Abstract
Objective
To examine the predictive significance of 2 pneumoproteins, surfactant protein D (SP-D) and CC-chemokine ligand 18 (CCL18), for the course of systemic sclerosis (SSc)-related interstitial lung disease.
Methods
The pneumoproteins were determined in the baseline plasma samples of 266 patients with early SSc enrolled in the GENISOS observational cohort. They also were measured in 83 followup patient samples. Pulmonary function tests were obtained annually. The primary outcome was decline in forced vital capacity (FVC percentage predicted) over time. The predictive significance for longterm change in FVC was investigated by a joint analysis of longitudinal measurements (sequentially obtained FVC percentage predicted) and survival data.
Results
SP-D and CCL18 levels were both higher in patients with SSc than in matched controls (p < 0.001 and p = 0.015, respectively). Baseline SP-D levels correlated with lower concomitantly obtained FVC (r = −0.27, p < 0.001), but did not predict the short-term decline in FVC at 1 year followup visit or its longterm decline rate. CCL18 showed a significant correlation with steeper short-term decline in FVC (p = 0.049), but was not a predictor of its longterm decline rate. Similarly, a composite score of SP-D and CCL18 was a significant predictor of short-term decline in FVC but did not predict its longterm decline rate. Further, the longitudinal change in these 2 pneumoproteins did not correlate with the concomitant percentage change in FVC.
Conclusion
SP-D correlated with concomitantly obtained FVC, while CCL18 was a predictor of short-term decline in FVC. However, neither SP-D nor CCL18 was a longterm predictor of FVC course in patients with early SSc.
Key Indexing Terms: SYSTEMIC SCLEROSIS, SURFACTANT D
INTERSTITIAL LUNG DISEASE CC-CHEMOKINE LIGAND 18
Systemic sclerosis (SSc; scleroderma) is associated with considerable morbidity and mortality. The overall pooled standardized mortality ratio of patients with SSc is 3.51, which is considerably higher than mortality ratios observed in other rheumatic diseases such as rheumatoid arthritis and Sjögren syndrome2. Pulmonary involvement is the leading cause of SSc-related death, with interstitial lung disease (ILD) being the most common cause3,4. However, the course of SSc-ILD is highly variable, ranging from a mild and stable involvement to a severe and rapidly progressive course5,6. Therefore, clinical and serological markers capable of predicting the course of SSc-ILD are crucial for more focused and effective monitoring and treatment of patients with SSc.
Pulmonary function tests (PFT) have long been used in patients with SSc to monitor lung function, but only forced vital capacity (FVC) has been validated as an outcome measure for the severity of SSc-ILD in randomized control trials7.
To date, there are few known predictors of pulmonary disease in patients with SSc. Presence of antitopoisomerase I antibody (ATA) was predictive of the rate of decline in FVC over time5. Additionally, fibrosis scores on high-resolution computed tomography (HRCT) are predictive of the decline in FVC8. However, both ATA and fibrosis scores are short-term predictors of decline in FVC. There are currently no clinically used longterm predictors of ILD course in patients with SSc. Finding additional biomarkers that can predict future lung disease is fundamental in identifying high-risk patients before irreversible fibrotic damage occurs. Pneumoprotein levels in blood are attractive candidates for ILD biomarkers because they are easily obtainable and lung-specific. Surfactant protein D (SP-D) and chemokine (C-C motif) ligand 18 (CCL18) are important pneumoproteins that are currently being investigated for use as biomarkers for SSc-related ILD.
SP-D is formed by type II alveolar cells in lung tissue and contributes to maintenance of pulmonary mechanics. Newer studies have found that surfactant also functions in pulmonary host defense and is part of the innate immunity. SP-D is able to coat bacteria and viruses and promotes phagocytosis by macrophages. Additionally, SP-D inhibits T cell proliferation9. Previous work with this pneumoprotein by several groups10,11,12,13 revealed high levels of SP-D in patients with SSc, and SP-D was higher in patients with ILD than in those without. Predictive qualities remain to be defined.
CCL18 [also known as pulmonary activation-regulated chemokine (PARC)] is mainly produced by alveolar macrophages (M2 phenotype). It is chemotactic for activated T cells and nonactivated lymphocytes. CCL18 also plays a role in the primary immune response by assisting in the trafficking of naive lymphocytes and immature dendritic cells14. In a study of 123 patients with SSc, CCL18 was associated with decreased vital capacity15. In another study, serum CCL18 correlated with its concentration in the bronchoalveolar lavage (BAL) fluid, as well as BAL eosinophil and neutrophil cell counts in patients with pulmonary fibrosis (including 12 patients with SSc). The change in the serum CCL18 levels over time correlated also with change in total lung capacity in this study16. In a sub-sequent study involving 83 patients with SSc, baseline CCL18 levels were predictive of time to occurrence of combined deleterious events of 10% decrease in lung capacity or FVC, or death17.
Our goal was to determine the predictive significance of either pneumoprotein, SP-D or CCL18, for the course of ILD (decline in percentage predicted FVC over time) in patients with SSc. Specifically, we examined whether SP-D or CCL18 correlated with percentage predicted FVC at the cross-sectional level. Subsequently, we investigated prospectively whether these pneumoproteins are predictive of either short-term or longterm change in percentage predicted FVC. We also examined whether percentage changes in these pneumoproteins over time correlate with change in lung function.
MATERIALS AND METHODS
The Genetics versus Environment in Scleroderma Outcome Study (GENISOS) is a prospective, observational cohort of patients with early SSc. The main goal of GENISOS is to identify clinical and molecular markers for various disease outcomes in patients with SSc. This prospective cohort study is a joint effort among 3 academic institutions in Texas, USA: the University of Texas Health Science Center at Houston, the University of Texas Medical Branch at Galveston, and the University of Texas Health Science Center at San Antonio. The study started in 1998, and recruitment and followup study visits are continuing. All patients enrolled in the GENISOS cohort at the time of analysis were included in this study.
Patient selection
Patients who fulfilled the following inclusion criteria were enrolled: diagnosis of SSc according to the American College of Rheumatology preliminary classification criteria or at least 3 of the 5 features of CREST (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia, with sclerodactyly being mandatory). Further, all patients were within 5 years of disease onset calculated from the first non-Raynaud phenomenon (RP) symptom. The study was approved by the institutional review board of all participating sites, and written informed consent was obtained from all study subjects. Further details about this cohort including the course of ILD have been published5.
Data plasma collection
SP-D and CCL18 (PARC) levels were determined in the baseline samples of 266 patients with SSc and 97 unaffected controls matched for age, ethnicity, and sex. In addition to the 266 samples collected at the study entry (baseline), we also examined 83 followup samples collected on an average followup time of 3.76 years after enrollment. Plasma was collected in EDTA blood collection tubes, centrifuged, aliquoted, and stored at −80°C. The plasma samples had undergone only 2 freeze/thaw cycles before protein measurements. All samples were measured in duplicates. The pneumoproteins were measured using human SP-D and CCL18 ELISA kits according to the manufacturer’s instructions (Cell Sciences). Coefficient of variation < 15% between the duplicate samples was considered acceptable. The upper limit of normal value based on the 95th percentile in controls was 2812 ng/ml for SP-D and 214 ng/ml for CCL18.
A normalized composite score of SP-D and CCL18 was also calculated. For each pneumoprotein, concentrations above the 95th percentile value in the dataset were assigned a value of 1.0. The remaining concentration values were then scaled to the 95th percentile value, and these normalized values of SP-D and CCL18 were summed to derive the final composite score for each subject (possible range 0–2).
Pulmonary function test
PFT tests were performed at the initial visit, and annually thereafter. FVC, expressed as the percentage predicted FVC, was used as the outcome measure for the severity of SSc-related ILD. Predicted FVC values were calculated according to the patient’s age, height, weight, sex, and ethnicity using consistent reference values. Similarly, predicted DLCO adjusted for the current hemoglobin levels were calculated. All PFT data were reviewed by a pulmonologist (RMEYM) and data that did not fulfill the American Thoracic Society/European Respiratory Society criteria for pulmonary function testing were excluded.
Statistical analysis
The pneumoprotein levels were log-transformed for all analyses because of the non-normal distribution of data in both control and SSc populations. First, the protein levels between patients and controls were compared by t test. In all subsequent analyses, FVC, expressed as percentage predicted values, was used. The correlation of protein levels at the baseline visit with concomitantly obtained FVC was investigated by linear regression.
Next, we investigated the predictive significance of baseline pneumoproteins for short-term change in FVC by linear regression. The short-term change in FVC was calculated based on percentage change in FVC between the baseline and 1-year followup visit [(FVC baseline – FVC 1 year)/FVC baseline].
A joint analysis of longitudinal measurements (sequentially obtained FVC) and survival data was also conducted to investigate the predictive significance of the 2 pneumoproteins for the change over time in the FVC value. This analysis allows inclusion of all FVC measurements and reflects the slope of decline in FVC better than other approaches such as annualized rate of decline and time to respiratory failure. Further, it accounts for the association between FVC and survival, and it reduces the bias resulting from the fact that patients with more rapid decline in FVC are more likely to die5. The longitudinal component consisted of a linear model with random effects. We accounted for baseline differences in FVC (random intercept). Predictors in the linear model included SP-D and CCL18 (separate models for each pneumoprotein) and followup time. The predictive value of SP-D and CCL18 on the decline rate in FVC was investigated by the interaction term between the protein levels and followup time in the longitudinal component18,19. Secondary models also included age, ethnicity, sex, and body mass index (BMI) as predictors in the linear model. The survival component fit a parametric Weibull model with SP-D and CCL18 as predictors.
Finally, the correlation between the longitudinal percentage change in pneumoproteins with the concomitant percentage change in FVC was investigated by linear regression. These analyses were performed only in the subgroup of patients with an available followup pneumoprotein measurement (n = 83), while the cross-sectional analyses and the analysis for predictive significance of pneumoproteins were performed in the entire cohort.
RESULTS
Between January 1998 and November 2009, 266 patients were enrolled in the GENISOS cohort. The average disease duration at time of enrollment was 2.5 years, based on the first non-RP symptom, while disease duration was 4.5 years based on the first symptom attributable to SSc; 156 patients (59%) had diffuse cutaneous involvement. The mean time-in-study was 4.4 years; 86 patients (32.3%) had died at the time of analysis. A total of 1016 FVC measurements from 248 patients fulfilled the American Thoracic Society/ European Respiratory Society criteria and were included in the analysis. Further, 191 patients had at least 2 FVC measurements that fulfilled these criteria. Only 8.3% of patients were treated with cyclophosphamide during the followup period. The demographic and baseline clinical characteristics of patients and matched unaffected controls are shown in Table 1.
Table 1.
Subject characteristics at baseline visit. Data are n (%) unless otherwise indicated.
| Characteristic | GENISOS Cohort | Control Subjects |
|---|---|---|
| Sex, female | 221 (83) | 78 (80) |
| Age at the time of first study visit, mean (SD) yrs |
48.6 (13.5) | 48 (12.7) |
| Ethnicity | ||
| White | 125 (47) | 48 (49) |
| African American | 54 (20) | 17 (18) |
| Latino | 77 (29) | 27 (29) |
| Asian | 10 (4) | 5 (5) |
| Diffuse cutaneous involvement | 156 (59) | |
| Disease duration 1, mean (SD)* | 2.5 (1.6) | |
| Disease duration 2, mean (SD)** | 4.5 (5.4) | |
| ACA | 32 (12) | |
| ATA | 49 (18) | |
| ARA | 61 (23) | |
| Forced vital capacty percentage predicted | ||
| > 80 | 132 (58.1) | |
| 50 to 80 | 81 (35.7) | |
| < 50 | 14 (6.2) | |
| Treatment with immunosuppressive agents |
82 (32) | |
Disease duration 1 was calculated from the onset of the first non-Raynaud syndrome.
Disease duration 2 was calculated from the onset of the first symptom attributable to systemic sclerosis including Raynaud. ACA: anticentromere antibodies; ATA: antitopoisomerase antibodies; ARA: anti-RNA polymerase III antibodies.
Comparison of pneumoprotein levels in patients and controls
As shown in Table 2, SP-D levels were significantly higher in patients than in matched controls (p < 0.001, log mean difference = 0.6, 95% CI 0.4–0.81). CCL18 levels also were higher in patients than in controls (p = 0.0146, log mean difference = 0.126, 95% CI 0.025–0.23).
Table 2.
Levels of SP-D and CCL18 in patients and controls.
| Pneumoproteins | Mean (± SD) in Patients*, ng/ml |
Mean (± SD) in Controls*, ng/ml |
Mean Difference† (95% CI††) |
p |
|---|---|---|---|---|
| SP-D | 1489.3 (1672.1) | 887.6 (1453.1) | 0.6 (0.4, 0.81) | < 0.001 |
| CCL18 | 133.3 (49.7) | 121.2 (54.8) | 0.13 (0.03, 0.23) | 0.015 |
Mean of raw data.
Log-transformed data.
Difference between the 2 groups using log-transformed data. SP-D: surfactant protein D; CCL18: CC-chemokine ligand 18.
Cross-sectional correlation of baseline pneumoprotein levels with FVC
As shown in Table 3 and Figure 1, there was a significant correlation between higher SP-D levels and lower concomitantly obtained FVC measurements at the baseline visit (r = −0.27, p < 0.001) in the GENISOS cohort. There was no significant correlation between plasma CCL18 concentrations and concomitantly obtained baseline FVC levels (r = −0.1, p = 0.175). The composite SP-D and CCL18 score correlated with lower concomitantly obtained FVC (r = −0.25, p = 0.001) but this relationship was not stronger than the observed correlation of FVC with SP-D alone. In the multivariable analysis, SP-D remained an independent correlate of concomitant FVC (p < 0.001) after adjustment for age, sex, ethnicity, and BMI. CCL18 did not correlate with concomitant FVC in the multivariable model (p = 0.283) after correction for the same potential confounding factors. The association of the composite SP-D and CCL18 score with the concomitantly obtained FVC was also independent of the above potential confounding factors (p = 0.001).
Table 3.
Correlation of SP-D and CCL18 with concomitantly obtained FVC.
| Pneumoproteins | r | punivariate | Mean Difference (95% CI) |
pmultivariate* |
|---|---|---|---|---|
| SP-D | −0.27 | < 0.001 | −7.09 (−10.55, −3.62) | < 0.001 |
| CCL18 | −0.10 | 0.175 | −6.41 (−15.69, 2.88) | 0.283 |
| SP-D and CCL18 composite score |
−0.25 | 0.001 | −17.85 (−28.76, −6.95) | 0.001 |
Adjusted for age, sex, ethnicity, and body mass index. SP-D: surfactant protein D; CCL18: CC-chemokine ligand 18; FVC: forced vital capacity.
Figure 1.
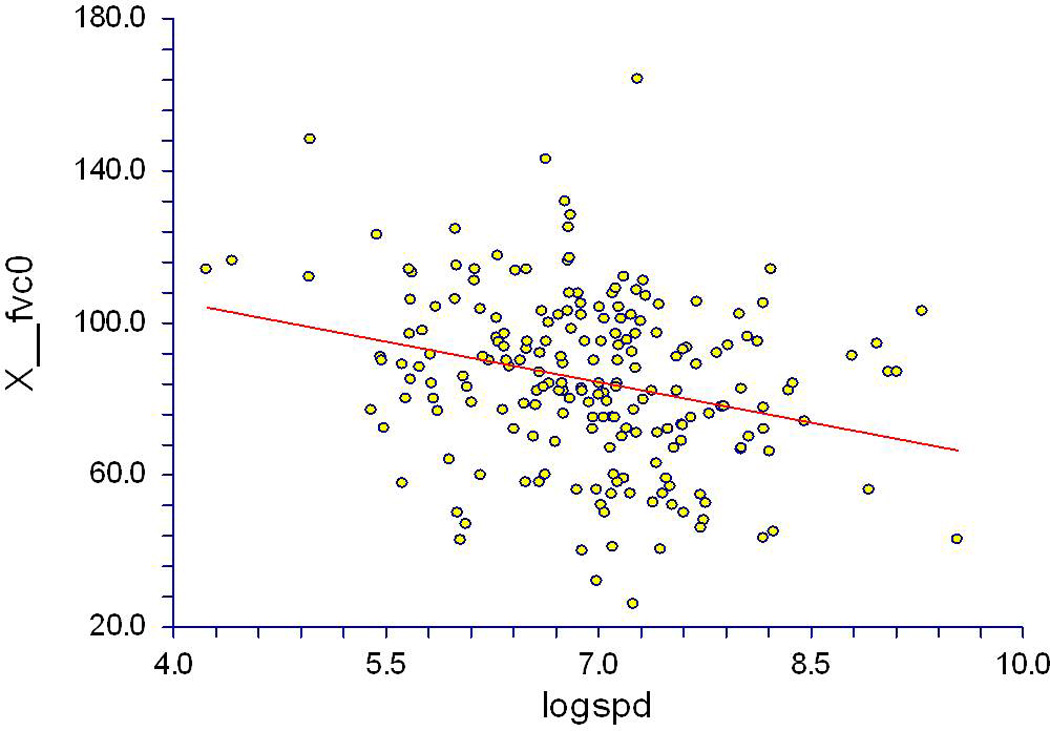
Correlation of surfactant protein D with concomitant forced vital capacity (FVC) at the baseline visit; n = 211, r = −0.27, p < 0.001.
Neither SP-D nor CCL18 correlated with the concomitantly obtained modified Rodnan Skin Score (r = 0.06, p = 0.351 and r = 0.09, p = 0.238, respectively). SP-D correlated negatively with the concomitantly obtained DLCO (r = −0.15, p = 0.032), although this relationship was weaker than the correlation seen with FVC. CCL18 did not correlate significantly with the concomitantly obtained DLCO (r = −0.14, p = 0.059).
Predictive significance of baseline pneumoprotein levels for short-term change in FVC
Baseline SP-D showed no predictive significance for short-term change in FVC in the univariable analysis (r = 0.05, p = 0.591) and multivariable analysis after adjustment for age, sex, ethnicity, and BMI (p = 0.437; Table 4).
Table 4.
Predictive significance of baseline SP-D and CCL18 for short-term change in FVC.
| Pneumoproteins | r | punivariable | Mean Difference (95% CI) |
pmultivariable* |
|---|---|---|---|---|
| SP-D | 0.05 | 0.591 | 0.01 (−0.02, 0.03) | 0.437 |
| CCL18 | 0.17 | 0.071 | 0.07 (0, 0.13) | 0.049 |
| SP-D and CCL18 composite score |
0.17 | 0.071 | 0.08 (0, 0.15) | 0.04 |
Adjusted for age, gender, ethnicity, and body mass index. SP-D: surfactant protein D; CCL18: CC-chemokine ligand 18; FVC: forced vital capacity.
Baseline CCL18 levels showed a trend for predicting short-term decline in FVC (r = 0.17, p = 0.071) in the uni -variable analysis, which became significant after adjustment for age, sex, ethnicity, and BMI (p = 0.049) with higher baseline pneumoprotein levels predicting higher decline in FVC on the 1-year followup visit (Table 4).
The composite SP-D and CCL18 score also showed a trend for predicting short-term decline in FVC (r = 0.17, p = 0.071) in the univariable analysis, which became significant after adjustment for potential confounders (p = 0.04).
Neither SP-D nor CCL18 was predictive of short-term decline in FVC when only patients were subgrouped based on the disease type (limited/diffuse) or ATA positivity (data not shown). Neither SP-D nor CCL18 predicted short-term change in percentage predicted DLCO (r = 0.04, p = 0.7 and r = −0.03, p = 0.784, respectively).
Predictive significance of baseline pneumoprotein levels for longterm change in FVC
Using a joint analysis of longitudinal measurements (sequentially obtained FVC) and survival data, we investigated the predictive significance of baseline pneumoproteins for longterm change in FVC accounting for mortality. As shown in Table 5, baseline SP-D was not predictive of longterm change in FVC (p = 0.937). Similarly, baseline CCL18 did not predict longterm change in FVC (p = 0.796; Table 5). These results did not change substantially after adjustment for age, sex, ethnicity, and BMI (p = 0.783 for SP-D and p = 0.753 for CCL18). Inclusion of treatment status as an independent variable did not show significant associations. Further, a subgroup analysis based on disease type (limited/diffuse) or ATA positivity did not yield significant results for longterm predictive value of SP-D or CCL18 (data not shown). The composite score of the SP-D and CCL18 also did not predict longterm course of FVC (Table 5). Neither SP-D nor CCL18 predicted longterm change in percentage predicted DLCO (p = 0.947 and p = 0.384, respectively).
Table 5.
Predictive significance of baseline SP-D and CCL18 for longterm change in FVC.
| Pneumoproteins | punivariable | b (95% CI) | pmultivariable* |
|---|---|---|---|
| SP-D | 0.937 | −0.016 (−0.41, 0.4) | 0.783 |
| CCL18 | 0.797 | −0.13 (−1.14, 0.88) | 0.753 |
| SP-D and CCL18 compositie score |
0.987 | 0.01 (−1.14, 1.16) | 0.987 |
Adjusted for age, sex, ethnicity, and body mass index. SP-D: surfactant protein D; CCL18: CC-chemokine ligand 18; FVC: forced vital capacity.
Correlation of longitudinal change in pneumoprotein levels
Finally, we investigated whether a percentage change in plasma pneumoprotein levels correlated with percentage change in FVC measurements. Repeat SP-D and CCL18 measurements were available in 83 patients on followup visits. The percentage change in SP-D and CCL18 did not correlate with concomitant percentage change in FVC (p = 0.388 and p = 0.666, respectively).
Effect of immunosuppression at baseline on the utility of SP-D and CCL18 as biomarkers
For this analysis, we excluded all patients treated with immunosuppressive agents at the baseline visit (except for patients treated with hydroxychloroquine or prednisone ≤ 5 mg per day). As shown in Appendices 1, 2, and 3, the results of these subgroup analyses (n = 184) were similar to those in the overall group. Baseline SP-D also correlated significantly with baseline FVC levels. Further, CCL18 showed a trend for correlation with the concomitantly obtained FVC (r = −0.17, p = 0.07). Similar to the overall cohort, the composite SP-D and CCL18 scores were predictive of short-term decline in FVC (r = 0.26, p = 0.019). However, neither of the 2 pneumoproteins nor the composite score predicted longterm decline in FVC.
DISCUSSION
In our study, we investigated the predictive significance of 2 pneumoproteins, SP-D and CCL18, for course of ILD in a large, well-characterized cohort of patients with early SSc, using advanced statistical modeling that allowed inclusion of all data points and accounted for dependence between the longitudinal FVC and survival. SP-D levels correlated with concomitantly obtained FVC and CCL18 correlated with short-term decline in FVC, but neither SP-D nor CCL18 was a longterm predictor of the course of ILD.
Pneumoproteins are more attractive for biomarker development in ILD than general markers of fibrosis, because they reflect the pathological processes specific to the pulmonary tissue. This is especially important in SSc because the skin fibrosis peaks early during the course of disease and improves afterward20, while the fibrosis in the pulmonary tissue continues to progress even in later stages of disease5. Therefore, general markers of fibrosis are not well suited to discerning the improving fibrosis in the skin tissue from progressive lung involvement. Despite this theoretical advantage, our data do not support that SP-D and CCL18 are reliable predictors for longterm course of ILD.
In previous studies of SP-D, there was a significant correlation between pneumoprotein levels and concomitant pulmonary volumes. Asano, et al found that serum levels of SP-D were significantly higher in patients with SSc than in healthy controls. SP-D levels also were significantly elevated in those patients with ILD versus those without11. Hant, et al reported that serum levels of SP-D were higher in patients with alveolitis than in those without this condition10. In this case, alveolitis was defined by either BAL fluid analysis or thoracic HRCT. SP-D also was positively correlated with concomitantly obtained maximum fibrosis scores on HRCT. This finding was confirmed by a subsequent independent study13. In agreement with previous studies, SP-D levels were higher in patients than controls in our study. Further, there was a significant but weak correlation (r = −0.27) between baseline SP-D measurements and lower concomitant FVC measurements. However, we found no correlation between SP-D levels and short-term or longterm change in lung function, as measured by decline in FVC.
Kodera, et al have reported that CCL18 was higher in patients with SSc than in controls and that its levels correlated inversely with vital capacity15. Tiev, et al reported in a prospective study of 83 patients with SSc that baseline CCL18 levels were predictive of time to occurrence of combined deleterious events of 10% decrease in total lung capacity, FVC, or death. The patients were followed longitudinally over a 4-year observation period in that study17. Both studies examined patients with relatively longstanding disease (mean disease duration 6.9 and 11.6 years, respectively). We also observed higher CCL18 levels in patients than in controls. However, there was no significant correlation between CCL18 levels and concomitantly obtained FVC, although there was a trend for this correlation after patients treated with immunosuppressive agents were excluded (Appendix 1). There was also a significant although weak correlation between baseline CCL18 levels and short-term change in FVC (r = 0.17), but this pneumoprotein was not a predictor of longterm decline in FVC. These findings indicate that CCL18 has some value for short-term prediction of course of ILD but cannot be used as a reliable longterm predictor. Some of the discrepancies between our findings and previous studies might have occurred because we examined patients with early disease; this approach is less prone to problems arising from survival bias. Further, the outcome measure investigated in our study was the longitudinal course of FVC, the only validated outcome measure in randomized controlled studies of SSc-ILD7,21. This outcome measure is different from time to development of 10% decline in total lung capacity or FVC, as used in the previous study17.
We also conducted subgroup analyses after exclusion of patients who were treated with immunosuppressive agents to avoid the potential confounding effect of immunosuppression. In these analyses, there was no substantial difference in predictive quality of SP-D or CCL18. Further, the combination of SP-D and CCL18 into a composite score did not result in substantially improved predictive quality.
The strengths of our study are the well-characterized and multiethnic cohort of patients and the large sample size. Additionally, all PFT measurements were obtained prospectively and reviewed according to uniform standards. Further, we used sophisticated methods that allowed joint analysis of longitudinal outcome and survival status. This joint model accounts for the dependence of serial FVC and survival while modeling the trajectory of FVC as a function of followup time.
Our study has some limitations. Our cohort contains a relatively high proportion of patients with diffuse disease, partly because it was based in 3 tertiary care centers. Further, the GENISOS cohort is an observation cohort that is well-suited for biomarkers that predict the natural course of disease, but we cannot exclude that these 2 pneumoproteins might be beneficial as predictors of response to treatment in randomized controlled trials of SSc-ILD.
Plasma SP-D and CCL18 levels are elevated in patients with SSc. SP-D correlates with the concomitantly obtained FVC and CCL18 has some value for predicting short-term decline in FVC. Neither SP-D nor CCL18 predicts the longterm course of ILD in patients with SSc.
Acknowledgments
Supported by the US National Institutes of Health (NIH)/US National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) P50 AR054144 (Dr. Mayes); NIH/NIAMS K23 AR061436 (Dr. Assassi); NIH N01 AR2251, R01 AR055258 (Dr. Mayes); NIH 1U01AI09090-01 (Dr. Zhou, Dr. Mayes); NIH T32 AR052283 (Reveille); University Clinic Research Center Grants M01 RR00073 (UTMB) and M01 RR01346 (UTHSC-SA); and NIH Clinical and Translational Sciences Award UL1 RR024148 and TL1 RR024147 from the National Center for Research Resources.
APPENDIX 1
Correlation of SP-D and CCL18 with concomitantly obtained FVC in patients not treated with immunosuppressive agents.
| Pneumoproteins | r | punivariate | Mean Difference (95% CI) |
pmultivariate* |
|---|---|---|---|---|
| SP-D | −0.33 | < 0.001 | −8.86 (−12.75, −4.98) | < 0.001 |
| CCL18 | −0.17 | 0.07 | −9 (−20.08, 2.09) | 0.111 |
| SP-D and CCL18 | −0.32 | 0.001 | −21.8 (−33.49, −10.12) | < 0.001 |
Adjusted for age, sex, ethnicity, and body mass index. SP-D: surfactant protein D; CCL18: CC-chemokine ligand 18; FVC: forced vital capacity.
APPENDIX 2
Predictive significance of baseline SP-D and CCL18 for short-term changes in FVC in patients not treated with immunosuppressive agents.
| Pneumoproteins | r | punivariate | Mean Difference (95% CI) |
pmultivariate* |
|---|---|---|---|---|
| SP-D | 0.12 | 0.255 | 0.02 (−0.01, 0.05) | 0.288 |
| CCL18 | 0.17 | 0.126 | 0.07 (−0.01, 0.16) | 0.1 |
| SP-D and CCL18 | 0.26 | 0.018 | 0.11 (0.02, 0.2) | 0.019 |
Adjusted for age, gender, ethnicity, and body mass index. SP-D: surfactant protein D; CCL18: CC-chemokine ligand 18; FVC: forced vital capacity.
APPENDIX 3
Predictive significance of baseline SP-D and CCL18 for longterm changes in FVC in patients not treated with immunosuppressive agents.
| Pneumoproteins | punivariate | b (95% CI) | pmultivariate* |
|---|---|---|---|
| SP-D | 0.742 | 0.08 (−0.39, 0.54) | 0.928 |
| CCL18 | 0.921 | 0.06 (−1.19, 1.31) | 0.628 |
| SP-D and CCL18 | 0.769 | −0.42 (−1.9, 1.06) | 0.571 |
Adjusted for age, sex, ethnicity, and body mass index. SP-D: surfactant protein D; CCL18: CC-chemokine ligand 18; FVC: forced vital capacity.
REFERENCES
- 1.Elhai M, Meune C, Avouac J, Kahan A, Allanore Y. Trends in mortality in patients with systemic sclerosis over 40 years: A systematic review and meta-analysis of cohort studies. Rheumatology. 2012;51:1017–1026. doi: 10.1093/rheumatology/ker269. [DOI] [PubMed] [Google Scholar]
- 2.Thomas E, Symmons DP, Brewster DH, Black RJ, Macfarlane GJ. National study of cause-specific mortality in rheumatoid arthritis, juvenile chronic arthritis, and other rheumatic conditions: A 20 year followup study. J Rheumatol. 2003;30:958–965. [PubMed] [Google Scholar]
- 3.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66:940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: A study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–1815. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 5.Assassi S, Sharif R, Lasky RE, McNearney TA, Estrada YMR, Draeger HT, et al. Predictors of interstitial lung disease in early systemic sclerosis: A prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther. 2010;12:R166. doi: 10.1186/ar3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steen VD, Conte C, Owens GR, Medsger TA., Jr Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994;37:1283–1289. doi: 10.1002/art.1780370903. [DOI] [PubMed] [Google Scholar]
- 7.Furst D, Khanna D, Matucci-Cerinic M, Clements P, Steen V, Pope J, et al. Systemic sclerosis — continuing progress in developing clinical measures of response. J Rheumatol. 2007;34:1194–1200. [PubMed] [Google Scholar]
- 8.Khanna D, Tseng CH, Farmani N, Steen V, Furst DE, Clements PJ, et al. Clinical course of lung physiology in patients with scleroderma and interstitial lung disease: Analysis of the Scleroderma Lung Study Placebo Group. Arthritis Rheum. 2011;63:3078–3085. doi: 10.1002/art.30467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 10.Hant FN, Ludwicka-Bradley A, Wang HJ, Li N, Elashoff R, Tashkin DP, et al. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol. 2009;36:773–780. doi: 10.3899/jrheum.080633. [DOI] [PubMed] [Google Scholar]
- 11.Asano Y, Ihn H, Yamane K, Yazawa N, Kubo M, Fujimoto M, et al. Clinical significance of surfactant protein D as a serum marker for evaluating pulmonary fibrosis in patients with systemic sclerosis. Arthritis Rheum. 2001;44:1363–1369. doi: 10.1002/1529-0131(200106)44:6<1363::AID-ART229>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Yanaba K, Hasegawa M, Takehara K, Sato S. Comparative study of serum surfactant protein-D and KL-6 concentrations in patients with systemic sclerosis as markers for monitoring the activity of pulmonary fibrosis. J Rheumatol. 2004;31:1112–1120. [PubMed] [Google Scholar]
- 13.Bonella F, Volpe A, Caramaschi P, Nava C, Ferrari P, Schenk K, et al. Surfactant protein D and KL-6 serum levels in systemic sclerosis: Correlation with lung and systemic involvement. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28:27–33. [PubMed] [Google Scholar]
- 14.Schutyser E, Richmond A, Van DJ. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J Leukoc Biol. 2005;78:14–26. doi: 10.1189/jlb.1204712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodera M, Hasegawa M, Komura K, Yanaba K, Takehara K, Sato S. Serum pulmonary and activation-regulated chemokine/CCL18 levels in patients with systemic sclerosis: A sensitive indicator of active pulmonary fibrosis. Arthritis Rheum. 2005;52:2889–2896. doi: 10.1002/art.21257. [DOI] [PubMed] [Google Scholar]
- 16.Prasse A, Pechkovsky DV, Toews GB, Schafer M, Eggeling S, Ludwig C, et al. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis Rheum. 2007;56:1685–1693. doi: 10.1002/art.22559. [DOI] [PubMed] [Google Scholar]
- 17.Tiev KP, Hua-Huy T, Kettaneh A, Gain M, Duong-Quy S, Toledano C, et al. Serum CC chemokine ligand-18 predicts lung disease worsening in systemic sclerosis. Eur Respir J. 2011;38:1355–1360. doi: 10.1183/09031936.00004711. [DOI] [PubMed] [Google Scholar]
- 18.Sweeting MJ, Thompson SG. Joint modelling of longitudinal and time-to-event data with application to predicting abdominal aortic aneurysm growth and rupture. Biom J. 2011;53:750–763. doi: 10.1002/bimj.201100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizopoulos D. JM: An R package for the joint modelling of longitudinal and time-to-event data. J Stat Softw. 2010;35:1–33. [Google Scholar]
- 20.Amjadi S, Maranian P, Furst DE, Clements PJ, Wong WK, Postlethwaite AE, et al. Course of the modified Rodnan skin thickness score in systemic sclerosis clinical trials: Analysis of three large multicenter, double-blind, randomized controlled trials. Arthritis Rheum. 2009;60:2490–2498. doi: 10.1002/art.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–2666. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]


