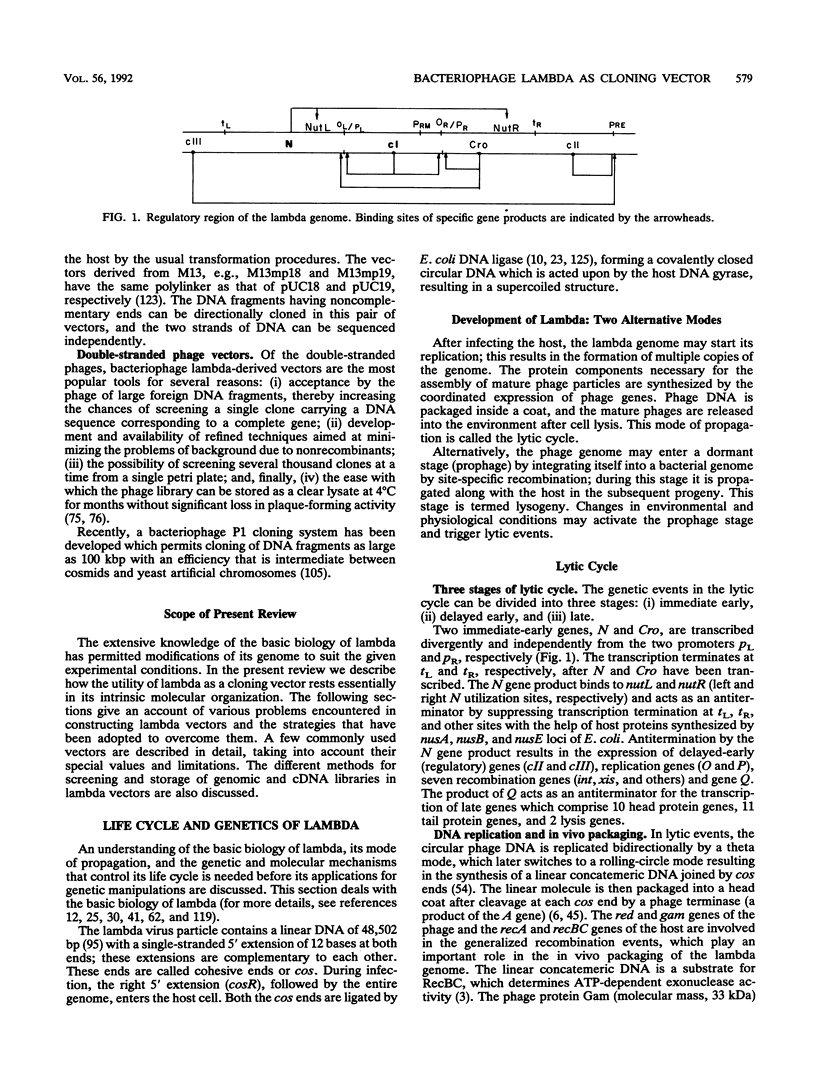
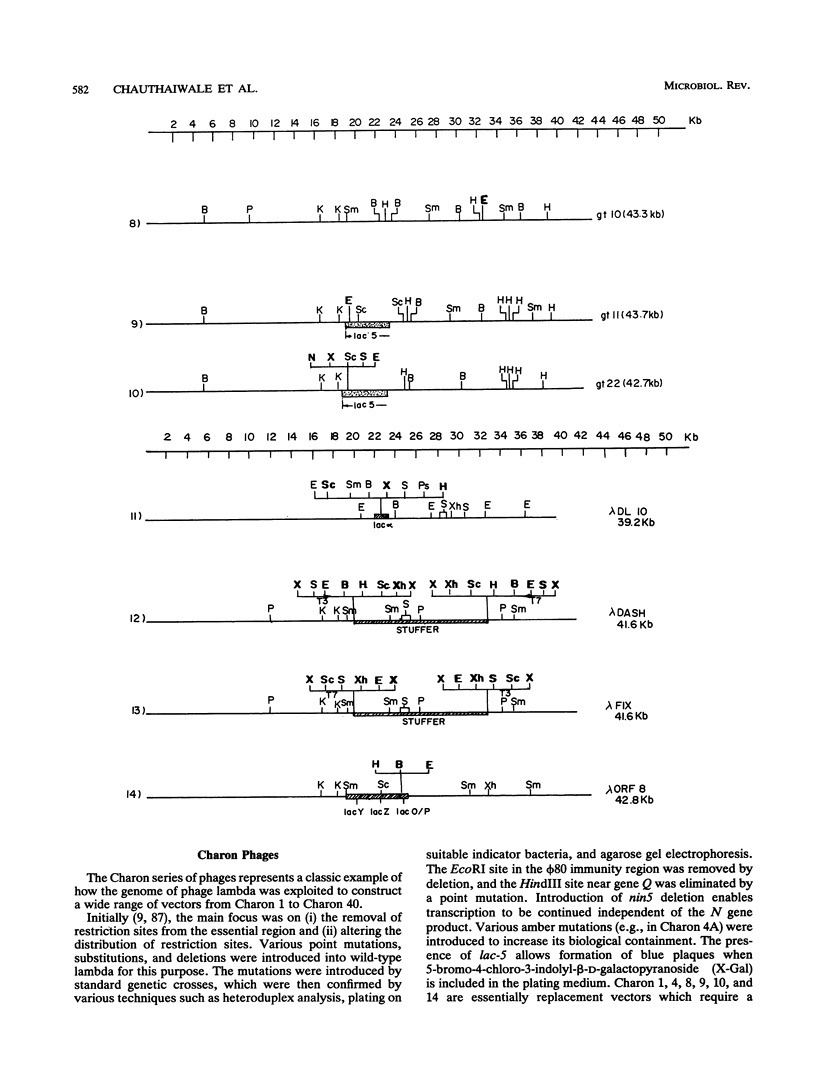
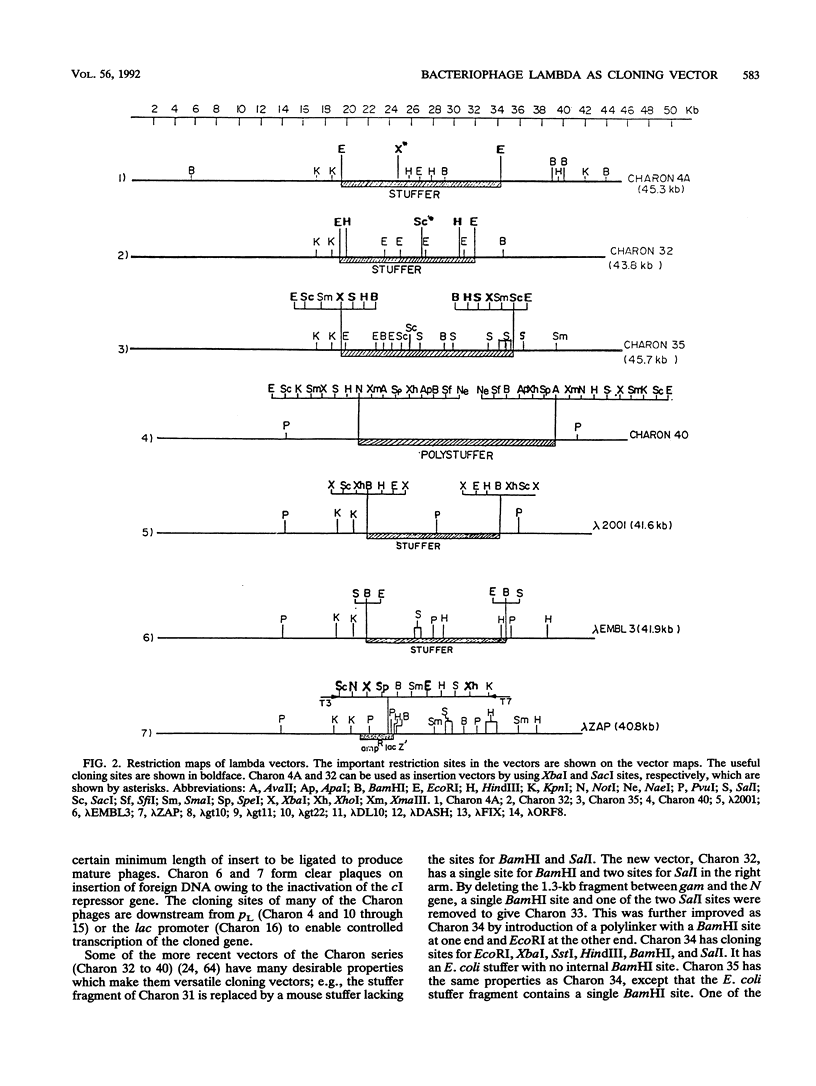
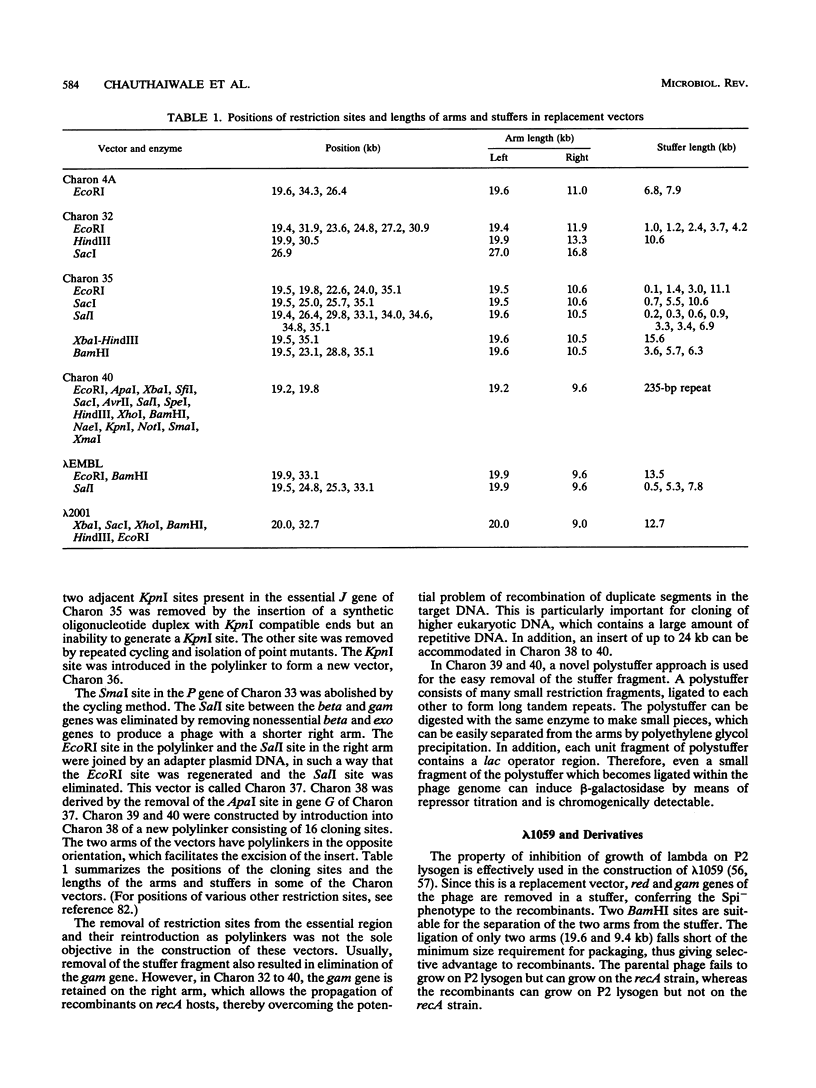
Abstract
Extensive research has been directed toward the development of multipurpose lambda vectors for cloning ever since the potential of using coliphage lambda as a cloning vector was recognized in the late 1970s. An understanding of the intrinsic molecular organization and of the genetic events which determine lysis or lysogeny in lambda has allowed investigators to modify it to suit the specific requirements of gene manipulations. Unwanted restriction sites have been altered and arranged together into suitable polylinkers. The development of a highly efficient in vitro packaging system has permitted the introduction of chimeric molecules into hosts. Biological containment of recombinants has been achieved by introducing amber mutations into the lambda genome and by using specific amber suppressor hosts. Taking advantage of the limited range of genome size (78 to 105% of the wild-type size) for its efficient packaging, an array of vectors has been devised to accommodate inserts of a wide size range, the limit being 24 kbp in Charon 40. The central dispensable fragment of the lambda genome can be replaced by a fragment of heterologous DNA, leading to the construction of replacement vectors such as Charon and EMBL. Alternatively, small DNA fragments can be inserted without removing the dispensable region of the lambda genome, as in lambda gt10 and lambda gt11 vectors. In addition, the introduction of many other desirable properties, such as NotI and SfiI sites in polylinkers (e.g., lambda gt22), T7 and T3 promoters for the in vitro transcription (e.g., lambda DASH), and the mechanism for in vivo excision of the intact insert (e.g., lambda ZAP), has facilitated both cloning and subsequent analysis. In most cases, the recombinants can be differentiated from the parental phages by their altered phenotype. Libraries constructed in lambda vectors are screened easily with antibody or nucleic acid probes since several thousand clones can be plated on a single petri dish. Besides the availability of a wide range of lambda vectors, many related techniques such as rapid isolation of lambda DNA, a high efficiency of commercially available in vitro packaging extracts, and in vitro amplification of DNA via the polymerase chain reaction have collectively contributed to lambda's becoming one of the most powerful and popular tools for molecular cloning.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguan K., Kusano T., Suzuki N., Kitagawa Y. An improved method for the construction of high efficiency cDNA library in plasmid or lambda vector. Nucleic Acids Res. 1990 Feb 25;18(4):1071–1071. doi: 10.1093/nar/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal O. B., Das R. H. A simple and rapid method for the isolation of plasmid and lambda phage DNAs. Nucleic Acids Res. 1989 Dec 11;17(23):10129–10129. doi: 10.1093/nar/17.23.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Sommer R., Auerswald E. A., Kurz C., Zink B., Osterburg G., Schaller H., Sugimoto K., Sugisaki H., Okamoto T. Nucleotide sequence of bacteriophage fd DNA. Nucleic Acids Res. 1978 Dec;5(12):4495–4503. doi: 10.1093/nar/5.12.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Zink B. Nucleotide sequence and genome organisation of filamentous bacteriophages fl and fd. Gene. 1981 Dec;16(1-3):35–58. doi: 10.1016/0378-1119(81)90059-7. [DOI] [PubMed] [Google Scholar]
- Becker A., Murialdo H. Bacteriophage lambda DNA: the beginning of the end. J Bacteriol. 1990 Jun;172(6):2819–2824. doi: 10.1128/jb.172.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A., Murialdo H., Lucko H., Morell J. Bacteriophage lambda DNA packaging. The product of the FI gene promotes the incorporation of the prohead to the DNA-terminase complex. J Mol Biol. 1988 Feb 20;199(4):597–607. doi: 10.1016/0022-2836(88)90304-x. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Bode V. C., Kaiser A. D. Changes in the structure and activity of lambda DNA in a superinfected immune bacterium. J Mol Biol. 1965 Dec;14(2):399–417. doi: 10.1016/s0022-2836(65)80190-5. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brammar W. J., Hadfield C. A programme for the construction of a lambda phage. J Embryol Exp Morphol. 1984 Nov;83 (Suppl):75–88. [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., Horn T., Kaiser A. D. Head assembly steps controlled by genes F and W in bacteriophage lambda. J Mol Biol. 1972 Mar 14;64(3):551–563. doi: 10.1016/0022-2836(72)90082-4. [DOI] [PubMed] [Google Scholar]
- Collins J., Hohn B. Cosmids: a type of plasmid gene-cloning vector that is packageable in vitro in bacteriophage lambda heads. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4242–4246. doi: 10.1073/pnas.75.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Parkinson J. S. Deletion mutants of bacteriophage lambda. 3. Physical structure of att-phi. J Mol Biol. 1971 Mar 14;56(2):403–423. doi: 10.1016/0022-2836(71)90473-6. [DOI] [PubMed] [Google Scholar]
- Deng Z. X., Kieser T., Hopwood D. A. Activity of a Streptomyces transcriptional terminator in Escherichia coli. Nucleic Acids Res. 1987 Mar 25;15(6):2665–2675. doi: 10.1093/nar/15.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman D. M., Zon L. I., Orkin S. H. Rapid amplification of lambda gt11 bacteriophage library inserts from plaques using the polymerase chain reaction (PCR). Biotechniques. 1989 Jun;7(6):568–570. [PubMed] [Google Scholar]
- Dotto G. P., Horiuchi K., Zinder N. D. The functional origin of bacteriophage f1 DNA replication. Its signals and domains. J Mol Biol. 1984 Feb 5;172(4):507–521. doi: 10.1016/s0022-2836(84)80020-0. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Zinder N. D. Increased intracellular concentration of an initiator protein markedly reduces the minimal sequence required for initiation of DNA synthesis. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1336–1340. doi: 10.1073/pnas.81.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., Zinder N. D. The morphogenetic signal of bacteriophage f1. Virology. 1983 Oct 15;130(1):252–256. doi: 10.1016/0042-6822(83)90136-8. [DOI] [PubMed] [Google Scholar]
- Dove W. F., Weigle J. J. Intracellular state of the chromosome of bacteriophage lambda. I. The eclipse of infectivity of the bacteriophage DNA. J Mol Biol. 1965 Jul;12(3):620–629. doi: 10.1016/s0022-2836(65)80316-3. [DOI] [PubMed] [Google Scholar]
- Dunn I. S., Blattner F. R. Charons 36 to 40: multi enzyme, high capacity, recombination deficient replacement vectors with polylinkers and polystuffers. Nucleic Acids Res. 1987 Mar 25;15(6):2677–2698. doi: 10.1093/nar/15.6.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Phasmid vectors for identification of genes by complementation of Escherichia coli mutants. J Bacteriol. 1985 May;162(2):777–783. doi: 10.1128/jb.162.2.777-783.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Enquist L., Tiemeier D., Leder P., Weisberg R., Sternberg N. Safer derivatives of bacteriophage lambdagt-lambdaC for use in cloning of recombinant DNA molecules. Nature. 1976 Feb 19;259(5544):596–598. doi: 10.1038/259596a0. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M. Construction and characterization of a genomic library in lambda. Methods Enzymol. 1987;152:190–199. doi: 10.1016/0076-6879(87)52020-1. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Murray N., Lehrach H. Lambda phage vectors--EMBL series. Methods Enzymol. 1987;153:103–115. doi: 10.1016/0076-6879(87)53051-8. [DOI] [PubMed] [Google Scholar]
- González A., Gómez-Márquez J. Purification of bacteriophage DNA by gel filtration chromatography. Genet Anal Tech Appl. 1990 Feb;7(1):2–4. doi: 10.1016/0735-0651(90)90037-g. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Grimaldi G., Manfioletti G., Schneider C. A lambda vector for directional cDNA cloning and in vitro transcription. Nucleic Acids Res. 1987 Nov 25;15(22):9608–9608. doi: 10.1093/nar/15.22.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. H., Rutter W. J. Lambda gt22, an improved lambda vector for the directional cloning of full-length cDNA. Nucleic Acids Res. 1987 Aug 11;15(15):6304–6304. doi: 10.1093/nar/15.15.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. H., Stratowa C., Rutter W. J. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987 Mar 24;26(6):1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- Hirano M., Shigesada K., Imai M. Construction and characterization of plasmid and lambda phage vector systems for study of transcriptional control in Escherichia coli. Gene. 1987;57(1):89–99. doi: 10.1016/0378-1119(87)90180-6. [DOI] [PubMed] [Google Scholar]
- Hohn B. DNA as substrate for packaging into bacteriophage lambda, in vitro. J Mol Biol. 1975 Oct 15;98(1):93–106. doi: 10.1016/s0022-2836(75)80103-3. [DOI] [PubMed] [Google Scholar]
- Hohn B., Hohn T. Activity of empty, headlike particles for packaging of DNA of bacteriophage lambda in vitro. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2372–2376. doi: 10.1073/pnas.71.6.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt M. A., Knight D. M., Das A., Miller H. I., Echols H. Control of phage lambda development by stability and synthesis of cII protein: role of the viral cIII and host hflA, himA and himD genes. Cell. 1982 Dec;31(3 Pt 2):565–573. doi: 10.1016/0092-8674(82)90312-9. [DOI] [PubMed] [Google Scholar]
- Jaurin B. A promoter probe vector (pJAC4) that utilizes the ampC beta-lactamase gene of Escherichia coli. Nucleic Acids Res. 1987 Oct 26;15(20):8567–8567. doi: 10.1093/nar/15.20.8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrisak J., Young R. A., Engel J. D. Cloning cDNA into lambda gt10 and lambda gt11. Methods Enzymol. 1987;152:359–371. doi: 10.1016/0076-6879(87)52043-2. [DOI] [PubMed] [Google Scholar]
- Jørgensen P., Mikkelsen T. lambda PJ4A, a lambda replacement vector carrying amber mutations for cloning of EcoRI fragments. Nucleic Acids Res. 1986 Dec 9;14(23):9538–9538. doi: 10.1093/nar/14.23.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D., Masuda T. In vitro assembly of bacteriophage Lambda heads. Proc Natl Acad Sci U S A. 1973 Jan;70(1):260–264. doi: 10.1073/pnas.70.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. Cell contact induces an increase in pinocytotic rate in cultured epithelial cells. Nature. 1976 Oct 14;263(5578):596–597. doi: 10.1038/263596a0. [DOI] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Matthes H. W., Gait M. J., Brenner S. A new selective phage cloning vector, lambda 2001, with sites for XbaI, BamHI, HindIII, EcoRI, SstI and XhoI. Gene. 1984 Dec;32(1-2):217–224. doi: 10.1016/0378-1119(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Cohen D. I. Preserving primary cDNA libraries. Anal Biochem. 1987 Feb 15;161(1):85–88. doi: 10.1016/0003-2697(87)90655-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Stahl M. M., Stahl F. W. The mechanism of the chi-cos interaction in RecA-RecBC-mediated recombination in phage lambda. Cold Spring Harb Symp Quant Biol. 1984;49:497–506. doi: 10.1101/sqb.1984.049.01.056. [DOI] [PubMed] [Google Scholar]
- Lathe R., Vilotte J. L., Clark A. J. Plasmid and bacteriophage vectors for excision of intact inserts. Gene. 1987;57(2-3):193–201. doi: 10.1016/0378-1119(87)90122-3. [DOI] [PubMed] [Google Scholar]
- Lindahl G., Sironi G., Bialy H., Calendar R. Bacteriophage lambda; abortive infection of bacteria lysogenic for phage P2. Proc Natl Acad Sci U S A. 1970 Jul;66(3):587–594. doi: 10.1073/pnas.66.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W. A., Blattner F. R. Lambda Charon vectors (Ch32, 33, 34 and 35) adapted for DNA cloning in recombination-deficient hosts. Gene. 1983 Dec;26(2-3):171–179. doi: 10.1016/0378-1119(83)90187-7. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin D. A., Wachtel E. J. Structure and assembly of filamentous bacterial viruses. Nature. 1975 Jan 3;253(5486):19–23. doi: 10.1038/253019a0. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Skorupa E. S., Kemper B. Single stranded DNA SP6 promoter plasmids for engineering mutant RNAs and proteins: synthesis of a 'stretched' preproparathyroid hormone. Nucleic Acids Res. 1985 Feb 25;13(4):1103–1118. doi: 10.1093/nar/13.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner P. S., Sisk W. P., Berman M. L. Bacteriophage lambda cloning system for the construction of directional cDNA libraries. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4171–4175. doi: 10.1073/pnas.84.12.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mel'nikov A. A., Chernov A. P., Fodor I. I. Plazmidofagi lambda i ikh svoistva. Mol Biol (Mosk) 1985 May-Jun;19(3):610–616. [PubMed] [Google Scholar]
- Meyer T. F., Geider K., Kurz C., Schaller H. Cleavage site of bacteriophage fd gene II-protein in the origin of viral strand replication. Nature. 1979 Mar 22;278(5702):365–367. doi: 10.1038/278365a0. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Becker A. Head morphogenesis of complex double-stranded deoxyribonucleic acid bacteriophages. Microbiol Rev. 1978 Sep;42(3):529–576. doi: 10.1128/mr.42.3.529-576.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Murray K. Manipulation of restriction targets in phage lambda to form receptor chromosomes for DNA fragments. Nature. 1974 Oct 11;251(5475):476–481. doi: 10.1038/251476a0. [DOI] [PubMed] [Google Scholar]
- Natt E., Scherer G. EMBL 12, a new lambda replacement vector with sites for SalI, XbaI, BamHI, SstI and EcoRI. Nucleic Acids Res. 1986 Sep 11;14(17):7128–7128. [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. Bacteriophage lambda vector for transducing a cDNA clone library into mammalian cells. Mol Cell Biol. 1985 May;5(5):1136–1142. doi: 10.1128/mcb.5.5.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo M. J., Meyerowitz E. M. A family of lambda phage cDNA cloning vectors, lambda SWAJ, allowing the amplification of RNA sequences. Gene. 1987;52(2-3):197–206. doi: 10.1016/0378-1119(87)90046-1. [DOI] [PubMed] [Google Scholar]
- Raleigh E. A., Murray N. E., Revel H., Blumenthal R. M., Westaway D., Reith A. D., Rigby P. W., Elhai J., Hanahan D. McrA and McrB restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 1988 Feb 25;16(4):1563–1575. doi: 10.1093/nar/16.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh E. A., Wilson G. Escherichia coli K-12 restricts DNA containing 5-methylcytosine. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9070–9074. doi: 10.1073/pnas.83.23.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambach A., Tiollais P. Bacteriophage lambda having EcoRI endonuclease sites only in the nonessential region of the genome. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3927–3930. doi: 10.1073/pnas.71.10.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos B. V. A method for the screening of fusion protein expression by lambda-GT11 recombinant clones without the preparation of lysogens. Nucleic Acids Res. 1989 Aug 11;17(15):6421–6421. doi: 10.1093/nar/17.15.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm D. L., Horness D., Kucera J., Blattner F. R. Construction of coliphage lambda Charon vectors with BamHI cloning sites. Gene. 1980 Dec;12(3-4):301–309. doi: 10.1016/0378-1119(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Roditi I., König E., Williams R. O. A rapid method for determining the orientation of inserts in bacteriophage lambda vectors. Nucleic Acids Res. 1989 Dec 25;17(24):10506–10506. doi: 10.1093/nar/17.24.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. M. Chi-stimulated patches are heteroduplex, with recombinant information on the phage lambda r chain. Cell. 1987 Mar 13;48(5):855–865. doi: 10.1016/0092-8674(87)90082-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. M. Improved in vitro packaging of lambda DNA. Methods Enzymol. 1987;153:95–103. doi: 10.1016/0076-6879(87)53050-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. M., Stahl M. M., Kobayashi I., Stahl F. W. Improved in vitro packaging of coliphage lambda DNA: a one-strain system free from endogenous phage. Gene. 1985;38(1-3):165–175. doi: 10.1016/0378-1119(85)90215-x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sakaki Y., Karu A. E., Linn S., Echols H. Purification and properties of the gamma-protein specified by bacteriophage lambda: an inhibitor of the host RecBC recombination enzyme. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2215–2219. doi: 10.1073/pnas.70.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Seed B. Purification of genomic sequences from bacteriophage libraries by recombination and selection in vivo. Nucleic Acids Res. 1983 Apr 25;11(8):2427–2445. doi: 10.1093/nar/11.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Amundsen S. K., Chaudhury A. M., Cheng K. C., Ponticelli A. S., Roberts C. M., Schultz D. W., Taylor A. F. Roles of RecBC enzyme and chi sites in homologous recombination. Cold Spring Harb Symp Quant Biol. 1984;49:485–495. doi: 10.1101/sqb.1984.049.01.055. [DOI] [PubMed] [Google Scholar]
- Snyder M., Elledge S., Sweetser D., Young R. A., Davis R. W. Lambda gt 11: gene isolation with antibody probes and other applications. Methods Enzymol. 1987;154:107–128. doi: 10.1016/0076-6879(87)54073-3. [DOI] [PubMed] [Google Scholar]
- Sprague K. U., Faulds D. H., Smith G. R. A single base-pair change creates a Chi recombinational hotspot in bacteriophage lambda. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6182–6186. doi: 10.1073/pnas.75.12.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W., Crasemann J. M., Stahl M. M. Rec-mediated recombinational hot spot activity in bacteriophage lambda. III. Chi mutations are site-mutations stimulating rec-mediated recombination. J Mol Biol. 1975 May 15;94(2):203–212. doi: 10.1016/0022-2836(75)90078-9. [DOI] [PubMed] [Google Scholar]
- Stahl F. W. Special sites in generalized recombination. Annu Rev Genet. 1979;13:7–24. doi: 10.1146/annurev.ge.13.120179.000255. [DOI] [PubMed] [Google Scholar]
- Stahl M. M., Kobayashi I., Stahl F. W., Huntington S. K. Activation of Chi, a recombinator, by the action of an endonuclease at a distant site. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2310–2313. doi: 10.1073/pnas.80.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N. Bacteriophage P1 cloning system for the isolation, amplification, and recovery of DNA fragments as large as 100 kilobase pairs. Proc Natl Acad Sci U S A. 1990 Jan;87(1):103–107. doi: 10.1073/pnas.87.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Grant K. A., Dockrell H. M., Howard C. R., Jouy N. F., McAdam K. P. High level expression of genes cloned in phage lambda gt11. Gene. 1989 May 15;78(1):93–99. doi: 10.1016/0378-1119(89)90317-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H. Genetic and physical mapping of the late region of bacteriophage T7 DNA by use of cloned fragments of T7 DNA. J Mol Biol. 1981 Dec 15;153(3):503–525. doi: 10.1016/0022-2836(81)90405-8. [DOI] [PubMed] [Google Scholar]
- Swaroop A., Weissman S. M. Charon BS(+) and (-), versatile lambda phage vectors for constructing directional cDNA libraries and their efficient transfer to plasmids. Nucleic Acids Res. 1988 Sep 12;16(17):8739–8739. doi: 10.1093/nar/16.17.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Cameron J. R., Davis R. W. Viable molecular hybrids of bacteriophage lambda and eukaryotic DNA. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4579–4583. doi: 10.1073/pnas.71.11.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. C., Echols H., Clark A. J. Interaction of the recombination pathways of bacteriophage lambda and host Escherichia coli: effects on lambda recombination. J Mol Biol. 1972 Oct 14;70(3):531–537. doi: 10.1016/0022-2836(72)90557-8. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Lewis K. A., Ruiz J. C., Rothenberg B., Zhao J., Evans G. A. Cosmid vectors for rapid genomic walking, restriction mapping, and gene transfer. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2160–2164. doi: 10.1073/pnas.84.8.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle J. Assembly of phage lambda in vitro. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1462–1466. doi: 10.1073/pnas.55.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle J. Studies on head-tail union in bacteriophage lambda. J Mol Biol. 1968 Apr 28;33(2):483–489. doi: 10.1016/0022-2836(68)90204-0. [DOI] [PubMed] [Google Scholar]
- Windle B. E. Phage lambda and plasmid expression vectors with multiple cloning sites and lacZ alpha-complementation. Gene. 1986;45(1):95–99. doi: 10.1016/0378-1119(86)90136-8. [DOI] [PubMed] [Google Scholar]
- YOUNG E. T., 2nd, SINSHEIMER R. L. NOVEL INTRA-CELLULAR FORMS OF LAMBDA DNA. J Mol Biol. 1964 Dec;10:562–564. doi: 10.1016/s0022-2836(64)80080-2. [DOI] [PubMed] [Google Scholar]
- Yagil E., Shtromas I. Rec-mediated recombinational activity of two adjacent Chi elements in bacteriophage lambda. Genet Res. 1985 Feb;45(1):1–8. doi: 10.1017/s0016672300021911. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabarovsky E. R., Allikmets R. L. An improved technique for the efficient construction of gene libraries by partial filling-in of cohesive ends. Gene. 1986;42(1):119–123. doi: 10.1016/0378-1119(86)90158-7. [DOI] [PubMed] [Google Scholar]
- Zagursky R. J., Berman M. L. Cloning vectors that yield high levels of single-stranded DNA for rapid DNA sequencing. Gene. 1984 Feb;27(2):183–191. doi: 10.1016/0378-1119(84)90139-2. [DOI] [PubMed] [Google Scholar]
- van Doorn L. J., van Belkum A., Kos T. Improved cDNA cloning into bacteriophage lambda gt11. Nucleic Acids Res. 1989 Nov 25;17(22):9496–9496. doi: 10.1093/nar/17.22.9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wezenbeek P. M., Hulsebos T. J., Schoenmakers J. G. Nucleotide sequence of the filamentous bacteriophage M13 DNA genome: comparison with phage fd. Gene. 1980 Oct;11(1-2):129–148. doi: 10.1016/0378-1119(80)90093-1. [DOI] [PubMed] [Google Scholar]



