Abstract
Environmental concerns have focused attention on natural forms of disease control as potentially safe and effective alternatives to chemical pesticides. This has led to increased efforts to develop control strategies that rely on natural predators and parasites or that involve genetically engineered microbial pest control agents. This review deals with a natural form of biological control in which the virulence of a fungal pathogen is attenuated by an endogenous viral RNA genetic element: the phenomenon of transmissible hypovirulence in the chestnut blight fungus, Cryphonectria parasitica. Recent progress in the molecular characterization of a hypovirulence-associated viral RNA has provided an emerging view of the genetic organization and basic expression strategy of this class of genetic elements. Several lines of evidence now suggest that specific hypovirulence-associated virus-encoded gene products selectively modulate the expression of subsets of fungal genes and the activity of specific regulatory pathways. The construction of an infectious cDNA clone of a hypovirulence-associated viral RNA represents a major advancement that provides exciting new opportunities for examining the molecular basis of transmissible hypovirulence and for engineering hypovirulent strains for improved biocontrol. These developments have significantly improved the prospects of using this system to identify molecular determinants of virulence and elucidate signal transduction pathways involved in pathogenic responses. In addition, novel approaches are now available for extending the application of transmissible hypovirulence for management of chestnut blight and possibly other fungal diseases.
Full text
PDF





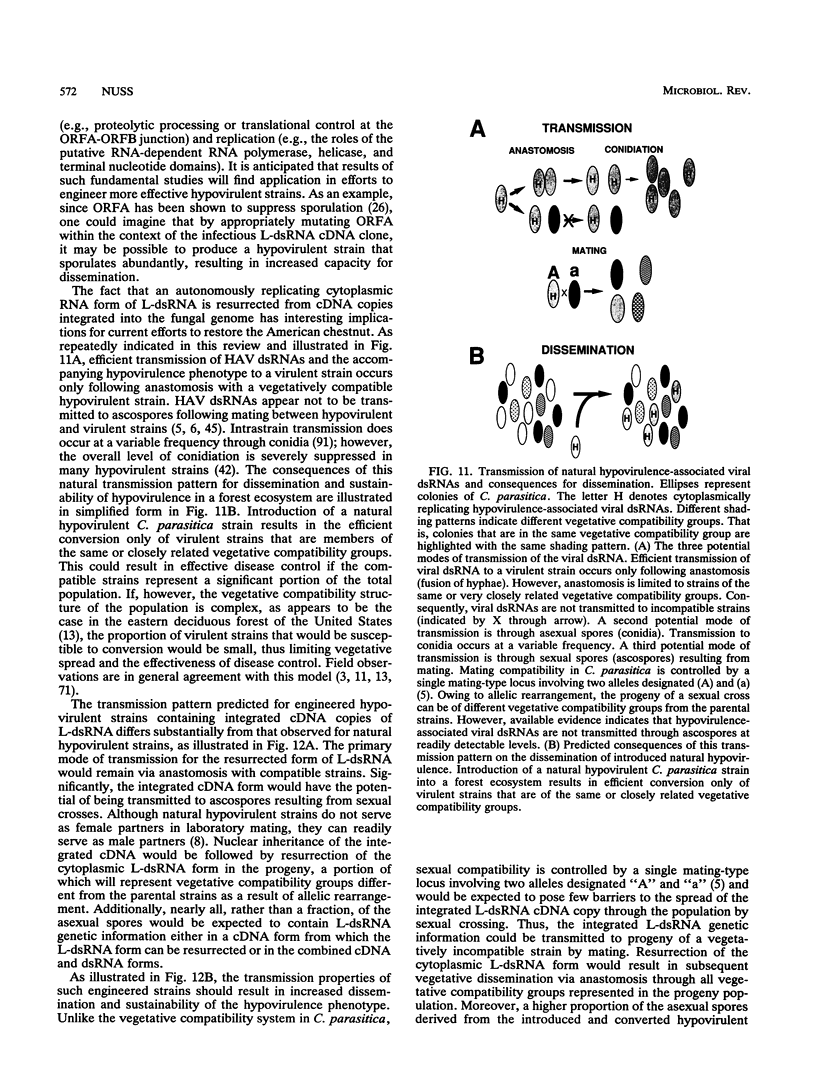



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., French R., Janda M., Loesch-Fries L. S. Multicomponent RNA plant virus infection derived from cloned viral cDNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7066–7070. doi: 10.1073/pnas.81.22.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostakis S. L. Biological control of chestnut blight. Science. 1982 Jan 29;215(4532):466–471. doi: 10.1126/science.215.4532.466. [DOI] [PubMed] [Google Scholar]
- Anagnostakis S. L. Genetic Analyses of ENDOTHIA PARASITICA: Linkage Data for Four Single Genes and Three Vegetative Compatibility Types. Genetics. 1982 Sep;102(1):25–28. doi: 10.1093/genetics/102.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- Bredenbeek P. J., Pachuk C. J., Noten A. F., Charité J., Luytjes W., Weiss S. R., Spaan W. J. The primary structure and expression of the second open reading frame of the polymerase gene of the coronavirus MHV-A59; a highly conserved polymerase is expressed by an efficient ribosomal frameshifting mechanism. Nucleic Acids Res. 1990 Apr 11;18(7):1825–1832. doi: 10.1093/nar/18.7.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J. A. Relationships among the positive strand and double-strand RNA viruses as viewed through their RNA-dependent RNA polymerases. Nucleic Acids Res. 1991 Jan 25;19(2):217–226. doi: 10.1093/nar/19.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. E., Mueller R. J., Kazmierczak P., Zhang L., Villalon D. K., Van Alfen N. K. Effect of a virus on accumulation of a tissue-specific cell-surface protein of the fungus Cryphonectria (Endothia) parasitica. Mol Plant Microbe Interact. 1992 Jan-Feb;5(1):55–61. doi: 10.1094/mpmi-5-055. [DOI] [PubMed] [Google Scholar]
- Carrington J. C., Cary S. M., Parks T. D., Dougherty W. G. A second proteinase encoded by a plant potyvirus genome. EMBO J. 1989 Feb;8(2):365–370. doi: 10.1002/j.1460-2075.1989.tb03386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Herndon K. L. Characterization of the potyviral HC-pro autoproteolytic cleavage site. Virology. 1992 Mar;187(1):308–315. doi: 10.1016/0042-6822(92)90319-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G. H., Larson T. G., Nuss D. L. Molecular analysis of the laccase gene from the chestnut blight fungus and selective suppression of its expression in an isogenic hypovirulent strain. Mol Plant Microbe Interact. 1992 Mar-Apr;5(2):119–128. doi: 10.1094/mpmi-5-119. [DOI] [PubMed] [Google Scholar]
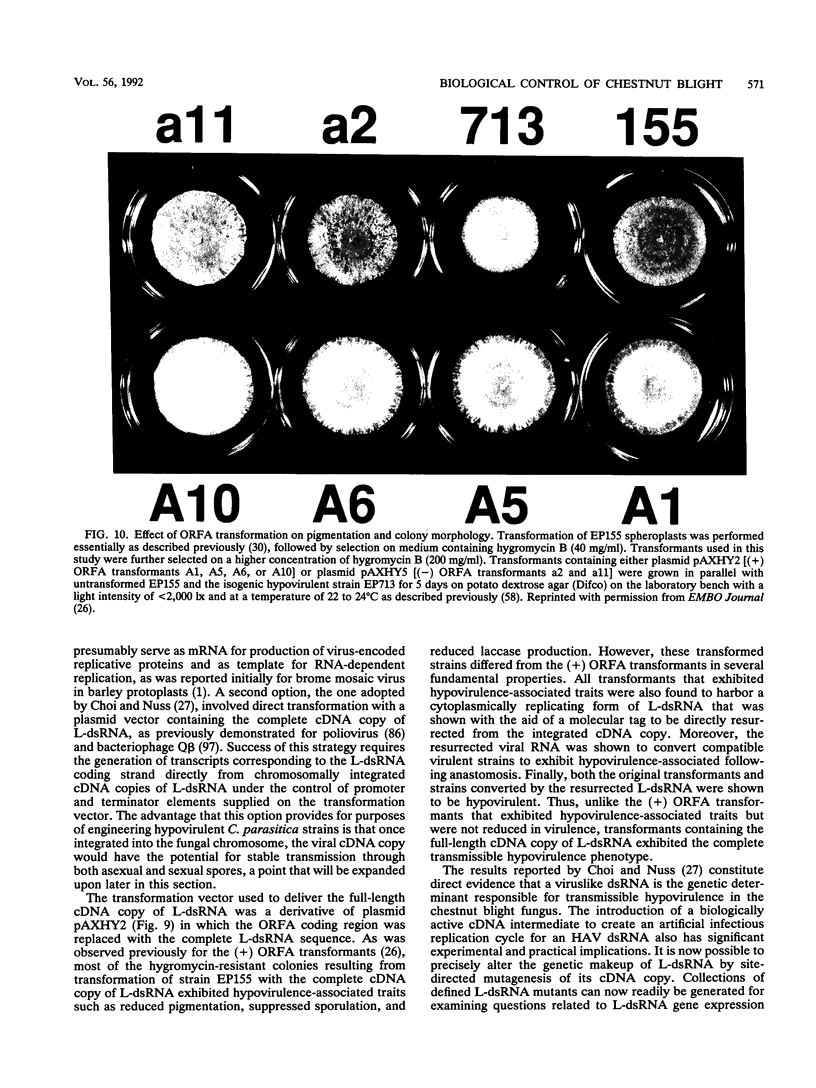
- Choi G. H., Nuss D. L. A viral gene confers hypovirulence-associated traits to the chestnut blight fungus. EMBO J. 1992 Feb;11(2):473–477. doi: 10.1002/j.1460-2075.1992.tb05077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G. H., Nuss D. L. Nucleotide sequence of the glyceraldehyde-3-phosphate dehydrogenase gene from Cryphonectria parasitica. Nucleic Acids Res. 1990 Sep 25;18(18):5566–5566. doi: 10.1093/nar/18.18.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G. H., Pawlyk D. M., Nuss D. L. The autocatalytic protease p29 encoded by a hypovirulence-associated virus of the chestnut blight fungus resembles the potyvirus-encoded protease HC-Pro. Virology. 1991 Aug;183(2):747–752. doi: 10.1016/0042-6822(91)91004-z. [DOI] [PubMed] [Google Scholar]
- Choi G. H., Shapira R., Nuss D. L. Cotranslational autoproteolysis involved in gene expression from a double-stranded RNA genetic element associated with hypovirulence of the chestnut blight fungus. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1167–1171. doi: 10.1073/pnas.88.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck A. J. Absence of laccase from yellow-spored mutants of Aspergillus nidulans. J Gen Microbiol. 1972 May;70(3):423–435. doi: 10.1099/00221287-70-3-423. [DOI] [PubMed] [Google Scholar]
- Cullen D., Leong S. A., Wilson L. J., Henner D. J. Transformation of Aspergillus nidulans with the hygromycin-resistance gene, hph. Gene. 1987;57(1):21–26. doi: 10.1016/0378-1119(87)90172-7. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Harrison M. J. Activation, structure, and organization of genes involved in microbial defense in plants. Adv Genet. 1990;28:165–234. doi: 10.1016/s0065-2660(08)60527-1. [DOI] [PubMed] [Google Scholar]
- Froehner S. C., Eriksson K. E. Induction of Neurospora crassa laccase with protein synthesis inhibitors. J Bacteriol. 1974 Oct;120(1):450–457. doi: 10.1128/jb.120.1.450-457.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. Coronavirus genome: prediction of putative functional domains in the non-structural polyprotein by comparative amino acid sequence analysis. Nucleic Acids Res. 1989 Jun 26;17(12):4847–4861. doi: 10.1093/nar/17.12.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman B. I., Tian Y., Bedker P. J., Brown M. P. A North American hypovirulent isolate of the chestnut blight fungus with European isolate-related dsRNA. J Gen Virol. 1992 Mar;73(Pt 3):681–686. doi: 10.1099/0022-1317-73-3-681. [DOI] [PubMed] [Google Scholar]
- Hiremath S., L'Hostis B., Ghabrial S. A., Rhoads R. E. Terminal structure of hypovirulence-associated dsRNAs in the chestnut blight fungus Endothia parasitica. Nucleic Acids Res. 1986 Dec 22;14(24):9877–9896. doi: 10.1093/nar/14.24.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath C. M., Williams M. A., Lamb R. A. Eukaryotic coupled translation of tandem cistrons: identification of the influenza B virus BM2 polypeptide. EMBO J. 1990 Aug;9(8):2639–2647. doi: 10.1002/j.1460-2075.1990.tb07446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., Choi G. H., Nuss D. L., Shapira R., Carrington J. C. Evidence for common ancestry of a chestnut blight hypovirulence-associated double-stranded RNA and a group of positive-strand RNA plant viruses. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10647–10651. doi: 10.1073/pnas.88.23.10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Selection of initiation sites by eucaryotic ribosomes: effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nucleic Acids Res. 1984 May 11;12(9):3873–3893. doi: 10.1093/nar/12.9.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Lamb C. J., Lawton M. A., Dron M., Dixon R. A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989 Jan 27;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Leonard T. J. Phenoloxidase activity and fruiting body formation Schizophyllum commune. J Bacteriol. 1971 Apr;106(1):162–167. doi: 10.1128/jb.106.1.162-167.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. P., Hinnebusch A. G. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986 Apr 25;45(2):201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
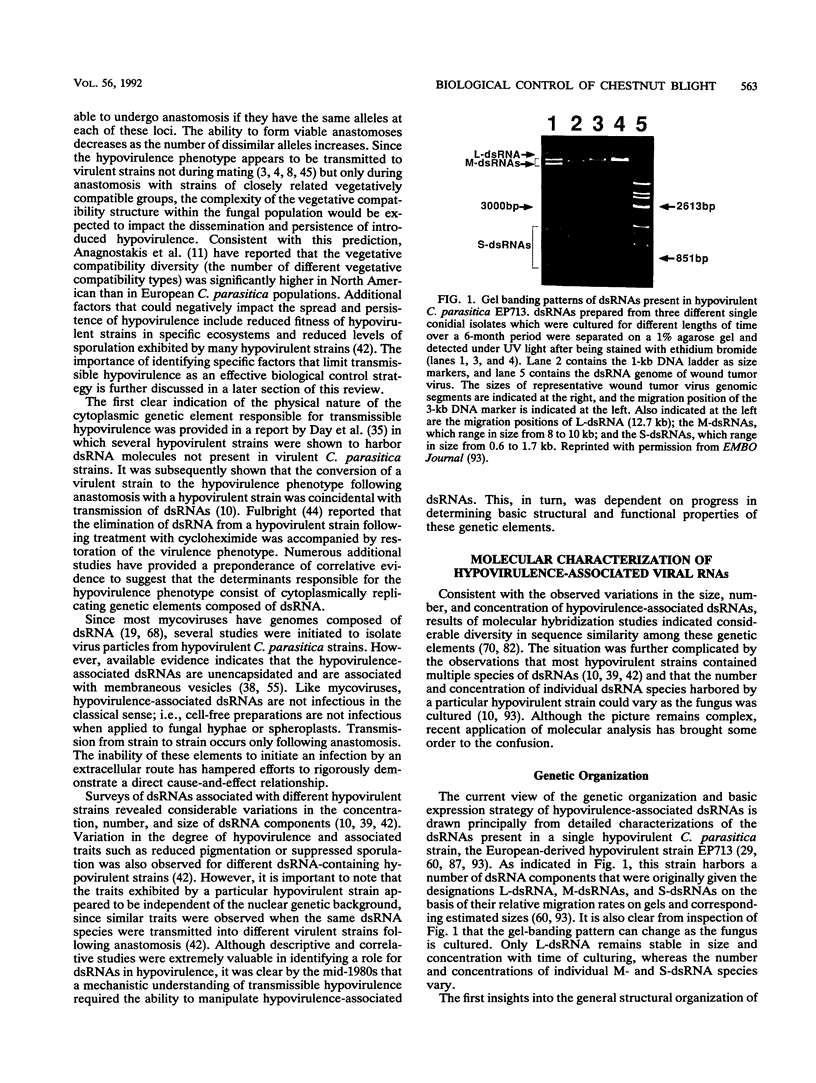
- Oh C. S., Carrington J. C. Identification of essential residues in potyvirus proteinase HC-Pro by site-directed mutagenesis. Virology. 1989 Dec;173(2):692–699. doi: 10.1016/0042-6822(89)90582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. A., Jr, Van Alfen N. K. Two nonhomologus viruses of Cryphonectria (Endothia) parasitica reduce accumulation of specific virulence-associated polypeptides. J Bacteriol. 1987 Nov;169(11):5324–5326. doi: 10.1128/jb.169.11.5324-5326.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. A., Van Alfen N. K. Differential accumulation of poly(A)+ RNA between virulent and double-stranded RNA-induced hypovirulent strains of Cryphonectria (Endothia) parasitica. Mol Cell Biol. 1987 Oct;7(10):3688–3693. doi: 10.1128/mcb.7.10.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science. 1981 Nov 20;214(4523):916–919. doi: 10.1126/science.6272391. [DOI] [PubMed] [Google Scholar]
- Rae B. P., Hillman B. I., Tartaglia J., Nuss D. L. Characterization of double-stranded RNA genetic elements associated with biological control of chestnut blight: organization of terminal domains and identification of gene products. EMBO J. 1989 Mar;8(3):657–663. doi: 10.1002/j.1460-2075.1989.tb03423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie J. The body against itself. Sci Am. 1990 Dec;263(6):106–115. doi: 10.1038/scientificamerican1290-106. [DOI] [PubMed] [Google Scholar]
- Rigling D., Van Alfen N. K. Regulation of laccase biosynthesis in the plant-pathogenic fungus Cryphonectria parasitica by double-stranded RNA. J Bacteriol. 1991 Dec;173(24):8000–8003. doi: 10.1128/jb.173.24.8000-8003.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira R., Choi G. H., Hillman B. I., Nuss D. L. The contribution of defective RNAs to the complexity of viral-encoded double-stranded RNA populations present in hypovirulent strains of the chestnut blight fungus Cryphonectria parasitica. EMBO J. 1991 Apr;10(4):741–746. doi: 10.1002/j.1460-2075.1991.tb08005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
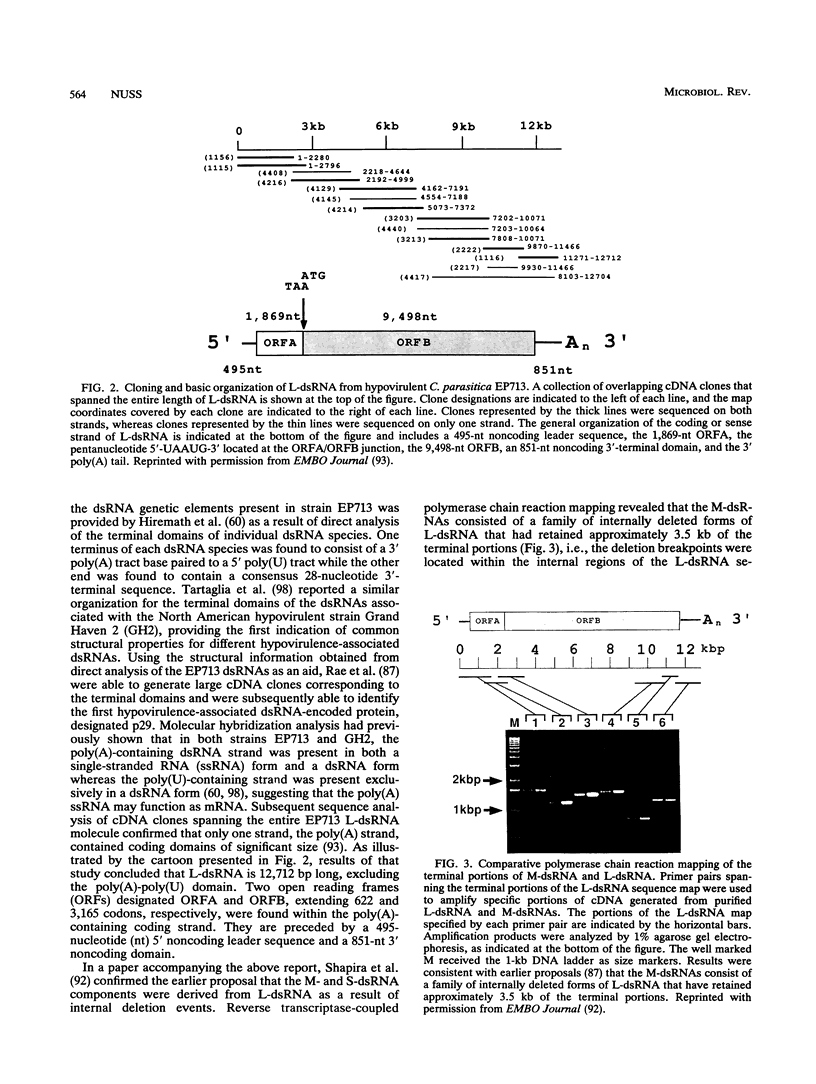
- Shapira R., Choi G. H., Nuss D. L. Virus-like genetic organization and expression strategy for a double-stranded RNA genetic element associated with biological control of chestnut blight. EMBO J. 1991 Apr;10(4):731–739. doi: 10.1002/j.1460-2075.1991.tb08004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira R., Nuss D. L. Gene expression by a hypovirulence-associated virus of the chestnut blight fungus involves two papain-like protease activities. Essential residues and cleavage site requirements for p48 autoproteolysis. J Biol Chem. 1991 Oct 15;266(29):19419–19425. [PubMed] [Google Scholar]
- Snijder E. J., den Boon J. A., Bredenbeek P. J., Horzinek M. C., Rijnbrand R., Spaan W. J. The carboxyl-terminal part of the putative Berne virus polymerase is expressed by ribosomal frameshifting and contains sequence motifs which indicate that toro- and coronaviruses are evolutionarily related. Nucleic Acids Res. 1990 Aug 11;18(15):4535–4542. doi: 10.1093/nar/18.15.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Palmieri M., Weissmann C. QB DNA-containing hybrid plasmids giving rise to QB phage formation in the bacterial host. Nature. 1978 Jul 20;274(5668):223–228. doi: 10.1038/274223a0. [DOI] [PubMed] [Google Scholar]
- Tartaglia J., Paul C. P., Fulbright D. W., Nuss D. L. Structural properties of double-stranded RNAs associated with biological control of chestnut blight fungus. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9109–9113. doi: 10.1073/pnas.83.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alfen N. K., Jaynes R. A., Anagnostakis S. L., Day P. R. Chestnut Blight: Biological Control by Transmissible Hypovirulence in Endothia parasitica. Science. 1975 Sep 12;189(4206):890–891. doi: 10.1126/science.189.4206.890. [DOI] [PubMed] [Google Scholar]
- Wellink J., van Kammen A. Proteases involved in the processing of viral polyproteins. Brief review. Arch Virol. 1988;98(1-2):1–26. doi: 10.1007/BF01321002. [DOI] [PubMed] [Google Scholar]