Abstract
Heart failure, caused by dilated cardiomyopathy and other cardiac disorders such as hypertension, is a major public health problem with high morbidity and mortality. Corin, a cardiac enzyme that cleaves natriuretic peptides, is a promising biomarker of cardiomyopathy and heart failure—but its functional role in these processes is not understood. We evaluated the potential effects of corin in mice with a well-characterized model of dilated cardiomyopathy. Mice with dilated cardiomyopathy developed heart failure, reduced contractile function, cardiac fibrosis and accelerated mortality in the setting of low corin expression. In wild-type mice, transgenic, cardiac-targeted, over-expression of corin enhanced cyclic guanosine monophosphate and blood pressure responses to pro-atrial natriuretic peptide, but did not affect heart size, contractility, body weights, survival and blood pressure. In mice with dilated cardiomyopathy, corin overexpression significantly reduced the development of myocardial fibrosis (p<0.05). Corin over-expression also enhanced heart contractile function (fractional shortening and ejection fraction (p<0.01) and it significantly reduced heart failure as assessed by lung water (p<0.05) and alveolar congestion (p<0.001). Consistent with these observations, corin over-expression significantly prolonged life in mice with dilated cardiomyopathy (p<0.0001). These results provide the first experimental evidence that corin expression plays a role in cardiomyopathy by modulating myocardial fibrosis, cardiac function, heart failure and survival.
Keywords: Corin, dilated cardiomyopathy, heart failure, natriuretic peptides
Heart failure (HF) is a syndrome of abnormal salt and water retention that frequently occurs in the setting of reduced cardiac function or cardiomyopathy. HF is a leading cause of morbidity and mortality; it affects more than 5.7 million Americans and ~670,000 new cases are diagnosed each year 1. Despite improvements in treatment, HF is a progressive process and nearly half of patients die within 5 years 2. The factors that modulate HF development and progression in patients with cardiomyopathy are still poorly understood.
Corin is a potential biomarker of HF and cardiomyopathy 3-5. Polymorphisms in corin are linked to more severe hypertension 6. Corin is a transmembrane serine protease expressed by cardiomyocytes that cleaves natriuretic pro-ANP to generate ANP; there is increasing evidence that it may also cleave pro-BNP 7-14. The natriuretic peptides (NPs) play a critical role in maintaining normal salt and water balance and arterial blood pressure; they are also important diagnostic and prognostic biomarkers for patients with HF 15. ANP and BNP interact with the natriuretic peptide receptor-A to regulate cGMP levels, vasodilation, natriuresis, fibrosis, etc 9, 16. As such the corin-NP system should protect against the development of progressive HF in patients with reduced systolic function 4.
One of the most common causes of progressive HF, cardiac transplantation and mortality is dilated cardiomyopathy (DCM) 17. DCM has several genetic and environmental causes in humans and mice 17, 18. One of the best characterized models of DCM in mice is caused by a phophorylation-resistant CREB mutant transgene (DCMc) 19-23. Mice with DCMc develop HF with features similar to human DCM including biventricular dilation, elevated NP levels, fibrosis, electrophysiologic abnormalities as well as progressive edema, dyspnea, hepatic congestion and early demise 19-23.
Through its positive effects on natriuresis, fibrosis and vascular resistance, the corin-NP system should delay the progression of DCM and HF. However, we and others have found that blood levels 3-5, 24 and cardiac transcripts for corin 25 are paradoxically reduced in patients with severe DCM. Similar to humans, mice with DCMc have reduced systolic function, enhanced cardiac fibrosis, elevated NP levels and accelerated mortality—all in the setting of decreased cardiac corin expression. Still, the contribution of corin to HF development remains controversial and poorly understood 3-5, 7, 24, 26-28. To examine this we genetically overexpressed corin in the hearts of mice with DCMc.
Methods
We analyzed corin transgenic (Tg) and DCMc mice in vivo and ex vivo. Experimental details are found in the online data supplement (please see http://hyper.ahajournals.org).
Statistical Analysis
Survival was analyzed by the Kaplan Meier method. Other statistical analyses were performed using non-parametric methods (unless otherwise indicated). Differences were considered to be significant if the two-tailed p ≤ 0.05. The number of animals (n) is indicated in the figure legends or results. Data are reported as mean ± SEM.
Results
Reduced corin expression in DCMc mice
Mice with cardiac transgenic expression of the CREB mutant develop a DCMc accompanied by frank HF with edema, ascites and shortened survival 19, 23. Although the promoter of the corin gene does not contain CREB binding sites 29, the DCMc mice showed reduced levels of corin transcripts (Fig. 1A) and protein (Figs. 1B-D) vs. wild-type litter mates. DCMc mice had higher ANP (2.1-fold, p<0.05, Fig. S1) and BNP transcripts (3.3-fold, p<0.01, Fig. S2).
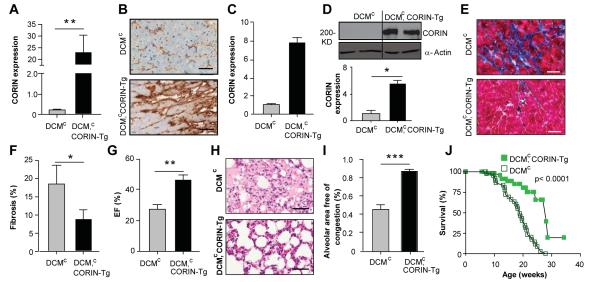
Figure 1. Corin expression is reduced in the hearts of mice with DCMc.

(A) Relative corin expression in DCM and wild-type (WT) assessed by qRT-PCR analysis. Transcripts are means of averages of triplicate measures in 7 mice. (B) Corin protein expression assessed by Western blotting under reducing conditions with anti-corin antibodies in WT and DCMc mice (upper panel). Densitometry analysis of corin expression relative to wild-type (bottom graph). (C, D) Corin protein expression assessed by immunohistochemical staining and densitometry analysis vs. wild-type. Representative staining of left ventricular sections (n = 2 per group) with anti-corin antibodies (40 X, bar =50 μm). Corin expression from image analyses of immunohistochemical staining (D). *p≤0.05. **p≤0.01.
Corin transgenic mice
To examine whether corin expression affects the progression of HF, we produced mice that selectively over-express corin in the heart due to the targeting effects of the alpha myosin heavy chain promoter. Corin-Tg mice were fertile, viable and indistinguishable from normal mice in appearance (Fig. 2A). Three corin-Tg lines were identified that displayed 1.3-13.5-fold increased levels of corin transcripts vs. wild-type mice (Tg1: 1.3±0.1-fold, Tg2: 6.9 ±0.5-fold; Tg3= 13.5±2.5-fold assessed by Northern blot). Corin protein was increased in the heart (Fig. 2B) and blood (1.4-fold, p<0.05; n=3 each group). There was no difference between wild-type mice and the corin-Tg mice in survival (n=701, Fig. 2C) or in ANP and BNP transcripts (not shown), thus we focused our studies on the transgenic line expressing the highest corin levels. Female wild-type and corin-Tg littermates had similar heart weights (WT 0.16±0.02g vs. Tg 0.17±0.02g) and body:heart weight ratios (WT 296.4±12.8 vs. 300.5±22.5; n=11-17 each group, Fig 2D); male mice were also similar to each other (11-20 each group). Indeed no differences in body weight were observed in mice up to 500-600 days old. There were no significant differences between wild-type and corin-Tg mice of the same gender in baseline systolic, diastolic or mean arterial blood pressure (MAP, Fig. 2E), heart rate or fractional shortening (31.9±1.2 vs. 32.4±2.3).
Figure 2. Comparison of wild-type and corin-Tg mice.
(A) Corin-Tg and wild-type female littermates and hearts are similar in appearance (15 weeks old, bar=2 mm). (B) Corin protein expression is increased in the heart of corin-Tg mice (female, 15 week old) assessed by Western blotting under reducing conditions with anti-corin antibodies (upper panel). Quantitation of corin expression (vs. Glut-4 expression, n=4 each group). (C) Survival in lines of corin-Tg mice and wild-type mice is similar (n=701). (D) Comparison of body weight (B), heart weight (H) and B:H values between corin-Tg and wild-type mice littermates (n=11-20 in each group; female data is shown). (E) Similar systolic (SBP), diastolic (DBP) or mean arterial blood pressure (MAP) in corin-Tg (n = 5 females, 15 males) and wild-type (n = 6 females, 16 males) mice.
Enhanced corin activity in corin-Tg mice
The cleavage of pro-ANP to ANP enhances cellular generation of cGMP and lowers blood pressures. In corin-Tg mice cGMP levels were slightly higher than in wild-type mice (Fig.3A) but there were no significant differences in mean arterial pressure, MAP (Fig. 3B) or heart rate. There was enhanced cleavage of recombinant pro-ANP by hearts from corin-Tg mice (Fig. S3). Bolus injection of pro-ANP increased cGMP levels in both corin-Tg and wild-type mice (Fig.3A). In response to pro-ANP injection, but not saline, MAP dropped significantly in corin-Tg but not wild-type mice (Fig 3B, p<0.05).
Figure 3. Enhanced corin activity and MAP in corin-Tg female mice.
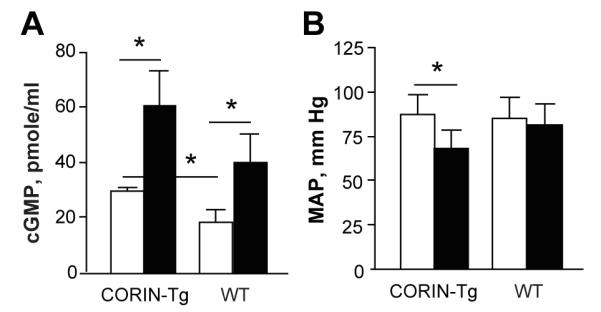
(A) Plasma levels of cGMP in corin-Tg and wild-type (WT) female mice before (open bars) and after (filled bars) bolus pro-ANP (10.5 ng/200 μl PBS) injection (n= 4-7 age matched per group). (B) MAP in Tgcorin (n = 6) and wild-type mice (n = 6) before (open bars) and after (filled bars) bolus pro-ANP containing medium injection. MAP was recorded using a Millar catheter and Power Lab software. * p≤0.05.
Corin modulates HF in mice with DCMc
To examine whether corin modulates HF, corin-Tg mice were backcrossed with DCMc mice on the same strain background. Female littermates were examined at 14-15 weeks. There was no significant difference in body weight or body:heart weight ratios (Fig. S4). CREB Tg transcript levels didn’t change after backcrossing (p=0.41). Corin transcripts were higher in DCMc, corin-Tg mice than in DCMc mice (Fig. 4A). Enhanced expression of corin protein was also found (Figs. 4B-D). Higher blood levels of soluble corin were detected in DCMc, corin-Tg mice than DCMc mice (p≤0.05, n=4-5 each group). Transcripts for ANP (1.7-fold, p≤0.05, Fig. S5) and BNP (1.4-fold, p≤0.001, Fig. S6) were higher in DCMc, corin-Tg than in DCMc mice. Consistent with this observation, levels of cGMP were significantly higher in DCMc, corin-Tg mice (Fig. S7; p≤0.05). DCMc, corin-Tg mice had reduced interstitial and perivascular cardiac fibrosis (54% lower, p≤0.05, n=4-5 each group; Fig. 4E,F) by Masson’s trichrome staining. Transcripts for collagen I (p≤0.01) and collagen III (p≤0.05) were lower in DCMc, corin Tg mice (Figs. S8, S9). There were a trend to lower TGF-beta levels, but CMA-1, MMP-9 and furin transcripts weren’t different between the two groups (Figs. S10-S13). DCMc, corin Tg mice had better contractile function with a higher EF% (p≤0.01, Fig 4G) and FS (23.0±2.4 vs. 12.9±1.3%, p≤0.01) than DCMc mice despite similar LV internal dimensions (Fig. S14). HF was significantly reduced in DCMc, corin Tg vs. DCMc mice as assessed by reduced alveolar edema and congestion (Figs. 4H, I , p<0.001) and reduced lung water (lung wet:dry ratio, p<0.05). Most importantly, the survival of DCMc, corin-Tg mice was significantly longer than the survival of DCMc mice (Fig. 4J, p≤0.0001).
Figure 4. Corin over-expression in DCMc mice reduces fibrosis, HF and increases survival.
(A) Cardiac expression of corin transcripts in DCMc and DCMc, corin-Tg mice assessed by qRT-PCR analysis, relative to wild-type. Transcripts are means of averages of triplicate measures in 7 mice. (B, C) Corin protein expression assessed by immunohistochemical staining. Representative immunoperoxidase-stained heart sections (n= 2 per group) probed with anti-corin antibody (40x magnification, bar = 50 um). Quantification of corin expression by image analyses. (D) Corin protein expression in heart assessed by Western blotting under reducing conditions with anti-corin and anti-actin antibodies. Relative corin levels normalized to actin. (E, F) Cardiac fibrosis in in representative heart ventricles sections (n=4-5 each group) stained with Masson’s trichrome (E, 40 × magnification, bar = 50 um). Quantification (F) of fibrosis by image analyses. (G) Cardiac EF% (n=6-7 per group). (H, I) Alveolar congestion in representative formalin-fixed lung sections (H, 40 × magnification, bar = 50 um) stained by hematoxylin and eosin from female DCMc and DCMc, corin-Tg mice. Bar graph (I) of total alveolar area free of edema and congestion per 20X field. Results are means of averages of 10 randomly selected fields from 6-7 mice of each group. (J) Kaplan-Meyer survival curves of DCMc (n= 56) and DCMc, corin-Tg (n=46) mice. *p≤0.05, **p≤0.01, *** p≤0.001.
Discussion
In patients with DCM, progressive HF is a major cause of morbidity and mortality with high social costs. As such, there is a critical need to discover mechanisms that regulate HF development and progression to create new diagnostic, treatment and prevention strategies. Corin’s cardiac-selective expression and its key role in regulating the NP system, make it a potential biomarker of acute HF in the setting of diminished systolic function 3-5. Cardiac transcripts 25 and circulating levels of corin 3-5, 24 are reduced in patients with HF and DCM but not in all cardiac conditions, particularly those involving hypertrophy 7, 26, 28. Still, the functional role of corin in DCM has not been established. In a well-characterized model of HF and DCM 19-23, we confirmed that myocardial corin transcripts (and protein levels) were reduced. Similar reductions in corin expression were observed in a model of HF induced by arterial venous shunting 27. Restoration of corin levels in DCMc mice markedly reduced development of cardiomyopathy and HF. There were significant reductions in myocardial fibrosis and improvements in contractile indices (FS, EF) in DCMc, corin-Tg vs. DCMc mice. HF was also improved in DCMc, corin-Tg mice as assessed by objective indices of lung water and congestion. Perhaps the most compelling finding was that restoration of corin levels significantly increased the survival of DCMc, coring –Tg mice vs. DCMc mice.
There are several potential mechanisms through which corin and the NP system may modulate the development of HF. Corin cleaves pro-ANP to ANP which has enhanced physiologic effects 9, 30. ANP increases salt and water excretion which should reduce the salt and water retention of HF 16, 31. We found low levels of circulating corin and impaired pro-ANP cleavage in patients with acute decompensated HF suggesting that low corin levels might contribute to this syndrome of salt and water retention in some patients 3, 4. Indeed, corin-deficiency reduces sodium excretion in response to high salt diets32. Our data shows that overexpression of corin increases physiologic responses to pro-ANP, increases cGMP levels, and reduces fluid retention in mice with DCMc. Although the relative contributions of cardiac and circulating corin to natriuretic peptide cleavage are still unknown, patients with HF respond to ANP infusions with increased cGMP levels and improved long term prognosis 33, 34. ANP also enhances vasodilation which can increase cardiac output in the presence of reduced cardiac function.
There is increasing evidence that corin also may cleave pro-BNP to BNP 10, 11, 13, 14. Recent studies have linked a hypo-functional polymorphism in corin to diminished pro-BNP cleavage and worse outcomes 35. Some patients with chronic HF appear to have abnormal processing of pro-BNP to BNP fragments with diminished biologic activity 36. Still, the therapeutic value of BNP (Natrecor/Nesiritide) therapy in HF patients is controversial and a large scale clinical trial showed no significant improvement in symptoms or mortality 37.
In addition to natriuretic and vasodilatory effects, ANP and BNP also affect apoptosis, inflammation and cardiac fibrosis—each of these mechanisms may affect the progression of cardiomyopathies 38, 39. Indeed, deletion of the receptor for ANP and BNP (NPR-A) accelerated mortality in mice with DCM 40. Cardiac fibrosis is significant in all DCMc mice by 8 weeks of age though no significant apoptosis or inflammation was appreciated 19. Cardiac fibrosis was also seen in knockout mice lacking ANP, BNP 41, 42. Cardiac fibrosis affects diastolic and systolic dysfunction and contributes to the development of HF 43. When analyzed at 14-15 weeks of age, hearts from DCMc, corin-Tg mice showed significantly less interstitial and perivascular ventricular fibrosis than DCM mice. In addition, the DCMc, corin-Tg mice had increased corin levels, cGMP levels, ANP and BNP transcripts. ANP and BNP inhibit collagen synthesis and proliferation of cardiac fibroblasts; which in turn inhibits cardiac fibrosis in vivo 39, 42. Thus, the reduced fibrosis seen in DCMc, corin-Tg mice may be attributable to increased activity of the natriuretic peptide system and may contribute to the improved ventricular function seen in these mice.
In summary, consistent with findings in humans with HF and DCM 25, we find that corin expression is significantly reduced in experimental DCMc and HF. In a just-published study, corin-deficient KitW-sh/W-sh mice developed rapidly progressive cardiac dilation and loss of cardiac function after aortic banding 44. These findings, in addition to corin’s cardiac-selective expression and, its role as regulator of the NP system, make corin an attractive biomarker for DCM and HF. Beyond its potential diagnostic value, corin appears to play a key functional role in DCM and HF where enhanced expression is associated with reduced myocardial fibrosis, enhanced contractility, prevention of HF and prolongation of life. Further studies of corin in other types of HF and cardiomyopathies, for instance – hypertensive heart disease and chronic myocardial infarction, will be necessary to determine the value of corin as a biomarker and potential therapeutic agent.
Supplementary Material
Perspectives.
Corin is a key regulator of the natriuretic peptide system which modulates salt and water balance in heart failure. However, levels of corin are unexpectedly reduced in humans and mice with dilated cardiomyopathy (DCM). Increasing cardiac corin expression in mice with DCM, enhances ANP and BNP expression, improves cardiac function, reduces cardiac fibrosis and prolongs survival. Thus in addition to its value as a potential biomarker, strategies for increasing corin levels in DCM may mitigate the progression of cardiac fibrosis, heart failure, systolic dysfunction and death.
Novelty and Significance.
What Is New?
- In a model of heart failure we found:
- ○ Reduced heart function and measurable heart failure
- ○ High levels of natriuretic peptides ANP and BNP
- ○ Reduced levels of corin, a novel heart protein
- Increasing corin in the heart of normal mice:
- ○ Reduced blood pressure in response to pro-ANP
- ○ Increased cGMP which regulates blood pressure
- Increasing the level of corin in mice with enlarged hearts:
- ○ Reduced heart scarring
- ○ Prevented heart failure
- ○ Increased heart function
- ○ Saved lives
What Is Relevant?
Hypertension often causes heart failure
Corin activates natriuretic peptides to reduce blood pressure
Coring polymorphisms may cause heart problems in patients with hypertension
Increasing corin levels in heart failure prevents loss of heart function, fluid retention, heart scarring, and early death
Summary
Corin is an attractive biomarker for heart failure and cardiomyopathy. These results also provide the first experimental evidence that corin expression may reduce heart scarring, improve heart function, prevent heart failure and increase survival.
Acknowledgments
We gratefully acknowledge the initial contributions of Brian Robinson, Roxanne Wadia and Natalia Zhidkova.
Sources of Funding Grants: NIH (HL58496, HL78562; PI-Reed; HL115036 PI-Gladysheva); Scientist Development Grant from the American Heart Association (0835376N, Gladysheva).
Footnotes
Disclosures None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, et al. Heart disease and stroke statistics--2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: Prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 3.Ibebuogu UN, Gladysheva IP, Reed GL. Is heart failure due to impaired clevage and activation of atrial natriuretic peptide? J Am Coll Cardiol. 2009;53:A467–A468. [Google Scholar]
- 4.Ibebuogu UN, Gladysheva IP, Houng AK, Reed GL. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ Heart Fail. 2011;4:114–120. doi: 10.1161/CIRCHEARTFAILURE.109.895581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong N, Chen S, Yang J, He L, Liu P, Zheng D, Li L, Zhou Y, Ruan C, Plow E, Wu Q. Plasma soluble corin in patients with heart failure. Circ Heart Fail. 2010;3:207–211. doi: 10.1161/CIRCHEARTFAILURE.109.903849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W, Drazner MH. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 7.Hooper JD, Scarman AL, Clarke BE, Normyle JF, Antalis TM. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur J Biochem. 2000;267:6931–6937. doi: 10.1046/j.1432-1033.2000.01806.x. [DOI] [PubMed] [Google Scholar]
- 8.Gladysheva IP, Robinson BR, Houng AK, Kovats T, King SM. Corin is co-expressed with pro-anp and localized on the cardiomyocyte surface in both zymogen and catalytically active forms. J Mol Cell Cardiol. 2008;44:131–142. doi: 10.1016/j.yjmcc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, Qin J, Wu Q. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103:502–508. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonne JM, Campbell JM, Cataliotti A, Ohmine S, Thatava T, Sakuma T, Macheret F, Huntley BK, Burnett JC, Jr., Ikeda Y. Secretion of glycosylated pro-b-type natriuretic peptide from normal cardiomyocytes. Clin Chem. 2011:57. doi: 10.1373/clinchem.2010.157438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng J, Jiang J, Wang W, Qi X, Sun XL, Wu Q. Glycosylation and processing of pro-b-type natriuretic peptide in cardiomyocytes. Biochem Biophys Res Commun. 2011;411:593–598. doi: 10.1016/j.bbrc.2011.06.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichiki T, Huntley BK, Heublein DM, Sandberg SM, McKie PM, Martin FL, Jougasaki M, Burnett JC., Jr. Corin is present in the normal human heart, kidney, and blood, with pro-b-type natriuretic peptide processing in the circulation. Clin Chem. 2011;57:40–47. doi: 10.1373/clinchem.2010.153908. [DOI] [PubMed] [Google Scholar]
- 14.Semenov AG, Tamm NN, Seferian KR, Postnikov AB, Karpova NS, Serebryanaya DV, Koshkina EV, Krasnoselsky MI, Katrukha AG. Processing of pro-b-type natriuretic peptide: Furin and corin as candidate convertases. Clin Chem. 2010;56:1166–1176. doi: 10.1373/clinchem.2010.143883. [DOI] [PubMed] [Google Scholar]
- 15.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 16.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocrine reviews. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 17.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. Contemporary definitions and classification of the cardiomyopathies: An american heart association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 18.Patten RD, Hall-Porter MR. Small animal models of heart failure: Development of novel therapies, past and present. Circ Heart Fail. 2009;2:138–144. doi: 10.1161/CIRCHEARTFAILURE.108.839761. [DOI] [PubMed] [Google Scholar]
- 19.Fentzke RC, Korcarz CE, Lang RM, Lin H, Leiden JM. Dilated cardiomyopathy in transgenic mice expressing a dominant-negative creb transcription factor in the heart. J Clin Invest. 1998;101:2415–2426. doi: 10.1172/JCI2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mor-Avi V, Korcarz C, Fentzke RC, Lin H, Leiden JM, Lang RM. Quantitative evaluation of left ventricular function in a transgenicmouse model of dilated cardiomyopathy with 2-dimensional contrast echocardiography. J Am Soc Echocardiogr. 1999;12:209–214. doi: 10.1016/s0894-7317(99)70137-9. [DOI] [PubMed] [Google Scholar]
- 21.Fentzke RC, Korcarz CE, Shroff SG, Lin H, Leiden JM, Lang RM. The left ventricular stress-velocity relation in transgenic mice expressing a dominant negative creb transgene in the heart. J Am Soc Echocardiogr. 2001;14:209–218. doi: 10.1067/mje.2001.111473. [DOI] [PubMed] [Google Scholar]
- 22.Zhu W, Saba S. Cardiac electrophysiologic abnormalities in the creba133 transgenic mouse model of idiopathic dilated cardiomyopathy. J Cardiovasc Electrophysiol. 2003;14:982–989. doi: 10.1046/j.1540-8167.2003.02002.x. [DOI] [PubMed] [Google Scholar]
- 23.Huggins GS, Lepore JJ, Greytak S, Patten R, McNamee R, Aronovitz M, Wang PJ, Reed GL. The creb leucine zipper regulates creb phosphorylation, cardiomyopathy, and lethality in a transgenic model of heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H1877–1882. doi: 10.1152/ajpheart.00516.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrestha K, Troughton RW, Borowski AG, Yandle TG, Richards AM, Klein AL, Tang WH. Plasma corin levels provide minimal prognostic utility incremental to natriuretic peptides in chronic systolic heart failure. J Card Fail. 2010;16:621–627. doi: 10.1016/j.cardfail.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Barth AS, Kuner R, Buness A, Ruschhaupt M, Merk S, Zwermann L, Kaab S, Kreuzer E, Steinbeck G, Mansmann U, Poustka A, Nabauer M, Sultmann H. Identification of a common gene expression signature in dilated cardiomyopathy across independent microarray studies. J Am Coll Cardiol. 2006;48:1610–1617. doi: 10.1016/j.jacc.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Tran KL, Lu X, Lei M, Feng Q, Wu Q. Upregulation of corin gene expression in hypertrophic cardiomyocytes and failing myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H1625–1631. doi: 10.1152/ajpheart.00298.2004. [DOI] [PubMed] [Google Scholar]
- 27.Langenickel TH, Pagel I, Buttgereit J, Tenner K, Lindner M, Dietz R, Willenbrock R, Bader M. Rat corin gene: Molecular cloning and reduced expression in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1516–1521. doi: 10.1152/ajpheart.00947.2003. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Sen S, Young D, Wang W, Moravec CS, Wu Q. Protease corin expression and activity in failing hearts. Am J Physiol Heart Circ Physiol. 2010;299:H1687–1692. doi: 10.1152/ajpheart.00399.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan J, Hinzmann B, Yan W, Wu F, Morser J, Wu Q. Genomic structures of the human and murine corin genes and functional gata elements in their promoters. J Biol Chem. 2002;277:38390–38398. doi: 10.1074/jbc.M205686200. [DOI] [PubMed] [Google Scholar]
- 30.Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102:785–790. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnett JC, Jr., Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Shen J, Cui Y, Jiang J, Chen S, Peng J, Wu Q. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int. 2012;82:26–33. doi: 10.1038/ki.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suwa M, Seino Y, Nomachi Y, Matsuki S, Funahashi K. Multicenter prospective investigation on efficacy and safety of carperitide for acute heart failure in the ‘real world’ of therapy. Circ J. 2005;69:283–290. doi: 10.1253/circj.69.283. [DOI] [PubMed] [Google Scholar]
- 34.Hata N, Seino Y, Tsutamoto T, Hiramitsu S, Kaneko N, Yoshikawa T, Yokoyama H, Tanaka K, Mizuno K, Nejima J, Kinoshita M. Effects of carperitide on the long-term prognosis of patients with acute decompensated chronic heart failure: The protect multicenter randomized controlled study. Circ J. 2008;72:1787–1793. doi: 10.1253/circj.cj-08-0130. [DOI] [PubMed] [Google Scholar]
- 35.Rame JE, Tam SW, McNamara D, Worcel M, Sabolinski ML, Wu AH, Dries DL. Dysfunctional corin i555(p568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: Results from the genetic risk assessment in heart failure substudy. Circ Heart Fail. 2009;2:541–548. doi: 10.1161/CIRCHEARTFAILURE.109.866822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller WL, Phelps MA, Wood CM, Schellenberger U, Van Le A, Perichon R, Jaffe AS. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of b-type natriuretic peptide in patients with chronic heart failure. Circ Heart Fail. 2011;4:355–360. doi: 10.1161/CIRCHEARTFAILURE.110.960260. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 38.Vollmar AM. The role of atrial natriuretic peptide in the immune system. Peptides. 2005;26:1086–1094. doi: 10.1016/j.peptides.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 39.Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006;69:318–328. doi: 10.1016/j.cardiores.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Yasuno S, Usami S, Kuwahara K, Nakanishi M, Arai Y, Kinoshita H, Nakagawa Y, Fujiwara M, Murakami M, Ueshima K, Harada M, Nakao K. Endogenous cardiac natriuretic peptides protect the heart in a mouse model of dilated cardiomyopathy and sudden death. Am J Physiol Heart Circ Physiol. 2009;296:H1804–1810. doi: 10.1152/ajpheart.01033.2008. [DOI] [PubMed] [Google Scholar]
- 41.Franco V, Chen YF, Oparil S, Feng JA, Wang D, Hage F, Perry G. Atrial natriuretic peptide dose-dependently inhibits pressure overload-induced cardiac remodeling. Hypertension. 2004;44:746–750. doi: 10.1161/01.HYP.0000144801.09557.4c. [DOI] [PubMed] [Google Scholar]
- 42.Ogawa Y, Tamura N, Chusho H, Nakao K. Brain natriuretic peptide appears to act locally as an antifibrotic factor in the heart. Can J Physiol Pharmacol. 2001;79:723–729. [PubMed] [Google Scholar]
- 43.Zannad F, Pitt B. Biomarkers of extracellular matrix turnover. Heart Fail Clin. 2009;5:589–599. doi: 10.1016/j.hfc.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Buckley CL, Stokes AJ. Corin-deficient w-sh mice poorly tolerate increased cardiac afterload. Regul Pept. 2011;172:44–50. doi: 10.1016/j.regpep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.