Abstract
During intra-erythrocytic development, malaria trophozoites digest hemoglobin, which leads to parasite growth and asexual replication while accumulating toxic heme. To avoid death, the parasite synthesizes insoluble hemozoin crystals in the digestive vacuole through polymerization of β-hematin dimers. In the process, the heme is converted to a high-spin ferriheme whose magnetic properties were studied as early as 1936 by Pauling et al. Here, by magnetophoretic cell motion analysis, we provide evidence for a graduated increase of live cell magnetic susceptibility with developing blood-stage parasites, compatible with the increase in hemozoin content and the mechanism used by P. falciparum to avoid heme toxicity. The measured magnetophoretic mobility of the erythrocyte infected with a late-stage schizont form was m = 2.94 × 10−6 mm3 s/kg, corresponding to the net volume magnetic susceptibility (relative to water) of Δχ = 1.80 × 10−6, significantly higher than that of the oxygenated erythrocyte (−0.18×10−6) but lower than that of the fully deoxygenated erythrocyte (3.33×10−6). The corresponding fraction of hemoglobin converted to hemozoin, calculated based on the known magnetic susceptibilities of hemoglobin heme and hemozoin ferriheme, was 0.50, in agreement with the published biochemical and crystallography data. Magnetophoretic analysis of live erythrocytes could become significant for antimalarial drug susceptibility and resistance determination.
Keywords: erythrocyte magnetic susceptibility, plasmodium falciparum, hemozoin, ferriheme, heme, toxicity
Blood-stage parasites are responsible for malarial disease that might include febrile illness, severe anemia, and cerebral malaria, and causes death of 2.5 million people annually (1–3). Plasmodium species of malaria parasites progress through liver and blood stages before gametocytes enable sexual reproduction to occur in the mosquito midgut.
Infection is introduced to humans in the bite of an infected female anopheline mosquito when sporozoites are introduced to the bloodstream with the salivary secretions of the mosquito. The sporozoites quickly enter the parenchymal cells of the liver, some in as little as 3 min (4) and most in less than 30 min (2). They multiply asexually in the liver cells by binary fission, referred to as hepatic schizogony. When these cells rupture, thousands of merozoites are released into the bloodstream where they proceed to invade the erythrocytes. Once an erythrocyte has been invaded, the parasite feeds on the hemoglobin, and this form is known as a trophozoite. In its early stage, it is commonly referred to as the ring form due to its characteristic appearance. The trophozoite metabolizes hemoglobin incompletely because it lacks the enzyme heme oxygenase, which is used by vertebrates for heme catabolism (5–9). Heme is highly toxic to the parasite. Rather than being excreted from the erythrocyte, it is sequestered in the cell by conversion to an insoluble form known as hemozoin, or malarial pigment. The hemozoin appears as characteristic brown crystals in the digestive vacuole of the parasite (6). As the parasite continues to grow, it enters the next stage, where the nuclear material fragments and becomes distributed throughout the organism. The cytoplasm divides to form around each fragment of nuclear material. This stage is known as the (erythrocytic) schizont and is composed of a number of individual merozoites. When the erythrocyte ultimately ruptures, the merozoites are released into the bloodstream where they proceed to infect other erythrocytes. The asexual proliferation of merozoites is known as erythrocytic schizogony, as opposed to the pre-erythrocytic, exoerythrocytic, or hepatic schizogony, which takes place in the parenchymal liver cells.
The number and the size of the hemozoin crystals in the erythrocyte depend on the stage of the parasite development, with the least amount of the hemozoin detected in the ring stage and the highest amount in the schizont stage. While the important property of hemozoin is its insolubility in the conditions of the parasite food vacuole, it is interesting to note that the hemozoin crystal structure is similar for primates and mouse (flat-faced cuboidal) but different for birds (barrelshaped with a waffle surface) (10). Because of its importance to the parasite survival inside the erythrocyte, hemozoin has been the subject of intensive physico-chemical and crystal studies. The spectroscopic and crystallographic analyses indicate that the hemozoin crystal structure is identical to that of a synthetic biomineral, β-hematin (11), which is the hematin dimer (ferriprotoporphyrin-IX)2 in which the propionate side chain on one hematin coordinated to the iron center of the other (12). Hemozoin is better described as a biomineral rather than a polymer and attains lengths on the order of 1 µm, which makes it clearly visible under a visible light microscope (10). Its molecular structure and composition is more complex than that of another biogenic magnetic particle, the magnetosome found in prokaryotes (such as Magnetospirillum magnetotacticum) that is typically a single domain crystal (~100 nm) of magnetite (Fe3O4) or greigite (Fe3S4) (13, 14). Although the hemozoin structure is known, the mechanism of its formation remains uncertain (15–17). The molecular structure of β-hematin has been resolved at the single atom level (18), and its magnetic properties have been determined using electron paramagnetic resonance (EPR) and Mössbauer spectroscopy (12). It has been shown that the Fe atom exists in a high-spin Fe(III) state, S = 5/2. The hemozoin heme electron configuration with five unpaired electrons corresponds to a ferriheme that is known to be a part of another high-spin hemoglobin species, methemoglobin (19). Methemoglobin-rich erythrocytes have been shown to possess higher magnetophoretic mobility than low-spin, oxygenated hemoglobin erythrocytes (S=0) (20).
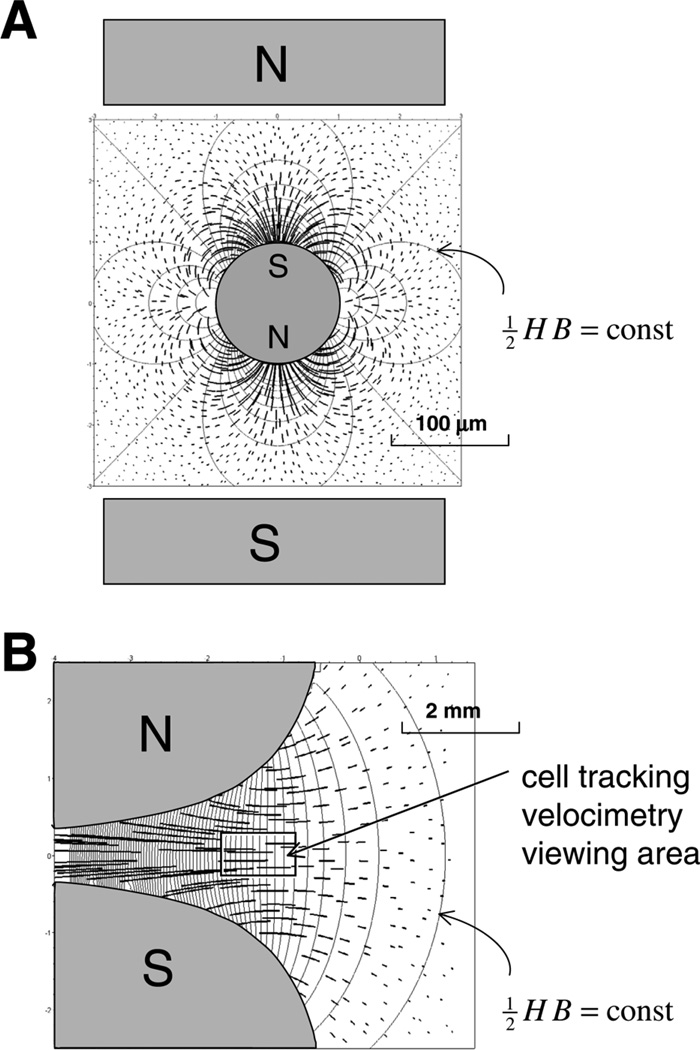
Attempts at using magnetic properties of malaria pigment, or hemozoin for concentration and capture of malaria infected cells, date back to 1946 when it was shown that positioning an electromagnet-generating field of 0.5 tesla (T) next to a tube containing a suspension of infected blood causes enrichment of the parasitized erythrocyte fraction from 0.17% to over 24% in the course of 6 to 12 h (21). Improved results were reported in the late 1970s using the technique of high-gradient magnetic separation (HGMS, illustrated in Fig. 1A) (22, 23). Commercially developed HGMS columns have been used recently to synchronize or enrich in vitro P. falciparum cultures or blood samples (24) and murine malaria parasite P. berghei ookinetes (that invade the mosquito midgut wall) for further in vitro studies (25). Although those studies identified early the presence of the paramagnetic hemozoin in the infected erythrocyte as the cause of the erythrocyte attraction by the magnetic field, Fig. 2, to date nothing has been reported on measuring the magnetic susceptibility of live erythrocytes in relation to malaria parasite physiology or development. Recently, such measurements became possible by single erythrocyte motion analysis using cell tracking velocimetry (CTV, Fig. 1B), which is sensitive to changes in the magnetic susceptibility of hemoglobin compounds (oxyhemoglobin, deoxyhemoglobin, and methemoglobin, Fig. 3A) in intact erythrocytes (20). The method is applied here to measure the fraction of hemoglobin converted to hemozoin at different stages of parasite development.
Figure 1.
Magnetic path lines (thick, calculated for equal time intervals) and Maxwell stress isolines (thin) that characterize two different field geometries used for magnetic erythrocyte capture and analysis. A) High-gradient magnetic separation (HGMS) geometry used for malaria-infected cell capture (22–25) and in the early studies on erythrocyte magnetophoresis (36). A high-gradient magnetic field is induced at the surface of a high-permeability filament (of a circular cross section) by an external magnetic field (represented by its source, rectangular pole pieces N and S, drawn not to scale). Paramagnetic erythrocytes are attracted to the filament in the direction of the N–S axis, depopulating the areas orthogonal to the N–S axis. An efficient capture requires filament packing (22, 23) or spherical bead packing (24, 25) in the form of filter beds. B) Field of hyperbolic pole pieces that provides a highly regular path line pattern in the neighborhood of the plane of pole symmetry [isodynamic field (29)], as indicated by only weak dependence of path lines on position. This improves the precision of the cell tracking velocimetry (CTV) method. Not shown is the erythrocyte gravity sedimentation component. Note difference in scale between (A) and (B).
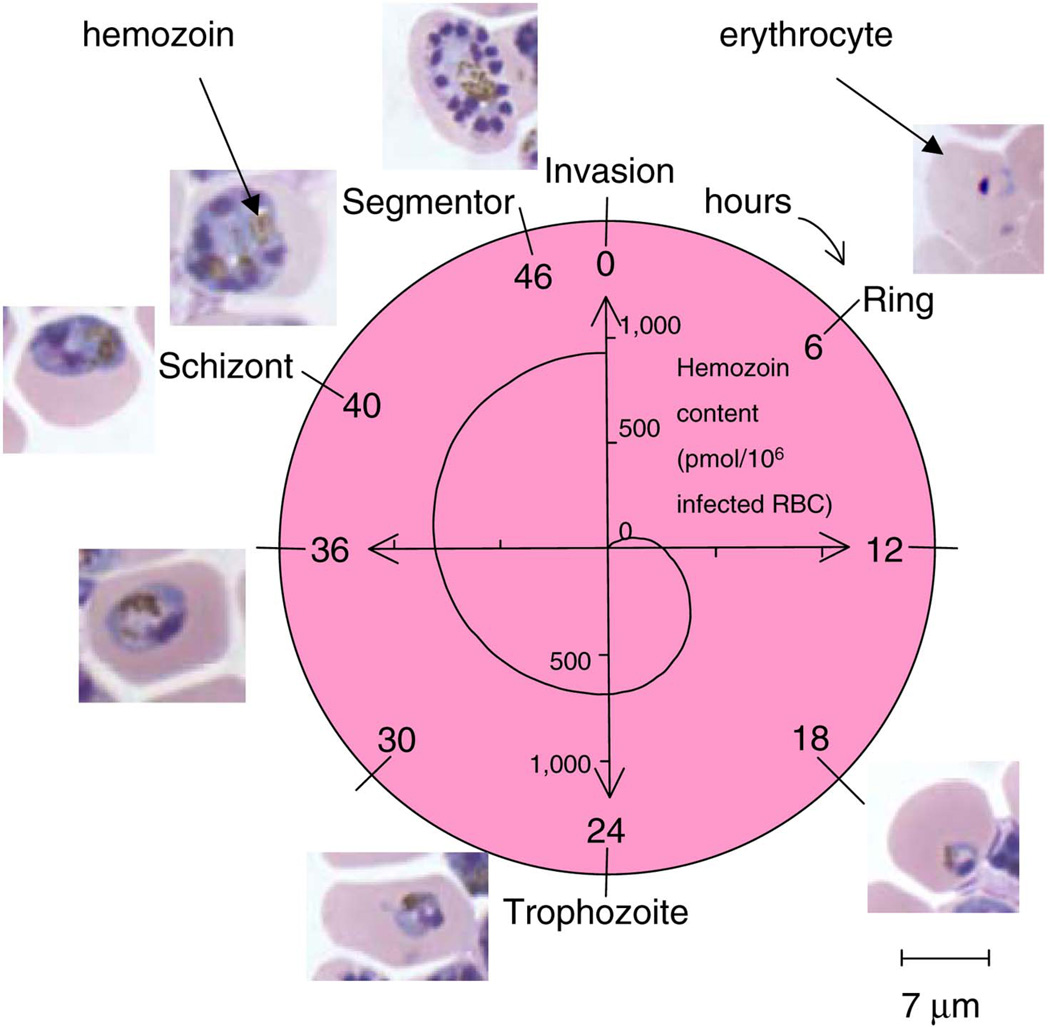
Figure 2.
P. falciparum parasite life cycle in the erythrocyte and the associated increase in the intra-erythrocytic hemozoin content. Note the hemozoin particle appearance as brown inclusions in the erythrocyte microscopic images. The hemozoin content values were taken from published literature, as discussed in the text.
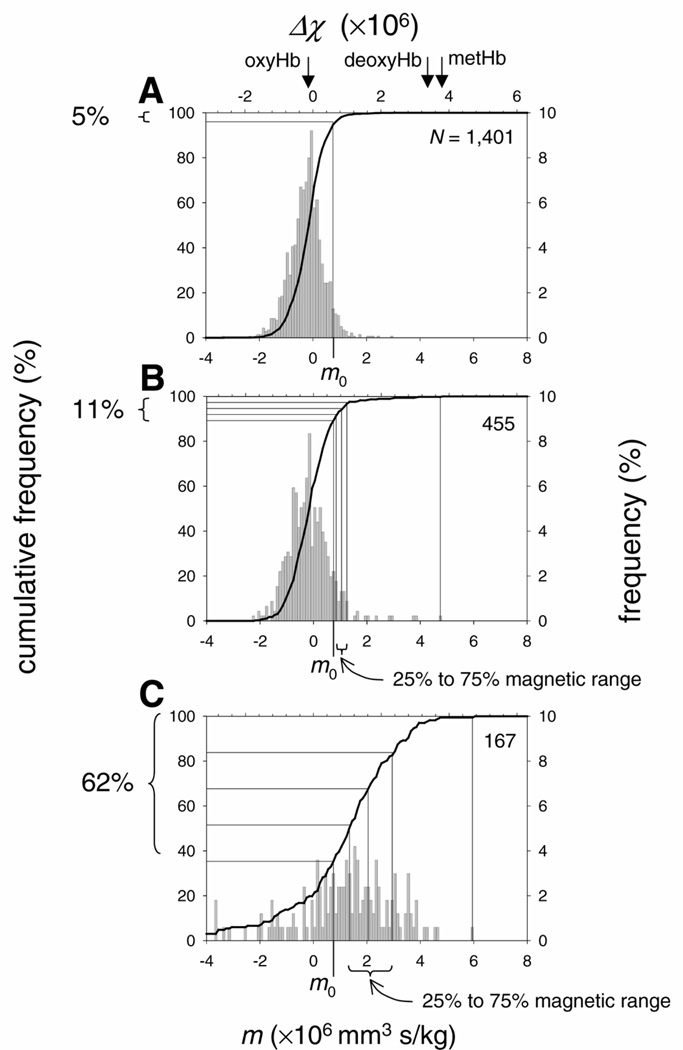
Figure 3.
A) Magnetophoretic mobility, m, and the corresponding net volume magnetic susceptibility, Δχ , histogram of the control (uninfected) erythrocyte suspension. The mobility is distributed normally around mean peak value of − 0.21 × 10−6 mm3 s/kg, R2 = 0.9696, at χ2 = 0.4230 and P < 0.0001. The cut-off mobility, m0 = 0.75 × 10−6 mm3 s/kg, was set at 95 percentile cumulative mobility (thick line and the left ordinate axis). For reference, the peak mobilities and susceptibilities of oxygenated, deoxygenated, and methemoglobin-converted erythrocytes are also shown (arrows) (20). The number of tracked cells is indicated by N. B) Magnetophoretic mobility, m, and the corresponding net volume magnetic susceptibility, Δχ , histogram of the erythrocyte suspension with predominantly early ring and trophozoite forms (Expt. #1, Table 1). C) Magnetophoretic mobility, m, and the corresponding net volume magnetic susceptibility, Δχ , histogram of the erythrocyte suspension with predominantly late schizont fraction (Expt. #5, Table 1).
MATERIALS AND METHODS
Parasite culture and sorbitol-synchronization of infected erythrocytes
Heparinized blood was washed with RPMI-1640 (Invitrogen/Gibco, Carlsbad, CA) and stored at 50% hematocrit at 4°C prior to use. Plasmodium falciparum HB3 clone in AA erythrocytes were cultivated at 5% hematocrit in albumax II complete medium (RPMI-1640 supplemented with 25 mg/mL HEPES, 2 mg/mL sodium bicarbonate, 5% albumax II (Invitrogen/Gibco, Carlsbad, CA) (26). All cultures were maintained at 37°C in an atmosphere of 5% CO2, 5% O2, and 90% N2, with once or twice daily medium changes. Parasitized erythrocytes with ring forms were treated with 5% D-sorbitol. This procedure was repeated 4 h after the first treatment to eliminate other forms and thus synchronize the culture (27). After treatment, the cells were washed twice with complete medium, resuspended, and cultured for 24, 36, 38, and 45 h to allow continued development to early trophozoite, late trophozoite, early schizont, and late schizont stages, respectively. A maximum expected level of parasitemia for these conditions is ~10%. Parasitized erythrocytes were counted per 104erythrocytes. We observed and evaluated 500 parasitized erythrocytes to estimate the developmental stage proportion, as listed in Table 1.
Table 1.
Percentages of P. falciparum parasite forms in synchronized erythrocyte cultures used in five independent CTV* experiments and the number of tracked cells in each experiment (N)
| Exp’t # | % Rings | %Trophozoites | % Schizonts | % Parasi- -temia† |
N | ||
|---|---|---|---|---|---|---|---|
| Early | Late | Early | Late | ||||
| Neg. Ctrl. | 0 | 0 | 0 | 0 | 0 | 0 | 1401 |
| 1 | 51.8 | 48.2 | 0 | 0 | 0 | 12.0 | 455 |
| 2 | 0 | 7.2 | 73.5 | 12.4 | 6.8 | 7.5 | 296 |
| 3 | 0 | 0 | 70.0 | 25.0 | 5.0 | 4.0 | 341 |
| 4 | 0 | 0 | 19.2 | 55.6 | 25.2 | 6.0 | 395 |
| 5 | 6.5 | 0 | 10.0 | 0 | 83.5 | 6.6 | 167 |
Cell-tracking velocimetry.
Cell number count corresponded to 500 infected cells in each sample.
Erythrocyte magnetophoresis in isodynamic magnetic field
The central part of the CTV apparatus is the permanent magnet assembly, with specially shaped pole pieces spaced 2.5 mm apart that produce a nearly constant Maxwell stress gradient, , in the microscope field of view, where H is the magnitude of the magnetic field strength, B is the magnitude of the magnetic field intensity, and ∇ is the gradient operator (28) (Fig. 1B). The microscope cell motion observation area is 1.72 × 1.27 mm and has a depth of view of ~20 µm, which is located inside a glass parallelepiped channel of internal dimensions of 0.6 × 1.7 mm, and 0.4-mm-thick walls that serve as a cell sample conduit. The orientation of the magnet and channel is such that the magnetic force is orthogonal to the force of gravity, in order to minimize the gravitational sedimentation interference with the magnetophoretic mobility. The geometry of the pole pieces and the placement of the channel ensure that the field is, essentially, two-dimensional. In computing mobilities, only the horizontal component of the gradient is used. In the viewing area, the average Maxwell stress gradient was 189.3 kg/(mm2 s2) ± 0.7%; the mean field value is 1.26 T, and the mean field gradient is 0.140 T/mm.
In order to produce an observable displacement on a large number of erythrocytes randomly distributed within a microscope field, an open-gradient, spatially independent [isodynamic (20, 29)] field configuration was used, as illustrated in Fig. 1B. The magnetophoretic mobility is defined as the erythrocyte velocity induced by the magnetic field, um, divided by the magnitude of the local Maxwell stress gradient, , resulting in the following expression:
| (1) |
The erythrocyte magnetic susceptibility relative to the water solution,Δχ , can be directly calculated from its magnetophoretic mobility, m:
| (2) |
where Δχ = χRBC − χH2O is the net volumetric magnetic susceptibility of the erythrocyte (relative to water), and χH2O = −0.719 × 10−6 is the magnetic susceptibility of water; f is the erythrocyte friction coefficient, and V is the erythrocyte volume (20). We observe that the erythrocyte velocity induced by the magnetic field is very small and, therefore, satisfies conditions of creeping flow, so that f = 6πηR , where η (= 0.955×10−3 kg/(m s) at 22°C) is the viscosity of the water solution and R is the hydrodynamic radius of the erythrocyte. Taking the volume and hydrodynamic radius of the erythrocyte to be 88.4 µm3 and 3.85 µm, respectively, and substituting into Eq. 2 one obtains:
| (3) |
Thus, the net volume magnetic susceptibility of the erythrocyte (relative to the fluid medium) is measured from its magnetophoretic mobility providing that the rheological factor f / V is known. The cell motion in the viewing area is observed with a 5× microscope objective and 2.5× photo eyepiece (Olympus, Tokyo, Japan). Light is supplied internally (epi-illumination) through the microscope. A Cohu (San Diego, CA) CCD 4915 camera is used with a µ-Tech Vision 1000 PCI Bus Frame Grabber (MuTech Corp., Billerica, MA) to convert the image into a 640 × 480 pixel array, where each pixel contains eight bits of gray-level information that ranges from 0 (black) to 255 (white). The CCD camera has a frame rate of 30 Hz, and the acquisition rate was set at every 180th frame to increase spatial resolution of slow moving objects, which results in the time between acquired frames of 6.0 s. The number of tracked frames was always 20 and, therefore, the time lapse between the first and last tracked frames was (20–1) × 6 = 114 s. In this time interval, the red cells moved a sufficient distance in the direction of the magnetic field gradient to be distinguished from zero. We have determined that the magnetic mobility discrimination power of CTV is below the noise introduced by the cell Brownian motion. The current code uses sets of five successive images to establish the most probable path for a specific particle. A linear fit of the total location-time dataset for each particle gives its velocity, um. The mobility frequency histograms were obtained by binning the magnetophoretic mobility values among 200 equally spaced intervals of 0.05 × 10−6 mm3 s/kg and calculating mobility frequencies normalized to the total number of erythrocytes tracked (29). The number of tracked cells ranged from 130 to 1400 per experiment. The frequency distributions were tested for normality using χ2 test and parameterized using usual statistical approaches, including mean and 95% confidence intervals. Also, non-parametric tests were used, as described below.
Calculation of hemoglobin fraction converted to hemozoin based on erythrocyte magnetophoresis
The calculations of erythrocyte magnetic susceptibility were based on the published data on hemoglobin susceptibilities by Pauling and Coryell (19, 30, 31), Savicki et al. (32), Cerdonio (33, 34), Spees et al. (35), and other groups (36–38), and recently available information on hemozoin crystal structure and susceptibility (12). Here we assume, after Pauling and, later, Spees, that there are no unpaired electrons in the oxyhemoglobin heme group; furthermore, that the hemozoin heme electron configuration corresponds to that of a ferriheme with 5 unpaired electrons, S = 5/2 (12). The various contributions to the erythrocyte volume magnetic susceptibility are
| (4) |
where ϕH2O and ϕHb are the volume fractions of water and hemoglobin in a normal (uninfected) erythrocyte, and χH2O and χglobin are the volumetric magnetic susceptibilities of water and the protein (globin) part of hemoglobin, respectively. Also, z is the fraction of hemoglobin heme converted to hemozoin heme in the infected erythrocyte, and χferri is the magnetic susceptibility of ferriheme. For an erythrocyte suspension in equilibrium with ambient air, the intact hemoglobin is fully saturated with oxygen and its four heme groups do not contribute to the erythrocyte magnetic susceptibility (19, 30, 35); therefore, the expected changes in the erythrocyte magnetic susceptibility with the parasite development stage depend on the hemozoin fraction alone, z. Also, ϕHb χferri = nHb χ’ferri, where χ’ferri = 57,428 × 10−6 cm3/mol, and nHb = 5.5 mM is the intracellular molar concentration of hemoglobin. Assuming that water and hemoglobin are the predominant components of the erythrocyte, ϕH2O = 1 – nHb νHb where νHb = 48,277 cm3/mol is the molar volume of hemoglobin, and ϕHbχglobin = nHbMHbχ”globin, where MHb = 64,450 is the molecular weight of hemoglobin, and χ”globin = −0.580×10−6 cm3/g is the specific susceptibility of globin in the hemoglobin molecule. By comparing the net magnetic susceptibility of the erythrocyte, Δχ (calculated from its magnetophoretic mobility, Eq. 3) to the value calculated from Eq. 4 above (and after taking into account the magnetic susceptibility of water,Δχ = χRBC − χH2O ), one finds the fraction of hemoglobin converted to hemozoin in the infected erythrocyte, z. The susceptibility values are given in the cgs unit system to facilitate comparison with the literature data; the conversion to values in the SI unit system requires multiplication by 4π.
Data analysis
The expected effect of hemozoin on erythrocyte motion in the magnetic field is small. It has been reported that in the extreme circumstances, only up to 80% of hemoglobin is converted to high-spin hemozoin in the late trophozoite stage (39, 40), and only up to 10% of all erythrocytes are infected in the P. falciparum culture (26). Therefore, for the un-enriched erythrocyte sample, the presence of the infected cells is expected to skew only slightly the magnetophoretic mobility distribution toward the high mobility values. Accordingly, we first measured the mobility distribution of a control, uninfected erythrocyte sample, and then used it to define a cut-off mobility, m0, to discriminate against the magnetic effect in the test samples. The cut-off mobility was defined as the 95th percentile mobility of the control sample. In addition, the magnetic erythrocyte population (classified by the mobility of its members being higher than the cut-off mobility, m0) was divided into quartiles and further characterized by the minimum (the lowest mobility that is higher than m0), 25th percentile, median, 75th percentile, and the maximum mobility of the magnetic erythrocyte fraction. Non-parametric tests were used to compare median mobilities from different experiments, including Spearman rank correlation coefficient (41).
The random error of the magnetophoretic mobility is caused by the large magnitude of the sedimentation velocity relative to its magnetic migration velocity um, as discussed in Results section. The observed random effects include the erythrocyte rotation, residual convective effects in different layers of the suspending fluid traveled by the erythrocyte, and occasionally the erythrocyte wandering off and on the microscope’s focal plane. Furthermore, it has been reported that the sedimentation velocity is higher for the uninfected and the early ring-infected erythrocytes than the erythrocytes infected with the schizont forms (42). This is expected to cause under-representation of the uninfected and the ring form-infected erythrocyte fraction in the magnetophoretic mobility histograms of mixed populations because a portion of those cells would sediment out of the microscope’s field of view during the time of image acquisition. However, the effect is not expected to affect the measured characteristic mobilities of the magnetic cell fraction, as defined above, because for the most part it stays in the field of view for the duration of the image acquisition procedure.
The experimental error of parameter z determination, δ z (where z is the fraction of hemoglobin heme converted to hemozoin heme in the infected erythrocyte, Eq. 4) is for the most part a function of the error of measurement of the erythrocyte magnetophoretic mobility, δ m
| (5) |
considering that the uncertainty associated with the measurement of the quantity m is higher than the uncertainties associated with the other quantities entering Eqs. 2, 3, and 4 (from which Eq. 5 follows). The estimated error of magnetic fraction mobility, δ m was taken as a half interval between 25th percentile and 75th percentile mobilities.
RESULTS
The magnetophoretic mobility and the corresponding volume magnetic susceptibility of uninfected erythrocytes were distributed normally (Fig. 3A). A small, negative value of the mean peak net susceptibility indicated that the erythrocytes were oxygenated and in equilibrium with the ambient air (20). A highly symmetrical distribution around the mean indicated contribution of random errors of measurement not related to the magnetic field, such as the effects of gravitational sedimentation (as discussed above). The cut-off mobility, set at the 95th percentile of cumulative mobility frequency distribution, was m0 = 0.75 × 10−6 mm3 s/kg (Fig. 3A).
With this analytical system we were interested in detecting an effect of P. falciparum developmental stages (Fig. 2). For these studies five separate synchronized parasite cultures were prepared by treatment with 5% D-sorbitol as described in Materials and Methods. Following synchronization, parasitized cells were analyzed directly (Expt. #1) or returned to culture for 24 (#2), 36 (#3), 38 (#4), and 45 (#5) h to allow continued development of the ring-stage parasites (Table 1). The magnetophoretic mobility and the corresponding net magnetic susceptibility of the sample were compared with that of the control.
Erythrocyte magnetophoretic mobility and the corresponding net magnetic susceptibility distributions differed markedly between predominantly ring and predominantly schizont cultures, Fig. 3B and C. In the predominantly rings and early trophozoites sample (Expt. #1, Table 1), the majority of the cells, 89%, had mobility below the cut-off mobility m0. The “magnetic” cell fraction, 11% in Fig. 3B, was comparable with the infected cell fraction in the sample, 12% as determined by the differential cell counting (listed as parasitemia in Expt. #1, Table 1). The unexpected presence of a few, highly mobile cells was explained as an artifact of the experimental procedure and considered outliers. The 25th to 75th percentile magnetic mobilities were grouped in a narrow range from 0.85 × 10−6 to 1.25 × 10−6 mm3 s/kg, Fig. 3B.
In comparison, the culture with predominantly late schizonts (Expt. #5, Fig. 3C) contained 62% of cells the increased magnetophoretic mobility higher than the cut-off mobility, m0. Interestingly, that percentage was higher than expected from the sample parasitemia, 6.6% (Table 1). This was consistent with the expected effects of the differential sedimentation rate between the uninfected and the infected erythrocytes on the erythrocyte mobility distribution, as discussed above. The effect was fortuitous in that it facilitated the analysis of the magnetic cell fraction by depleting the sample of the faster sedimenting, low-mobility fraction. The 25th to 75th percentile mobility range was from 1.35 × 10−6 to 2.95 × 10−6 mm3 s/kg, Fig. 3C, and was significantly higher than that in the sample devoid of schizonts shown in Fig. 3B. The magnetophoretic mobility of the erythrocyte enriched in the schizont form did not exceed that of the normal, deoxygenated erythrocytes and that of the erythrocytes with the hemoglobin converted to high-spin methemoglobin, as indicated in Fig. 3A, determined during our previous studies (20). This observation agrees with the published magnetic susceptibility data for the constituents of the infected erythrocyte, as discussed in relation to Eq. 4.
Cultures from experiments 2 to 4, populated by increasing percentages of mature trophozoites and schizonts, were also analyzed as described above (Fig. 4). The results were consistent with the results of experiments 1 and 5, but less pronounced, as explained by only incremental progression in the parasite development toward schizont and late schizont forms (compare Table 1).
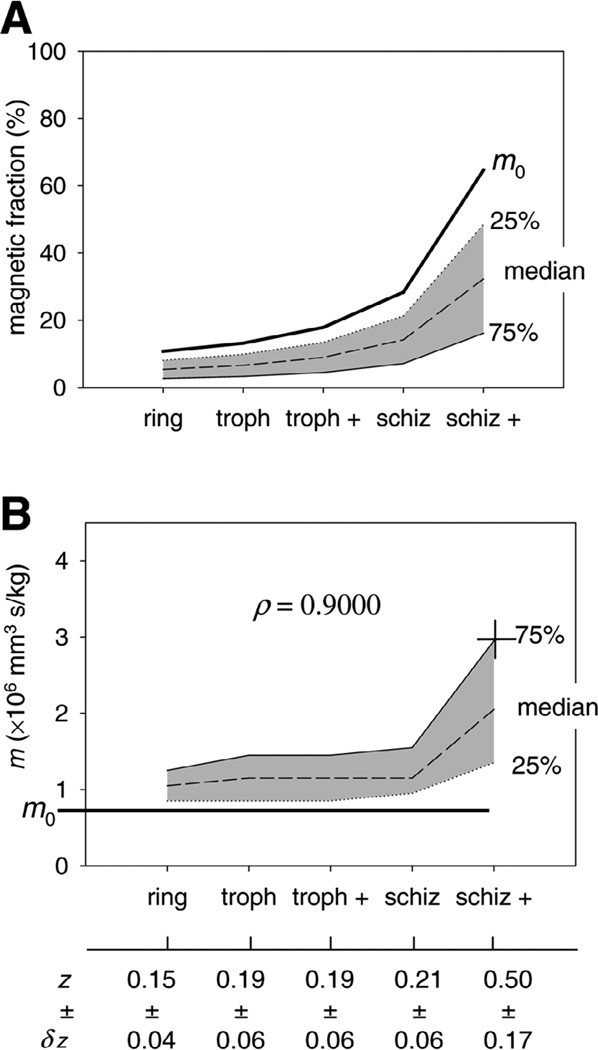
Figure 4.
A) Magnetic erythrocyte fraction plotted as a function of the predominant P. falciparum form in the erythrocyte culture for all samples listed in Table 1. The apparent increase in the magnetic fraction is not correlated to the parasitemia values listed in Table 1 but is an artifact of the increasing differential sedimentation rate between early stage and late stage forms, as discussed in the text. B) Magnetophoretic mobility as a function of the predominant P. falciparum form showing monotonic increase in mobility with progression from early to late parasite stage. The correlation is significant, as indicated by Spearman rank correlation coefficient,ρ, at P = 0.05%. The fractions of hemoglobin converted to hemozoin in the infected erythrocytes, z, calculated from Equation 4, and the error δz, are also shown.
By comparing the measured and calculated net magnetic susceptibilities, one finds the fraction of hemoglobin converted to hemozoin in the infected erythrocyte, z, from Eq. 4. Thus, for 75th percentile mobility of the schizont-rich magnetic fraction, equal to m = 2.94 × 10−6 mm3 s/kg, as indicated by a cross in Fig. 4B, one obtains Δχ = 1.80 × 10−6, corresponding to the hemozoin-converted hemoglobin fraction of z = 0.50 (also indicated in Fig. 4B). In other words, the results show that 75% of schizont-rich erythrocytes that move in the magnetic field faster than the uninfected control contain at least 50% hemoglobin heme groups converted to a high-spin form characteristic of hemozoin. The respective values calculated for the ring/early trophozoite culture, 0.15 (Expt. #1 in Table 1), and cultures containing maturing trophozoites/schizonts (0.19–0.21 for Expts. #2 to #4) indicate monotonic increase of the high-spin heme fraction associated with hemozoin, Fig. 4B, consistent with the schematics shown in Fig. 2. The results are consistent with numerical values reported from biochemical and morphological analyses of fixed erythrocyte specimens showing that the molar fraction of hemoglobin converted to hemozoin is the highest for the mature trophozoite stage, for which it can reach z = 0.50 (39) or higher (40).
DISCUSSION
Magnetic cell separation has become an important tool in research and clinical laboratories (43, 44). The current magnetic cell separation strategies rely on attaching synthetic, magnetic beads to cells in order to pull cells from suspension (45). The specificity of the separation is based on cell immunophenotype and use of antibodies that distinguish between characteristic cell surface antigen markers. In this respect, the current practice of the magnetic cell separation relies on immunocytochemistry.
Here, we describe quantitative measurement of cell motion induced by the magnetic field that does not require attachment of the synthetic beads to the cell but relies solely on the intrinsic magnetic properties of the cell. We selected a model of a malaria parasite-infected erythrocyte because of a number of important factors: 1) the model is pertinent to research on malaria and finding a cure; 2) the physical properties of the erythrocyte are well known, including magnetic susceptibility of hemoglobins from the pioneering work of Pauling in 1936 and later studies, such as functional nuclear magnetic resonance imaging (37), which provided a firm basis for the physical explanation of the observed effect; 3) the crystal structure of the malaria pigment, hemozoin, has been recently elucidated and its relation to the magnetic susceptibility of the hemozoin is now well established; 4) the accumulation of the hemozoin crystals over time of the parasite blood development has been clearly correlated to distinct parasite forms, which facilitates quantitative correlation between the magnetic migration velocity of the infected erythrocyte and the hemozoin load.
The work describes a physical effect of the parasite biology and its host, the erythrocyte, on cell motion in the magnetic field. The effect does not require cell manipulation or chemical treatment to achieve magnetization that could alter cell biology. We describe novel findings that correlate the amount of accumulated hemozoin in the infected erythrocyte to its velocity induced by the magnetic field. Practical applications of the strategies presented here may lead to magnetic fractionation tools of live erythrocytes that are sensitive to cell biology and improve our understanding of parasite heme management in relation to anti-malarial drug susceptibility and resistance. It has been reported that within infected erythrocytes, P. falciparum consumes from 50% (39) to 80% (40) of the cytosolic hemoglobin (molar concentration of 5 mM in the erythrocyte’s volume of 88.4 µm3) during a 48 h time period releasing an equivalent of 10–16 mM free heme, a potent biological toxin (46). For survival, the parasite compartmentalizes free heme in the digestive vacuole [volume of 4 µm3, resulting in an estimated 22-fold increase in concentration reaching 350 to 400 mM (8, 40)], and polymerizes this toxin into insoluble hemozoin (5, 10, 11, 16, 18) (Fig. 2). Indeed, without conversion to hemozoin, free heme would be increasing to an intra-erythrocytic concentration of at least 3 mM in culture analyzed in Expts. #1 and 10 mM in culture analyzed in Expts. #5 that would greatly exceed the 100 µM toxic level observed in other studies (40, 47).
There has been some debate in the literature on the fate of the considerable amount of released heme. Orjih and Fitch (48) reported that, of the 1800 pmol of ferriprotoporphyrin IX (FP) per 106 erythrocytes present in the form of hemoglobin, they found that a chloroquine-susceptible strain had converted 750 ± 140 pmol/106 parasitized cells to insoluble material at 24 h, and 960 ± 118 pmol/106 cells at 44 h. A chloroquine-resistant strain had sequestered somewhat less. Egan et al. (15) quantified the iron present in the erythrocyte, the P. falciparum parasite, and the food vacuole. They found that parasitized erythrocytes contain 95 ± 8 fg Fe/cell, the same as unparasitized cells. The trophozoites contained 58 ± 2 fg/cell, of which 53 ± 3 fg was in the food vacuole, and 47 ± 5 fg of this was in the form of hemozoin. At the trophozoite stage, they deduced that 61 ± 2% of the hemoglobin had been consumed, 92 ± 6% of the iron from the consumed hemoglobin was in the food vacuole, and of this 88 ± 9% was in the form of hemozoin. These results are in disagreement with the earlier hypothesis that 70 to 80% of the heme is broken down outside the food vacuole (49, 50). Tekwani and co-workers (51) have recently pointed out that aggregates of monomeric heme and hemozoin have differential solubility in organic and aprotic solvents and in sodium dodecyl sulfate and mildly alkaline bicarbonate solutions; therefore, the discrepancies in the hemozoin assays from infected erythrocytes reported earlier may be due to some of the heme in the food vacuoles being sequestered as aggregates rather than in the form of hemozoin.
The calculated parameter z, the fraction of hemoglobin degraded as a result of parasite metabolic activity, was based on the high spin state of iron ion in the hemozoin heme, S = 5/2 (12). The magnitude of iron spin in free heme or in amorphous heme aggregates is less certain. Coryell at al. (19) have shown that the spin of iron ion varies with the variation of oxidation state and chemical environment, such that S = 5/2 in ferrihemoglobin, or “acid methemoglobin” and S = 3/2 in ferrihemoglobin hydroxide, or “alkaline methemoglobin” (with no spin contribution from the globin part). The iron ion of hemin, a ferric protoporphyrin IX chloride, exists in two states, S = 5/2 and 1/2 (hematin is the ferric protoporphyrin IX hydroxide) (52). Thus, the erythrocyte magnetic susceptibility may be a function of not only the distribution of heme molecules between oxyhemoglobin (S=0) and hemozoin (S=5/2) but also the presence of free heme and amorphous heme aggregates (for which S may vary from S=0 to S=5/2). It is this latter and a rather ill-defined heme fraction that contributes to error in the calculated value of z. However, that fraction is relatively small and does not exceed 5% (15) and, therefore, in the final analysis has little effect on the error of z.
The observed increase in the magnetophoretic mobility of the infected erythrocyte with the parasite development suggests a slower rate of hemoglobin breakdown in the early stages than in the late stages, Fig. 4B. This approximately matches the reported decline in hemoglobin content of infected cells during parasite maturation for the HB3 strain (same as used in this study) (53), and an apparently bi-phasic rate of production of hemozoin during HB3 development cycle shown by others (50). However, due to a rather broad mobility distribution of the magnetic fraction, Fig. 3, and the resulting broad distribution of the parameter z, for each parasite development stage, Fig. 4B, the exact nature of the dependence of z on time cannot be established here. Rather, our data show a monotonic increase of hemoglobin degradation with the parasite development cycle and a significant difference between schizont and the ring stages. The confidence in the results is increased by the fact that they were obtained using live erythrocytes with minimum sample manipulation and, therefore, few artifacts.
Using the current magnetic field configuration, the observed effect of hemozoin accumulation on the erythrocyte magnetic migration velocity is small, as illustrated by the following calculation. By inserting the value of m = 2.94 × 10−6 mm3 s/kg, representative of schizont-laden erythrocyte, Fig. 3C, into Eq. 1, one calculates the erythrocyte velocity um, induced by the Maxwell stress gradient One thus obtains um = 0.56 µm/s, a fraction of erythrocyte sedimentation velocity, which in an aqueous isoosmotic electrolyte solution is 1.2 µm/s. Current efforts are directed toward increasing the Maxwell stress gradient of the cell tracking velocimetry in order to increase the magnetic migration velocity and the resolution of the method. This should lead to a greater precision in determining the exact dependence of the amount of degraded hemoglobin on the parasite life cycle.
We have demonstrated that changes of the magnetic properties of live, infected erythrocytes are consistent with the conversion of low-spin heme associated with oxyhemoglobin to high-spin heme associated with hemozoin in the course of parasite development from ring to schizont forms. Our results suggest that, upon further refinement, the method could be used to study the molar ratio of free heme to hemozoin-sequestered heme in live erythrocytes, a parameter that is of vital importance to parasite survival inside the erythrocyte (8, 47). The anticipated refinement to the strategies presented here might lead to magnetic fractionation tools (54) of live erythrocytes that are sensitive to hemoglobin conversion to hemozoin during P. falciparum ring and early trophozoite stages and could improve our understanding of parasite heme management in relation to anti-malarial drug susceptibility and resistance (47).
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health (AI-523213 to P.A.Z., CA-062349 to M.Z.) and the National Science Foundation (BES-9731059 and BES-0124897 to J.J.C.). We thank Rajeev Mehlotra, Jodi Thomson, and Kiet Dan Luc for technical assistance, helpful comments, and criticisms during the preparation of the manuscript and Dr. Alan Schechter, Dr. Graciela Ostera, and Dr. Thomas E. Wellems for the initial impetus for the study.
References
- 1.Greenwood B, Mutabingwa T. Malaria in 2002. Nature. 2002;415:670–672. doi: 10.1038/415670a. [DOI] [PubMed] [Google Scholar]
- 2.Taylor TE, Strickland GT. Infections of the blood and reticuloendothelial system. In: Strickland GT, editor. Hunter's Tropical Medicine and Emerging Infectious Diseases. Philadelphia, PA: W. B. Saunders Company; 2000. pp. 614–643. [Google Scholar]
- 3.Krogstad DF. Malaria. In: Guerrant RL, Walker DH, Weller PF, editors. Essentials of Tropical Infectious Diseases. Philadelphia, PA: Churchill Livingstone; 2001. pp. 341–355. [Google Scholar]
- 4.Gutierrez Y. Apicomplexia of the Blood. In: Gutierrez Y, editor. Diagnostic Pathology of Parsitic Infections with Clinical Correlations. 2nd Edition. Vol. New York, NY: Oxford University Press; 2000. pp. 235–262. [Google Scholar]
- 5.Goldberg DE, Slater AFG, Cerami A, Henderson GB. Hemoglobin degradation in the malaria parasite Plasmodium falciparum: An ordered process in a unique organelle. Proc. Natl. Acad. USA. 1990;87:2931–2935. doi: 10.1073/pnas.87.8.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg DE, Slater AF, Beavis R, Chait B, Cerami A, Henderson GB. Hemoglobin degradation in the human malaria pathogen Plasmodium falciparum: a catabolic pathway initiated by a specific aspartic protease. J. Exp. Med. 1991;173:961–969. doi: 10.1084/jem.173.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan DJ, Jr, Gluzman IY, Goldberg DE. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science. 1996;271:219–222. doi: 10.1126/science.271.5246.219. [DOI] [PubMed] [Google Scholar]
- 8.Francis SE, Sullivan DJ, Jr, Goldberg DE. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 1997;51:97–123. doi: 10.1146/annurev.micro.51.1.97. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee R, Liu J, Beatty W, Pelosof L, Klemba M, Goldberg DE. Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc. Natl. Acad. Sci. USA. 2002;99:990–995. doi: 10.1073/pnas.022630099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noland GS, Briones N, Sullivan DJ., Jr The shape and size of hemozoin crystals distinguishes diverse Plasmodium species. Mol. Biochem. Parasitol. 2003;130:91–99. doi: 10.1016/s0166-6851(03)00163-4. [DOI] [PubMed] [Google Scholar]
- 11.Egan TJ. Physico-chemical aspects of hemozoin (malaria pigment) structure and formation. J. Inorg. Biochem. 2002;91:19–26. doi: 10.1016/s0162-0134(02)00372-0. [DOI] [PubMed] [Google Scholar]
- 12.Bohle DS, Debrunner P, Jordan PA, Madsen SK, Schulz CE. Aggregated heme detoxification byproducts in malarial trophozoites: beta-hematin and malaria pigment have a single S=5/2 iron environment in the bulk phase as determined by EPR and magnetic Moessbauer spectroscopy. J. Am. Chem. Soc. 1998;120:8255–8256. [Google Scholar]
- 13.Dunin-Borkowski RE, McCartney MR, Frankel RB, Bazylinski DA, Posfai M, Buseck PR. Magnetic microstructure of magnetotactic bacteria by electron holography. Science. 1998;282:1868–1870. doi: 10.1126/science.282.5395.1868. [DOI] [PubMed] [Google Scholar]
- 14.Bazylinski DA, Frankel RB. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2004;2:217–230. doi: 10.1038/nrmicro842. [DOI] [PubMed] [Google Scholar]
- 15.Egan TJ, Combrinck JM, Egan J, Hearne GR, Marques HM, Ntenteni S, Sewell BT, Smith PJ, Taylor D, van Schalkwyk DA, Walden JC. Fate of haem iron in the malaria parasite Plasmodium falciparum. Biochem. J. 2002;365:343–347. doi: 10.1042/BJ20020793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendrat K, Berger BJ, Cerami A. Haem polymerization in malaria. Nature. 1995;378:138–139. doi: 10.1038/378138a0. [DOI] [PubMed] [Google Scholar]
- 17.Ridley RG. Malaria: to kill a parasite. Nature. 2003;424:887–889. doi: 10.1038/424887a. [DOI] [PubMed] [Google Scholar]
- 18.Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. The structure of malaria pigment beta-haematin. Nature. 2000;404:307–310. doi: 10.1038/35005132. [DOI] [PubMed] [Google Scholar]
- 19.Coryell C, Stitt F, Pauling L. The magnetic properties and structure of ferrihemoglobin (methemoglobin) and some of its compounds. J. Am. Chem. Soc. 1937;59:633–642. [Google Scholar]
- 20.Zborowski M, Ostera GR, Moore LR, Milliron S, Chalmers JJ, Schechter AN. Red blood cell magnetophoresis. Biophys. J. 2003;84:2638–2645. doi: 10.1016/S0006-3495(03)75069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidelberger M, Mayer MM, Demarest CR. Studies in human malaria. I. The preparation of vaccines and suspensions containing plasmodia. J. Immunol. 1946;52:325–330. [PubMed] [Google Scholar]
- 22.Melville D, Paul F, Roath S. Direct magnetic separation of red cells from whole blood. Nature. 1975;255:706. doi: 10.1038/255706a0. [DOI] [PubMed] [Google Scholar]
- 23.Paul F, Melville D, Roath S, Warhurst D, Osisanya JOS. Separation of malaria-infected erythrocytes from whole blood: Use of a selective high-gradient magnetic separaiton technique. The Lancet. 1981;2:70–71. doi: 10.1016/s0140-6736(81)90414-1. [DOI] [PubMed] [Google Scholar]
- 24.Uhlemann AC, Staalsoe T, Klinkert MO, Hviid L. Analysis of Plasmodium falciparum-infected red blood cells. MACS&more. 2000;4:7–8. [Google Scholar]
- 25.Carter V, Cable HC, Underhill BA, Williams J, Hurd H. Isolation of Plasmodium berghei ookinetes in culture using Nycodenz density gradient columns and magnetic isolation. Malar. J. 2003;2:35. doi: 10.1186/1475-2875-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cranmer SL, Magowan C, Liang J, Coppel RL, Cooke BM. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 1997;91:363–365. doi: 10.1016/s0035-9203(97)90110-3. [DOI] [PubMed] [Google Scholar]
- 27.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 28.Becker R. Electromagnetic Fields and Interactions. New York: Dover Publications, Inc.; 1982. [Google Scholar]
- 29.Moore LR, Milliron S, Williams PS, Chalmers JJ, Margel S, Zborowski M. Control of magnetophoretic mobility by susceptibility-modified solutions as evaluated by cell tracking velocimetry and continuous magnetic sorting. Anal Chem. 2004;76:3899–3907. doi: 10.1021/ac049910f. [DOI] [PubMed] [Google Scholar]
- 30.Pauling L, Coryell C. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc. Natl. Acad. Sci. USA. 1936;22:159–163. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pauling L. Magnetic properties and structure of oxyhemoglobin. Proc. Natl. Acad. Sci. USA. 1977;74:2612–2613. doi: 10.1073/pnas.74.7.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savicki JP, Lang G, Ikeda-Saito M. Magnetic susceptibility of oxy- and carbonmonoxyhemoglobins. Proc. Natl. Acad. Sci. USA. 1984;81:5417–5419. doi: 10.1073/pnas.81.17.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerdonio M, Congiu-Castellano A, Calabrese L, Morante S, Pispisa B, Vitale S. Room-temperature magnetic properties of oxy- and corbonmonoxyhemoglobin. Proc. Natl. Acad. Sci. USA. 1978;75:4916–4919. doi: 10.1073/pnas.75.10.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerdonio M, Morante S, Torresani D, Vitale S, DeYoung A, Noble RW. Reexamination of the evidence for paramagnetism in oxy- and carbonmonoxyhemoglobins. Proc. Natl. Acad. Sci. USA. 1985;82:102–103. doi: 10.1073/pnas.82.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spees WM, Yablonskiy DA, Oswood MC, Ackerman JJ. Water proton MR properties of human blood at 1.5 Tesla: magnetic susceptibility, T(1), T(2), T*(2), and non-Lorentzian signal behavior. Magn. Reson. Med. 2001;45:533–542. doi: 10.1002/mrm.1072. [DOI] [PubMed] [Google Scholar]
- 36.Plyavin YA, Blum EY. Magnetic parameters of blood cells and high gradient paramagnetic and diamagnetic phoresis. Magnetohydrodynamics. 1983;19:349–359. [Google Scholar]
- 37.Fabry ME, San George RC. Effect of magnetic susceptibility on nuclear magnetic resonance signals arising from red cells: a warning. Biochemistry. 1983;22:4119–4125. doi: 10.1021/bi00286a020. [DOI] [PubMed] [Google Scholar]
- 38.Weisskoff RM, Kiihne S. MRI susceptometry: image-based measurement of absolute susceptibility of MR contrast agents and human blood. Magn. Reson. Med. 1992;24:375–383. doi: 10.1002/mrm.1910240219. [DOI] [PubMed] [Google Scholar]
- 39.Orjih AU, Ryerse JS, Fitch CD. Hemoglobin catabolism and the killing of intraerythrocytic Plasmodium falciparum by chloroquine. Experientia. 1994;50:34–39. doi: 10.1007/BF01992046. [DOI] [PubMed] [Google Scholar]
- 40.Becker K, Tilley L, Vennerstrom JL, Roberts D, Rogerson S, Ginsburg H. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Inl. J. Parasitology. 2004;34:163–189. doi: 10.1016/j.ijpara.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Miller JC, Miller JN. Statistics for Analytical Chemistry. Ellis Horwood Limited, Chichester, West Sussex, England: 1992. [Google Scholar]
- 42.Lelievre J, Berry A, Benoit-Vical F. An alternative method for Plasmodium culture synchronization. Exp. Parasitol. 2005;109:195–197. doi: 10.1016/j.exppara.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Recktenwald D, Radbruch A. Cell Separation Methods and Applications. New York, NY: Marcel Dekker, Inc.; 1998. [Google Scholar]
- 44.Zborowski M, Chalmers JJ. Magnetic cell sorting. Methods Mol. Biol. 2005;295:291–300. doi: 10.1385/1-59259-873-0:291. [DOI] [PubMed] [Google Scholar]
- 45.McCloskey KE, Chalmers JJ, Zborowski M. Magnetic cell separation: characterization of magnetophoretic mobility. Anal. Chem. 2003;75:6868–6874. doi: 10.1021/ac034315j. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005;157:175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Dzekunov SM, Ursos LM, Roepe PD. Digestive vacuolar pH of intact intraerythrocytic P. falciparum either sensitive or resistant to chloroquine. Mol. Biochem. Parasitol. 2000;110:107–124. doi: 10.1016/s0166-6851(00)00261-9. [DOI] [PubMed] [Google Scholar]
- 48.Orjih AU, Fitch CD. Hemozoin production by Plasmodium falciparum: variation with strain and exposure to chloroquine. Biochim. Biophys. Acta. 1993;1157:270–s274. doi: 10.1016/0304-4165(93)90109-l. [DOI] [PubMed] [Google Scholar]
- 49.Ginsburg H, Famin O, Zhang J, Krugliak M. Inhibition of glutathione-dependent degradation of heme by chloroquine and amodiaquine as a possible basis for their antimalarial mode of action. Biochem. Pharmacol. 1998;56:1305–1313. doi: 10.1016/s0006-2952(98)00184-1. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, Krugliak M, Ginsburg H. The fate of ferriprotorphyrin IX in malaria infected erythrocytes in conjunction with the mode of action of antimalarial drugs. Mol. Biochem. Parasitol. 1999;99:129–141. doi: 10.1016/s0166-6851(99)00008-0. [DOI] [PubMed] [Google Scholar]
- 51.Tripathi AK, Khan SI, Walker LA, Tekwani BL. Spectrophotometric determination of de novo hemozoin/beta-hematin formation in an in vitro assay. Anal. Biochem. 2004;325:85–91. doi: 10.1016/j.ab.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 52.Bartoszek M, Balanda M, Skrzypek D, Drzazga Z. Magnetic field effect on hemin. Physica B. 2001;307:217–223. [Google Scholar]
- 53.Krugliak M, Zhang J, Ginsburg H. Intraerythrocytic Plasmodium falciparum utilizes only a fraction of the amino acids derived from the digestion of host cell cytosol for the biosynthesis of its proteins. Mol. Biochem. Parasitol. 2002;119:249–256. doi: 10.1016/s0166-6851(01)00427-3. [DOI] [PubMed] [Google Scholar]
- 54.Carpino F, Moore LR, Zborowski M, Chalmers JJ, Williams PS. Analysis of magnetic nanoparticles using quadrupole magnetic field-flow fractionation. J. Magnetism Magnetic Mater. 2005;293:546–552. [Google Scholar]