Abstract
More than 40 years after their introduction in therapy, 1,4-dihydropyridines (DHPs) are still amongst the most prescribed drugs in the world. Though they all share a similar mechanism of action blocking L-type voltage-gated Ca2+ channels, DHPs differ in crucial pharmacological properties like tissue selectivity and cardiodepressant activity. This review examines how changes in the DHP structure can modify the pharmacological properties of these drugs and how some of these chemical manipulations have been exploited to obtain clinically more effective molecules. Special emphasis is given to the evidence that L-type Ca2+ channels are an heterogeneous family and that DHPs with different pharmacological properties differ in their affinity for the different isoforms of this class of channels. Data showing that DHP pharmacological heterogeneity could be in part dependent on the interaction of some of these molecules with ion channels different from the L-type Ca2+ channels is reviewed as well.
Keywords: dihydropyridines, voltage-gated Ca2+ channels, enantiomers, L-type channels, T-type channels
I. INTRODUCTION
Since their introduction in therapy more than 40 years ago [1], 1,4-dihydropyridines (DHPs) have been amongst the most successful drugs ever used in humans. Testifying this undeniable success amlodipine ranks amongst the 10 most prescribed drugs in the United States [2] as in the rest of the world. Like phenylalkilamines and benzothiazepines, DHPs act by blocking L-type voltage gated Ca2+[3]. These ion channels are multimeric protein composed by a pore forming α1 subunit and by accessory α2δ, β and, variably, γ subunits [4,5]. Among their many functional roles, L-type Ca2+ channels are crucial in controlling heart contractility and excitability [6], vascular tone [7] and the generation of spontaneous depolarizations in cardiac, neuronal or endocrine cells with pacemaking activity [8–10].
DHPs differ from the other Ca2+ channel blockers because of their marked selectivity for vascular smooth cells respect to myocardium. This selectivity confers to DHPs the property of being good antihypertensive drugs with small or no cardiodepressant activity [11]. Two different mechanisms have been proposed to explain the higher DHP activity on blood vessels as compared with the heart: 1- an higher state-dependent affinity for the inactivated forms of L-type channels in vascular smooth muscle cells, and 2- the existence of two different isoforms of these channels, a cardiac isoform, exuisitely sensitive to phenylalkilamine inhibition, and a vascular smooth muscle cell isoform, preferentially inhibited by DHPs. The latter hypothesis has been formally demonstrated when different splicing variants of the CaV1.2 channel gene expressed in the heart and in vascular smooth muscle cells were identified [12–14]. The cardiac (CaV1.2a) and smooth muscle (CaV1.2b) isoforms differ in four different splicing loci: exon 1/1a in the N-terminus, exon 8/8a in the transmembrane segment IS6, exon 31/32 in the transmembrane segment IVS3, and exon 9* in the loop that connects domains I and II. Specifically, the exon composition of the cardiac and vascular smooth muscle cell isoform are the following: 1a/8a/Δ9*/31/33 * [15], and 1/8/9*/ 32/33 [16], respectively. Importantly, when expressed in heterologous systems these two isoforms showed the different sensitivity to DHPs and other Ca2+ channel blockers observed in the heart and in blood vessels; in addition, mutagenesis studies showed that exon 8 in the IS6 region of the channel dictates DHPs selectivity [13–14].
Concerning the first hypothesis, i.e. that DHP could more active on vascular ion channels because they preferentially block inactivated Ca2+ channels in vascular smooth muscle cells, experiments performed with the cloned cardiac and vascular isoforms showed that gating differences cannot explain DHP tissue selectivity [17]. However, more recently, a splice variant that differs from the canonical CaV1.2b isoform because it lacks exon 33 (CaV1.2SM: 1/8/9*/ 32/Δ33) was identified in vascular smooth muscle cells and it was shown to be specifically sensitive to state-dependent inhibition by nifedipine [18]. Therefore, the vascular districts expressing the CaV1.2SM isoform, DHPs could specifically display a more marked state/dependent block of L-type Ca2+ channels.
A number of excellent reviews have been published on the structure, mechanism of action and clinical uses of DHPs [for instance, 19–20]. Here, instead, we will focus on an interesting characteristic of this drug family, that has not been so extensively addressed in the literature: its pharmacological heterogeneity. While, indeed, all DHPs share a common molecular backbone and act on similar molecular targets, important differences do exist among them both in their pharmacokinetic and pharmacodynamic properties. It has been proposed that the choice of the more appropriate DHP in specific clinical settings should take into account these differences that could also could influence the safety of each of these molecules.
A first relevant pharmacodynamic difference among DHPs pertains tissue selectivity as classical observations showed that some DHPs could be more effective in relaxing some vascular beds than others. In particular, manidipine is relatively specific for renal vessels and seems to be a good choice in patients in which the preservation of a deranged renal function is the primary concern [21–22] and the same has been reported also for benidipine, efonidipine and nivaldipine [23]. In addition, nimodipine has been proposed to be quite selective for brain vessels and is recommended for the treatment of the vasospasm caused by subarhacnoid hemorragy [24–29], flunarizine is much more active on mesenteric artery than on the aorta [30] while nisoldipine is thought to be a relatively specific coronary vasorelaxant drug [31]. A second relevant pharmacodynamic difference concerns the effect on heart rate. Because of their predominant effect on blood vessels and of the fall in blood pressure that they cause, DHPs induce reflex tachycardia. However, important differences have been reported amongst the different members of this drug family in the entity of this tachycardic response. Specifically, isradipine and amplodipine are considered as the less tachycardic DHPs [32–34] and more recently cilnidipine was added to this list [35].
Finally, some DHPs retain some negative inotropic activity that can be harmful especially when significant plasma concentrations are rapidly reached [36]. This eventuality appears more consistent with drugs of short half-life like nifedipine whose clinical use has found to be associated to an increase in the incidence of sudden deaths or cerebral ischemia [37–38]. DHPs differ, indeed, also in their pharmacokinetics and, in particular, in their half life. Taking into account both their half-life and cardiodepressant properties, DHPs have been traditionally classified in three different groups, or generations: cardiodepressant DHPs with a short half-life, cardiodepressant DHPs with a long half-life and non cardiodepressant DHPs with a long half life [39–40]. In Tab. 1 this classification has been modified to introduce a new group of non-cardiodepressant DHPs with ultra-short half-life that is exemplified by clevidipine. This new DHP, which has been approved by FDA in 2008, represents an important advancement in the treatment of hypertensive emergencies because it couples the lack of cardiodepressant activity with a very favourable pharmacokinetic profile with a rapid onset and disappearance of its effects [41–43].
Table I.
CLASSIFICATION OF DHPs
| Drug | Half-life | Negative Inotropic Activity | |
|---|---|---|---|
| 1st GENERATION | Nifedipine | Short | + |
| 2nd GENERATION | Felodipine, Nisoldipine | Long | +/− |
| 3rd GENERATION | Amlodipine, Lacidipine | Long | − |
| 4th GENERATION | Clevidipine | Very short | − |
Considering that all DHPs share a similar mechanism of action as they block L-type Ca2+ channels, the question arises of establishing how and why different DHPs could be pharmacologically different. While this question is still open and object of investigation by several groups, the (few) data available seem to suggest that specific substitutions at key residues of the DHP backbone could affect the specificity of these drugs for CaV1.2 channels. Intriguingly, DHP pharmacological profile can be significantly affected by structural changes lowering drug specificity an conferring them some activity on ion channels different from CaV1.2.
II. DIFFERENT PHARMACOLOGICAL PROPERTIES OF DIFFERENT DHPs: JUST A MATTER OF ION CHANNEL SELECTIVITY?
A-. THE CASE OF NON-TACHYCARDIC DHPS
Isradipine, amlodipine and cilnidipine are a good example of how a relative loss of selectivity could be responsible for the better pharmacological profile of some DHPs as compared with the other members of this family. As mentioned above, these DHPs have been described as less tachycardic than classical DHPs. This important pharmacological property can be explained on the basis of their interaction with ion channels other than those encoded by the CaV1.2 gene. In particular, two other voltage-gated Ca2+ channel subtypes seem to be involved, the CaV1.3 and the CaV2.2-encoded channels. The CaV1.3 gene encodes for the pore forming subunit of the endocrine form of L-type Ca2+ channels, formerly known as α1D[44]. CaV1.3 has a different distribution in the heart as compared with CaV1.2. While, indeed, CaV1.2 channels localize both in atria and in ventricles, CaV1.3 channels are expressed only in atria [45] and represent the main L-type channel form in sinoatrial node [46]. CaV1.3 channels differ from CaV1.2 channels because of a more negative threshold for activation and of a less marked voltage-dependent inactivation [46–47]. Because of these biophysical properties CaV1.3 channels are well suited to take part in the generation of repetitive depolarizations in pacemaker cells. Consistent with these data, severe bradyarrhythmias do occurr in CaV1.3 knock-out mice suggesting that this channel isoform is critical for cardiac pacemaking and for maintaining sinusal rhythm [10, 46, 48–50]. Importantly, this hypothesis is also supported by the results of electrophysiological studies performed on isolated SAN and AVN cells [10, 46, 48–50]. While CaV1.3 channels have been shown to be less sensitive to DHPs than CaV1.2 channels [47], significant differences do exist among the different members of the DHP family as isradipine, amlodipine and azidopine are at least 3–4 times more effective than nifedipine or nitrendipine in blocking CaV1.3 channels in vitro [51]. These results suggest that L-type Ca2+ currents in the sinoatrial and atrioventricular nodes are significantly blocked when either isradipine or, possibly, amlodipine are administered to hypertensive patients and this could explain why these drugs do not cause a significant reflex tachycardia (Fig. 1). Thus, specific DHPs may affect heart rate without exerting a significant negative inotropic activity. Confirming the hypothesis that negative inotropic and negative chronotropic effects could be dissociated in DHPs, among the different imidazo[2,1-b]thiazole DHP derivatives synthesized by Budriesi et al. [52] some have a marked negative inotropic activity others a marked negative chronotropic activity and others both. Interestingly, the ability to differentially affect contractility and rhythmicity in the myocardium has been demonstrated also in non-DHP compounds with L-type Ca2+channel blocking properties [53].
Fig. 1.
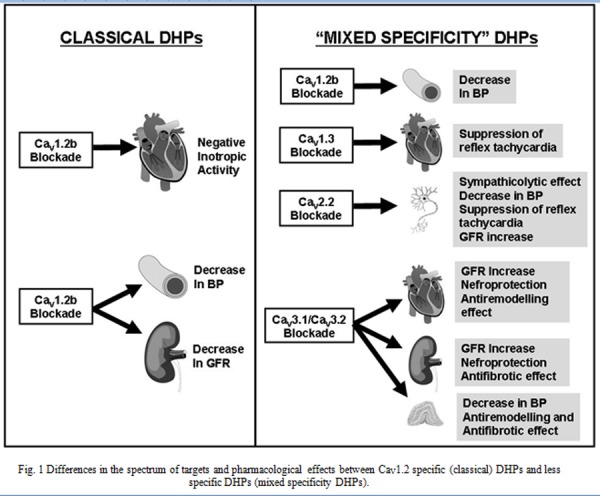
Differences in the spectrum of targets and pharmacological effects between CaV1.2 specific (classical) DHPs and less specific DHPs (mixed specificity DHPs).
N-type channels Ca2+ channels whose pore forming unit is encoded by the CaV2.2 gene are the other channel type whose blockade could explain why some DHPs are less tachycardic than others. These Ca2+ channel subclass, is expressed in neuronal axon terminals where it controls the release of neurotransmitter including glutamate [54], γ-aminobutyric acid [55], acetylcholine [56], dopamine [57], and noradrenaline [58]. They have a special relevance in cardiovascular physiology as they control noradrenaline release in the orthosympathetic nervous system. This is demonstrated by the ability of the N/type blockers ω-conotoxin GVIA and ω-conotoxin MVIIA (SNX-111) to inhibit noradrenaline release from superior cervical ganglia in vitro [59] and to exert a sympatholytic activity in vivo [60], respectively. A formal demonstration that N-type channel activity is essential for the orthosympathetic control of the cardiovascular system came from the evidence of its significant impairment in CaV 2.2 knock-out mice [61].
As shown by patch clamp experiments performed in heterologous expression systems with cloned CaV2.2 channels the vast majority of DHPs are virtually ineffective on N-type channels. However, a small DHP subgroup consisting of amlodipine, benidipine, cilnidipine, nicardipine, and barnidipine seems to stand apart from the others for it exerts some blockade of this channel type [62]. N-type channel blocking activity seems to be relevant for amlodipine ad cilnidipine with IC50s close to those for L-type channel blockade and in the low micromolar range [62]. Importantly, evidence that the orthosympathetic system is actually blocked in vivo has been reported for amlodipine and cilnidipine as these DHPs both decrease serum catecholamine levels and decrease heart rate variability in humans [63–66]. The ability of some DHPs like cilnidipine or amlodipine to exert a sympathicolytic activity could be extremely helpful in improving the antihypertensive activity of these drugs as it not only prevents reflex tachycardia but also other unwanted consequences of reflex authonomic activation like secondary hypereninemia of reflex peripheral vasoconstriction (Fig. 1). While the real relevance of N-type Ca2+ channel blockade in determining the clinical activity of amlodipine remains doubtful, some evidence that it could be crucial in the case of cilnidipine has been provided [35]. Before proceeding further we have, however, to remind that drug pharmacokinetic could also greatly influence the tachycardic reflex response elicited by DHP. DHPs with a long half-life only slowly reach effective plasma concentrations and, therefore, do not cause the sudden changes in blood pressure as those needed to trigger reflex tachycardia. This may be important in the case of amplodipine, which has a very long half-life of about 30 hours [67].
B-. THE CASE OF “NEFROPROTECTIVE”DHPS
The small group of “nefroprotective” DHPs, including efonidipine, benidipine or manidipine, gives us an additional example of how some loss of selectivity could lead to DHPs with pharmacological properties different from those observed in more CaV1.2-selective members of this family. Compelling experimental evidences suggest, indeed, that the ability of this group of DHPs to preferentially increase renal blood flow is the consequence of their ability to significantly block another subclass of voltage/gated Ca2+ channels, the T-type channels. Also known as low-voltage activated Ca2+ channels this channel family differs from HVA channels because of their permeation properties and because they open at much more negative potentials, inactivate more rapidly and deactivate more slowly [68–69]. Three different isoforms of T-type channels have been identified, CaV3.1, CaV3.2 and CaV3.3 which differ in their electrophysiological properties and tissue d istribution [69]. T-type channels also differ from L-type channels in their pharmacology. Though really selective T-type blockers are still missing, these channels are blocked by insect toxins like kurtoxins and Pro/Tx-I [70–71], by neuroleptics [72], kinase inhibitors like imatinib mesylate [73] and non-steroidal anti-inflammatory drugs [74]. In addition, their gating behavior is profoundly affected by transition metals like Zn2+[75]. While the majority of the DHPs are ineffective or only minimally effective on the different T-type isoforms, some of them strongly block these channels at concentrations close to those effective on L-type channels. Furukawa et al. [76] performed a systematic analysis of the effects of several DHPs on CaV3.1, CaV3.2 and CaV3.3 channels heterologously expressed in Xenopus oocytes. Among the many DHPs tested, the most potent in blocking T/type channels were barnidipine, manidipine and amlodipine that, at 10 μM concentrations, caused a 50% or higher current blockade; though less potent, also efonidipine, benidipine, and the old DHPs nifedipine and nicardipine displayed some T-type blocking effect [76]. Important differences were noted in the effect of these DHPs on the different CaV3 isoforms. In general, the above mentioned DHPs were more effective on CaV3.1 and CaV3.2 channels whereas they induced only a slight CaV3.3 blockade [76].
In addition, some DHPs like azelnipidine were more effective on CaV3.1 whereas others like manidipine were significantly more effective on CaV3.2 than on CaV3.1 channels. Interestingly, nimodipine was almost ineffective on CaV3.1 but significantly inhibited CaV3.2 channels. The rank of T-type blocking activity by different DHPs reported by Furukawa, however, may be highly dependent on the expression system used because different results were obtained when DHP effect on these channels were evaluated in HEK cells [77]. In particular, in these cells efonidipine, felodipine, isradipine, and nitrendipine behaved as potent T-type channel blockers and were almost tenfold more potent than amlodipine and nifedipine [77]. The ability of selected DHPs to significantly inhibit T-type channel activity has relevant pathophysiological implications in the kidney. It has been shown, indeed, that whereas L-type Ca2+ channels are preferentially located at the afferent arterioles, T-type channels are highly represented also at the efferent arterioles where they control vascular tone [78–80]. Specifically, by immunocitochemistry Poulsen et al. [80] showed that CaV3.1 is the prevalent T-type isoform in human glomerular vasculature. Consistent with the location of T-type but not L-type Ca2+ channels in the efferent arterioles, “pure” L-type blockers only dilate afferent arterioles whereas T-type blockers like mibefradil dilate both afferent and efferent arterioles [81–82] (Fig. 1). Thus, filtration pressure, tends to increase when a pure L-type channel blockade is applied though this effect may be masked by the fall in systemic pressure [83–86]. Conversely, it is markedly reduced by T-type channel blockers [79]. Therefore, according to the hyperfiltration theory [87], which states that glumerular hypertension is a causative factor for the progression of chronic kidney disease (CKD), drugs with T-type channel blocker properties are expected to display nefroprotective effects [88] and could be helpful in delaying CKD progression [89]. Importantly, it has been observed that DHPs with a relevant T-type channel blocking activity exert favorable effects on the progression of CKD both in preclinical models of this disease and in humans. For instance, this has been reported for efonidipine [90–97], benidipine [98–104] and manidipine [105–111].
Non-hemodynamic effects may have a role in conferring nefroprotective properties to T-type blocking DHPs. T-type blockade [79]. Specifically, T-type channels control aldosterone synthesis in adrenocortical cells [112–113] and T-type blocking DHPs decrease aldosterone synthesis in vitro [114–115] (Fig. 1) and circulating aldosterone concentrations in hypertensive patients [91, 116–117]. In addition, T-type channels seem to control mesangial proliferation [118]. Intriguingly, it has been reported that efonidipine significantly decreases tubulo-interstitial fibrosis in an experimental model of chronic unilateral ureteral obstruction in the rat, a pharmacological effect that has been attribute either to the decrease of aldosterone concentrations caused by these drugs or to their antioxidant properties [119].
Considering the rich innervation by the noradrenergic system of both afferent and efferent arterioles, it is expected that they are both vasodilated upon orthosympathetic pharmacological blockade. As a matter of fact, DHPs with combined N- and L-type Ca2+ channel blocking properties showed similar effect in a number of preclinical models in vivo and in vitro [120–123] and some evidence that they could exert nefroprotective effects also in human patients have been reported [124–125] (Fig. 1). However, the entity of this effect seem to be considerably smaller than those of combined T- and L-type blocking DHPs as showns by the few comparative studies available. For instance, benidipine performed better in nefroprotection than cinildipine or amlodipine [126–127].
A mixed T- and L-type blockade could be a much more effective pharmacological approach than pure L-type blockade also in other clinical conditions. For instance, T-type channels are implicated in cardiac hypertrophy and in preclinical models mixed T- and L-type Ca2+ channel blockers proved to be effective as antiremodelling agents [128–131] (Fig. 1). Concluding this section we would like to emphasize thanks to a small loss of selectivity, DHPs acting also on T-type channels acquire relevant new pharmacological properties and, paradoxically, tissue-selective effects.
C-. FURTHER MECHANISMS FOR SELECTIVE EFFECTS ON SPECIFIC VASCULAR BEDS?
While the data reported in the previous section explain how some DHPs and not others may exert specific pharmacological effects in specific tissues, many pieces of information are still missing and we still do not have a definite explanation for other tissue-selective effects. However, a number of additional factors could be involved. In particular, it is now clear that the CaV1.2 L-type Ca2+ channel family is much more complex that initially believed and that it encompasses much more members than just a cardiac and a smooth mucle cell isoform. As the CaV1.2 gene is composed by 55 exons, 19 of which can be alternatively spliced, theoretically 219 CaV1.2 L-type channel variants could be generated and approximately 40 of them have been actually identified [132]. The possibility that different splicing variants could be differently distributed in blood vessels of different tissues appears intriguing also considering that something similar has been demonstrated for other ion channels like voltage-gated K+ channels [133]. Unfortunately, a detailed mapping of their distribution of in different vascular beds is still missing and we ignore whether they differ in DHP sensitivity as well. However, we would like to mention that, as we mentioned above, Liao et al. [18] identified a splice variant that differs from CaV1.2 because it lacks the 33 exon and showed that it activates at more negative potentials than CaV1.2b, generates a window current and displays state-dependent block by nifedipine.
Another possibility that remains unexplored is that the CaV1.3 channels subunit could be differentally expressed in different vascular compartments. It has been demonstrated, indeed, that this Ca2+ channels subtype, which, as reported above, differs from CaV1.2 in its sensitivity to DHPs, is expressed in vascular smooth muscle cells but its distribution in different vascular beds has not been systematically explored yet [134].
Finally, T-type channels are also expressed in other vascular districts besides the kidney such as the brain resistance vessels [134–136] and the pulmonary arterioles [137–138]. The pharmacological and clinical implications of these observations are still unclear and will represent, indubitably, a rich area for future investigations.
III. STRUCTURAL BASIS OF DHP FUNCTIONAL DIVERSITY
The evidence reported in the previous section suggests that the functional diversity of DHPs could be determined by subtle differences in the affinity either for channels other than CaV1.2 or, possibly, for different isoforms of CaV1.2 channels. They also raise, however, the question of how molecules with a similar structure could display such differences in target affinity. All the DHPs share, indeed, a similar basic structure consisting of an N-heterocyclyc pyridine ring bearing a phenyl substituent on its para-position. However, while the general three-dimensional structure of the molecule is believed to be preserved, both the N-heterocyclic ring and the benzene ring can be substituted in different positions giving rise to structurally different compounds (Fig. ). Since the early ‘80s it has been known that the nature of the substituents both on the heterocylic and on the benzene ring affect potency and tissue selectivity of DHPs. Specifically, using the Hansch analysis for Quantitative Structure-Activity Analysis (QSAR) Rodenkirken et al. [139] showed that the position of phenyl ring substitution affects the cardiodepressant potency of DHPs in the cat papillary muscle preparation. In particular, the DHPs whose phenyl ring is substituted in position ortho are the most cardiodepressant, those with substituents in position para the less cardiodepressant whereas metha-substituted compounds rank in-between [139]. The effect of the phenyl-substituents depends also on steric factors since the cardiodepressant activity increases with the width of the substituent. Similar results were obtained when the potency in relaxing guinea pig ileal preparations was measured [140].
While phenyl ring substitutions seem to affect mainly DHP potency, ester chain substitutions on the heterocylic ring influence not only the potency but also the selectivity of these drugs. In particular, vascular selectivity seems to increase with the size of the substituent. So niludipine [141] and nisoldipine [142], not only have much bigger side chain substituents on the heterocyclic ring but also a much higher vascular selectivity than nifedipine. Similarly, benidipine [143] and nicardipine [144], two DHPs with very large side chains, have a very favorable pharmacological profile with high vascular selectivity. Kojda et al. [145] explored the effect of lengthening the side chain ester substituents in nitrendipine derivatives and found that a small increase in side chain length (up to 3-I-propil-ester) determines an increase in vasodilator potency which did not correlate with the lipophylicity but with the charge of the side chain.
The substitution of the heterocyclic ring on position 3 and 5 has also another important structural consequence which can affect DHP potency and selectivity: whenever the residues on these two positions are not identical the DHP molecule bocomes chiral. In fact, the carbon 4 becomes a chiral center and two different optical enantiomers will exist for this DHP molecule. Starting from the observation that verapamil, a calcium channel blocker belonging to the family of phenylalkilamine, is also a chiral molecule and that its two enantiomers display a different potency in blocking L-type calcium channel [146], a few years after the synthesis of the first asymmetrically substituted DHPs, Towart et al. [147] looked at the potency of the enantiomers of a series of chiral DHPs. They found that exactly as in the case of verapamil, the two enantiomers of chiral DHPs display markedly different potencies. Since there, a number of studies confirmed and extended this observation. Clear differences in the potency of the two optical enantiomers of chiral DHPs have been described in a number of studies. For example, Shibanuma et al. [148] showed that two enantiomers of nicardipine display a different potency in lowering the blood pressure in the dog. Amlodipine [149] and nimodipine [150] enantiomers differ in their ability to induce the relaxation of high potassium-precontracted aorta strips in vitro. Similarly, manidipine [151] and benidipine (Muto et al., 1988) enantiomers display a different potency in lowering the blood pressure in rats. Inagaki et al. [152] examined in anestesized dogs the pharmacological activity of a the optical enantiomers of barnidipine, a DHP with two chiral centers, and found differences in the potency of its four stereoisomers with a potency order that agreed with that reported in vitro. A limited number of studies suggest that differences in potency between the different enantiomers of chiral DHPs similar to those observed in experimental animals could also exist in humans. For instance, Mikus et al. [153] examined the blood pressure lowering activity of the two nitrendipine enantiomers in healty volunteers and confirmed that the pharmacological activity of this drug is almost completely due to the S-enantiomer. Soons et al have obtained similar results [154] comparing the effects of the two enantiomers of felodipine and nitrendipine and of their racemic mixtures in young, healthy males.
Very limited data suggest that DHP chirality could also influence tissue selectivity. In particular, this has been observed for the two optical isomers of isradipine [155] because in anesthesized cats the S-enantiomer of this DHP is much more effective in lowering systemic pressure and heart rate than R-enantiomer, which, instead, is more potent in increasing subendocardial flux. Intriguingly, evidence of a role of the enantiomeric configuration in influencing tissue selectivity has been reported also for non-DHP chiral calcium channel blockers like verapamil [156].
Several mechanisms could determine the differences in potency and/or tissue selectivity observed among different enatiomers of the same DHPs. First, differences in potency in couples of DHP enantiomers could be determined by differences in the affinity for L-type channels. For instance, it has been shown that in radioligand studies the less potent enantiomer of nicardipine [157], manidipine [151], niguldipine [158] and benidipine [159] show a reduced affinity for these channels. As we mentioned above, some DHPs may also interact with CaV channel isoform different from CaV1.2 L-type channels and this could be relevant in determining tissue-specific effects of these compounds. In this perspective, it interesting to emphasize that T-type channel blockade both by efonidipine and by benidipine is stereoselective with R-efonidipine and S-benidipine being much more potent than their enantiomeric counterparts [160–161]. This suggests that differences in tissue selectivity among DHP enantiomers could mirror differences in the choreography of the ion channels blocked by these drugs.
It is also intriguing that relevant differences in the kinetics of Ca2+ channel blockade have been reported among different DHP enantiomers. For instance, great differences were observed in the time of onset and disappearance of the increase in coronary blood flow observed after the intracoronary administration of the four enantiomers of barnidipine [152]. While this represents only a very indirect indication of a different activity on L-type Ca2+ channels more direct evidences have bee reported. In particular, we used a microfluorimetric approach in fura-2 loaded pituitary GH3 cells to evaluate the kinetics of the inhibitory effect of the enantiomers of nitrendipine and manidipine on the increase in [Ca2+]i elicited by membrane depolarization with an high K+ solution [162]. In control conditions this stimulus elicited a biphasic [Ca2+]i response consisting of a first fast spike flowed by a long lasting plateau (Fig. 2). Nitrendipine and manidipine affected both these phases by lowering the spike and accelerating the decay of the plateau. However, in both cases, the more active S enantiomer almost exclusively blocked the fast spike without significantly affect the plateau phase channels whereas the less active R enantiomers only accelerated the decay of the plateau without affect the spike [162] (Fig. 2). In patch clamp experiments, this corresponded to a significantly longer time to maximal current inhibition in R as compared with S/enantiomers. Similar results were also obtained with the two optical isomers of lercanidipine [163] (Fig. 2). The differences that we observed could be possibly explained in terms of a differential affinity of the two optical isomers of the tested DHPs for open and inactivated Ca2+ channels according to the guarded receptor hypothesis similarly to what observed by Handrock et al. [164] for the two enantiomers of isradipine. By whole cell electrophysiology, they found, indeed, that the less potent enantiomer of isradipine, (-)-isradipine, preferentially blocks the Ba2+ currents flowing through L-type Ca2+ channels in the late phases of a depolarizing pulse when many channels are inactivated whereas the more active (+)-isradipine preferentially induces a block during the early phase of the pulse when the majority of the channels are in an open state.
Fig. 2.
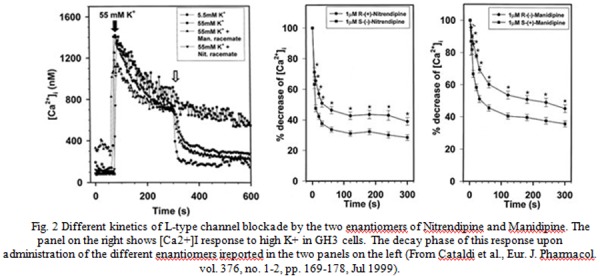
Different kinetics of L-type channel blockade by the two enantiomers of Nitrendipine and Manidipine. The panel on the right shows [Ca2+]I response to high K+ in GH3 cells. The decay phase of this response upon administration of the different enantiomers ireported in the two panels on the left (From Cataldi et al., Eur. J. Pharmacol. vol. 376, no. 1–2, pp. 169–178, Jul 1999).
IV. CONCLUSIONS AND FUTURE PERSPECTIVES
In conclusion, though sharing a similar mechanism of action and a common main binding site on voltage gated CaV1.2 Ca2+ channels, DHPs are actually an heterogeneous drug family with relevant differences in potency, tissue selectivity and pharmacokinetics. Here, we put the emphasis on the evidence that part of this heterogeneity originates from activities exerted on ion channels diverse from CaV1.2. However, additional “unintended” pharmacological activities have been described in selected DHPs [165–166]. For instance, a number of marketed DHPs like nimodipine, felodipine and benidipine may have mineralcorticoid antagonistic properties [167–168]. Interestingly, this pharmacological activity is markedly dependent on the enantiomeric configuration [169] and it could be relevant in conferring to these drugs the ability to exert antiremodelling effects [170] (Fig. 1). Moreover, selected DHPs including amlodipine, azelnidipine, benidipine or cinildipine enhance the release of nitric oxide from the endothelium and exert antioxidant effects [171–179]. Additional pharmacological properties such as a combined α and β-adrenergic antagonistic activity [180–181] or NO-donor properties [182] may be conferred to DHPs by rationally designed modifications. In conclusion, modifications of the backbone of CaV1.2 blocking DHPs can confer to these drugs additional, clinically relevant pharmacological activities. By improving our understanding of the mechanisms involved more and more effective new compounds can be designed so rejuvenating this evergreen drug family.
REFERENCES
- [1].Bossert F, Vater W. “Dihydropyridines, a new group of strongly effective coronary therapeutic agents”. Naturwissenschaften. 1971 Nov;58(11):578. doi: 10.1007/BF00598745. [DOI] [PubMed] [Google Scholar]
- [2].IHII_Medicines_in_U.S_Report_2011: http://www.imshealth.com/ims/Global/Content/Insights/IMS%20Institute%20for%20Healthcare%20Informatics/IHII_Medicines_in_U.S_Report_2011.pdf
- [3].Hockerman GH, Peterson BZ, Johnson BD, Catterall WA. “Molecular determinants of drug binding and action on L-type calcium channels”. Annu Rev Pharmacol Toxicol. 1997;37:361–396. doi: 10.1146/annurev.pharmtox.37.1.361. [DOI] [PubMed] [Google Scholar]
- [4].Catterall WA. “Structure and regulation of voltage-gated Ca2+channels”. Annu Rev Cell Dev Biol. 16:521–55. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- [5].Dolphin AC. “Calcium channel diversity: multiple roles of calcium channel subunits”. Curr Opin Neurobiol. 2009 Jun;19(3):237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- [6].Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. “The L-type calcium channel in the heart: the beat goes on”. J Clin Invest. 2005 Dec;115(12):3306–3317. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ. “Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications”. Vascul Pharmacol. 2006 Mar;44(3):131–142. doi: 10.1016/j.vph.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cataldi M, Taglialatela M, Guerriero S, Amoroso S, Lombardi G, Di Renzo GF, Annunziato L. “Protein-tyrosine kinases activate while protein-tyrosine phosphatases inhibit L-type calcium channel activity in pituitary GH3 cells. J. Biol. Chem. 1996 Apr;271(16):9441–6. doi: 10.1074/jbc.271.16.9441. [DOI] [PubMed] [Google Scholar]
- [9].Cataldi M, Secondo A, D’Alessio A, Sarnacchiaro F, Colao AM, Amoroso S, Di Renzo GF, Annunziato L. “Involvement of phosphodiesterase-cGMP-PKG pathway in intracellular Ca2+ oscillations in pituitary GH3 cells”. Biochim Biophys Acta. 1999 Mar;1449(2):186–193. doi: 10.1016/s0167-4889(99)00013-0. [DOI] [PubMed] [Google Scholar]
- [10].Mangoni ME, Couette B, Marger L, Bourinet E, Striessnig J, Nargeot J.“Voltage-dependent calcium channels and cardiac pacemaker activity: from ionic currents to genes” Prog Biophys Mol Biol 901–338–63.Jan.–Apr2006 [DOI] [PubMed] [Google Scholar]
- [11].Triggle DJ. “Calcium-channel drugs: structure-function relationships and selectivity of action”. J Cardiovasc Pharmacol. 1991;18(suppl. 10):S1–6. [PubMed] [Google Scholar]
- [12].Liao P, Yong TF, Liang MC, Yue DT, Soong TW. Splicing for alternative structures of Cav1.2 Ca2+channels in cardiac and smooth muscles. Cardiovasc. Re.s. 2005 Nov;68(2):197–203. doi: 10.1016/j.cardiores.2005.06.024. [DOI] [PubMed] [Google Scholar]
- [13].Welling A, Kwan YW, Bosse E, Flockerzi V, Hofmann F, Kass RS. “Subunit-dependent modulation of recombinant L-type calcium channels. Molecular basis for dihydropyridine tissue selectivity”. Circ Res. 1993 Nov;73(5):974–980. doi: 10.1161/01.res.73.5.974. [DOI] [PubMed] [Google Scholar]
- [14].Welling A, Zimmer Ludwig S, Klugbauer N, Flockerzi V, Hofmann F. “Alternatively spliced IS6 segments of the alpha 1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+channels”. Circ Res. 1997 Oct;81(4):526–532. doi: 10.1161/01.res.81.4.526. [DOI] [PubMed] [Google Scholar]
- [15].Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. “Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel”. Nature. 1989 Jul;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- [16].Biel M, Ruth P, Bosse E, Hullin R, Stuhmer W, Flockerzi V, Hofmann F. “Primary structure and functional expression of a high voltage activated calcium channel from rabbit lung”. FEBS Lett. 1990 Sep;269(2):409–412. doi: 10.1016/0014-5793(90)81205-3. [DOI] [PubMed] [Google Scholar]
- [17].Hu H, Marban E. “Isoform-specific inhibition of L-type calcium channels by dihydropyridines is independent of isoform-specific gating properties“. Mol. Pharmacol. 1998 May;53(5):902–907. [PubMed] [Google Scholar]
- [18].Liao P, Yu D, Li G, Yong TF, Soon JL, Chua YL, Soong TW. “A smooth muscle Cav1.2 calcium channel splice variant underlies hyperpolarized window current and enhanced state-dependent inhibition by nifedipine”. J Biol Chem. 2007 Nov;282(48):35133–35142. doi: 10.1074/jbc.M705478200. [DOI] [PubMed] [Google Scholar]
- [19].Chen N, Zhou M, Yang M, Guo J, Zhu C, Yang J, Wang Y, Yang X, He L. “Calcium channel blockers versus other classes of drugs for hypertension”. Cochrane Database Syst Rev. 2010 Aug;8:CD003654. doi: 10.1002/14651858.CD003654.pub4. [DOI] [PubMed] [Google Scholar]
- [20].Budriesi review
- [21].Kobayashi S, Hishida A. Effects of a calcium antagonist, manidipine, on progressive renal injury associated with mild hypertension in remnant kidneys. J Lab Clin Med. 1995 May;125(5):572–580. [PubMed] [Google Scholar]
- [22].Wenzel RR. Renal protection in hypertensive patients: selection of antihypertensive therapy. Drugs. 2005;65(Suppl 2):29–39. doi: 10.2165/00003495-200565002-00005. [DOI] [PubMed] [Google Scholar]
- [23].Hayashi K, Ozawa Y, Fujiwara K, Wakino S, Kumagai H, Saruta T.Role of actions of calcium antagonists on efferent arterioles--with special references to glomerular hypertension Am J Nephrol 234229–244.July–Aug2003 [DOI] [PubMed] [Google Scholar]
- [24].Hongo K, Kobayashi S. “Calcium antagonists for the treatment of vasospasm following subarachnoid haemorrhage”. Neurol Res. 1993 Aug;15(4):218–24. doi: 10.1080/01616412.1993.11740140. [DOI] [PubMed] [Google Scholar]
- [25].Kazda S, Garthoff B, Krause HP, Schlossmann K. ”Cerebrovascular effects of the calcium antagonistic dihydropyridine derivative nimodipine in animal experiments”. Arzneimittelforschung. 1982;32(4):331–338. [PubMed] [Google Scholar]
- [26].Schmid-Elsaesser R, Kunz M, Zausinger S, Prueckner S, Briegel J, Steiger HJ. “Intravenous magnesium versus nimodipine in the treatment of patients with aneurysmal subarachnoid hemorrhage: a randomized study“. Neurosurgery. 2006 Jun;58(6):1054–65. doi: 10.1227/01.NEU.0000215868.40441.D9. [DOI] [PubMed] [Google Scholar]
- [27].Langley MS, Sorkin EM. “Nimodipine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in cerebrovascular disease”. Drugs. 1989 May;37(5):669–99. doi: 10.2165/00003495-198937050-00004. [DOI] [PubMed] [Google Scholar]
- [28].Towart R, Perzborn E. “Nimodipine inhibits carbocyclic thromboxane-induced contractions of cerebral arteries”. Eur J Pharmacol. 1981 Jan;69(2):213–215. doi: 10.1016/0014-2999(81)90417-9. [DOI] [PubMed] [Google Scholar]
- [29].Dorhout Mees SM, Rinkel GJ, Feigin VL, Algra A, van den Bergh WM, Vermeulen M, van Gijn J. “Calcium antagonists for aneurysmal subarachnoid haemorrhage”. Cochrane Database Syst Rev. 2007 Jul;18(3):1–50. CD000277. [Google Scholar]
- [30].Godfraind T, Egleme C, Finet M, Jaumin P. “The actions of nifedipine and nisoldipine on the contractile activity of human coronary arteries and human cardiac tissue in vitro”. Pharmacol Toxicol. 1987 Aug;61(2):79–84. doi: 10.1111/j.1600-0773.1987.tb01779.x. [DOI] [PubMed] [Google Scholar]
- [31].Godfraind T, Morel N, Wibo M. “Tissue specificity of dihydropyridine-type calcium antagonists in human isolated tissues” (1988) Trends Pharmacol Sci. 1988 Jan;9(1):37–39. doi: 10.1016/0165-6147(88)90241-6. [DOI] [PubMed] [Google Scholar]
- [32].Duprez D, De Backer T, De Pue N, Hermans L, De Buyzere M, Clement DL. Effects of isradipine on peripheral hemodynamic reflex responses in mild-to-moderate essential hypertension. Am J Hypertens. 1991 Feb;4(2 Pt 2):194S–196S. doi: 10.1093/ajh/4.2.194s. [DOI] [PubMed] [Google Scholar]
- [33].Grossman E, Messerli FH, Oren S, Nunez B, Garavaglia GE. Cardiovascular effects of isradipine in essential hypertension. Am J Cardiol. 1991 Jul;68(1):65–70. doi: 10.1016/0002-9149(91)90712-t. [DOI] [PubMed] [Google Scholar]
- [34].Mauser M, Voelker W, Ickrath O, Karsch KR. “Myocardial properties of the new dihydropyridine calcium antagonist isradipine compared to nifedipine with or without additional beta blockade in coronary artery disease”. Am J Cardiol. 1989 Jan;63(1):40–44. doi: 10.1016/0002-9149(89)91073-4. [DOI] [PubMed] [Google Scholar]
- [35].Takahara A. “Cilnidipine: a new generation Ca channel blocker with inhibitory action on sympathetic neurotransmitter release”. Cardiovasc Ther. 2009 Summer;27(2):124–139. doi: 10.1111/j.1755-5922.2009.00079.x. [DOI] [PubMed] [Google Scholar]
- [36].Böhm M, Schwinger RH, Erdmann E. “Different cardiodepressant potency of various calcium antagonists in human myocardium”. Am J Cardiol. 1990 Apr;65(15):1039–41. doi: 10.1016/0002-9149(90)91013-v. [DOI] [PubMed] [Google Scholar]
- [37].Maxwell CJ, Hogan DB, Campbell NR, Ebly EM. “Nifedipine and mortality risk in the elderly: relevance of drug formulation, dose and duration”. Pharmacoepidemiol Drug Saf. 2000 Jan;9(1):11–23. doi: 10.1002/(SICI)1099-1557(200001/02)9:1<11::AID-PDS468>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- [38].Jung SY, Choi NK, Kim JY, Chang Y, Song HJ, Lee J, Park BJ. “Short-acting nifedipine and risk of stroke in elderly hypertensive patients”. Neurology. 2011 Sep;77(13):1229–1234. doi: 10.1212/WNL.0b013e318230201a. [DOI] [PubMed] [Google Scholar]
- [39].Aouam K, Berdeaux A.“Dihydropyridines from the first to the fourth generation: better effects and safety” Therapie 584333–339.July–Aug2003 [DOI] [PubMed] [Google Scholar]
- [40].Toyo-oka T, Nayler WG. “Third generation calcium entry blockers”. Blood Press. 1996 Jul;5(4):206–208. doi: 10.3109/08037059609079672. [DOI] [PubMed] [Google Scholar]
- [41].Kenyon KW. “Clevidipine: an ultra short-acting calcium channel antagonist for acute hypertension”. Ann Pharmacother. 2009 Jul;43(7):1258–1265. doi: 10.1345/aph.1L610. [DOI] [PubMed] [Google Scholar]
- [42].Espina IM, Varon J. “Clevidipine : a state-of-the-art antihypertensive drug under the scope”. Expert Opin Pharmacother. 2012 Feb;13(3):387–393. doi: 10.1517/14656566.2012.651126. [DOI] [PubMed] [Google Scholar]
- [43].Awad AS, Goldberg ME. “Role of clevidipine butyrate in the treatment of acute hypertension in the critical care setting: a review. Vasc. Health Risk. Manag. 2010 Aug;6:457–464. doi: 10.2147/vhrm.s5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB, Harpold MM. “Structure and functional expression of alpha 1, alpha 2, and beta subunits of a novel human neuronal calcium channel subtype”. Neuron. 1992 Jan;8(1):71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- [45].Takimoto K, Li D, Nerbonne JM, Levitan ES. “Distribution, splicing and glucocorticoid-induced expression of cardiac alpha 1C and alpha 1D voltage-gated Ca2+channel mRNAs”. J Mol Cell Cardiol. 1997 Nov;29(11):3035–3042. doi: 10.1006/jmcc.1997.0532. [DOI] [PubMed] [Google Scholar]
- [46].Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. “Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+channels”. Cell. 2000 Jul;102(1):89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- [47].Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. “alpha 1D (Cav1.3) subunits can form L-type Ca2+channels activating at negative voltages”. J. Biol. Chem. 2001 Jun;276(25):22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- [48].Zuccotti A, Clementi S, Reinbothe T, Torrente A, Vandael DH, Pirone A. “Structural and functional differences between L-type calcium channels: crucial issues for future selective targeting. Trends Pharmacol. Sci. 2011 Jun;32(6):366–375. doi: 10.1016/j.tips.2011.02.012. [DOI] [PubMed] [Google Scholar]
- [49].Zhang Z, He Y, Tuteja D, Xu D, Timofeyev V, Zhang Q, Glatter KA, Xu Y, Shin HS, Low R, Chiamvimonvat N. “Functional roles of Cav1.3(alpha1D) calcium channels in atria: insights gained from gene-targeted null mutant mice”. Circulation. 2005 Sep;112(13):1936–1944. doi: 10.1161/CIRCULATIONAHA.105.540070. [DOI] [PubMed] [Google Scholar]
- [50].Zhang Q, Timofeyev V, Qiu H, Lu L, Li N, Singapuri A, Torado CL, Shin HS, Chiamvimonvat N. “Expression and roles of Cav1.3 (α1D) L-type Ca2+ channel in atrioventricular node automaticity”. J Mol Cell Cardiol. 2011 Jan;50(1):194–202. doi: 10.1016/j.yjmcc.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, Hoda JC, Sartori SB, Striessnig J. “Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms”. Mol Pharmacol. 2009 Feb;75(2):407–414. doi: 10.1124/mol.108.049981. [DOI] [PubMed] [Google Scholar]
- [52].Budriesi R, Ioan P, Locatelli A, Cosconati S, Leoni A, Ugenti MP, Andreani A, Di Toro R, Bedini A, Spampinato S, Marinelli L, Novellino E, Chiarini A. “Imidazo[2,1-b]thiazole system: a scaffold endowing dihydropyridines with selective cardiodepressant activity”. J Med Chem. 2008 Mar;51(6):1592–1600. doi: 10.1021/jm070681+. [DOI] [PubMed] [Google Scholar]
- [53].Cosimelli B, Severi E, Novellino E, Cavaccini A, Cataldi M, Budriesi R, Micucci M, Chiarini A, Ioan P. Preliminary finding on a new calcium channel entry blocker chemotype: 5,6-diamino-4-hydroxy-2-mercaptopyrimidine derivatives. J. Med. Chem. 54(15):5597–5601. doi: 10.1021/jm200414s. [DOI] [PubMed] [Google Scholar]
- [54].Luebke JI, Dunlap K, Turner TJ. “Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus”. Neuron. 1993 Nov;11(5):895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- [55].Horne AL, Kemp JA. “The effect of ω-conotoxin GVIA on synaptic transmission within the nucleus accumbens and hippocampus of the rat in vitro”. Br J Pharmacol. 1991 Jul;103(3):1733–1739. doi: 10.1111/j.1476-5381.1991.tb09855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wessler I, Dooley DJ, Werhand J, Schlemmer F. “Differential effects of calcium channel antagonists (ω-conotoxin GVIA, nifedipine, verapamil) on the electrically-evoked release of [3H]acetylcholine from the myenteric plexus, phrenic nerve and neocortex of rats”. Naunyn Schmiedebergs Arch Pharmacol. 1990 Apr;341(4):288–294. doi: 10.1007/BF00180653. [DOI] [PubMed] [Google Scholar]
- [57].Woodward JJ, Rezazadeh SM, Leslie SW. “Differential sensitivity of synaptosomal calcium entry and endogenous dopamine release to ω-conotoxin”. Brain Res. 1988 Dec;475(1):141–145. doi: 10.1016/0006-8993(88)90207-7. [DOI] [PubMed] [Google Scholar]
- [58].Dooley DJ, Lupp A, Hertting G, Osswald H. “ω-Conotoxin GVIA and pharmacological modulation of hippocampal noradrenaline release”. Eur. J. Pharmacol. 1988 Mar;148(2):261–267. doi: 10.1016/0014-2999(88)90572-9. [DOI] [PubMed] [Google Scholar]
- [59].Hirning LD, Fox AP, McCleskey EW, Olivera BM, Thayer SA, Miller RJ, Tsien RW. “Dominant role of N-type Ca2+channels in evoked release of norepinephrine from sympathetic neurons”. Science. 1988 Jan;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- [60].McGuire D, Bowersox S, Fellmann JD, Luther RR. “Sympatholysis after neuron-specific, N-type, voltage-sensitive calcium channel blockade: first demonstration of N-channel function in humans”. J Cardiovasc Pharmacol. 1997 Sep;30(3):400–403. doi: 10.1097/00005344-199709000-00019. [DOI] [PubMed] [Google Scholar]
- [61].Ino M, Yoshinaga T, Wakamori M, Miyamoto N, Takahashi E, Sonoda J, Kagaya T, Oki T, Nagasu T, Nishizawa Y, Tanaka I, Imoto K, Aizawa S, Koch S, Schwartz A, Niidome T, Sawada K, Mori Y. “Functional disorders of the sympathetic nervous system in mice lacking the α1B subunit (Cav 2.2) of N-type calcium channels”. Proc Natl Acad Sci USA. 2001 Apr;98(9):5323–5328. doi: 10.1073/pnas.081089398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Furukawa T, Yamakawa T, Midera T, Sagawa T, Mori Y, Nukada T. “Selectivities of Dihydropyridine Derivatives in Blocking Ca2+Channel Subtypes Expressed in Xenopus Oocytes”. J Pharmacol Exp Ther. 1999 Nov;291(2):464–473. [PubMed] [Google Scholar]
- [63].Abernethy DR, Gutkowska J, Lambert MD. “Amlodipine in elderly hypertensive patients: pharmacokinetics and pharmacodynamics”. J Cardiovasc Pharmacol. 1988;12(suppl 7):S67–S71. doi: 10.1097/00005344-198812007-00015. [DOI] [PubMed] [Google Scholar]
- [64].Hamada T, Watanabe M, Kaneda T, Ohtahara A, Kinugawa T, Hisatome I, Fujimoto Y, Yoshida A, Shigemasa C. “Evaluation of changes in sympathetic nerve activity and heart rate in essential hypertensive patients induced by amlodipine and nifedipine”. J Hypertens. 1998 Jan;16(1):111–118. doi: 10.1097/00004872-199816010-00016. [DOI] [PubMed] [Google Scholar]
- [65].Minami J, Ishimitsu T, Kawano Y, Matsuoka H.“Effects of amlodipine and nifedipine retard on autonomic nerve activity in hypertensive patients” Clin Exp Pharmacol Physiol 257–8572–576.Jul.–Aug1998 (a) [DOI] [PubMed] [Google Scholar]
- [66].Minami J, Ishimitsu T, Kawano Y, Numabe A, Matsuoka H. “Comparison of 24-hour blood pressure, heart rate, and autonomic nerve activity in hypertensive patients treated with cilnidipine or nifedipine retard”. J Cardiovasc Pharmacol. 1998b Aug;32(2):331–336. doi: 10.1097/00005344-199808000-00023. [DOI] [PubMed] [Google Scholar]
- [67].Faulkner JK, McGibney D, Chasseaud LF, Perry JL, Taylor IW. “The pharmacokinetics of amlodipine in healthy volunteers after single intravenous and oral doses and after 14 repeated oral doses given once daily. Br J Clin Pharmacol. 1986 Jul;22(1):21–25. doi: 10.1111/j.1365-2125.1986.tb02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cataldi M, Perez-Reyes E, Tsien RW. “Differences in apparent pore sizes of low and high voltage-activated Ca2+ channels”. J Biol Chem. 2002 Nov;277(48):45969–45976. doi: 10.1074/jbc.M203922200. [DOI] [PubMed] [Google Scholar]
- [69].Perez-Reyes E. “Molecular physiology of low-voltage-activated T-type calcium channels”. Physiol Rev. 2003 Jan;83(1):117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- [70].Chuang RS, Jaffe H, Cribbs L, Perez-Reyes E, Swartz KJ. “Inhibition of T-type voltage-gated calcium channels by a new scorpion toxin”. Nat Neurosci. 1998 Dec;1(8):668–674. doi: 10.1038/3669. [DOI] [PubMed] [Google Scholar]
- [71].Middleton RE, Warren VA, Kraus RL, Hwang JC, Liu CJ, Dai G, Brochu RM, Kohler MG, Gao YD, Garsky VM, Bogusky MJ, Mehl JT, Cohen CJ, Smith MM. “Two tarantula peptides inhibit activation of multiple sodium channels”. Biochemistry. 2002 Dec;41(50):14734–14747. doi: 10.1021/bi026546a. [DOI] [PubMed] [Google Scholar]
- [72].Santi CM, Cayabyab FS, Sutton KG, McRory JE, Mezeyova J, Hamming KS, Parker D, Stea A, Snutch TP. “Differential inhibition of T-type calcium channels by neuroleptics”. J Neurosci. 2002 Jan;22(2):396–403. doi: 10.1523/JNEUROSCI.22-02-00396.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cataldi M, Gaudino A, Lariccia V, Russo M, Amoroso A, di Renzo G G, Annunziato L. “Imatinib-mesylate blocks recombinant T-type calcium channels expressed in human embryonic kidney-293 cells by a protein tyrosine kinase-independent mechanism”. J Pharmacol Exp Ther. 2004 Apr;309(1):208–215. doi: 10.1124/jpet.103.061184. [DOI] [PubMed] [Google Scholar]
- [74].Rimoli MG, Russo E, Cataldi M, Citraro R, Ambrosino P, Melisi D, Curcio A, De Lucia S, Patrignani P, De Sarro G, Abignente E. “T-type channel blocking properties and antiabsence activity of two imidazo[1,2-b]pyridazine derivatives structurally related to indomethacin”. Neuropharmacology. 2009 Mar;56(3):637–646. doi: 10.1016/j.neuropharm.2008.11.003. [DOI] [PubMed] [Google Scholar]
- [75].Cataldi M, Lariccia V, Marzaioli V, Cavaccini A, Curia G, Viggiano D, Canzoniero LM, di Renzo G, Avoli M, Annunziato L. “Zn(2+) slows down Ca(V)3.3 gating kinetics: implications for thalamocortical activity”. J Neurophysiol. 2007 Oct;98(4):2274–2284. doi: 10.1152/jn.00889.2006. [DOI] [PubMed] [Google Scholar]
- [76].Furukawa T, Nukada T, Namiki Y, Miyashita Y, Hatsuno K, Ueno Y, Yamakawa T, Isshiki T. “Five different profiles of dihydropyridines in blocking T-type Ca(2+) channel subtypes (Ca(v)3.1 (alpha(1G)), Ca(v)3.2 (alpha(1H)), and Ca(v)3.3 (alpha(1I))) expressed in Xenopus oocytes”. Eur J Pharmacol. 2009 Jun;613(1–3):100–107. doi: 10.1016/j.ejphar.2009.04.036. [DOI] [PubMed] [Google Scholar]
- [77].Perez-Reyes E, Van Deusen AL, Vitko I. “Molecular pharmacology of human Cav3.2 T-type Ca2+ channels: block by antihypertensives, antiarrhythmics, and their analogs”. J Pharmacol Exp Ther. 2009 Feb;328(2):621–627. doi: 10.1124/jpet.108.145672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hayashi K, Wakino S, Homma K, Sugano N, Saruta T. “Pathophysiological significance of T-type Ca2+ channels: role of T-type Ca2+ channels in renal microcirculation”. J Pharmacol Sci. 2005 Nov;99(3):221–227. doi: 10.1254/jphs.fmj05002x6. [DOI] [PubMed] [Google Scholar]
- [79].Hayashi K, Wakino S, Sugano N, Ozawa Y, Homma K, Saruta T. “Ca2+ channel subtypes and pharmacology in the kidney”. Circ Res. 2007 Feb;100(3):342–353. doi: 10.1161/01.RES.0000256155.31133.49. [DOI] [PubMed] [Google Scholar]
- [80].Poulsen CB, Al-Mashhadi RH, Cribbs LL, Skøtt O, Hansen PB. “T-type voltage-gated calcium channels regulate the tone of mouse efferent arterioles”. Kidney Int. 2011 Feb;79(4):443–451. doi: 10.1038/ki.2010.429. [DOI] [PubMed] [Google Scholar]
- [81].Feng MG, Li M, Navar LG. “T-type calcium channels in the regulation of afferent and efferent arterioles in rats”. Am J Physiol Renal Physiol. 2004 Feb;286(2):F331–F337. doi: 10.1152/ajprenal.00251.2003. [DOI] [PubMed] [Google Scholar]
- [82].Ozawa Y, Hayashi K, Nagahama T, Fujiwara K, Saruta T. “Effect of T-type selective calcium antagonist on renal microcirculation: studies in the isolated perfused hydronephrotic kidney”. Hypertension. 2001 Sep;38(3):343–7. doi: 10.1161/01.hyp.38.3.343. [DOI] [PubMed] [Google Scholar]
- [83].Abe Y Y, Komori T, Miura K, Takada T, Imanishi M, Okahara T, Yamamoto K. “Effects of the calcium antagonist nicardipine on renal function and renin release in dogs”. J Cardiovasc Pharmacol. 1983 Mar.apr;5(2):254–259. doi: 10.1097/00005344-198303000-00015. [DOI] [PubMed] [Google Scholar]
- [84].Dietz JR, Davis JO, Freeman RH, Villarreal D, Echtenkamp SF.“Effects of intrarenal infusion of calcium entry blockers in anesthetized dogs” Hypertension 54482–488.Jul.–Aug.1983 [DOI] [PubMed] [Google Scholar]
- [85].Heller J, Horacek V. “The effect of two different calcium antagonists on the glomerular haemodynamics in dogs”. Pflugers Arch. 1990 Mar;415(6):751–755. doi: 10.1007/BF02584016. [DOI] [PubMed] [Google Scholar]
- [86].Roy M, Guthrie GP, Holladay FP, Kotchen TA. “Effects of verapamil on renin and aldosterone in the dog and rat”. Am J Physiol. 1983 Oct;245(4):E410–E416. doi: 10.1152/ajpendo.1983.245.4.E410. [DOI] [PubMed] [Google Scholar]
- [87].Brenner BM, Lawler EV, Mackenzie HS. “The hyperfiltration theory: a paradigm shift in nephrology”. Kidney Int. 1996 Jun;49(6):1774–1777. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- [88].Hayashi K, Homma K, Wakino S, Tokuyama H, Sugano N, Saruta T, Itoh H. “T-type Ca channel blockade as a determinant of kidney protection”. Keio J. Med. 2010;59(3):84–95. doi: 10.2302/kjm.59.84. [DOI] [PubMed] [Google Scholar]
- [89].Derwa A, Peeters P, Vanholder R.“Calcium channel blockers in the prevention of end stage renal disease: a review” Acta Clin Belg 59144–56.January–Feb2004 [DOI] [PubMed] [Google Scholar]
- [90].Fujiwara K, Kanno Y, Hayashi K, Takenaka T, Saruta T. Renal protective effects of efonidipine in partially nephrectomized spontaneously hypertensive rats. Clin. Exp. Hypertens. 1998 Apr;20(3):295–312. doi: 10.3109/10641969809052123. [DOI] [PubMed] [Google Scholar]
- [91].Ishimitsu T, Kameda T, Akashiba A, Takahashi T, Ohta S, Yoshii M, Minami J, Ono H, Numabe A, Matsuoka H. “Efonidipine reduces proteinuria and plasma aldosterone in patients with chronic glomerulonephritis”. Hypertens Res. 2007 Jul;30(7):621–626. doi: 10.1291/hypres.30.621. [DOI] [PubMed] [Google Scholar]
- [92].Kawabata M, Ogawa T, Han WH, Takabatake T. “Renal effects of efonidipine hydrochloride, a new calcium antagonist, in spontaneously hypertensive rats with glomerular injury”. Clin Exp Pharmacol Physiol. 1999 Sep;26(9):674–679. doi: 10.1046/j.1440-1681.1999.03114.x. [DOI] [PubMed] [Google Scholar]
- [93].Nakamura M, Notoya M, Kohda Y, Yamashita J, Takashita Y, Gemba M. “Effects of efonidipine hydrochloride on renal arteriolar diameters in spontaneously hypertensive rats”. Hypertens Res. 2002 Sep;25(5):751–755. doi: 10.1291/hypres.25.751. [DOI] [PubMed] [Google Scholar]
- [94].Okura T. “Efonidipine improves renal function and decreases proteinuria in elderly hypertensive patients in the JATOS study”. Hypertens Res. 2010 Nov;33(11):1112–1113. doi: 10.1038/hr.2010.150. [DOI] [PubMed] [Google Scholar]
- [95].Sasaki H, Saiki A, Endo K, Ban N, Yamaguchi T, Kawana H, Nagayama D, Ohhira M, Oyama T, Miyashita Y, Shirai K. “Protective effects of efonidipine, a T- and L-type calcium channel blocker, on renal function and arterial stiffness in type 2 diabetic patients with hypertension and nephropathy”. J Atheroscler Thromb. 2009 Oct;16(5):568–575. doi: 10.5551/jat.1628. [DOI] [PubMed] [Google Scholar]
- [96].Shudo C, Masuda Y, Sugita H, Furukawa S, Hayashi K, Hirata H, Tanaka S, Tomita K. “Renal protective effect of efonidipine hydrochloride (NZ-105), a new calcium antagonist, in spontaneously hypertensive rats”. Gen Pharmacol. 1994 Dec;25(8):1567–1575. doi: 10.1016/0306-3623(94)90356-5. [DOI] [PubMed] [Google Scholar]
- [97].Takeda M, Shou I, Tomino Y. “Effects of the antihypertensive drug efonidipine hydrochloride on albuminuria and renal histopathology in young spontaneously hypertensive rats with diabetes. Gen. Pharmacol. 1998 May;30(5):749–752. doi: 10.1016/s0306-3623(97)00337-6. [DOI] [PubMed] [Google Scholar]
- [98].Abe M, Okada K, Maruyama N, Matsumoto S, Maruyama T, Fujita T, Matsumoto K, Soma M. “Benidipine reduces albuminuria and plasma aldosterone in mild-to-moderate stage chronic kidney disease with albuminuria”. Hypertens Res. 2011 Feb;34(2):268–273. doi: 10.1038/hr.2010.221. [DOI] [PubMed] [Google Scholar]
- [99].Nakamura T, Obata JE, Onitsuka M, Shimada Y, Yoshida Y, Kawachi H, Shimizu F. “Benidipine, a long-acting calcium-channel blocker, prevents the progression to end-stage renal failure in a rat mesangioproliferative glomerulonephritis”. Nephron. 2000 Nov;86(3):315–326. doi: 10.1159/000045787. [DOI] [PubMed] [Google Scholar]
- [100].Nito M, Sato H, Hara T, Karasawa A, Yamada K, Ohta Y. “Suppressive effects of benidipine on the development of hypertension and renal lesions in salt-loaded stroke-prone spontaneously hypertensive rats”. Clin Exp Pharmacol Physiol Suppl. 1995 Dec;22(1):S337–s338. doi: 10.1111/j.1440-1681.1995.tb02944.x. [DOI] [PubMed] [Google Scholar]
- [101].Shirakura S, Sano J, Karasawa A, Kubo K. “Protective effect of benidipine against the development of glomerular sclerosis in experimental nephrotic syndrome”. Jpn J Pharmacol. 1992 Aug;59(4):461–467. doi: 10.1254/jjp.59.461. [DOI] [PubMed] [Google Scholar]
- [102].Saito F, Fujita H, Takahashi A, Ichiyama I, Harasawa S, Oiwa K, Takahashi N, Otsuka Y, Uchiyama T, Kanmatsuse K, Kushiro T. “Renoprotective effect and cost-effectiveness of using benidipine, a calcium channel blocker, to lower the dose of angiotensin receptor blocker in hypertensive patients with albuminuria”. Hypertens Res. 2007 Jan;30(1):39–47. doi: 10.1291/hypres.30.39. [DOI] [PubMed] [Google Scholar]
- [103].Tomino Y, Shimizu Y, Hamada C, Kurusu A, Ohsawa I, Suzuki Y, Tsuge T, Io H, Kobayashi N, Takeda Y, Asanuma K, Tanaka Y, Suzuki H, Nakata J, Takara K, Horikoshi S.“One-year results of an open-label study on antiproteinuric effect of benidipine in elderly patients with chronic kidney disease” J Nephrol 246756–763.November–Dec2011 [DOI] [PubMed] [Google Scholar]
- [104].Yamamoto E, Kataoka K, Dong YF, Nakamura T, Fukuda M, Nako H, Ogawa H, Kim-Mitsuyama S. “Benidipine, a dihydropyridine L-type/T-type calcium channel blocker, affords additive benefits for prevention of cardiorenal injury in hypertensive rats”. J Hypertens. 2010 Jun;28(6):1321–1329. doi: 10.1097/HJH.0b013e3283388045. [DOI] [PubMed] [Google Scholar]
- [105].Bellinghieri G, Mazzaglia G, Savica V, Santoro D. “Effects of manidipine and nifedipine on blood pressure and renal function in patients with chronic renal failure: a multicenter randomized controlled trial. Ren. Fail. 2003 Sep;25(5):681–689. doi: 10.1081/jdi-120024284. [DOI] [PubMed] [Google Scholar]
- [106].Del Vecchio L, Pozzi M, Salvetti A, Maschio G, Fusaroli M, Rovati C, Antonucci F, Cascone C, Scanferla F, Panichi V, Sturani A, Locatelli F, the Manidipine Study Group “Efficacy and tolerability of manidipine in the treatment of hypertension in patients with non-diabetic chronic kidney disease without glomerular disease. Prospective, randomized, double-blind study of parallel groups in comparison with enalapril” J Nephrol 172261–269.Mar.–Apr.2004 [PubMed] [Google Scholar]
- [107].He H, Tamaki T, Aki Y, Kiyomoto H, Iwao H, Miyatake A, Abe Y. “Effects of the calcium antagonist manidipine on renal hemodynamics and function in dogs: comparison with nifedipine”. Blood Press. 1992;3(Suppl):68–74. [PubMed] [Google Scholar]
- [108].Ikenaga H, Suzuki H, Saruta T. “Effects of manidipine on blood pressure and renal function in salt-loaded, spontaneously hypertensive rats”. Blood Press Suppl. 1992;3:75–79. [PubMed] [Google Scholar]
- [109].Kobayashi S, Hishida A. “Effects of a calcium antagonist, manidipine, on progressive renal injury associated with mild hypertension in remnant kidneys. J. Lab. Clin. Med. 1995 May;125(5):572–580. [PubMed] [Google Scholar]
- [110].Takabatake T, Ushiogi Y, Ise T, Kobayashi K. “Effect of calcium antagonist, manidipine hydrochloride, on renal hemodynamics and tubuloglomerular feedback in spontaneously hypertensive rats”. Am Heart J. 1993 Feb;125(2 Pt 2):578–581. doi: 10.1016/0002-8703(93)90206-o. [DOI] [PubMed] [Google Scholar]
- [111].Tojo A, Kimura K, Matsuoka H, Sugimoto T. “Effects of manidipine hydrochloride on the renal microcirculation in spontaneously hypertensive rats”. J Cardiovasc Pharmacol. 1992 Dec;20(6):895–899. doi: 10.1097/00005344-199212000-00008. [DOI] [PubMed] [Google Scholar]
- [112].Chen XL, Bayliss DA, Fern RJ, Barrett PQ. “A role for T-type Ca2+ channels in the synergistic control of aldosterone production by ANG II and K+”. Am J Physiol. 1999 May;276(5 Pt 2):F674–F683. doi: 10.1152/ajprenal.1999.276.5.F674. [DOI] [PubMed] [Google Scholar]
- [113].Rossier MF, Burnay MM, Vallotton MB, Capponi AM. “Distinct functions of T- and L-type calcium channels during activation of bovine adrenal glomerulosa cells”. Endocrinology. 1996 Nov;137(11):4817–4826. doi: 10.1210/endo.137.11.8895352. [DOI] [PubMed] [Google Scholar]
- [114].Akizuki O, Inayoshi A, Kitayama T, Yao K, Shirakura S, Sasaki K, Kusaka H, Matsubara M. “Blockade of T-type voltage-dependent Ca2+ channels by benidipine, a dihydropyridine calcium channel blocker, inhibits aldosterone production in human adrenocortical cell line NCI-H295R”. Eur J Pharmacol. 2008 Apr;584(2–3):424–34. doi: 10.1016/j.ejphar.2008.02.001. [DOI] [PubMed] [Google Scholar]
- [115].Imagawa K, Okayama S, Takaoka M, Kawata H, Naya N, Nakajima T, Horii M, Uemura S, Saito Y. “Inhibitory effect of efonidipine on aldosterone synthesis and secretion in human adrenocarcinoma (H295R) cells. J. Cardiovasc. Pharmacol. 2006 Jan;47(1):133–138. doi: 10.1097/01.fjc.0000197539.12685.f5. [DOI] [PubMed] [Google Scholar]
- [116].Okayama S, Imagawa K, Naya N, Iwama H, Somekawa S, Kawata H, Horii M, Nakajima T, Uemura S, Saito Y. “Blocking T-type Ca2+ channels with efonidipine decreased plasma aldosterone concentration in healthy volunteers”. Hypertens Res. 2006 Jul;29(7):493–497. doi: 10.1291/hypres.29.493. [DOI] [PubMed] [Google Scholar]
- [117].Tanaka T, Tsutamoto T, Sakai H, Fujii M, Yamamoto T, Horie M. “Comparison of the effects of efonidipine and amlodipine on aldosterone in patients with hypertension”. Hypertens Res. 2007 Aug;30(8):691–697. doi: 10.1291/hypres.30.691. [DOI] [PubMed] [Google Scholar]
- [118].Mulgrew CJ, Cove-Smith A, McLatchie LM, Brooks G, Shattock MJ, Hendry BM. “Inhibition of human mesangial cell proliferation by targeting T-type calcium channels”. Nephron Exp Nephrol. 2009;113(2):e77–e88. doi: 10.1159/000232590. [DOI] [PubMed] [Google Scholar]
- [119].Matsuda H, Mori T, Kurumazuka D, Kitada K, Hayashi T, Nagatoya K, Inoue T, Ukimura A, Matsumura Y, Ishizaka N, Kitaura Y. “Inhibitory effects of T/L-type calcium channel blockers on tubulointerstitial fibrosis in obstructed kidneys in rats”. Urology. 2011 Jan;77(1):249.e9–249.e15. doi: 10.1016/j.urology.2010.07.496. [DOI] [PubMed] [Google Scholar]
- [120].Fan YY, Kohno M, Nakano D, Ohsaki H, Kobori H, Suwarni D, Ohashi N, Hitomi H, Asanuma K, Noma T, Tomino Y, Fujita T, Nishiyama A. “Cilnidipine suppresses podocyte injury and proteinuria in metabolic syndrome rats: possible involvement of N-type calcium channel in podocyte”. J Hypertens. 2010 May;28(5):1034–1043. doi: 10.1097/hjh.0b013e328336ade3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Konda T, Enomoto A, Takahara A, Yamamoto H. “Effects of L/N-type calcium channel antagonist, cilnidipine on progressive renal injuries in Dahl salt-sensitive rats”. Biol Pharm Bull. 2006 May;29(5):933–7. doi: 10.1248/bpb.29.933. [DOI] [PubMed] [Google Scholar]
- [122].Takemori K, Ishida H, Dote K, Yamamoto K, Ito H.“Prophylactic effects of an N- and L-type Ca2+ antagonist, cilnidipine, against cardiac hypertrophy and dysfunction in stroke-prone, spontaneously hypertensive rats Can. J. Physiol. Pharmacol 838–9785–790.Aug.–Sep2005 [DOI] [PubMed] [Google Scholar]
- [123].Zhou X, Ono H, Ono Y, Frohlich ED. “N- and L-type calcium channel antagonist improves glomerular dynamics, reverses severe nephrosclerosis, and inhibits apoptosis and proliferation in an l-NAME/SHR model. J. Hypertens. 2000 May;20(5):993–1000. doi: 10.1097/00004872-200205000-00035. [DOI] [PubMed] [Google Scholar]
- [124].Morimoto S, Yano Y, Maki K, Iwasaka T. “Renal and vascular protective effects of cilnidipine in patients with essential hypertension”. J Hypertens. 2007 Oct;25(10):2178–2183. doi: 10.1097/HJH.0b013e3282c2fa62. [DOI] [PubMed] [Google Scholar]
- [125].Toba H, Yoshida M, Tojo C, Nakano A, Oshima Y, Kojima Y, Noda K, Wang J, Kobara M, Nakata T. “L/N-type calcium channel blocker cilnidipine ameliorates proteinuria and inhibits the renal renin-angiotensin-aldosterone system in deoxycorticosterone acetate-salt hypertensive rats”. Hypertens Res. 2011 Apr;34(4):521–529. doi: 10.1038/hr.2010.279. [DOI] [PubMed] [Google Scholar]
- [126].Abe M, Okada K, Maruyama T, Maruyama N, Matsumoto K. “Comparison of the antiproteinuric effects of the calcium channel blockers benidipine and amlodipine administered in combination with angiotensin receptor blockers to hypertensive patients with stage 3–5 chronic kidney disease. Hypertens Res. 2009 Apr;32(4):270–275. doi: 10.1038/hr.2009.11. [DOI] [PubMed] [Google Scholar]
- [127].Ohishi M, Takagi T, Ito N, Terai M, Tatara Y, Hayashi N, Shiota A, Katsuya T, Rakugi H, Ogihara T. “Renal-protective effect of T-and L-type calcium channel blockers in hypertensive patients: an Amlodipine-to-Benidipine Changeover (ABC) study. Hypertens. Res. 2007 Sep;30(9):797–806. doi: 10.1291/hypres.30.797. [DOI] [PubMed] [Google Scholar]
- [128].Chiang CS, Huang CH, Chieng H, Chang YT, Chang D, Chen JJ, Chen YC, Chen YH, Shin HS, Campbell KP, Chen CC. “The Ca(v)3.2 T-type Ca(2+) channel is required for pressure overload-induced cardiac hypertrophy in mice”. Circ Res. 2009 Feb;104(4):522–530. doi: 10.1161/CIRCRESAHA.108.184051. [DOI] [PubMed] [Google Scholar]
- [129].Horiba M, Muto T, Ueda N, Opthof T, Miwa K, Hojo M, Lee JK, Kamiya K, Kodama I, Yasui K. “T-type Ca2+ channel blockers prevent cardiac cell hypertrophy through an inhibition of calcineurin-NFAT3 activation as well as L-type Ca2+ channel blockers. Life Sci. 2008 Mar;82(11–12):554–560. doi: 10.1016/j.lfs.2007.11.010. [DOI] [PubMed] [Google Scholar]
- [130].Huang B, Qin D, Deng L, Boutjdir M, E1-Sherif N. “Reexpression of T-type Ca2+ channel gene and current in post-infarction remodeled rat left ventricle”. Cardiovasc Res. 2000 Jun;46(3):442–449. doi: 10.1016/s0008-6363(00)00017-1. [DOI] [PubMed] [Google Scholar]
- [131].Takebayashi S, Li Y, Kaku T, Inagaki S, Hashimoto Y, Kimura K, Miyamoto S, Hadama T, Ono K. “Remodeling excitation-contraction coupling of hypertrophied ventricular myocytes is dependent on T-type calcium channels expression. Biochem. Biophys. Res. Commun. 2006 Jun;345(2):766–773. doi: 10.1016/j.bbrc.2006.04.146. [DOI] [PubMed] [Google Scholar]
- [132].Tang ZZ, Liang MC, Lu S, Yu D, Yu CY, Yue DT, Soong TW. “Transcript scanning reveals novel and extensive splice variations in human l-type voltage-gated calcium channel, Cav1.2 alpha1 subunit. J. Biol. Chem. 2004 Oct;279(43):44335–4443. doi: 10.1074/jbc.M407023200. [DOI] [PubMed] [Google Scholar]
- [133].Fisher SA. “Vascular smooth muscle phenotypic diversity and function”. Physiol. Genomics. 2010 Nov;42A(3):169–187. doi: 10.1152/physiolgenomics.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Nikitina E, Jahromi BS, Bouryi VA, Takahashi M, Xie A, Zhang ZD, Macdonald RL. Voltage-dependent calcium channels of dog basilar artery. J Physiol. 2007 Apr;580(2):523–541. doi: 10.1113/jphysiol.2006.126128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kuo IY, Ellis A, Seymour VA, Sandow SL, Hill CE. “Dihydropyridine-insensitive calcium currents contribute to function of small cerebral arteries”. J Cereb Blood Flow Metab. 2010 Jun;30(6):1226–1239. doi: 10.1038/jcbfm.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Navarro-Gonzalez MF, Grayson TH, Meaney KR, Cribbs LL, Hill CE. “Non-L-type voltage-dependent calcium channels control vascular tone of the rat basilar artery. Clin. Exp. Pharmacol. Physiol. 2009 Jan;36(1):55–66. doi: 10.1111/j.1440-1681.2008.05035.x. [DOI] [PubMed] [Google Scholar]
- [137].Pluteanu F, Cribbs LL. “Regulation and function of Cav3.1 T-type calcium channels in IGF-I-stimulated pulmonary artery smooth muscle cells”. Am J Physiol Cell Physiol. 2011 Mar;300(3):C517–C525. doi: 10.1152/ajpcell.00107.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Rodman DM, Reese K, Harral J, Fouty B, Wu S, West J, Hoedt-Miller M, Tada Y, Li KX, Cool C, Fagan K, Cribbs L. “Low-voltage-activated (T-type) calcium channels control proliferation of human pulmonary artery myocytes”. Circ Res. 2005 Apr;96(8):864–872. doi: 10.1161/01.RES.0000163066.07472.ff. [DOI] [PubMed] [Google Scholar]
- [139].Rodenkirchen R, Bayer R, Steiner R, Bossert F, Meyer H, Moller E. “Structure-activity studies on nifedipine in isolated cardiac muscle”. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(1):69–78. doi: 10.1007/BF00499876. [DOI] [PubMed] [Google Scholar]
- [140].Mahmoudian M, Richards WG. “QSAR of binding of dihydropyridine-type calcium antagonists to their receptor on ileal smooth muscle preparations”. J Pharm Pharmacol. 1986 Apr;38(4):272–276. doi: 10.1111/j.2042-7158.1986.tb04565.x. [DOI] [PubMed] [Google Scholar]
- [141].Hashimoto K, Takeda K, Katano Y, Nakagawa Y, Tsukada T, Hashimoto T, Shimamoto N, Sakai K, Otorii T, Imai S. “Effects of niludipine (Bay a 7168) on the cardiovascular system with a note on its calcium-antagonistic effects”. Arzneimittelforschung. 1979;29(9):1368–1373. [PubMed] [Google Scholar]
- [142].Kazda S, Garthoff B, Meyer H, Schlossmann K, Stoepel K, Towart R, Vater W, Wehinger E. “Pharmacology of a new calcium antagonistic compound, isobutyl methyl 1,4-dihydro-2,6-dimethyl-4(2-nitrophenyl)-3,5-pyridinedicarboxylate (Nisoldipine, Bay k 5552)“. Arzneimittelforschung. 1980;30(12):2144–2162. [PubMed] [Google Scholar]
- [143].Karasawa A, Kubo K, Shuto K, Oka T, Nakamizo N. “Pharmacological actions of benidipine hydrochloride in several isolated smooth muscles and myocardium”. Arzneimittelforschung. 1988 Nov;38(11A):1722–1730. [PubMed] [Google Scholar]
- [144].Pepine A. “Nicardipine, a new calcium channel blocker: role for vascular selectivity”. Clin Cardiol. 1989 May;12(5):240–246. doi: 10.1002/clc.4960120503. [DOI] [PubMed] [Google Scholar]
- [145].Kojda G, Klaus W, Werner G, Fricke U. “The influence of 3-ester side chain variation on the cardiovascular profile of nitrendipine in porcine isolated trabeculae and coronary arteries”. Naunyn Schmiedebergs Arch Pharmacol. 1991;344(4):488–494. doi: 10.1007/BF00172590. [DOI] [PubMed] [Google Scholar]
- [146].Bayer R, Kalusche D, Kaufmann R, Mannhold R. “Inotropic and electrophysiological actions of verapamil and D 600 in mammalian myocardium. III. Effects of the optical isomers on transmembrane action potentials”. Naunyn Schmiedebergs Arch. Pharmacol. 1975;290(1):81–97. doi: 10.1007/BF00499991. [DOI] [PubMed] [Google Scholar]
- [147].Towart R, Wehinger E, Meyer H. “Effects of unsymmetrical ester substituted 1,4-dihydropyridine derivatives and their optical isomers on contraction of smooth muscle”. Naunyn Schmiedebergs Arch Pharmacol. 1981 Sep;317(2):183–185. doi: 10.1007/BF00500079. [DOI] [PubMed] [Google Scholar]
- [148].Shibanuma T, Iwanani M, Okuda K, Takenaka T, Murakami M. “Synthesis of optically active 2-(N-benzyl-N-methylamino)ethyl methyl 2,6-dimethyl-4-(m-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate (Nicardipine)”. Chem Pharm Bull. 1980 Sep;28(9):2809–2812. doi: 10.1248/cpb.28.2809. [DOI] [PubMed] [Google Scholar]
- [149].Arrowsmith JE, Campbell SF, Cross PE, Stubbs JK, Burges RA, Gardiner DG, Blackburn KJ. “Long-acting dihydropyridine calcium antagonists. 1. 2-Alkoxymethyl derivatives incorporating basic substituents”. J Med Chem. 1986;29(9):1696–1702. doi: 10.1021/jm00159a022. [DOI] [PubMed] [Google Scholar]
- [150].Towart R, Wehinger E, Meyer H, Kazda S. “The effects of nimodipine, its optical isomers and metabolites on isolated vascular smooth muscle”. Arzneimittelforschung. 1982;32(4):338–346. [PubMed] [Google Scholar]
- [151].Kajino M, Wada Y, Nagai Y, Nagaoka A, Meguro K. “Synthesis and biological activities of optical isomers of 2-(4-diphenylmethyl-1-piperazinyl)ethyl methyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate (manidipine) dihydrochloride”. Chem Pharm Bull. 1989 Aug;37(8):2225–2228. doi: 10.1248/cpb.37.2225. [DOI] [PubMed] [Google Scholar]
- [152].Inagaki O, Asano M, Takenaka T. “In vitro and in vivo vasodilatory activity of barnidipine and its enantiomers”. Biol Pharm Bull. 1999 Feb;22(2):151–156. doi: 10.1248/bpb.22.151. [DOI] [PubMed] [Google Scholar]
- [153].Mikus G, Mast V, Ratge D, Wisser H, Eichelbaum M. “Stereoselectivity in cardiovascular and biochemical action of calcium antagonists: studies with the enantiomers of the dihydropyridine nitrendipine”. Clin Pharmacol Ther. 1995 Jan;57(1):52–61. doi: 10.1016/0009-9236(95)90265-1. [DOI] [PubMed] [Google Scholar]
- [154].Soons PA, Cohen AF, Breimer DD. “Comparative effects of felodipine, nitrendipine and nifedipine in healthy subjects: concentration-effect relationships of racemic drugs and enantiomers”. Eur J Clin Pharmacol. 1993;44(2):113–120. doi: 10.1007/BF00315467. [DOI] [PubMed] [Google Scholar]
- [155].Hof RP, Hof A, Ruegg UT, Cook NS, Vogel A. “Stereoselectivity at the calcium channel: different profiles of hemodynamic activity of the enantiomers of the dihydropyridine derivative PN 200-110”. J Cardiovasc Pharmacol. 1986 Apr;8(2):221–226. doi: 10.1097/00005344-198603000-00001. [DOI] [PubMed] [Google Scholar]
- [156].van Amsterdam FT, Zaagsma J. “Stereoisomers of calcium antagonists discriminate between coronary vascular and myocardial sites”. Naunyn Schmiedebergs Arch Pharmacol. 1988 Feb;337(2):213–219. doi: 10.1007/BF00169250. [DOI] [PubMed] [Google Scholar]
- [157].Marciniak G, Delgado A, Leclerc G, Velly J, Decker N, Schwartz J. “New 1,4-dihydropyridine derivatives combining calcium antagonism and alpha-adrenolytic properties”. J Med Chem. 1989 Jun;32(6):1402–1407. doi: 10.1021/jm00126a042. [DOI] [PubMed] [Google Scholar]
- [158].Boer R, Grassegger A, Schudt C, Glossmann H. “(+)-Niguldipine binds with very high affinity to Ca2+channels and to a subtype of alpha 1-adrenoceptors”. Eur. J. Pharmacol. 1989 May;172(2):131–145. doi: 10.1016/0922-4106(89)90004-7. [DOI] [PubMed] [Google Scholar]
- [159].Muto K, Kuroda T, Kawato H, Karasawa A, Kubo K, Nakamizo N. “Synthesis and pharmacological activity of stereoisomers of 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridine-dicarboxylic acid methyl 1-(phenylmethyl)-3-piperidinyl ester”. Arzneimittelforschung. 1988 Nov;38(11A):1662–1665. [PubMed] [Google Scholar]
- [160].Furukawa T, Miura R, Honda M, Kamiya N, Mori Y, Takeshita S, Isshiki T, Nukada T. “Identification of R(-)-isomer of efonidipine as a selective blocker of T-type Ca2+channels”. Br J Pharmacol. 2004 Dec;143(8):1050–1057. doi: 10.1038/sj.bjp.0705944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Inayoshi A, Sugimoto Y, Funahashi J, Takahashi S, Matsubara M, Kusaka H. “Mechanism underlying the block of human Cav3.2 T-type Ca2+channels by benidipine, a dihydropyridine Ca2+channel blocker”. Life Sci. 2011 May;88(19–20):898–907. doi: 10.1016/j.lfs.2011.03.019. [DOI] [PubMed] [Google Scholar]
- [162].Cataldi M, Taglialatela M, Palagiano F, Secondo A, de Caprariis P, Amoroso S, di Renzo G, Annunziato L. “Effects of manidipine and nitrendipine enantiomers on the plateau phase of K+-induced intracellular Ca2+ increase in GH3 cells. Eur. J. Pharmacol. 1999 Jul;376(1–2):169–178. doi: 10.1016/s0014-2999(99)00149-1. [DOI] [PubMed] [Google Scholar]
- [163].De Caprariis P, Boatto G, Nieddu M, Cataldi M, Melisi D, Amoroso S. “Separazione e analisi delle propriet‡ farmacologiche delle diidropiridine chirali: valutazione comparativa degli isomeri ottici della amlodipina e della lercanidipina” in Istituto Superiore di Sanità Third Research Project. Chemical-physical properties of drugs and their safety. Edited E.Ciranni. 2002:102. iii. Rapporti ISTISAN 02/42. [Google Scholar]
- [164].Handrock R, Rao-Schymanski R, Klugbauer N, Hofmann F, Herzig S. “ Dihydropyridine enantiomers block recombinant L-type Ca2+channels by two different mechanisms”. J. Physiol. 1999 Nov;521(1):31–42. doi: 10.1111/j.1469-7793.1999.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Triggle DJ. “1,4-Dihydropyridines as calcium channel ligands and privileged structures”. Cell. Mo.l Neurobiol. 2003 Jun;23(3):293–303. doi: 10.1023/A:1023632419813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Carosati E, Ioan P, Micucci M, Broccatelli F, Cruciani G, Zhorov BS, Chiarini A, Budriesi R. “1,4-Dihydropyridine Scaffold in Medicinal Chemistry, The Story so Far And Perspectives (Part 2): Action in Other Targets and Antitargets”. Curr. Med. Chem. 2012 Jun 18; doi: 10.2174/092986712802884204. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [167].Dietz JD, Du S, Bolten CW, Payne MA, Xia C, Blinn JR, Funder JW, Hu X. “A number of marketed dihydropyridine calcium channel blockers have mineralocorticoid receptor antagonist activity”. Hypertension. 2008 Mar;51(3):742–8. doi: 10.1161/HYPERTENSIONAHA.107.103580. [DOI] [PubMed] [Google Scholar]
- [168].Kosaka H, Hirayama K, Yoda N, Sasaki K, Kitayama T, Kusaka H, Matsubara M. “The L-, N-, and T-type triple calcium channel blocker benidipine acts as an antagonist of mineralocorticoid receptor, a member of nuclear receptor family. Eur. J. Pharmacol. 2010 Jun;635(1–3):49–55. doi: 10.1016/j.ejphar.2010.03.018. [DOI] [PubMed] [Google Scholar]
- [169].Arhancet GB, Woodard SS, Dietz JD, Garland DJ, Wagner GM, Iyanar K, Collins JT, Blinn JR, Numann RE, Hu X, Huang HC. “Stereochemical requirements for the mineralocorticoid receptor antagonist activity of dihydropyridines”. J Med Chem. 2010 May;53(10):4300–4304. doi: 10.1021/jm1002827. [DOI] [PubMed] [Google Scholar]
- [170].Matsui T, Takeuchi M, Yamagishi S. “Nifedipine, a calcium channel blocker, inhibits inflammatory and fibrogenic gene expressions in advanced glycation end product (AGE)-exposed fibroblasts via mineralocorticoid receptor antagonistic activity. Biochem. Biophys. Res. Commun. 2010 May;396(2):566–570. doi: 10.1016/j.bbrc.2010.04.149. [DOI] [PubMed] [Google Scholar]
- [171].Berkels R, Roesen R, Bartels H, Purol-Schnabel S, Kirmiziguel I, Farmer H, Born GV, Klaus W. “Nisoldipine increases the bioavailability of endothelial NO”. Naunyn Schmiedebergs Arch Pharmacol. 2001 Aug;364(2):110–116. doi: 10.1007/s002100100429. [DOI] [PubMed] [Google Scholar]
- [172].Berkels R, Taubert D, Rosenkranz A, Rösen R. “Vascular protective effects of dihydropyridine calcium antagonists. Involvement of endothelial nitric oxide”. Pharmacology. 2003 Dec;69(4):171–176. doi: 10.1159/000073659. [DOI] [PubMed] [Google Scholar]
- [173].Dhein S, Salameh A, Berkels R, Klaus W. “Dual mode of action of dihydropyridine calcium antagonists: a role for nitric oxide. Drugs. 1999 Sep;58(3):397–404. doi: 10.2165/00003495-199958030-00002. [DOI] [PubMed] [Google Scholar]
- [174].Leung HS, Yao X, Leung FP, Ko WH, Chen ZY, Gollasch M, Huang Y. “Cilnidipine, a slow-acting Ca2+ channel blocker, induces relaxation in porcine coronary artery: role of endothelial nitric oxide and [Ca2+]i. Br. J. Pharmacol. 2006 Jan;147(1):55–63. doi: 10.1038/sj.bjp.0706450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Ma J, Kishida S, Wang GQ, Meguro K, Imuta H, Oonuma H, Iida H, Jo T, Takano H, Morita T, Nagai R, Nakajima T. “Comparative effects of azelnidipine and other Ca2+-channel blockers on the induction of inducible nitric oxide synthase in vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2006 Feb;47(2):314–321. doi: 10.1097/01.fjc.0000205497.90765.b0. [DOI] [PubMed] [Google Scholar]
- [176].Matsubara M, Yao K, Hasegawa K. “Benidipine, a dihydropyridine-calcium channel blocker, inhibits lysophosphatidylcholine-induced endothelial injury via stimulation of nitric oxide release. Pharmacol. Res. 2006 Jan;53(1):35–43. doi: 10.1016/j.phrs.2005.08.006. [DOI] [PubMed] [Google Scholar]
- [177].Matsubara M, Akizuki O, Ikeda J, Saeki K, Yao K, Sasaki K. “Benidipine, an anti-hypertensive drug, inhibits reactive oxygen species production in polymorphonuclear leukocytes and oxidative stress in salt-loaded stroke-prone spontaneously hypertensive rats. Eur. J Pharmacol. 2008 Feb;580(1–2):201–213. doi: 10.1016/j.ejphar.2007.10.072. [DOI] [PubMed] [Google Scholar]
- [178].Rosenkranz C, Lob T, Breitenbach T, Berkels R, Roesen R. “Endothelial antioxidant actions of dihydropyridines and angiotensin converting enzyme inhibitors. Eur. J. Pharmacol. 2006 Jan;529(1–3):55–62. doi: 10.1016/j.ejphar.2005.10.046. [DOI] [PubMed] [Google Scholar]
- [179].Zhang XP, Loke KE, Mital S, Chahwala S, Hintze TH. “Paradoxical release of nitric oxide by an L-type calcium channel antagonist, the R+ enantiomer of amlodipine”. J Cardiovasc Pharmacol. 2002 Feb;39(2):208–214. doi: 10.1097/00005344-200202000-00007. [DOI] [PubMed] [Google Scholar]
- [180].Liang JC, Yeh JL, Wang CS, Liou SF, Tsai CH, Chen IJ. “The new generation dihydropyridine type calcium blockers, bearing 4-phenyl oxypropanolamine, display alpha-/beta-adrenoceptor antagonist and long-acting antihypertensive activities”. Bioorg Med Chem. 2002 Mar;10(3):719–730. doi: 10.1016/s0968-0896(01)00318-2. [DOI] [PubMed] [Google Scholar]
- [181].Yeh JL, Liou SF, Liang JC, Lee CH, Chiu CC, Lin YT, Chen IJ. “Labedipinedilol-C: a third-generation dihydropyridine-type calcium channel antagonist displaying K+ channel opening, NO-dependent and adrenergic antagonist activities”. J Cardiovasc Pharmacol. 2005 Aug;46(2):130–140. doi: 10.1097/01.fjc.0000167016.61845.c8. [DOI] [PubMed] [Google Scholar]
- [182].Visentin S, Rolando B, Di Stilo A, Fruttero R, Novara M, Carbone E, Roussel C, Vanthuyne N, Gasco A. New 1,4-dihydropyridines endowed with NO-donor and calcium channel agonist properties. J Med Chem. 2004 May 6;47(10):2688–93. doi: 10.1021/jm031109v. [DOI] [PubMed] [Google Scholar]


