fixed by transcardial infusion of paraformaldehyde. After gross sectioning and cryoprotection, we snap freeze the brain and section on a cryostat. These brain sections are stained with Cresyl violet and imaged on a light microscope.
Induce anesthesia by placing the animal into the induction chamber and filling with 30% oxygen/ 70% nitrous oxide with 5% isoflurane. Intubate the animal and ventilate at 30% oxygen/ 70% nitrous oxide with 5% isoflurane.
Prepare the material for the perfusion procedure. Fill one beaker with 100 ml of 1x phosphate buffered saline (PBS) at 4 °C and one beaker with 100 ml of 4% paraformaldehyde (PFA) at 4 °C. The brain is flushed with PBS followed by PFA, through a perfusion pump. Tubing will be placed into each beaker and connected by a three-way stopcock to allow a smooth transition from PBS to PFA perfusion. Place a blunted 18 gauge needle onto the tubing and fill the line with PBS. Ensure there are no bubbles in the needle and tubing as air may form an embolism, which could prevent perfusion. Also, ready a small container with PFA for immersion fixation of the brain.
Use a fluid collection tray, with enough capacity to hold at least 200 ml of fluid. At this point the animal should have been ventilated with isoflurane for long enough (at least 3 min) to reach deep anesthesia.
Important: Ensure surgical plane of anesthesia is reached.
Proceed by making an incision to access the thoracic cavity through the abdomen. Clamp the descending aorta. When the heart is exposed, insert the blunted 18 gauge needle through the apex of the heart into the aorta. Make a small cut in the right auricle to allow perfusate to exit the animal.
Turn on the pump at 50 ml/min, beginning with PBS. After pumping 100 ml of PBS, switch the stopcock to perfuse 100 ml of PFA.
Remove the brain, taking care not to damage the tissue.
Place the brain into a tissue matrix and cut between the cerebellum and cerebrum. Section the forebrain; ~ 3 millimeters from the front and back of the cerebral cortex. This will produce three sections, the middle section will contain the hippocampus.
Place the brain sections into a jar with enough PFA for complete immersion. Immersion fix the brain for exactly 2 hr.
To protect the tissue from damage caused by freezing, the brain is cryoproteced by replacing the PFA with 30% sucrose in PBS. Cryoprotection is complete with the tissue samples sink in the sucrose solution (~24-48 hr).
To snap freeze the brain sections, place a beaker in dry ice and fill with 2-methylbutanol for ten minutes. Place the brain slices into the methylbutanol for 5 min, then transfer into a sealable container and store at -80 °C until cryostat sectioning.
Cryostat section the brain at 20 μm, collecting at least 3 sections at 3 brain atlas coordinates (The Rat Brain in Stereotaxic Coordinates. Paxinos and Watson, plate sections 29, 31, 33 or Bregma -2.8 mm, -3.3 mm, and -3.8 mm). The sections are placed on charged microscope slides and allowed to dry before storing at -80 °C.
Cresyl violet (0.1% at pH 3.5) stain 9 brain sections, 3 at each brain atlas coordinate.
Image the CA1 region of the hippocampus at 40x with a microscope mounted camera and insert a 300 μm scale bar parallel with the CA1 neuronal plane. Save images as TIFF files.
6. Brain Damage Quantification
ImageJ, a free program offered by the National Institute of Health, can be used to count and quantify the viable neurons, which will represent the extent of brain damage.
Open microscope TIFF images with the 'cell counter' plugin of ImageJ. Select a marking color and count neurons within the boundaries of the scale bar. Neurons are counted based on simple inclusion criteria based on neuronal morphology (large, pyramidal shape) or exclusion criteria for microglial/astrocyte morphology (small round or long tubular shape). Important: Be consistent with inclusion /exclusion criteria between slides, and individuals when counting neurons.
Record neuron counts and save the cell counter window.
Neuron counts should be completed by two separate people to attain consistent, accurate neuron counts.
The mean of all 9 images from each animal will provide a single mean "neuron count", which is the quantitative measure of CA1 hippocampal damage to be used in statistical analysis. Groups can be compared statically using a one-way ANOVA followed by a Tukey's HSD test for post hoc analysis, or, where normality test fails, a Kruskal-Wallis one-way ANOVA on ranks followed by Dunn's post hoc analysis to statistically evaluate differences between groups. Specific statistical analysis should be dictated by the individual study design, the one presented here may not be appropriate for the specific hypotheses being tested.
Representative Results
The 2VOH model of global brain ischemia/reperfusion causes neuronal death in the CA1 region of the hippocampus. Figure 2 represents the injury produced by 8 min of global brain ischemia, processed 14 days after reperfusion. Figures 2A and 2B compare the hippocampi from sham and post-ischemic brains, stained with Cresyl violet. Figure 2A shows a hippocampus from a sham-operated rat which exhibits normal morphology, including an intact CA1. Figure 2B demonstrates the hippocampus subject to ischemia/reperfusion, where the dentate gyrus, CA2 and CA3 have minimally affected morphology. The selectively vulnerable neurons of the CA1 hippocampus do not survive ischemia/reperfusion injury and only scattered neurons remain. However, dramatic increases in infiltrating macrophages and/or microglia (Iba-1-positive cells), and reactive astrocytes (GFAP-positive cells) are evident in the CA1 hippocampal field. Specific immunofluorescent labeling of neurons with NeuN, macrophage/microglia labeling with Iba-1, and astrocyte labeling with GFAP corroborate these findings.
Figure 2C presents sample neuron counting windows for microscope images of the CA1 at 40x magnification. The top panel shows CA1 from a sham-operated control and the lower panel shows the CA1 following ischemia/reperfusion. The injured CA1 displays a staining that includes microglia and astrocytes, which appear small and tubular or teardrop shaped. These can be distinguished from neurons by their shape. Intact hippocampal neurons, whether in the dentate gyrus, CA2 or the CA1, are large with a circular or pyramidal shape.
Figure 2D is a graphical analysis of the 2VOH induced injury quantified by CA1 neuronal counts. It can be concluded that 8 min of ischemia produced by the 2VOH model can cause a consistent and reproducible injury that is primarily isolated to the CA1 hippocampus.
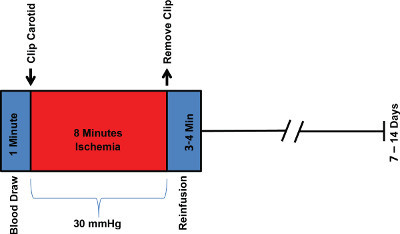 Figure 1. Experimental Timeline. The experimental timeline is a representation of the surgical steps and tissue processing.
Figure 1. Experimental Timeline. The experimental timeline is a representation of the surgical steps and tissue processing.
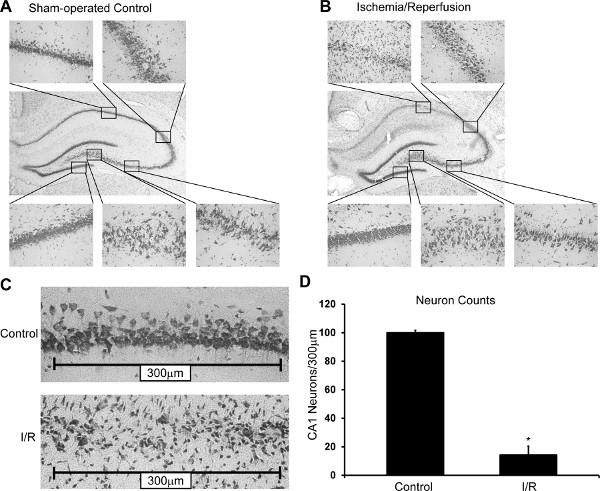 Figure 2. Representative Results. Hippocampal damage in an untreated rat subjected to I/R (B) compared to a sham-operated control rat (A). Cresyl violet stained hippocampus (10X) [Surround] 40X magnifications of the CA1, CA2, CA3, hilus, and DG (top left clockwise). (B) Damage is localized to the CA1 hippocampus. (C) Representative counting windows used for neuron counting and damage quantification in a sham-operated control animal (top, control) and an animal exposed to global brain ischemia (bottom, I/R). (D) Representative quantitation of CA1 hippocampal neuron counts from an unpublished study (Mean + Standard Deviation; n = 8, p<0.05).
Figure 2. Representative Results. Hippocampal damage in an untreated rat subjected to I/R (B) compared to a sham-operated control rat (A). Cresyl violet stained hippocampus (10X) [Surround] 40X magnifications of the CA1, CA2, CA3, hilus, and DG (top left clockwise). (B) Damage is localized to the CA1 hippocampus. (C) Representative counting windows used for neuron counting and damage quantification in a sham-operated control animal (top, control) and an animal exposed to global brain ischemia (bottom, I/R). (D) Representative quantitation of CA1 hippocampal neuron counts from an unpublished study (Mean + Standard Deviation; n = 8, p<0.05).
Discussion
The model described here produces an ischemic insult to the brain that can occur as a result of cardiac arrest and resuscitation, providing an injury similar to that found in humans. This method for producing global brain ischemia is one of multiple protocols. We utilize this protocol foremost for its comparably low mortality rate, rapid recovery, and reproducible results. The cardiac arrest/resuscitation model is arguably the most clinically relevant model, however technically the most difficult to constantly reproduce. The 4VO model of global brain ischemia is another commonly used protocol, valuable in the fact that all four contributing vessels are occluded during ischemia. This model also has drawbacks, which include multiple surgeries and possibility of neuroprotective preconditioning. The 4VO model has been adapted, allowing transient occlusion of all four vessels during one procedure to produce ischemia, however the surgery remains technically demanding 30.
Variations on 2VOH have been shown to produce a range of results 31,32 . Duration of ischemia and degree of hypotension are the major variables that can affect outcome. Reports have shown that if blood pressure is not reduced enough the injury produced can be inconsistent or unilateral in rats 30. We have found 30 mmHg to be an optimal degree of hypotension because it produces reliable ischemia without causing notable peripheral injury. This paradigm produces ischemia characterized as severe, however following our protocol will reduce the otherwise increased chance of complications. The severe nature of the insult may be the reason slight variation in physiological parameters between animals does not typically affect injury consistency.
Disclosures
The authors have no financial conflicts of interest.
References
- Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathologica. 1984;62:201–208. doi: 10.1007/BF00691853. [DOI] [PubMed] [Google Scholar]
- Smith ML, Auer RN, Siesjo BK. The density and distribution of ischemic brain injury in the rat following 2-10 min of forebrain ischemia. Acta Neuropathologica. 1984;64:319–332. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]
- Sanderson TH, Kumar R, Sullivan JM, Krause GS. Insulin blocks cytochrome c release in the reperfused brain through PI3-K signaling and by promoting Bax/Bcl-XL binding. Journal of Neurochemistry. 2008;106:1248–1258. doi: 10.1111/j.1471-4159.2008.05473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson TH, et al. Insulin activates the PI3K-Akt survival pathway in vulnerable neurons following global brain ischemia. Neurological Research. 2009;31:947–958. doi: 10.1179/174313209X382449. [DOI] [PubMed] [Google Scholar]
- Sanderson TH, et al. PKR-like endoplasmic reticulum kinase (PERK) activation following brain ischemia is independent of unfolded nascent proteins. Neuroscience. 2010;169:1307–1314. doi: 10.1016/j.neuroscience.2010.05.076. [DOI] [PubMed] [Google Scholar]
- Hazelton JL, et al. Hyperoxic reperfusion after global cerebral ischemia promotes inflammation and long-term hippocampal neuronal death. Journal of Neurotrauma. 2010;27:753–762. doi: 10.1089/neu.2009.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Krause GS, Kumar K, White BC, Aust SD, Wiegenstein JG. Ischemia, resuscitation, and reperfusion: mechanisms of tissue injury and prospects for protection. American Heart Journal. 1986;111:768–780. doi: 10.1016/0002-8703(86)90114-6. [DOI] [PubMed] [Google Scholar]
- Krause GS, White BC, Aust SD, Nayini NR, Kumar K. Brain cell death following ischemia and reperfusion: a proposed biochemical sequence. Critical Care Medicine. 1988;16:714–726. doi: 10.1097/00003246-198807000-00015. [DOI] [PubMed] [Google Scholar]
- Bloom HL, et al. Long-term survival after successful inhospital cardiac arrest resuscitation. American Heart Journal. 2007;153:831–836. doi: 10.1016/j.ahj.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke; a Journal of Cerebral Circulation. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Uluc K, Miranpuri A, Kujoth GC, Akture E, Baskaya MK. Focal cerebral ischemia model by endovascular suture occlusion of the middle cerebral artery in the rat. J. Vis. Exp. 2011. p. e1978. [DOI] [PMC free article] [PubMed]
- Neumar RW, et al. Calpain mediates eukaryotic initiation factor 4G degradation during global brain ischemia. J. Cereb. Blood Flow Metab. 1998;18:876–881. doi: 10.1097/00004647-199808000-00007. [DOI] [PubMed] [Google Scholar]
- Paine MG, Che D, Li L, Neumar RW. Cerebellar Purkinje Cell Neurodegeneration After Cardiac Arrest: Effect of Therapeutic Hypothermia. Resuscitation. 2012. [DOI] [PubMed]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Annals of Neurology. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Current Opinion in Cell Biology. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Research. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Yager JY, Brucklacher RM, Vannucci RC. Cerebral energy metabolism during hypoxia-ischemia and early recovery in immature rats. The American Journal of Physiology. 1992;262:672–677. doi: 10.1152/ajpheart.1992.262.3.H672. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. J. Neurosci. 1999;19:RC39. doi: 10.1523/JNEUROSCI.19-22-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino H, et al. Pathophysiological process after transient ischemia of the middle cerebral artery in the rat. Brain Research Bulletin. 1994;35:51–56. doi: 10.1016/0361-9230(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Ross DT, Ebner FF. Thalamic retrograde degeneration following cortical injury: an excitotoxic process. Neuroscience. 1990;35:525–550. doi: 10.1016/0306-4522(90)90327-z. [DOI] [PubMed] [Google Scholar]
- Soriano MA, Ferrer I, Rodriguez-Farre E, Planas AM. Apoptosis and c-Jun in the thalamus of the rat following cortical infarction. Neuroreport. 1996;7:425–428. doi: 10.1097/00001756-199601310-00012. [DOI] [PubMed] [Google Scholar]
- Watanabe H, et al. Protein synthesis inhibitor transiently reduces neuronal death in the thalamus of spontaneously hypertensive rats following cortical infarction. Neuroscience Letters. 1997;233:25–28. doi: 10.1016/s0304-3940(97)00617-4. [DOI] [PubMed] [Google Scholar]
- Wei L, Ying DJ, Cui L, Langsdorf J, Yu SP. Necrosis, apoptosis and hybrid death in the cortex and thalamus after barrel cortex ischemia in rats. Brain Research. 2004;1022:54–61. doi: 10.1016/j.brainres.2004.06.080. [DOI] [PubMed] [Google Scholar]
- Zong WX, Thompson CB. Necrotic death as a cell fate. Genes & Development. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- Ito U, Spatz M, Walker JT, Klatzo I. Experimental cerebral ischemia in mongolian gerbils. I. Light microscopic observations. Acta Neuropathologica. 1975;32:209–223. doi: 10.1007/BF00696570. [DOI] [PubMed] [Google Scholar]
- Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M. Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke; a Journal of Cerebral Circulation. 2009;40:2594–2600. doi: 10.1161/STROKEAHA.109.552554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busto R, Dietrich WD, Globus MY, Ginsberg MD. The importance of brain temperature in cerebral ischemic injury. Stroke; a Journal of Cerebral Circulation. 1989;20:1113–1114. doi: 10.1161/01.str.20.8.1113. [DOI] [PubMed] [Google Scholar]
- Voll CL, Auer RN. Postischemic seizures and necrotizing ischemic brain damage: neuroprotective effect of postischemic diazepam and insulin. Neurology. 1991;41:423–428. doi: 10.1212/wnl.41.3.423. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Calvert JW, Kusaka G, Zhang JH. One-stage anterior approach for four-vessel occlusion in rat. Stroke; a Journal of Cerebral Circulation. 2005;36:2212–2214. doi: 10.1161/01.STR.0000182238.08510.c5. [DOI] [PubMed] [Google Scholar]
- Gionet TX, Warner DS, Verhaegen M, Thomas JD, Todd MM. Effects of intra-ischemic blood pressure on outcome from 2-vessel occlusion forebrain ischemia in the rat. Brain Research. 1992;586:188–194. doi: 10.1016/0006-8993(92)91626-p. [DOI] [PubMed] [Google Scholar]
- Sugawara T, et al. Effect of hypotension severity on hippocampal CA1 neurons in a rat global ischemia model. Brain Research. 2000;877:281–287. doi: 10.1016/s0006-8993(00)02684-6. [DOI] [PubMed] [Google Scholar]


