Abstract
The article describes a readily easy adaptive in vitro model to investigate macrophage polarization. In the presence of GM-CSF/M-CSF, hematopoietic stem/progenitor cells from the bone marrow are directed into monocytic differentiation, followed by M1 or M2 stimulation. The activation status can be tracked by changes in cell surface antigens, gene expression and cell signaling pathways.
Keywords: Immunology, Issue 76, Cellular Biology, Molecular Biology, Medicine, Genetics, Biomedical Engineering, biology (general), genetics (animal and plant), immunology, life sciences, Life Sciences (General), macrophage polarization, bone marrow derived macrophage, flow cytometry, PCR, animal model
Introduction
Distinct from classical inflammatory responses, macrophages that infiltrate tissues often display polarized activation status that plays a crucial role in regulating host tissue physiological functions1-8. Upon stimulation, macrophage activation can be sorted into classic (M1) and alternative (M2) activation2, 4, 9 . M1 macrophage activation depends on Toll-like receptors (TLRs) and activation of nuclear factor kappa B (NFκB)/c-Jun N-terminal kinase 1(JNK1), leading to production of inflammatory cytokines, such as TNF-α and IL-1β and activation of iNOS that results in increased production of reactive oxygen species such as nitride oxide (NO) 10, 11 . In contrast, M2 macrophage activation recruits PPARγ, PPARδ, or IL-4-STAT6 pathways, leading to alternative, anti-inflammatory (M2) activation that is associated with upregulation of mannose receptor CD206, and arginase 1(Arg1) 6, 12-14 .
Bone marrow derived macrophages (BMDM) present an ideal in vitro model to understand the mechanisms controlling polarization of activated macrophages15. Specifically, activation of M1 macrophages can be induced by lipopolysaccharides (LPS) stimulation, while polarization of M2 macrophages can be induced by IL-4 and/or IL-13. Mature bone marrow derived macrophages and activated macrophages can be identified through flow cytometry analysis for expression of surface antigens, including CD11b, F4/80, CD11c, CD206, CD69, CD80 and CD869, 16, 17. In addition, changes in cytokine production and cell signaling pathways associated with macrophage polarization can be measured by quantitative RT-PCR and western blotting, respectively. In summary, mouse bone marrow derived macrophages can serve as a relevant model to study macrophage polarization in vitro.
Protocol
1. Isolation of Bone Marrow Cells
Isolate femur and tibia bones from 6-8 week old mice, rinse off hair and then cut open the bone.
Use a 21G needle and 10 ml syringe to flush out marrow into cold PBS+2% heat inactivated Fetal Bovine Serum (FBS) (3-5 ml/mouse).
Pass marrow through a 21G needle 4-6 times to dissociate the cells.
Pass cells through a 70 μm cell strainer to remove cell clumps, bone, hair and other cells/tissues.
Add 3 volume of NH4Cl solution (0.8% NH4Cl solution, Stemcell Technology), and incubate on ice for 10 min to remove red blood cells.
Spin down cells at 500 x g for 5 min at 4 °C.
Resuspend the cell pellet in cold PBS+2%FBS (20-50 ml, depending on the quantity of cells).
2. Induction BMDM Formation
Resuspend the isolated bone marrow cells in BMDM growth medium (2x106 cells/ml).
BMDM growth medium:
Iscove's Modified Dulbecco's Medium (IMDM) + 10% FBS + 15% filtered (0.2 μm) L-929 cell (ATCC, CCL-1) culture supernatant (containing monocyte-colony stimulating factor, M-CSF) or 10 ng/ml M-CSF.
Note: L-929 cell supernatant contains M-CSF18. To ensure the effective activity of conditioned medium, 5 X 105 L-929 cells are seeded in T75 cm2 flasks for 6-7 days, conditioned medium is collected and passed through a 0.45 μM filter before use. Medium aliquots can be used immediately or stored in -80 °C for 1-2 months.
Seed cells in 6 or 12 well tissue culture plates (depending on the experimental design) (Corning Costar).
Change fresh BMDM growth medium on day 3.
On day 7, formation of mature BMDM is evaluated using flow cytometry analysis and fluorophore conjugated antibodies to detect cells expressing CD11b and F4/80.
3. BMDM Polarized Activation
On day 7, change to fresh stimulation medium: for M1 activation, use IMDM containing 10% FBS and 100 ng/ml LPS or 100 ng/ml LPS with 50 ng/ml IFNγ; for M2 activation, use IMDM containing 10% FBS with 10 ng/ml IL-4 and/or 10 ng/ml IL-13.
Collect stimulated BMDMs by detaching them from the dish using warm 0.05% trypsin, followed by washing the cells twice with PBS containing 10% FBS.
Note: To detach and resuspend mature macrophages after differentiation, 0.05% Trypsin solution (containing 0.48 mM EDTA, Invitrogen) or 2-5 mM EDTA in Ca- and Mg-free PBS or Hank's balanced buffer (HBSS) can be used. When using digestive medium containing trypsin, cells are treated at 37 °C for less than 10 min to avoid loss of surface proteins due to over-digestion.
Use antibodies to detect expression of cell surface antigens, including CD11b, F4/80, CD11c, CD206, CD69, CD80 or CD86 at various time points using standard flow cytometry staining procedures.
Determine expression of genes characteristic of activated M1 and M2 macrophages including IL-1β, TNF-α and IL-6 (M1 activation) or IL-10, IL-13, arginase1 and PPARγ (M2 activation) using qRT-PCR. Determine activation of cell signaling pathways involved in activation of M1 or M2 macrophages by western blotting analysis.
Representative Results
A schematic description of the BMDM generation procedure is presented (Figure 1). High purity of mature macrophages can be observed on day 7 when they represent 95 to 99% of CD11b+F4/80+ cells (Figure 2). Polarized macrophages can be examined using antibodies against CD11b, F4/80, CD11c and CD206 followed by flow cytometry analysis. As shown in Figure 3, M1 macrophages are detected as CD11b+F4/80+CD11c+CD206- cells (Q2), whereas M2 macrophages are CD11b+F4/80+CD11c-CD206+ cells (Q4). The BMDM activation status can be confirmed by increased cellular size (Figure 4A, right shift in FSC-A) and increased abundance of surface antigens CD69, CD80, or CD86 on macrophages (Gate: CD11b+F4/80+) as shown in Figure 4B. Cytokine production, gene expression and cell signaling pathway activation in M1 or M2 macrophages can be evaluated using quantitative RT-PCR or western blotting. As shown in Figure 5, arginase 1(Arg1) and PPARγ levels were increased in M2 macrophages upon 10 ng/ml IL-4 stimulation; whereas IL-1β and TNFα production were increased in macrophages (M1) stimulated with 100 ng/ml LPS, which is accompanied by p65 activation (Figure 6).
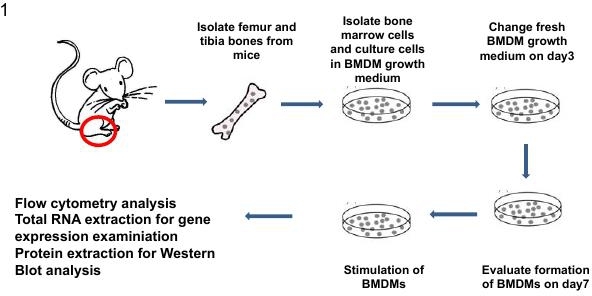 Figure 1. Scheme for the isolation, formation and stimulation of mouse BMDMs. Step by step procedures are described in the text. In brief, femur and tibia bones are collected from 6-8 weeks mice and bone marrow cells flushed out using PBS supplemented with 2% heat inactivated FBS. After red blood cells are lysed with NH4Cl solution, cells are cultured in BMDM growth medium for 7 days followed by maturation and analysis for purity of the cell population. For analysis of macrophage polarization, cells were stimulated with LPS or LPS+IFNγ for M1, or IL-4 and/or IL-13 for M2 activation. Polarized macrophages can be evaluated on the basis of changes in cell morphology, surface marker presentation, cytokine production and cell signaling pathway activated.
Figure 1. Scheme for the isolation, formation and stimulation of mouse BMDMs. Step by step procedures are described in the text. In brief, femur and tibia bones are collected from 6-8 weeks mice and bone marrow cells flushed out using PBS supplemented with 2% heat inactivated FBS. After red blood cells are lysed with NH4Cl solution, cells are cultured in BMDM growth medium for 7 days followed by maturation and analysis for purity of the cell population. For analysis of macrophage polarization, cells were stimulated with LPS or LPS+IFNγ for M1, or IL-4 and/or IL-13 for M2 activation. Polarized macrophages can be evaluated on the basis of changes in cell morphology, surface marker presentation, cytokine production and cell signaling pathway activated.
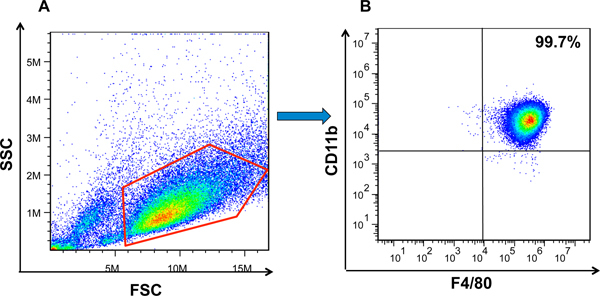 Figure 2. Flow cytometry analysis of BMDM formation. (A) BMDMs were first gated on FSC and SSC to remove debris and conjugates. (B) Mature BMDMs were defined as CD11b+F4/80+ subpopulations (upper right) with the purity displayed as percentage of parent population gated on FSC/SSC.
Figure 2. Flow cytometry analysis of BMDM formation. (A) BMDMs were first gated on FSC and SSC to remove debris and conjugates. (B) Mature BMDMs were defined as CD11b+F4/80+ subpopulations (upper right) with the purity displayed as percentage of parent population gated on FSC/SSC.
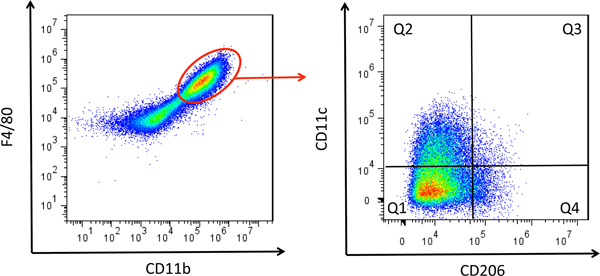 Figure 3. Macrophage polarization analysis. Tissue infiltrated macrophages are defined as CD11b+F4/80+ cells; M1 macrophages are CD11b+F4/80+CD11c+CD206- cells, whereas M2 macrophages are CD11b+F4/80+CD11c-CD206+ cells.
Figure 3. Macrophage polarization analysis. Tissue infiltrated macrophages are defined as CD11b+F4/80+ cells; M1 macrophages are CD11b+F4/80+CD11c+CD206- cells, whereas M2 macrophages are CD11b+F4/80+CD11c-CD206+ cells.
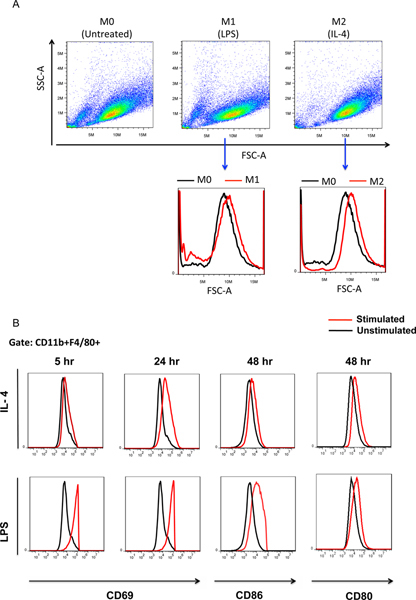 Figure 4. Macrophage activation analysis by flow cytometry.(A) Upon activation, the size of macrophages increase as evidenced by the shift of FSC-A of M1 or M2 macrophages at 24 hr after stimulation compared to M0 (untreated) macrophages. (B) The activation-related surface markers were analyzed by flow cytometry after IL4 or LPS stimulation. The early responding marker CD69 was evaluated at 5 or 24 hr post-stimulation; CD80 and CD86 markers were analyzed at 48 hr after stimulation. Click here to view larger figure.
Figure 4. Macrophage activation analysis by flow cytometry.(A) Upon activation, the size of macrophages increase as evidenced by the shift of FSC-A of M1 or M2 macrophages at 24 hr after stimulation compared to M0 (untreated) macrophages. (B) The activation-related surface markers were analyzed by flow cytometry after IL4 or LPS stimulation. The early responding marker CD69 was evaluated at 5 or 24 hr post-stimulation; CD80 and CD86 markers were analyzed at 48 hr after stimulation. Click here to view larger figure.
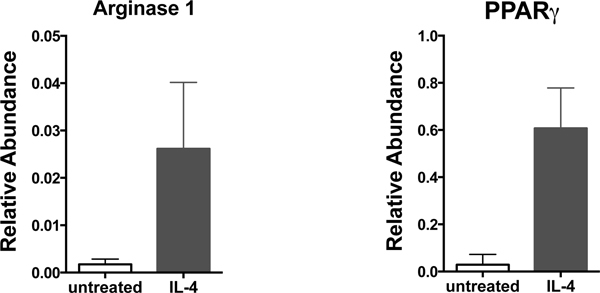 Figure 5. Alternative activation of macrophages. Macrophages stimulated with 10 ng/ml IL-4 for 24 hr were collected and total RNA extracted. Elevated arginase 1 (Arg1) and PPARγ expression was detected in M2 macrophages as compared to untreated macrophages using quantitative RT-PCR analysis.
Figure 5. Alternative activation of macrophages. Macrophages stimulated with 10 ng/ml IL-4 for 24 hr were collected and total RNA extracted. Elevated arginase 1 (Arg1) and PPARγ expression was detected in M2 macrophages as compared to untreated macrophages using quantitative RT-PCR analysis.
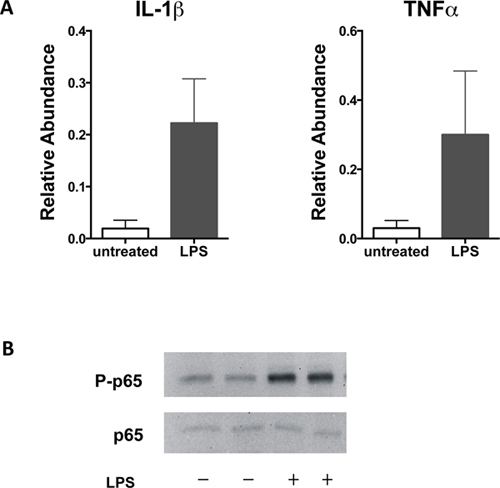 Figure 6. Classical activation of macrophages. (A) Total RNA was extracted from macrophages stimulated with 100 ng/ml LPS for 24 hr and used in quantitative RT-PCR analyses. Expression of proinflammatory cytokines including IL-1β and TNFα increased in M1 macrophages. (B) Activation of the NFκB pathway was induced by LPS as determined using antibodies to p65 and phosphorylated p65 in western blotting analyses.
Figure 6. Classical activation of macrophages. (A) Total RNA was extracted from macrophages stimulated with 100 ng/ml LPS for 24 hr and used in quantitative RT-PCR analyses. Expression of proinflammatory cytokines including IL-1β and TNFα increased in M1 macrophages. (B) Activation of the NFκB pathway was induced by LPS as determined using antibodies to p65 and phosphorylated p65 in western blotting analyses.
Discussion
We report here a simple and readily adaptable in vitro procedure to induce activation of macrophages derived from bone marrow progenitor cells. This procedure can be used for investigation of mechanisms responsible for polarization of macrophages. The purity of mature macrophages obtained using this protocol averages 95 to 99%, and no additional purification procedures are required. To investigate the function of specific genes of interests in the context of macrophage polarization, ectopic expression or gene specific knockdown can be conducted following transfection of the cells on day 7. This protocol will also provide a 7-day culture window to investigate the impacts of certain factors or genes that affect the formation, maturation and phenotype of macrophages.
Activated macrophages display complicated cellular and molecular profiles with great plasticity in response to various stimuli3, 9, 19 . For example, LPS elicits potent pro-inflammatory M1 responses which can be further enhanced in the presence of IFNγ or tumor-necrosis factor (TNF) and IL-4 and IL-13 both stimulate M2 activation; however, the activation profiles do not completely overlap 3, 4, 9, 15, 19, 20 . The results presented in this protocol only represent typical outcomes of experiments with bone marrow derived macrophages analyzed by flow cytometry, quantitative RT-PCR and western blotting. Surface antigen presentation and cell signaling pathways associated with activation of macrophages vary as noted in the extensive literature on macrophage polarization1-8.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by the American Heart Association (BGIA 7850037 to Dr. Beiyan Zhou).
References
- Meng ZX, Wang GX, Lin JD. A microrna circuitry links macrophage polarization to metabolic homeostasis. Circulation. 2012. [DOI] [PubMed]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific pparg controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative m2 activation of kupffer cells by ppard ameliorates obesity-induced insulin resistance. Cell Metabolism. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and pgc-1[beta] attenuate macrophage-mediated inflammation. Cell Metabolism. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li Z-W, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. Ikk-b links inflammation to obesity-induced insulin resistance. Nat. Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Saberi M, Woods N-B, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee C-H. Adipocyte-derived th2 cytokines and myeloid ppard regulate macrophage polarization and insulin sensitivity. Cell Metabolism. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. Pparg activation primes human monocytes into alternative m2 macrophages with anti-inflammatory properties. Cell Metabolism. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Bronte V, Zanovello P. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, Li H, Wang G, Evans AR, Safe S, Wu C, Zhou B. A novel regulator of macrophage activation: Mir-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125:2892–2903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- Kradin RL, McCarthy KM, Preffer FI, Schneeberger EE. Flow-cytometric and ultrastructural analysis of alveolar macrophage maturation. J. Leukoc. Biol. 1986;40:407–417. doi: 10.1002/jlb.40.4.407. [DOI] [PubMed] [Google Scholar]
- Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassmann G, Bertolini DR, Kerby SB, Fong M. Regulation of murine mononuclear phagocyte inflammatory products by macrophage colony-stimulating factor. Lack of il-1 and prostaglandin e2 production and generation of a specific il-1 inhibitor. J. Immunol. 1991;147:1279–1285. [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]


