Abstract
Stem cell transplantation therapy has emerged as a promising regenerative medicine for ischemic stroke and other neurodegenerative disorders. However, many issues and problems remain to be resolved before successful clinical applications of the cell-based therapy. To this end, some recent investigations have sought to benefit from well-known mechanisms of ischemic/hypoxic preconditioning. Ischemic/hypoxic preconditioning activates endogenous defense mechanisms that show marked protective effects against multiple insults found in ischemic stroke and other acute attacks. As in many other cell types, a sub-lethal hypoxic exposure significantly increases the tolerance and regenerative properties of stem cells and progenitor cells. So far, a variety of preconditioning triggers have been tested on different stem cells and progenitor cells. Preconditioned stem cells and progenitors generally show much better cell survival, increased neuronal differentiation, enhanced paracrine effects leading to increased trophic support, and improved homing to the lesion site. Transplantation of preconditioned cells helps to suppress inflammatory factors and immune responses, and promote functional recovery. Although the preconditioning strategy in stem cell therapy is still an emerging research area, accumulating information from reports over the last few years already indicates it as an attractive, if not essential, prerequisite for transplanted cells. It is expected that stem cell preconditioning and its clinical applications will attract more attention in both the basic research field of preconditioning as well as in the field of stem cell translational research. This review summarizes the most important findings in this active research area, covering the preconditioning triggers, potential mechanisms, mediators, and functional benefits for stem cell transplant therapy.
Keywords: Stem cell preconditioning, Stroke, Ischemia, Neurodegenerative disorder, Heart attack
Introduction
Stem cell transplant is a rapidly developing potential regenerative therapy for stroke, heart attack, wound healing, kidney failure and other degenerative disorders. Stem cells and progenitor cells may promote tissue repair and functional recovery via enforcing trophic support and cell replacement mechanisms [1]. So far, several cell-based therapies using different stem cells and progenitors such as mesenchymal stem/stromal cells (MSCs), endothelial progenitor cells (EPCs), embryonic or hematopoietic stem cells and c-kit+ cells have been under extensive pre-clinical and clinical investigations for a variety of disorders [2]. However, a number of issues and problems remain unresolved and need specific attention in order to develop successful clinical treatments. These may include, but are not limited to: appropriate cell source in consideration of therapeutic value and ethical concerns, cell type specific differentiation, survival of transplanted cells in the pathological environment subjected to multiple insults including ischemia/hypoxia, excitotoxicity, reactive oxygen species (ROS), inflammatory response, apoptotic cascade activation, excessive autophagy and so on. In addition, homing of transplanted cells to the lesion site, integration/engraftment of grafted cells with host cells/tissues, and, finally, desirable neural network repair and functional recovery require the development of target specific strategies [2–4]. Although many in the field may ignore these dilemmas and move forward to clinical trials, we believe that it is critically important to recognize and address these issues in order to avoid potential risks and failures in clinical application of the cell-based therapy.
Ischemic or hypoxic preconditioning has been extensively investigated in various cell types, organs, animal models and humans [4–9]. Although the benefits of ischemic/hypoxic preconditioning by mobilizing the endogenous defense mechanism are consistently demonstrated in cell cultures, animal models and human organs, the conventional preconditioning in vivo approach or the in vitro mechanistic investigation are not clinically feasible except in a few cases such as remote ischemic preconditioning (RIPC) that uses a similar ischemic insult applied to limbs or legs for a protective effect on the CNS [4, 10–12]. However, the safety and therapeutic range of the ischemic insult in RIPC have not been well investigated; preconditioning stimuli still potentially cause structural damage in patients [13]. A recent development in combining the preconditioning strategy with stem cell therapy opens a door for a broader opportunity of clinical applications of hypoxic preconditioning [14–17]. Work from us and a few other groups showed that exposure of stem cells or progenitor cells to sub-lethal hypoxia or other preconditioning insults increased the tolerance of these cells to multiple injurious insults and protected them against the harsh environment after transplantation. Since then, there has been a surge of research papers on this topic (Tables 1 and 2).
Table 1.
Preconditioning triggers in stem cells and their benefits in the heart
| Types and Triggers | Cells | Benefits | References | |
|---|---|---|---|---|
| In vitro | In vivo after transplantation | |||
| Ischemia/Hypoxia/Anoxia | rat and mouse mesenchymal stem cells; rat H9c2 cardiomyoblast; rat L6 skeletal myoblast; mouse bone marrow-derived endothelial progenitor cells | improve survival; | [14, 19, 26, 35, 78] | |
| improve survival; increase paracrine factors; reduce infarct size; enhance angiogenesis, vascularization or myogenesis; promote functional recovery | [14, 18, 21, 24–26, 40, 137, 156] | |||
| Preconditioning mediators: H2S, IGF-1, FGF-2, TGF-α, TNF-α | rat MSCs; mouse SCA-1+ stem cells | improve survival; increase paracrine factors | [27, 117] | |
| improve survival; increase paracrine factors; reduce infarct size; show anti-inflammatory effects; promote functional recovery | [27, 88, 117, 119] | |||
| Pharmacological agents: diazoxide, isoflurane, LiCl, CsA, sildenafil, LPS, NS1619, N6-cyclopentyladenosine, TMZ, OT, CoPP | mouse adipose tissure-derived stem cells; rat and mouse mesenchymal stem cells; human Nkx2.5+ cardiac progenitor cell; rat skeletal myoblast; rat H9c2 cardiomyoblast; c-Kit+ human cardiac stem cells; human iPSC-derived cardiomyocyte | improve survival; increase paracrine factors; increase migration | [42–44, 80, 97, 118, 152, 157] | |
| improve survival; increase paracrine factors; recovery cardiac function | [37, 40, 43, 49, 105, 118, 158] | |||
| Injury insults: heat shock, CO | C2C12 myogenic cells; rat H9c2 cardiomyoblast | improve survival | [29, 36] | |
Table 2.
Preconditioning triggers in stem cells and their benefits in the brain
| Types and Triggers | Cells | Benefits | References | |
|---|---|---|---|---|
| In vitro | In vivo after transplantation | |||
| Hypoxia | neural stem cells; bone marrow-derived mesenchymal stem cells | improve survival; increase paracrine factors; reduce inflammatory genes | improve survival; increase paracrine factors; promote anti-inflammatory effects; reduce infarct size; enhance angiogenesis, vascularization or neural differentiation; improve neurological performance | [15, 16, 64, 75] |
| Pharmacological agent: minocycline | neural stem cells | increase paracrine factors | improve survival; increase paracrine factors; promote anti-inflammatory effects; reduce infarct size; enhance angiogenesis, vascularization or neural differentiation; improve neurological performance | [75] |
Up to now, a number of preconditioning triggers have been tested in stem cells and stem cell-derived progenitor cells. These triggers are often sublethal insults such as ischemia [18], hypoxia [19–23], anoxia [24–26], hydrogen sulfide (H2S) [27], hydrogen dioxide (H2O2) [28], and carbon monoxide (CO) [29]. Alternatively, preconditioning can be achieved using preconditioning mediators such as erythropoietin (EPO) [16, 30], stromal-derived factor-1 (SDF-1) [31–33], insulin-like growth factor-1 (IGF-1) [34], heat shock proteins (HSPs) [35, 36] or pharmacological agents such as diazoxide [37–40], apelin [41], isoflurane [42], lipopolysaccharide (LPS) [43], and cobalt protoporphyrin (CoPP) [44] (Tables 1 and 2).
The following review highlights some advances in preconditioned stem/progenitor cells, the mechanism of preconditioning and its benefits, which are bringing new approaches for enhanced cell quality/adaptability and improved transplantation therapy for human diseases.
A. Pathways Involved in Preconditioning Stem Cells and Other Cells
As energy metabolism dysfunction and glutamate excitotoxicity occur in ischemic brain injury, mass cell death is induced in hours to days with additional injury from increased free radicals, inflammatory responses, activation of apoptotic cascades and other pathological processes. Preconditioning treatments applied to stem cells have been shown to enhanced resistance to those insults by increasing survival signals [45, 46]. Many survival and protective molecules including hypoxia-inducible factor-1 (HIF-1) [47], trophic/growth factors [2], Akt [43, 48], extracellular signal-regulated kinase (ERK) [48], glycogen synthase kinase-3β (GSK-3β), matrix metalloproteinase-2 (MMP-2) [25], survivin [49] and Bcl-2 are involved in response to preconditioning stimuli. Selective upregulation of these molecules coupled to enhancing protective signaling, is adequently controlled in both preconditioned stem cells and the cells adjacent to injury regions [50].
Central roles of HIF-1 in hypoxic/ischemic preconditioning
In ischemic/hypoxic preconditioning and neuroprotection against ischemic injury, HIF-1α and HIF-1β play central roles [51, 52]. As a nuclear factor for transcriptional activation in response to hypoxia, HIF-1 acts as a low-oxygen sensor. Its translocation and activation in the nucleus result in production of several downstream genes such as vascular endothelial growth factor (VEGF), EPO, sodium-calcium exchanger-1 (NCX-1), pyruvate dehydrogenase kinase-1 (PDK-1), lactate dehydrogenase A (LDHA), and uncoupling protein-2 (UCP-2). These protein molecules act as survival signals, maintain cellular ion homeostasis, regulate the balance between oxidative stress and glycolytic metabolism in mitochondria and regulate many other stress-induced responses [52–55]. VEGF and EPO stimulate endogenous mechanisms for angiogenesis and neurogenesis, which are vital processes for wound healing and functional repairs of injuried brains [56–59]. Some other HIF-1 targeted genes such as CXCR4 and extracellular matrix proteins (MMPs) are key factors for cell migration [60]. Besides hypoxia, HIF-1 also responds to many inflammatory mediators such as ROS, NO, LPS and cytokines, and may further increase inducible nitric oxide synthase (iNOS) and antioxidant genes that are involved in regulation of cell fate in the inflammatory microenvironment [53, 61, 62]. Inhibition of HIF-1 degradation by prolyl hydroxylase inhibitor Dimethyloxalylglycine (DMOG) enhances MSC survival after exposure to serum deprivation (SD) and oxygen-glucose deprivation (OGD) [51, 63].
In stem cells, HIF-1 also plays a role in preconditioning and shows benefit for transplantation therapy (Figure 1). HIF-1 induces the cysteine glutamate exchanger system Xc- of NSCs by increasing expression of the light-chain subunit xCT [64], which is a rate-limiting step for brain antioxidant glutathione (GSH) production [65]. HIF-1 overexpression in MSCs upregulates a set of genes that contribute to cell adhesion, migration and paracrine effect. Transplantation of these cells into the myocardium of rats shortly after induction of myocardial infarction (MI) enhances the recovery of cardiac functions and angiogenesis [66]. A clinical drug for the treatment of angina pectoris, Trimetazidine (TMZ), has been used to precondition MSCs and shows cardioprotection mediated by HIF-1 [49]. These results have suggested that HIF-1 is an important mediator in stem cell preconditioning (Figure 1).
Figure 1. Central roles of HIF-1, mitochondria and signaling pathways in stem cell preconditioning.
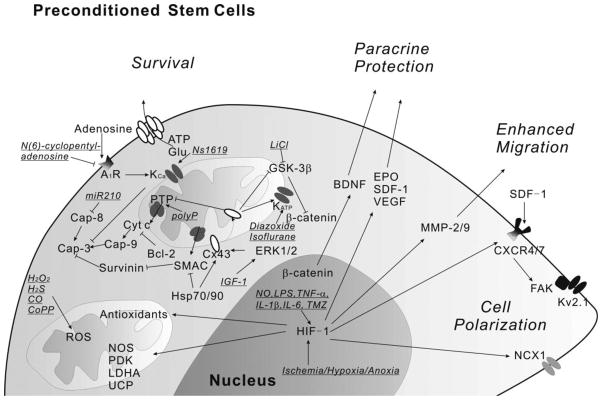
Ischemia, hypoxia, anoxia and some other insults increase HIF-1 expression. HIF-1 then regulates antioxidants, survival signals and many other genes related to cell adhesion, polarization, migration and paracrine protection. Mitochondria also play essential roles for improving cell viability responding to preconditioning insults. The underlined indicates the insults used.
Abbreviations: A1R, adenosine A1 receptor; BDNF, brain-derived neurotrophic factor; Cap, caspase; CoPP, cobalt protoporphyrin; Cx43, connexin-43; CXCR, CXC chemokine receptor; Cyt c, cytochrome c; EPO, erythropoietin; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; GSK-3β, glycogen synthase kinase-3β; HIF-1, hypoxia-inducible factor-1; Hsp, heat shock protein; IGF-1, insulin-like growth factor-1; IL-1β, interleukin-1beta; IL-6, interleukin-6; LPS, lipopolysaccharide; miR, micro-RNA; MMP, matrix metalloproteinase; NCX-1, sodium-calcium exchanger-1; NOS, nitric oxide synthase; PDK, pyruvate dehydrogenase kinase; polyP, polyphosphate; PTP, permeability transition pore; ROS, reactive oxygen species; SDF-1, stromal-derived factor-1; SMAC, second mitochondria-derived activator of caspase; TMZ, Trimetazidine; TNF-α, tumor necrosis factor-alpha; UCP, uncoupling protein; VEGF, vascular endothelial growth factor.
Preconditioning induced changes in mitochondria
Mitochondria play an important role in cytoprotection and preconditioning of cells [67, 68] (Figure 1). Generation of ROS in mitochondria is one of the main triggers that induce ischemic tolerance in brain and heart [69, 70]. Many insults, including H2O2 [71], H2S [72] and CO [29] exert protective effects by inducing mitochondrial ROS production. Appropriate ROS production in mitochondria can effectively protect H9c2 cells and MSCs against consequent injurious oxidative stress induced by severe hypoxia [21, 22]. Preconditioning c-kit+ human cardiac stem cells with cobalt protoporphyrin (CoPP) induces oxygenase 1 to generate endogenous CO and increases H2O2. This preconditioning effect is attenuated by knocking down HO-1, COX-2, or Nrf2 antioxidant genes [44]. Preconditioning BMSCs with O2 can also increase the antioxidant capacity by upregulating stanniocalcin-1 [73].
Exposure to sublethal OGD can precondition neuronal cultures via increased H2O2 [74]. Minocycline-preconditioned NSCs upregulate antioxidant genes such as NQO1 and HO-1, induced by the stress-inducible transcription factor Nrf2 [75]. Coenzyme Q10 (CoQ10), essential for mitochondrial electron transport, is shown to protect NSCs against hypoxia [67]. Upregulated antioxidants may further protect transplanted NSCs against ischemic reperfusion injury and sustained inflammation.
The opening of mitochondrial permeability transition pores (mPTPs) leads to release of cytochrome c and apoptosis-inducing factor (AIF) into the cytoplasm and activation of apoptotic cascades. Overloaded Ca2+ and its interaction with polyphosphate (polyP) in the inner mitochondrial membrane can induce mPTP to open and thus increase mitochondrial membrane permeability in cardiomyocytes, neurons and astrocytes [76, 77]. This event, which is a leading cause of cardiac/brain damage and cell death, again, can trigger either a preconditioning or an injurious reponse depending on the severity, duration, and timing of the event. A sublethal hypoxia-induced preconditioning is able to stabilize mitochondrial membrane potentials [78]. In another report, the inhalation anesthetic isoflurane-induced preconditioning in cardiomyocytes was mediated by depolarized mitochondria membrane potentials and delayed opening of mPTPs [79].
Ca2+ and/or ATP sensitive potassium channels exist on the mitochondrial membrane, which play key roles in regulating the mitochondrial potential. A large-conductance Ca2+-activated potassium channel in mitochondrial fractions of H9c2 cells was shown to be involved in pharmacological preconditioning using N(6)-cyclopentyladenosine (adenosine A1 receptor agonist) and NS1619 (Ca2+-activated K+ channel opener), which protects the cardiomyoblasts against in vitro hypoxia, glucose and serum deprivations [80].
Investigations on neuronal cells and cardiomyocytes showed that activation of mitochondrial ATP-sensitive potassium (mitoKATP) channels are involved in cytoprotection by attenuating mitochondrial Ca2+ overload thus preventing mPTP induction [81]. Diazoxide, a pharmalogical agent to open mitoKATP, can increase the survival of skeletal myoblasts [38, 39] and MSCs [37]. Isoflurane-induced preconditioning that increases the survival of cardiac progenitor cells (CPCs) has revealed similar mechanisms through targeting the mitoKATP [42].
Heat shock proteins are a group of conserved proteins that are upregulated during stress conditions including ischemia/hypoxia. The upregulated Hsp70 and Hsp90 after preconditioning in myocardium is reported to inhibit the mitochondrial release of second mitochondria-derived activator of caspase (SMAC) and prevent activation of caspase-9 and caspase-3 [36]. Hsp70 is also upregulated in other cells and tissues exposed to preconditioning insults including sublethal OGD, transient focal ischemia and intermittent hypoxia. The Hsp70 upregulation have protective effects on cortical neuronal cultures [82], PC12 adrenal medulla pheochromocytoma cells [83], and renal tubular cells [84]. Additionally, Hsp90 and Hsp70 may form a complex with Cx43 and facilitate the translocase of the outer membrane 20 (TOM20)-mediated translocation of Cx43 onto inner mitochondrial membranes [85], regulating another important molecular mechanism in preconditioning.
Gap-junction protein Cx43
Connexins are a group of gap-junction proteins that facilitate communications between adjacent cells in the form of hemichannels. Connexin-43 (Cx43) is highly expressed in heart and is a potential target for anti-arrhythmogenic therapy [86, 87]. RNAi-mediated Cx43 inhibition reduces survival of antigen-1-positive (SCA-1+) stem cells in vitro under OGD and in vivo after transplantation to infarct heart [88]. In the brain, astrocytes also express Cx43 hemichannels through which ATP and glutamate can be released to cause neuronal toxicity. Treatment with cytokines including TNF-α and IL-1β can reduce glial membrane Cx43 level. This effect contributes to membrane permeability changes in response to inflammatory stimuli [89]. Some studies have suggested that preconditioning can reduce the degradation of Cx43 in astrocyte and other cells, thus markedly increasing the Cx43 hemichannels in the plasma membrane [90]. Opening of Cx43 hemichannels releases ATP and accumulates extracellular adenosine, which has been shown to activate purine signaling to have neuroprotective and cardioprotective effects via suppression of metabolism. Hypoxic preconditioning of NSCs increases Cx43 and enhances hemi-channel functions after transplantation, which may be important for early communications between transplanted stem cells and host cells [91].
Recent data show that expression of Cx43 in mitochondrial membranes improves SCA-1+ stem cell survival after transplantation, consistent with the roles of Cx43 in cytoprotection via mitochondrial pathways [92]. IGF-1-activated ERK1/2 can induce the translocation of Cx43 onto the mitochondrial inner membrane (mito-Cx43), where mito-Cx43 may interact with Bcl-2 and reduce cytochrome c release from mitochondrial inner membrane, leading to anti-apoptotic effects [92, 93]. Supporting a relationship between mito-Cx43 and apoptotic signaling, genetically modified MSCs overexpressing Cx43 show increased Bcl-2 and phosphorylated Akt. This provides a potential anti-apoptotic mechanism for hypoxic tolerance either in vitro or ex vivo [94]. Furthermore, the KATP channel Kir6.1 can interact with Cx43 in mouse cardiomyocytes and embryo fibroblasts, implicating a role for Cx43 in ischemic/hypoxia preconditioning via KATP channels [95, 96]. Lithium chloride (LiCl) preconditioning of skeletal myoblasts can also upregulate the expression of Cx43 and promote skeletal myoblast proliferation through interactions with canonical Wnt signaling [97]. A possible molecular mechanism may involve inactivation of GSK-3β, stabilization of β-catenin, and nuclear translocation to promote gene transcriptions.
SDF1-CXCR4 axis
Stromal-derived factor-1 (SDF-1), or CXCL12, is a CXC chemokine family member. Two major isoforms, SDF-1α and SDF-1β, generated by alternative gene splicing have been identified in membranes of various cell types [98]. In bone marrow SDF-1 binding to its receptor CXC chemokine receptor 4 (CXCR4), which is known as the SDF-1/CXCR4 axis, plays critical roles for mobilization, homing and engraftment of HSCs [99, 100]. Functional activities of the SDF-1/CXCR4 axis can also be elevated under hypoxic/anoxic exposures [101]. Upregulation of SDF-1 and/or CXCR4 genes under stress conditions has been confirmed in several stem cell types including MSCs [33, 47, 102], peripheral blood mononuclear cells (PBMNCs) [103], cardiosphere-derived Lin- c-kit+ progenitors (CLK) [104], bone marrow c-kit+ cells [105] and hemangioblasts [106].
Hypoxia induces CXCR4 and CXCR7 expression in BMSCs via upregulated HIF-1α [47]. EPO, a cytokine regulating haematopoiesis and neuroprotection, is also shown to upregulate SDF-1 in ischemic heart myocardium and recruit CD34+/CXCR4+ cells from blood [107]. Blocking EPOR can reduce CD34+/CXCR4+ cells in the heart. These highlight the endogenous preconditioning mechanisms in mobilization of stem/progenitor cells and homing of these cells to lesion sites in heart. In the ischemic neonatal brain, upregulated SDF-1 helps to recruit BMSCs to the injuried regions [108]. Interestingly, both in vivo tumor cells and tumor cell-conditioned medium can recruit MSCs by increasing production of SDF-1 in MSCs. SDF-1 can bind to CXCR4 and CXCR7 to activate focal adhesion kinase (FAK) mainly through JAK2/STAT3 signaling to promote MSC migration [109]. Alternatively, SDF-1-induced migration of EPCs has been shown to be mediated by PI3K/Akt/eNOS [110].
Regulation by microRNA and cfDNA
MicroRNAs (miRs) are a group of short RNA molecules that are involved in post-transcriptional downregulation via complementary binding to target mRNA transcripts. Through targeting caspase 8-associated protein-2 and programmed cell death-10 mRNAs respectively, miR-210 and miR-107 exert significant anti-apoptotic effects in BMSCs [111]. Differentiation-related functions of HIF-1β after hypoxic induction can be partially inhibited by miR-107 in bone marrow-derived EPCs [112]. During ischemia-reperfusion (I/R), endogenous protective miRs are upregulated [113]. Pharmacological agents, including diazoxide [113], can induce protective miR expressions. Except for microRNAs, cell-free DNAs (cfDNAs) in human blood plasma are higher in patients suffering from many co-morbidities [114]. A recent investigation explored the preconditioning of MSCs with specific cfDNA for increased cell survival via Toll-like receptor 9 (TLR9) and translocation of nuclear factor-kappa B (NFκB) [114]. This evidence highlights the possibility that miRs and cfDNAs may be potential new targets in stem cell preconditioning to promote their survival after transplantation.
B. Preconditioning-Induced Therapeutic Benefits in Stem Cell Therapies
Enhanced cell survival in vitro and after transplantation
The surviving quality of transplanted cells is the primary issue after the cells are transplanted into the ischemic brain or heart. The preconditioning triggers mentioned in this review generally show better survival of stem cells and progenitors in vitro and/or after transplantation (Tables 1 and 2).
In our investigations, preconditioning using sublethal hypoxia and EPO significantly increased the tolerance of treated cells to apoptotic and other insults in vitro as well as in the harsh environments of the ischemic core and peri-infarct regions [14–16]. BMSCs and embryonic stem cell-derived neural progenitor cells (ES-NPCs) survived better after sublethal exposure to low oxygen (1% O2). There was 40–50% reduction in cell death and caspase-3 activation assays. The protective effects on cultured cells lasted for at least 6 days. Hypoxic preconditioning increased secretion of EPO and upregulated expression of Bcl-2, HIF-1α, EPO receptor (EPOR), neurofilament (NF), and synaptophysin in ES-NPCs. The cytoprotective effect was diminished by blocking EPOR, while pretreatment of ES-NPCs with recombinant human EPO mimicked the hypoxic preconditioning effect. After transplantation into the ischemic brain, there was 30–40% reduction in cell death of hypoxic preconditioned ES-NPCs 3 days after transplantation compared to non-hypoxic cells. These survived ES-NPCs also exhibited extensive neuronal differentiation in the ischemic brain and enhanced recovery of sensorimotor function [16]. A similar pro-survival effect of hypoxia pretreatment was seen in human ES-NPCs [115]. Our earlier report revealed that ES-NPCs had potentials for peripheral nerve injury repair as well [116]. Consequently, enhancing the stem cell survival by preconditioning is a logic approach for application of cell-based therapies for tissue repairs [17].
Enhanced paracrine protective effects
Preconditioned MSCs treated with growth factors (such as TGF-α, IGF-1 and FGF-2), pharmacological agents or ischemia/hypoxia show increased paracrine potentials. Upregulated factors may include angiopoietin-I [117, 118], VEGF [20, 117–119], fibroblast growth factor-2 (FGF-2) [118], hepatocyte growth factor (HGF) [120, 121], placental growth factor (PlGF) [120], brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) [75]. Treatment using some of these recombinant proteins or induced endogenous expression reduces neuronal death, promotes angiogenesis and attenuates many pathophysiological changes [122, 123]. Our previous reports show that a sublethal hypoxic exposure increases expression of SDF-1 and CXCR4, together with BDNF, GDNF, VEGF, FIK-1, EPO and EPOR [14–16, 124]. Those factors have been shown to share same trophic mechanisms that are essential for the follow-up roles of grafted stem/progenitor cells that succeed in survival. Other cytokines, such as inducible nitric oxide synthase (iNOS) isoforms, are upregulated in bone marrow-derived EPCs from ischemic mice, mediating the recruitment to rescue the infarct myocardium after bone marrow transplantation [18].
Enhanced migration and homing of transplanted cells
Migration and homing to the ischemic/peri-infarct regions are clinically relevant to the efficacy of exogenous cell delivery. BMSCs are recruited to ischemic heart via regulation of increasing VEGF and SDF-1 in peripheral blood [125]. H2O2-induced preconditioning is reported to increase the migration of MSCs through upregulation of CXCR4 and activation of extracellular signal-regulated kinase (ERK) [126]. Hypoxic preconditioning enhances migration of MSCs via induced expression of cMet which binds to HGF and facilitates the recruitment of MSCs [127]. Preconditioning HSCs with SDF-1 or dextran sulfate enhances their homing efficiency to bone marrow, which is mediated via several genes including CXCR4 and MMP-9 [128].
In our recent investigations, we have shown that hypoxic preconditioning of BMSCs upregulates migration-associated proteins and enhances cell mobility [14, 15, 129]. Our earlier work revealed a novel mechanism underlying FAK phosphorylation/activation via formation of a FAK-Kv2.1 complex [130]. We showed that the voltage-gated potassium channel, Kv2.1, interacted with FAK as an integrin protein. The formation of the FAK-Kv2.1 complex increased FAK phosphorylation in neurons and Kv2.1-expressing CHO cells, which played a key role in cellular polarization and directed migration towards the wounded area in a traumatic injury model and an in vitro wound healing test [131]. Our recent data further show that hypoxic preconditioning significantly promotes the FAK-Kv2.1 complex formation, increases FAK phosphorylation and upregulates CXCR4 in BMSCs. All of these hypoxic effects reinforce migration capacity and homing of transplanted cells to the lesion sites [14, 15, 129].
Preconditioning human amniotic fluid stem cells with GDNF greatly ameliorates renal tubular injury [132]. In this study, increased stem cell homing was demonstrated. In pigs, ischemia preconditioned by balloon occlusion/reperfusion, recruitment of HSCs and MSCs to infarcted regions is greatly promoted and circulating VEGF, TNF-α and IL-8 are increased [133]. A human study also showed increasing blood IL-8 concentration and CD34+ progenitor cells after transient ischemia of lower limbs [134]. In ischemia preconditioned mice that are subjected to unilateral renal artery clamping, circulating c-Kit+/Tie-2+ EPCs increased. Moreover, isolation and transplantation of this cell population to mice with acute renal ischemia show successful mobilization of the splenic pool and EPC enrichment in the renal medullopapillary region [135]. VEGF and its receptor Flk-1 are essential for the mobilization.
In a very recent investigation, we explored the novel intranasal delivery of stem cells after ischemic stroke. Hoechst dye-labeled normoxic or hypoxic pre-treated BMSCs (1×106 cells/animal) were delivered intranasally 24 hrs after stroke. Cells reached the ischemic cortex and deposited outside of blood vessels as early as 1.5 hrs after administration. Hypoxia-treated BMSCs showed increased levels of proteins associated with migration, including CXCR4, MMP-2, and MMP-9. These cells survive much better and have dramatically enhanced homing efficiency to the infarct cortex when compared with normoxic cultured BMSCs.
Increased regenerative and repair potentials of preconditioned cells
Increased regenerative and repair potentials are found following enhanced migration and homing of stem/progenitor cells to the lesion sites. Many chemokine and angiogenic genes are upregulated after hypoxic induction on bone marrow-derived hemangioblasts, which promote their differentiation toward endothelial lineage [106]. Hypoxia enhances the differentiation of EPC-like attaching cells, which in vivo promote neovascularization [136]. The function is highly dependent on its releasing VEGF and increasing VEGF2R expression in response to hypoxia [57]. Hypoxia preconditioned MSCs increase expressions of Wnt4 and stronger vascular regenerative properties of these cells were observed in a hindlimb ischemia model of the mouse [137]. Another important signaling molecule during development, sonic hedgehog (SHH), may be involved in EPC-mediated angiogenesis and neovascularization induced by VEGF, SDF-1 and angiopoietin-1 [138]. Hypoxic preconditioning of hMSCs can effectively restore the osteogenic differentiation, which shows benefit for transplantation therapy for bone regeneration [139]. Treatment on hMSCs with polyP, the mPTP activator, also promotes differentiation of hMSCs into osteoblastic cells through activation of FGF-2 and other related genes at the early and later stages of osteoblastic differentiation [140, 141].
Some trophic/growth factors such as VEGF and BDNF have been used for stem cell cultures. They promote differentiation of human fetal CD133+ liver cells into myogenic and endothelial progenitors, thus enhancing angiomyogenesis [142]. Low-intensity ultrasound and/or TGF-β1 treatment induces in vitro chondrogenic differentiation of MSCs [143]. A recent study using ultraviolet B to precondition ADSCs significantly promotes hair regeneration after transplantation into C(3)H/HeN mice. The regenerative mechanism has been shown to include a contribution from Nox4 generated ROS. Preconditioning using sevoflurane, a volatile anesthetic, promotes the proliferation of circulating CD134+ CD34+ and CD34+ flk-1+ EPCs, leading to enhanced vascular healing and myocardial regeneration [144, 145]. An in vitro study on EPCs preconditioned with SDF-1 demonstrated induced secretion of FGF-2 and MMP2, enhanced cell adhesion and increased differentiation into vascular tubes. All these effects contribute to the stimulating angiogenesis in ischemic hind limb [32]. These effects were significantly attenuated after incubation with a CXCR4 antagonist.
Transplantation of preconditioned MSCs leads to enhanced revascularization and skeletal muscle regeneration, which is observed in the hind limb ischemia model [127, 137, 146], myocardial infarction model [14] and ischemic stroke model [15]. In our investigations, hypoxia-preconditioned BMSCs can significantly reduce infarct size of infarcted heart compared with non-hypoxia-preconditioned MSCs. Hypoxic preconditioned BMSCs showed increased expression and release of angiogenic factors and enhanced angiogenesis, vascularization and myogenesis.
Suppressed inflammatory and immune responses after cell transplantation
Inflammatory and immune responses in host tissues may impose secondary and continuous danger to transplanted cells. Autologous availability from the transplant recipient makes the stem cell transplantation therapy free of immunosuppressive drugs [3]. Transplantations of BMSCs and adipose tissue-derived poietic preadipocyte cells for treating MI also suppress inflammatory responses via significantly decreasing myocardial proinflammatory signaling molecules including TNF-α and IL-6 [147]. NSC transplantation after stroke causes downregulation of many inflammation-related genes (TNF-α, IL-1β, IL-6, IFN-γ) in the brain, and attenuates the glial scar formation by inhibiting the activation of astrocytes [148].
Ischemia or low dose TNF-α preconditioned C2C12 myoblasts show tolerance to ischemic injury by affecting phosphorylation and translocation of cytosolic phospholipase A2 (cPLA2) to the nucleus [149]. Preconditioned cells show inhibitory effects on COX I and COX II production and inflammation. In human MSCs, in response to interferon-gamma (IFN-γ) released by activated T cells and NK cells, indoleamine 2,3-dioxygenase (IDO) inhibits immune responses via decreasing proliferation of those immunocytes [2, 150]. These observations suggest that preconditioning stem cells with cytokines such as TNF-α and IFN-γ may enhance their immunosuppressive effects after transplantation.
We have examined expression of many pro-inflammatory cytokines/chemokines in BMSCs subjected to hypoxia treatment and observed down-regulated genes such as CC3, CC5, CC17, CCL4, CXCR3 and CXCL10 [15]. In hypoxic preconditioned BMSCs, expression of IL-1β, IL-6, TNF-α and OX-42 is noticeably reduced. After intravenous injection into adult rats 24 h after ischemic stroke, compared to normoxia-treated cells, hypoxia-preconditioned BMSCs show a greater ability to suppress microglial activity in the brain.
Enhanced functional recovery by preconditioned cells
The ultimate goal of tissue repair in regenerative medicine is the restoration or recovery of functional activities. Increasing evidence supports that preconditioned cells show markedly better ability in tissue repair and functional recovery. We have demonstrated that transplantation of hypoxia-preconditioned MSCs can significantly improve left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP) and rate of pressure rise of the infarcted heart compared with non-hypoxia-preconditioned MSCs transplantation [14]. Our study on CNS disorders reveals improved motor functions for stroke animals that receive hypoxia-preconditioned MSCs transplantation [15, 16]. In an investigation on transplantation of anoxia preconditioned MSCs in a myocardial ischemia model of diabetic mouse, transplanted cells increased capillary density and attenuated myocardial fibrosis. Fractional shortening (FS) of the post-ischemic myocardium was markedly improved in the animals that received preconditioned MSCs [25]. Following traumatic brain injury (TBI), application of secretome from hypoxia-preconditioned MSCs has lead to better recovery of motor and cognitive functions in rats [121].
In the investigations on ischemic stroke models, we showed significantly more NeuN-positive and Glut1-positive cells in the ischemic core and peri-infarct regions of animals that received hypoxia preconditioned BMSC transplantation than in those that received normoxia treated BMSCs. Some NeuN-positive and Glut-1-positive cells showed eGFP or BrdU immunoflourescent reactivity, suggesting differentiation from exogenous BMSCs into neuronal and vascular endothelial cells. In Rotarod testing performed 15 days after stroke, animals that received hypoxia preconditioned BMSCs showed better locomotion recovery compared with stroke control and non-hypoxic BMSC groups [15]. In our recent investigation of intranasal delivery of BMSCs, we tested sensorimotor functional recovery after the barrel cortex stroke. In adhesive-removal testing performed 3 days after transplantation, stroke mice that received hypoxia-preconditioned BMSCs behaved significantly better than normoxic BMSC and vehicle-treated animals.
C. Further Directions
It is well-known that ischemic/hypoxic preconditioning can generate a cross tolerance and that an individual trigger can increase resistance of preconditioned cells to different insults. The experimental approach that synthesizes the benefits of preconditioning stem cells and progenitor cells has already shown promising therapeutic efficacy [151]. However, it has been assumed that in patients suffering stroke or myocardial ischemia combined with other diseases such as diabetes, one type of preconditioning strategy or preconditioning alone may not induce enough protection [152]. Very recent studies have attempted to combine different preconditioning triggers to increase efficacy. For example, combination of apelin-13 treatment and hypoxia-preconditioned BMSCs has been proposed as a therapeutic strategy for diabetic stroke. We have previously shown that apelin-13 is neuroprotective against apoptosis and ischemic brain damage [48, 153]. In addition, apelin-13 preconditioned BMSCs showed enhanced resistance to apoptotic stimulation through activation of surviving signals. Apelin-13 can also help myocardial progenitor cells survive to repair postmyocardial infarction [41]. Combined pre-treatment with hypoxia may activate more protective mechanisms for synergetic effects. Another preconditioning strategy may involve targeting specific treatment to enhance the survival of implanted cells and paracrine effects on endogenous regeneration. For example, co-transplanted BMSCs can enhance islet graft survival and promote revascularization via paracrine effects [154]. Another recent trial on co-transplantation of NSCs with hypoxia preconditioned ADMSCs showed improved survival of NSCs after implantation into spinal cord injury sites [155].
Acknowledgments
This work was supported by NIH grants NS062097, NS058710, NS057255 and AHA Established Invistigator Award 0840110N. Zheng Wei was an exchange PhD student from Neuroscience Research Institute and Department of Neurobiology, School of Basic Medical Sciences, Peking University, Beijing 100191, China.
Footnotes
Conflict of Interest Statement
All authors declare that they have no conflict of interest.
References
- 1.Deveau T, Yu SP, Wei L. Cellular Therapy for Ischemic Stroke. In: Lapchak PA, Zhang JH, editors. Translational Stroke Research: from Target Selection to Clinical Trials. Springer; New York: 2012. pp. 777–814. [Google Scholar]
- 2.Doorn J, Moll G, Le Blanc K, van Blitterswijk C, de Boer J. Therapeutic Applications of Mesenchymal Stromal Cells: Paracrine Effects and Potential Improvements. Tissue Engineering Part B-Reviews. 2012;18:101–115. doi: 10.1089/ten.TEB.2011.0488. [DOI] [PubMed] [Google Scholar]
- 3.Haider KH, Ashraf M. Preconditioning approach in stem cell therapy for the treatment of infarcted heart. Progress in molecular biology and translational science. 2012;111:323–56. doi: 10.1016/B978-0-12-398459-3.00015-0. [DOI] [PubMed] [Google Scholar]
- 4.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: Underlying mechanisms and clinical application. Atherosclerosis. 2009;204:334–341. doi: 10.1016/j.atherosclerosis.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Pinzon MA. Mechanisms of neuroprotection during ischemic preconditioning: lessons from anoxic tolerance. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:291–9. doi: 10.1016/j.cbpa.2006.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Reme CE. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–24. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- 7.Trendelenburg G, Dirnagl U. Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning. Glia. 2005;50:307–20. doi: 10.1002/glia.20204. [DOI] [PubMed] [Google Scholar]
- 8.Ran R, Xu H, Lu A, Bernaudin M, Sharp FR. Hypoxia preconditioning in the brain. Dev Neurosci. 2005;27:87–92. doi: 10.1159/000085979. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Yu SP, Mohamad O, Genetta T, Wei L. Translational Stroke Research. Springer; New York: 2010. Sublethal Transient Global Ischemia Stimulates Migration of Neuroblasts and Neurogenesis in Mice; pp. 184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Przyklenk K, Whittaker P. Remote Ischemic Preconditioning: Current Knowledge, Unresolved Questions, and Future Priorities. Journal of Cardiovascular Pharmacology and Therapeutics. 2011;16:255–259. doi: 10.1177/1074248411409040. [DOI] [PubMed] [Google Scholar]
- 11.Fairbanks SL, Brambrink AM. Preconditioning and postconditioning for neuroprotection: the most recent evidence. Best practice & research Clinical anaesthesiology. 2010;24:521–34. doi: 10.1016/j.bpa.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Koch S, Katsnelson M, Dong CH, Perez-Pinzon M. Remote Ischemic Limb Preconditioning After Subarachnoid Hemorrhage A Phase Ib Study of Safety and Feasibility. Stroke. 2011;42:1387–1391. doi: 10.1161/STROKEAHA.110.605840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurology. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu XY, Yu SP, Fraser JL, Lu ZY, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. Journal of Thoracic and Cardiovascular Surgery. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 15.Wei L, Fraser JL, Lu ZY, Hu XY, Yu SP. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiology of Disease. 2012;46:635–645. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theus MH, Wei L, Cui L, Francis K, Hu XY, Keogh C, Yu SP. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Experimental Neurology. 2008;210:656–670. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Ogle ME, Yu SP, Wei L. Primed for lethal battle: A step forward to enhance the efficacy and efficiency of stem cell transplantation therapy. Journal of Thoracic and Cardiovascular Surgery. 2009;138:527–527. doi: 10.1016/j.jtcvs.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T, Losordo DW. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation. 2005;111:1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 19.Yan FD, Yao YY, Chen LJ, Li YF, Sheng ZL, Ma GS. Hypoxic Preconditioning Improves Survival of Cardiac Progenitor Cells: Role of Stromal Cell Derived Factor-1 alpha-CXCR4 Axis. Plos One. 2012;7:9. doi: 10.1371/journal.pone.0037948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stubbs SL, Hsiao STF, Peshavariya HM, Lim SY, Dusting GJ, Dilley RJ. Hypoxic Preconditioning Enhances Survival of Human Adipose-Derived Stem Cells and Conditions Endothelial Cells In Vitro. Stem Cells and Development. 2012;21:1887–1896. doi: 10.1089/scd.2011.0289. [DOI] [PubMed] [Google Scholar]
- 21.Aly A, Peterson KM, Lerman A, Lerman LO, Rodriguez-Porcel M. Role of oxidative stress in hypoxia preconditioning of cells transplanted to the myocardium: a molecular imaging study. Journal of Cardiovascular Surgery. 2011;52:579–585. [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson KM, Aly A, Lerman A, Lerman LO, Rodriguez-Porcel M. Improved survival of mesenchymal stromal cell after hypoxia preconditioning: Role of oxidative stress. Life Sciences. 2011;88:65–73. doi: 10.1016/j.lfs.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das R, Jahr H, van Osch G, Farrell E. The Role of Hypoxia in Bone Marrow-Derived Mesenchymal Stem Cells: Considerations for Regenerative Medicine Approaches. Tissue Engineering Part B-Reviews. 2010;16:159–168. doi: 10.1089/ten.TEB.2009.0296. [DOI] [PubMed] [Google Scholar]
- 24.Wang JA, He A, Hu XY, Jiang Y, Sun Y, Jiang J, Gui C, Wang YP, Chen H. Anoxic preconditioning: A way to enhance the cardioprotection of mesenchymal stem cells. International Journal of Cardiology. 2009;133:410–412. doi: 10.1016/j.ijcard.2007.11.096. [DOI] [PubMed] [Google Scholar]
- 25.Li JH, Zhang N, Wang JA. Improved anti-apoptotic and anti-remodeling potency of bone marrow mesenchyma stem cells by anoxic pre-conditioning in diabetic cardiomyopathy. Journal of Endocrinological Investigation. 2008;31:103–110. doi: 10.1007/BF03345575. [DOI] [PubMed] [Google Scholar]
- 26.He AN, Jiang Y, Gui C, Sun Y, Li JH, Wang JA. The antiapoptotic effect of mesenchymal stem cell transplantation on ischemic myocardium is enhanced by anoxic preconditioning. Canadian Journal of Cardiology. 2009;25:353–358. doi: 10.1016/s0828-282x(09)70094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie XX, Sun AJ, Zhu WQ, Huang ZY, Hu XY, Jia JG, Zou YZ, Ge JB. Transplantation of Mesenchymal Stem Cells Preconditioned with Hydrogen Sulfide Enhances Repair of Myocardial Infarction in Rats. Tohoku Journal of Experimental Medicine. 2012;226:29–36. doi: 10.1620/tjem.226.29. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Chen GH, Wang YW, Zhao J, Duan HF, Liao LM, Zhang XZ, Chen YD, Chen H. Hydrogen peroxide preconditioning enhances the therapeutic efficacy of Wharton’s Jelly mesenchymal stem cells after myocardial infarction. Chinese medical journal. 2012;125:3472–8. [PubMed] [Google Scholar]
- 29.Kondo-Nakamura M, Shintani-Ishida K, Uemura K, Yoshida K. Brief exposure to carbon monoxide preconditions cardiomyogenic cells against apoptosis in ischemia-reperfusion. Biochemical and Biophysical Research Communications. 2010;393:449–454. doi: 10.1016/j.bbrc.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Lu ZY, Keogh CL, Yu SP, Wei L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. Journal of Cerebral Blood Flow and Metabolism. 2007;27:1043–1054. doi: 10.1038/sj.jcbfm.9600417. [DOI] [PubMed] [Google Scholar]
- 31.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovascular Research. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 32.Zemani F, Silvestre JS, Fauvel-Lafeve F, Bruel A, Vilar J, Bieche I, Laurendeau I, Galy-Fauroux I, Fischer AM, Boisson-Vidal C. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arteriosclerosis Thrombosis and Vascular Biology. 2008;28:644–650. doi: 10.1161/ATVBAHA.107.160044. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Du XL, Zhang KL. Effects of stromal-derived factor 1 preconditioning on apoptosis of rat bone mesenchymal stem cells. Journal of Huazhong University of Science and Technology-Medical Sciences. 2009;29:423–426. doi: 10.1007/s11596-009-0406-8. [DOI] [PubMed] [Google Scholar]
- 34.Lu G, Ashraf M, Haider KH. Insulin-Like Growth Factor-1 Preconditioning Accentuates Intrinsic Survival Mechanism in Stem Cells to Resist Ischemic Injury by Orchestrating Protein Kinase C alpha-Erk1/2 Activation. Antioxidants & Redox Signaling. 2012;16:217–227. doi: 10.1089/ars.2011.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tilkorn DJ, Davies EM, Keramidaris E, Dingle AM, Gerrand YW, Taylor CJ, Han XL, Palmer JA, Penington AJ, Mitchell CA, Morrison WA, Dusting GJ, Mitchell GM. The in vitro preconditioning of myoblasts to enhance subsequent survival in an in vivo tissue engineering chamber model. Biomaterials. 2012;33:3868–3879. doi: 10.1016/j.biomaterials.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Jiang BM, Xiao WM, Shi YZ, Liu MD, Xiao XZ. Heat shock pretreatment inhibited the release of Smac/DIABLO from mitochondria and apoptosis induced by hydrogen peroxide in cardiomyocytes and C2C12 myogenic cells. Cell Stress & Chaperones. 2005;10:252–262. doi: 10.1379/CSC-124R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui XJ, Wang HJ, Guo HD, Wang C, Ao H, Liu XQ, Tan YZ. Transplantation of Mesenchymal Stem Cells Preconditioned with Diazoxide, a Mitochondrial ATP-Sensitive Potassium Channel Opener, Promotes Repair of Myocardial Infarction in Rats. Tohoku Journal of Experimental Medicine. 2010;220:139–147. doi: 10.1620/tjem.220.139. [DOI] [PubMed] [Google Scholar]
- 38.Niagara MI, Haider HK, Jiang SJ, Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circulation Research. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 39.Idris NM, Ashraf M, Ahmed RPH, Jiang SJ, Haider KH. Activation of IL-11/STAT3 pathway in preconditioned human skeletal myoblasts blocks apoptotic cascade under oxidant stress. Regenerative Medicine. 2012;7:47–57. doi: 10.2217/rme.11.109. [DOI] [PubMed] [Google Scholar]
- 40.Afzal MR, Haider HK, Idris NM, Jiang SJ, Ahmed RPH, Ashraf M. Preconditioning Promotes Survival and Angiomyogenic Potential of Mesenchymal Stem Cells in the Infarcted Heart via NF-kappa B Signaling. Antioxidants & Redox Signaling. 2010;12:693–702. doi: 10.1089/ars.2009.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li LF, Zeng H, Chen JX. Apelin-13 increases myocardial progenitor cells and improves repair postmyocardial infarction. American Journal of Physiology-Heart and Circulatory Physiology. 2012;303:H605–H618. doi: 10.1152/ajpheart.00366.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JH, Oh AY, Choi YM, Ku SY, Kim YY, Lee NJ, Sepac A, Bosnjak ZJ. Isoflurane decreases death of human embryonic stem cell-derived, transcriptional marker Nkx2.5(+) cardiac progenitor cells. Acta Anaesthesiologica Scandinavica. 2011;55:1124–1131. doi: 10.1111/j.1399-6576.2011.02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao YW, Zhang FM, Wang LS, Zhang GH, Wang ZJ, Chen JM, Gao X. Lipopolysaccharide preconditioning enhances the efficacy of mesenchymal stem cells transplantation in a rat model of acute myocardial infarction. Journal of Biomedical Science. 2009;16:11. doi: 10.1186/1423-0127-16-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai C, Teng L, Vu D, He JQ, Guo Y, Li Q, Tang XL, Rokosh G, Bhatnagar A, Bolli R. The Heme Oxygenase 1 Inducer (CoPP) Protects Human Cardiac Stem Cells against Apoptosis through Activation of the Extracellular Signal-regulated Kinase (ERK)/NRF2 Signaling Pathway and Cytokine Release. The Journal of biological chemistry. 2012;287:33720–32. doi: 10.1074/jbc.M112.385542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haider HK, Ashraf M. Strategies to promote donor cell survival: Combining preconditioning approach with stem cell transplantation. Journal of Molecular and Cellular Cardiology. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haider HK, Ashraf M. Preconditioning and Stem Cell Survival. Journal of Cardiovascular Translational Research. 2010;3:89–102. doi: 10.1007/s12265-009-9161-2. [DOI] [PubMed] [Google Scholar]
- 47.Liu HB, Xue WJ, Ge GQ, Luo XH, Li Y, Xiang HL, Ding XM, Tian PX, Tian XH. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1 alpha in MSCs. Biochemical and Biophysical Research Communications. 2010;401:509–515. doi: 10.1016/j.bbrc.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 48.Zeng XJ, Yu SP, Taylor T, Ogle M, Wei L. Protective effect of apelin on cultured rat bone marrow mesenchymal stem cells against apoptosis. Stem Cell Research. 2012;8:357–367. doi: 10.1016/j.scr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, Sun BC, Hideg K, Kuppusamy P. Pharmacological Preconditioning of Mesenchymal Stem Cells with Trimetazidine (1-2,3,4-Trimethoxybenzyl piperazine) Protects Hypoxic Cells against Oxidative Stress and Enhances Recovery of Myocardial Function in Infarcted Heart through Bcl-2 Expression. Journal of Pharmacology and Experimental Therapeutics. 2009;329:543–550. doi: 10.1124/jpet.109.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi KE, Hall CL, Sun JM, Wei L, Mohamad O, Dix TA, Yu SP. A novel stroke therapy of pharmacologically induced hypothermia after focal cerebral ischemia in mice. Faseb Journal. 2012;26:2799–2810. doi: 10.1096/fj.11-201822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogle ME, Gu XH, Espinera AR, Wei L. Inhibition of prolyl hydroxylases by dimethyloxaloylglycine after stroke reduces ischemic brain injury and requires hypoxia inducible factor-1 alpha. Neurobiology of Disease. 2012;45:733–742. doi: 10.1016/j.nbd.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Semenza GL. Hypoxia-inducible factor 1: Regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochimica Et Biophysica Acta-Molecular Cell Research. 2011;1813:1263–1268. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dehne N, Brune B. HIF-1 in the inflammatory microenvironment. Experimental Cell Research. 2009;315:1791–1797. doi: 10.1016/j.yexcr.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 54.Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. Embo Journal. 2012;31:2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valsecchi V, Pignataro G, Del Prete A, Sirabella R, Matrone C, Boscia F, Scorziello A, Sisalli MJ, Esposito E, Zambrano N, Di Renzo G, Annunziato L. NCX1 Is a Novel Target Gene for Hypoxia-Inducible Factor-1 in Ischemic Brain Preconditioning. Stroke. 2011;42:754–763. doi: 10.1161/STROKEAHA.110.597583. [DOI] [PubMed] [Google Scholar]
- 56.Keogh CL, Yu SP, Wei L. The effect of recombinant human erythropoietin on neurovasculature repair after focal ischemic stroke in neonatal rats. Journal of Pharmacology and Experimental Therapeutics. 2007;322:521–528. doi: 10.1124/jpet.107.121392. [DOI] [PubMed] [Google Scholar]
- 57.Li WL, Fraser JL, Yu SP, Zhu J, Jiang YJ, Wei L. The role of VEGF/VEGFR2 signaling in peripheral stimulation-induced cerebral neurovascular regeneration after ischemic stroke in mice. Experimental Brain Research. 2011;214:503–513. doi: 10.1007/s00221-011-2849-y. [DOI] [PubMed] [Google Scholar]
- 58.Li WL, Yu SP, Ogle ME, Ding XS, Wei L. Enhanced Neurogenesis and Cell Migration Following Focal Ischemia and Peripheral Stimulation in Mice. Developmental Neurobiology. 2008;68:1474–1486. doi: 10.1002/dneu.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitaker VR, Cui L, Miller S, Yu SP, Wei L. Whisker stimulation enhances angiogenesis in the barrel cortex following focal ischemia in mice. Journal of Cerebral Blood Flow and Metabolism. 2007;27:57–68. doi: 10.1038/sj.jcbfm.9600318. [DOI] [PubMed] [Google Scholar]
- 60.Peng H, Wu Y, Duan Z, Ciborowski P, Zheng JC. Proteolytic processing of SDF-1alpha by matrix metalloproteinase-2 impairs CXCR4 signaling and reduces neural progenitor cell migration. Protein & cell. 2012;3:875–82. doi: 10.1007/s13238-012-2092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahfoudh-Boussaid A, Zaouali MA, Hadj-Ayed K, Miled AH, Saidane-Mosbahi D, Rosello-Catafau J, Ben Abdennebi H. Ischemic preconditioning reduces endoplasmic reticulum stress and upregulates hypoxia inducible factor-1 alpha in ischemic kidney: the role of nitric oxide. Journal of Biomedical Science. 2011;19:8. doi: 10.1186/1423-0127-19-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson MA, Baumgardner JE, Otto CM. Oxygen-dependent regulation of nitric oxide production by inducible nitric oxide synthase. Free Radical Biology and Medicine. 2011;51:1952–1965. doi: 10.1016/j.freeradbiomed.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 63.Liu XB, Wang JA, Ogle ME, Wei L. Prolyl Hydroxylase Inhibitor Dimethyloxalylglycine Enhances Mesenchymal Stem Cell Survival. Journal of Cellular Biochemistry. 2009;106:903–911. doi: 10.1002/jcb.22064. [DOI] [PubMed] [Google Scholar]
- 64.Sims B, Clarke M, Francillion L, Kindred E, Hopkins ES, Sontheimer H. Hypoxic preconditioning involves system Xc(-) regulation in mouse neural stem cells. Stem Cell Research. 2012;8:285–291. doi: 10.1016/j.scr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. Journal of Neuroscience. 2006;26:10514–10523. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cerrada I, Ruiz-Sauri A, Carrero R, Trigueros C, Dorronsoro A, Sanchez-Puelles JM, Diez-Juan A, Montero JA, Sepulveda P. Hypoxia-Inducible Factor 1 Alpha Contributes to Cardiac Healing in Mesenchymal Stem Cells-Mediated Cardiac Repair. Stem Cells Dev. doi: 10.1089/scd.2012.0340. [DOI] [PubMed] [Google Scholar]
- 67.Park J, Park H-H, Choi H, Seo Kim Y, Yu H-J, Lee K-Y, Joo Lee Y, Hyun Kim S, Koh S-H. Coenzyme Q10 protects neural stem cells against hypoxia by enhancing survival signals. Brain research. 2012;1478:64–73. doi: 10.1016/j.brainres.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 68.Dirnagl U, Meisel A. Endogenous neuroprotection: Mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55:334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 69.Ravati A, Ahlemeyer B, Becker A, Klumpp S, Krieglstein J. Preconditioning-induced neuroprotection is mediated by reactive oxygen species and activation of the transcription factor nuclear factor-kappa B. Journal of Neurochemistry. 2001;78:909–919. doi: 10.1046/j.1471-4159.2001.00463.x. [DOI] [PubMed] [Google Scholar]
- 70.Jou MJ. Pathophysiological and pharmacological implications of mitochondria-targeted reactive oxygen species generation in astrocytes. Advanced Drug Delivery Reviews. 2008;60:1512–1526. doi: 10.1016/j.addr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Tang XQ, Feng JQ, Chen J, Chen PX, Zhi JL, Cui Y, Guo RX, Yu HM. Protection of oxidative preconditioning against apoptosis induced by H2O2 in PC12 cells: Mechanisms via MMP, ROS, and Bcl-2. Brain Research. 2005;1057:57–64. doi: 10.1016/j.brainres.2005.07.072. [DOI] [PubMed] [Google Scholar]
- 72.Xiao L, Lan A, Mo L, Xu W, Jiang N, Hu F, Feng J, Zhang C. Hydrogen sulfide protects PC12 cells against reactive oxygen species and extracellular signal-regulated kinase 1/2-mediated downregulation of glutamate transporter-1 expression induced by chemical hypoxia. International journal of molecular medicine. 2012;30:1126–32. doi: 10.3892/ijmm.2012.1090. [DOI] [PubMed] [Google Scholar]
- 73.Waszak P, Alphonse R, Vadivel A, Ionescu L, Eaton F, Thebaud B. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem cells and development. 2012;21:2789–97. doi: 10.1089/scd.2010.0566. [DOI] [PubMed] [Google Scholar]
- 74.Furuichi T, Liu WL, Shi HL, Miyake M, Liu KJ. Generation of hydrogen peroxide during brief oxygen-glucose deprivation induces preconditioning neuronal protection in primary cultured neurons. Journal of Neuroscience Research. 2005;79:816–824. doi: 10.1002/jnr.20402. [DOI] [PubMed] [Google Scholar]
- 75.Sakata H, Niizuma K, Yoshioka H, Kim GS, Jung JE, Katsu M, Narasimhan P, Maier CM, Nishiyama Y, Chan PH. Minocycline-Preconditioned Neural Stem Cells Enhance Neuroprotection after Ischemic Stroke in Rats. Journal of Neuroscience. 2012;32:3462–3473. doi: 10.1523/JNEUROSCI.5686-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seidlmayer LK, Gomez-Garcia MR, Blatter LA, Pavlov E, Dedkova EN. Inorganic polyphosphate is a potent activator of the mitochondrial permeability transition pore in cardiac myocytes. Journal of General Physiology. 2012;139:321–331. doi: 10.1085/jgp.201210788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abramov AY, Fraley C, Diao CT, Winkfein R, Colicos MA, Duchen MR, French RJ, Pavlov E. Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18091–18096. doi: 10.1073/pnas.0708959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang JA, Chen TL, Jiang J, Shi H, Gui C, Luo RH, Xie XJ, Xiang MX, Zhang X. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacologica Sinica. 2008;29:74–82. doi: 10.1111/j.1745-7254.2008.00716.x. [DOI] [PubMed] [Google Scholar]
- 79.Sepac A, Sedlic F, Si-Tayeb K, Lough J, Duncan SA, Bienengraeber M, Park F, Kim J, Bosnjak ZJ. Isoflurane Preconditioning Elicits Competent Endogenous Mechanisms of Protection from Oxidative Stress in Cardiomyocytes Derived from Human Embryonic Stem Cells. Anesthesiology. 2010;113:906–916. doi: 10.1097/ALN.0b013e3181eff6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fretwell L, Dickenson JM. Role of large-conductance Ca2+-activated potassium channels in adenosine A(1) receptor-mediated pharmacological preconditioning in H9c2 cells. European Journal of Pharmacology. 2009;618:37–44. doi: 10.1016/j.ejphar.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 81.Simerabet M, Robin E, Aristi I, Adamczyk S, Tavernier B, Vallet B, Bordet R, Lebuffe G. Preconditioning by an in situ administration of hydrogen peroxide: Involvement of reactive oxygen species and mitochondrial ATP-dependent potassium channel in a cerebral ischemia-reperfusion model. Brain Research. 2008;1240:177–184. doi: 10.1016/j.brainres.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 82.Sheng R, Liu XQ, Zhang LS, Gao B, Han R, Wu YQ, Zhang XY, Qin ZH. Autophagy regulates endoplasmic reticulum stress in ischemic preconditioning. Autophagy. 2012;8:310–325. doi: 10.4161/auto.18673. [DOI] [PubMed] [Google Scholar]
- 83.Park HK, Chu K, Jung KH, Lee ST, Bahn JJ, Kim M, Lee SK, Roh JK. Autophagy is involved in the ischemic preconditioning. Neuroscience Letters. 2009;451:16–19. doi: 10.1016/j.neulet.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 84.Yeh CH, Hsu SP, Yang CC, Chien CT, Wang NP. Hypoxic preconditioning reinforces HIF-alpha-dependent HSP70 signaling to reduce ischemic renal failure-induced renal tubular apoptosis and autophagy. Life Sciences. 2010;86:115–123. doi: 10.1016/j.lfs.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 85.Rodriguez-Sinovas A, Boengler K, Cabestrero A, Gres P, Morente M, Ruiz-Meana M, Konietzka I, Miro E, Totzeck A, Heusch G, Schulz R, Garcia-Dorado D. Translocation of connexin 43 to the inner mitochondrial membrane of cardiomyocytes through the heat shock protein 90-dependent TOM pathway and its importance for cardioprotection. Circulation Research. 2006;99:93–101. doi: 10.1161/01.RES.0000230315.56904.de. [DOI] [PubMed] [Google Scholar]
- 86.Fontes MSC, van Veen LAB, de Bakker JMT, van Rijen HVM. Functional consequences of abnormal Cx43 expression in the heart. Biochimica Et Biophysica Acta-Biomembranes. 2012;1818:2020–2029. doi: 10.1016/j.bbamem.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 87.Axelsen LN, Stahlhut M, Mohammed S, Larsen BD, Nielsen MS, Holstein-Rathlou NH, Andersen S, Jensen ON, Hennan JK, Kjolbye AL. Identification of ischemia-regulated phosphorylation sites in connexin43: A possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123) Journal of Molecular and Cellular Cardiology. 2006;40:790–798. doi: 10.1016/j.yjmcc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 88.Lu G, Haider HK, Jiang SJ, Ashraf M. Sca-1(+) Stem Cell Survival and Engraftment in the Infarcted Heart Dual Role for Preconditioning-Induced Connexin-43. Circulation. 2009;119:2587–U107. doi: 10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MVL, Naus CC, Giaume C, Saez JC. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. Journal of Neurochemistry. 2011;118:826–840. doi: 10.1111/j.1471-4159.2011.07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin JHC, Lou N, Kang N, Takano T, Hu F, Han XN, Xu QQ, Lovatt D, Torres A, Willecke K, Yang J, Kang J, Nedergaard M. A central role of connexin 43 in hypoxic preconditioning. Journal of Neuroscience. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jaderstad J, Brismar H, Herlenius E. Hypoxic preconditioning increases gap-junctional graft and host communication. Neuroreport. 2010;21:1126–1132. doi: 10.1097/WNR.0b013e328340a77b. [DOI] [PubMed] [Google Scholar]
- 92.Lu G, Jiang SJ, Ashraf M, Haider KH. Subcellular preconditioning of stem cells: mito-Cx43 gene targeting is cytoprotective via shift of mitochondrial Bak and Bcl-xL balance. Regenerative Medicine. 2012;7:323–334. doi: 10.2217/rme.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu G, Haider HK, Porollo A, Ashraf M. Mitochondria-specific transgenic overexpression of connexin-43 simulates preconditioning-induced cytoprotection of stem cells. Cardiovascular Research. 2010;88:277–286. doi: 10.1093/cvr/cvq293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang DG, Shen WZ, Zhang FX, Chen ML, Chen HW, Cao KJ. Connexin43 promotes survival of mesenchymal stem cells in ischaemic heart. Cell Biology International. 2010;34:415–423. doi: 10.1042/CBI20090118. [DOI] [PubMed] [Google Scholar]
- 95.Ahmad Waza A, Andrabi K, Ul Hussain M. Adenosine-triphosphate-sensitive K(+)channel (Kir6.1): A novel phosphospecific interaction partner of connexin 43 (Cx43) Experimental cell research. 2012;318:2559–66. doi: 10.1016/j.yexcr.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 96.Rottlaender D, Boengler K, Wolny M, Michels G, Endres-Becker J, Motloch LJ, Schwaiger A, Buechert A, Schulz R, Heusch G, Hoppe UC. Connexin 43 acts as a cytoprotective mediator of signal transduction by stimulating mitochondrial K-ATP channels in mouse cardiomyocytes. Journal of Clinical Investigation. 2010;120:1441–1453. doi: 10.1172/JCI40927. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97.Du WJ, Li JK, Wang QY, Hou JB, Yu B. Lithium Chloride Preconditioning Optimizes Skeletal Myoblast Functions for Cellular Cardiomyoplasty in vitro via Glycogen Synthase Kinase-3 beta/beta-Catenin Signaling. Cells Tissues Organs. 2009;190:11–19. doi: 10.1159/000167699. [DOI] [PubMed] [Google Scholar]
- 98.Sierra MD, Yang FQ, Narazaki M, Salvucci O, Davis D, Yarchoan R, Zhang HWH, Fales H, Tosato G. Differential processing of strornal-derived factor-1 alpha and strornal-derived factor-1 beta explains functional diversity. Blood. 2004;103:2452–2459. doi: 10.1182/blood-2003-08-2857. [DOI] [PubMed] [Google Scholar]
- 99.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nature Immunology. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 100.Sharma M, Afrin F, Satija N, Tripathi RP, Gangenahalli GU. Stromal-Derived Factor-1/CXCR4 Signaling: Indispensable Role in Homing and Engraftment of Hematopoietic Stem Cells in Bone Marrow. Stem Cells and Development. 2011;20:933–946. doi: 10.1089/scd.2010.0263. [DOI] [PubMed] [Google Scholar]
- 101.Zhao DH, Najbauer J, Garcia E, Metz MZ, Gutova M, Glackin CA, Kim SU, Aboody KS. Neural Stem Cell Tropism to Glioma: Critical Role of Tumor Hypoxia. Molecular Cancer Research. 2008;6:1819–1829. doi: 10.1158/1541-7786.MCR-08-0146. [DOI] [PubMed] [Google Scholar]
- 102.Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, Prockop DJ. Short-Term Exposure of Multipotent Stromal Cells to Low Oxygen Increases Their Expression of CX3CR1 and CXCR4 and Their Engraftment In Vivo. Plos One. 2007;2:11. doi: 10.1371/journal.pone.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kubo M, Li TS, Kamota T, Ohshima M, Qin SL, Hamano K. Increased Expression of CXCR4 and Integrin alpha M in Hypoxia-Preconditioned Cells Contributes to Improved Cell Retention and Angiogenic Potency. Journal of Cellular Physiology. 2009;220:508–514. doi: 10.1002/jcp.21803. [DOI] [PubMed] [Google Scholar]
- 104.Tang YL, Zhu WQ, Cheng M, Chen LJ, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin GJ. Hypoxic Preconditioning Enhances the Benefit of Cardiac Progenitor Cell Therapy for Treatment of Myocardial Infarction by Inducing CXCR4 Expression. Circulation Research. 2009;104:1209–U218. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cencioni C, Capogrossi MC, Napolitano M. The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovascular Research. 2012;94:400–407. doi: 10.1093/cvr/cvs132. [DOI] [PubMed] [Google Scholar]
- 106.Ong LL, Li WZ, Oldigs JK, Kaminski A, Gerstmayer B, Piechaczek C, Wagner W, Li RK, Ma N, Steinhoff G. Hypoxic/Normoxic Preconditioning Increases Endothelial Differentiation Potential of Human Bone Marrow CD133+Cells. Tissue Engineering Part C-Methods. 2010;16:1069–1081. doi: 10.1089/ten.TEC.2009.0641. [DOI] [PubMed] [Google Scholar]
- 107.Lin JS, Chen YS, Chiang HS, Ma MC. Hypoxic preconditioning protects rat hearts against ischaemia-reperfusion injury: role of erythropoietin on progenitor cell mobilization. Journal of Physiology-London. 2008;586:5757–5769. doi: 10.1113/jphysiol.2008.160887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miller JT, Bartley JH, Wimborne HJC, Walker AL, Hess DC, Hill WD, Carroll JE. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. Bmc Neuroscience. 2005;6:11. doi: 10.1186/1471-2202-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao H, Priebe W, Glod J, Banerjee D. Activation of Signal Transducers and Activators of Transcription 3 and Focal Adhesion Kinase by Stromal Cell-Derived Factor 1 Is Required for Migration of Human Mesenchymal Stem Cells in Response to Tumor Cell-Conditioned Medium. Stem Cells. 2009;27:857–865. doi: 10.1002/stem.23. [DOI] [PubMed] [Google Scholar]
- 110.Zheng H, Fu GS, Dai T, Huang H. Migration of endothelial progenitor cells mediated by stromal cell-derived Factor-1 alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. Journal of Cardiovascular Pharmacology. 2007;50:274–280. doi: 10.1097/FJC.0b013e318093ec8f. [DOI] [PubMed] [Google Scholar]
- 111.Kim HW, Mallick F, Durrani S, Ashraf M, Jiang SJ, Haider KH. Concomitant Activation of miR-107/PDCD10 and Hypoxamir-210/Casp8ap2 and Their Role in Cytoprotection During Ischemic Preconditioning of Stem Cells. Antioxidants & Redox Signaling. 2012;17:1053–1065. doi: 10.1089/ars.2012.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meng S, Cao JT, Wang LS, Zhou Q, Li YG, Shen CX, Zhang XP, Wang CQ. MicroRNA 107 Partly Inhibits Endothelial Progenitor Cells Differentiation via HIF-1 beta. Plos One. 2012;7:7. doi: 10.1371/journal.pone.0040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suzuki Y, Kim HW, Ashraf M, Haider HK. Diazoxide potentiates mesenchymal stem cell survival via NF-kappa B-dependent miR-146a expression by targeting Fas. American Journal of Physiology-Heart and Circulatory Physiology. 2010;299:H1077–H1082. doi: 10.1152/ajpheart.00212.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kostjuk S, Loseva P, Chvartatskaya O, Ershova E, Smirnova T, Malinovskaya E, Roginko O, Kuzmin V, Izhevskaia V, Baranova A, Ginter E, Veiko N. Extracellular GC-rich DNA activates TLR9-and NF-kB-dependent signaling pathways in human adipose-derived mesenchymal stem cells (haMSCs) Expert Opinion on Biological Therapy. 2012;12:S99–S111. doi: 10.1517/14712598.2012.690028. [DOI] [PubMed] [Google Scholar]
- 115.Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death & Disease. 2010;1:11. doi: 10.1038/cddis.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin C, Jun J, Ling W, Xin Z, Fraser JL, Snider BJ, Yu SP. Transplantation of embryonic stem cells improves nerve repair and functional recovery after severe sciatic nerve axotomy in rats. Stem Cells. 2008;26:1356–1365. doi: 10.1634/stemcells.2007-0333. [DOI] [PubMed] [Google Scholar]
- 117.Khan M, Akhtar S, Mohsin S, Khan SN, Riazuddin S. Growth Factor Preconditioning Increases the Function of Diabetes-Impaired Mesenchymal Stem Cells. Stem Cells and Development. 2011;20:67–75. doi: 10.1089/scd.2009.0397. [DOI] [PubMed] [Google Scholar]
- 118.Hoke NN, Salloum FN, Kass DA, Das A, Kukreja RC. Preconditioning by Phosphodiesterase-5 Inhibition Improves Therapeutic Efficacy of Adipose-Derived Stem Cells Following Myocardial Infarction in Mice. Stem Cells. 2012;30:326–335. doi: 10.1002/stem.789. [DOI] [PubMed] [Google Scholar]
- 119.Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan JN, Meldrum DR. Preconditiong mesenchymal stem cells with transforming growth factor-α improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010;33:24–30. doi: 10.1097/SHK.0b013e3181b7d137. [DOI] [PubMed] [Google Scholar]
- 120.Efimenko A, Starostina E, Kalinina N, Stolzing A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. Journal of Translational Medicine. 2011;9:13. doi: 10.1186/1479-5876-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang CP, Chio CC, Cheong CU, Chao CM, Cheng BC, Lin MT. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clinical science (London, England: 1979) 2013;124:165–76. doi: 10.1042/CS20120226. [DOI] [PubMed] [Google Scholar]
- 122.Mohamad O, Chen DD, Zhang LL, Hofmann C, Wei L, Yu SP. Erythropoietin reduces neuronal cell death and hyperalgesia induced by peripheral inflammatory pain in neonatal rats. Molecular Pain. 2011;7:15. doi: 10.1186/1744-8069-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li JM, Li JP, Zhang X, Lu ZY, Yu SP, Wei L. Expression of heparanase in vascular cells and astrocytes of the mouse brain after focal cerebral ischemia. Brain Research. 2012;1433:137–144. doi: 10.1016/j.brainres.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hu X, Wei L, Taylor TM, Wei J, Zhou X, Wang JA, Yu SP. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. American Journal of Physiology-Cell Physiology. 2011;301:C362–C372. doi: 10.1152/ajpcell.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kamota T, Li TS, Morikage N, Murakami M, Ohshima M, Kubo M, Kobayashi T, Mikamo A, Ikeda Y, Matsuzaki M, Hamano K. Ischemic Pre-Conditioning Enhances the Mobilization and Recruitment of Bone Marrow Stem Cells to Protect Against Ischemia/Reperfusion Injury in the Late Phase. Journal of the American College of Cardiology. 2009;53:1814–1822. doi: 10.1016/j.jacc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 126.Li SY, Deng YB, Feng JQ, Ye WB. Oxidative preconditioning promotes bone marrow mesenchymal stem cells migration and prevents apoptosis. Cell Biology International. 2009;33:411–418. doi: 10.1016/j.cellbi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 127.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hayakawa J, Migita M, Ueda T, Fukazawa R, Adachi K, Ooue Y, Hayakawa M, Shimada T, Fukunaga Y. Dextran Sulfate and Stromal Cell Derived Factor-1 Promote CXCR4 Expression and Improve Bone Marrow Homing Efficiency of Infused Hematopoietic Stem Cells. Journal of Nippon Medical School. 2009;76:198–208. doi: 10.1272/jnms.76.198. [DOI] [PubMed] [Google Scholar]
- 129.Hu X, Wei L, Taylor TM, Wei J, Zhou X, Wang J-A, Yu SP. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. American Journal of Physiology-Cell Physiology. 2011;301 doi: 10.1152/ajpcell.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wei JF, Wei L, Zhou X, Lu ZY, Francis K, Hu XY, Liu Y, Xiong WC, Zhang X, Banik NL, Zheng SS, Yu SP. Formation of Kv2.1-FAK complex as a mechanism of FAK activation, cell polarization and enhanced motility. Journal of Cellular Physiology. 2008;217:544–557. doi: 10.1002/jcp.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wei J-F, Wei L, Zhou X, Lu Z-Y, Francis K, Hu X-Y, Liu Y, Xiong W-C, Zhang X, Banik NL, Zheng S-S, Yu SP. Formation of Kv2.1-FAK complex as a mechanism of FAK activation, cell polarization and enhanced motility. Journal of Cellular Physiology. 2008;217 doi: 10.1002/jcp.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rota C, Imberti B, Pozzobon M, Piccoli M, De Coppi P, Atala A, Gagliardini E, Xinaris C, Benedetti V, Fabricio ASC, Squarcina E, Abbate M, Benigni A, Remuzzi G, Morigi M. Human Amniotic Fluid Stem Cell Preconditioning Improves Their Regenerative Potential. Stem Cells and Development. 2012;21:1911–1923. doi: 10.1089/scd.2011.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gyongyosi M, Posa A, Pavo N, Hemetsberger R, Kvakan H, Steiner-Boker S, Zsolt P, Ferenc M, Pavo IJ, Edes IF, Wojta J, Glogar D, Huber K. Differential effect of ischaemic preconditioning on mobilisation and recruitment of haematopoietic and mesenchymal stem cells in porcine myocardial ischaemia-reperfusion. Thrombosis and Haemostasis. 2010;104:376–384. doi: 10.1160/TH09-08-0558. [DOI] [PubMed] [Google Scholar]
- 134.Czeiger D, Dukhno O, Douvdevani A, Porat Y, Shimoni D, Fulga V, Ament JD, Shaked G. Transient Extremity Ischemia Augments CD34+Progenitor Cell Availability. Stem Cell Reviews and Reports. 2011;7:639–645. doi: 10.1007/s12015-011-9234-x. [DOI] [PubMed] [Google Scholar]
- 135.Patschan D, Krupincza K, Patschan S, Zhang ZT, Hamby C, Goligorsky MS. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. American Journal of Physiology-Renal Physiology. 2006;291:F176–F185. doi: 10.1152/ajprenal.00454.2005. [DOI] [PubMed] [Google Scholar]
- 136.Akita T, Murohara T, Ikeda H, Sasaki KI, Shimada T, Egami K, Imaizumi T. Hypoxic preconditioning augments efficacy of human endothelial progenitor cells for therapeutic neovascularization. Laboratory Investigation. 2003;83:65–73. doi: 10.1097/01.lab.0000050761.67879.e4. [DOI] [PubMed] [Google Scholar]
- 137.Leroux L, Descamps B, Tojais NF, Seguy B, Oses P, Moreau C, Daret D, Ivanovic Z, Boiron JM, Lamaziere JMD, Dufourcq P, Couffinhal T, Duplaa C. Hypoxia Preconditioned Mesenchymal Stem Cells Improve Vascular and Skeletal Muscle Fiber Regeneration After Ischemia Through a Wnt4-dependent Pathway. Molecular Therapy. 2010;18:1545–1552. doi: 10.1038/mt.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yamazaki M, Nakamura K, Mizukami Y, Ii M, Sasajima J, Sugiyama Y, Nishikawa T, Nakano Y, Yanagawa N, Sato K, Maemoto A, Tanno S, Okumura T, Karasaki H, Kono T, Fujiya M, Ashida T, Chung DC, Kohgo Y. Sonic hedgehog derived from human pancreatic cancer cells augments angiogenic function of endothelial progenitor cells. Cancer Science. 2008;99:1131–1138. doi: 10.1111/j.1349-7006.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Volkmer E, Kallukalam BC, Maertz J, Otto S, Drosse I, Polzer H, Bocker W, Stengele M, Docheva D, Mutschler W, Schieker M. Hypoxic Preconditioning of Human Mesenchymal Stem Cells Overcomes Hypoxia-Induced Inhibition of Osteogenic Differentiation. Tissue Engineering Part A. 2010;16:153–164. doi: 10.1089/ten.TEA.2009.0021. [DOI] [PubMed] [Google Scholar]
- 140.Morimoto D, Tomita T, Kuroda S, Higuchi C, Kato S, Shiba T, Nakagami H, Morishita R, Yoshikawa H. Inorganic polyphosphate differentiates human mesenchymal stem cells into osteoblastic cells. Journal of Bone and Mineral Metabolism. 2010;28:418–423. doi: 10.1007/s00774-010-0157-4. [DOI] [PubMed] [Google Scholar]
- 141.Kawazoe Y, Katoh S, Onodera Y, Kohgo T, Shindoh M, Shiba T. Activation of the FGF signaling pathway and subsequent induction of mesenchymal stem cell differentiation by inorganic polyphosphate. International Journal of Biological Sciences. 2008;4:37–47. doi: 10.7150/ijbs.4.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shmelkov SV, Meeus S, Moussazadeh N, Kermani P, Rashbaum WK, Rabbany SY, Hanson MA, Lane WJ, St Clair R, Walsh KA, Dias S, Jacobson JT, Hempstead BL, Edelberg JM, Rafii S. Cytokine preconditioning promotes codifferentiation of human fetal liver CD133(+) stem cells into angiomyogenic tissue. Circulation. 2005;111:1175–1183. doi: 10.1161/01.CIR.0000157155.44008.0F. [DOI] [PubMed] [Google Scholar]
- 143.Cui JH, Park SR, Park K, Choi BH, Min BH. Preconditioning of mesenchymal stem cells with low-intensity ultrasound for cartilage formation in vivo. Tissue Engineering. 2007;13:351–360. doi: 10.1089/ten.2006.0080. [DOI] [PubMed] [Google Scholar]
- 144.Lucchinetti E, Zeisberger SM, Baruscotti I, Wacker J, Feng JH, Zaugg K, Dubey R, Zisch AH, Zaugg M. Stem Cell-Like Human Endothelial Progenitors Show Enhanced Colony-Forming Capacity After Brief Sevoflurane Exposure: Preconditioning of Angiogenic Cells by Volatile Anesthetics. Anesthesia and Analgesia. 2009;109:1117–1126. doi: 10.1213/ANE.0b013e3181b5a277. [DOI] [PubMed] [Google Scholar]
- 145.Popescu M, Munteanu A, Isvoranu G, Suciu L, Pavel B, Marinescu B, Zagrean L. Dynamics of endothelial progenitor cells following sevoflurane preconditioning. Roumanian archives of microbiology and immunology. 2011;70:109–13. [PubMed] [Google Scholar]
- 146.Kubo M, Li TS, Kurazumi H, Takemoto Y, Ohshima M, Murata T, Katsura S, Morikage N, Furutani A, Haman K. Hypoxic Preconditioning Enhances Angiogenic Potential of Bone Marrow Cells With Aging-Related Functional Impairment. Circulation Journal. 2012;76:986–994. doi: 10.1253/circj.cj-11-0605. [DOI] [PubMed] [Google Scholar]
- 147.Wang MJ, Tsai BM, Crisostomo PR, Meldrum DR. Pretreatment with adult progenitor cells improves recovery and decreases native myocardial proinflammatory signaling after ischemia. Shock. 2006;25:454–459. doi: 10.1097/01.shk.0000209536.68682.90. [DOI] [PubMed] [Google Scholar]
- 148.Bacigaluppi M, Pluchino S, Jametti LP, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- 149.Loos B, Smith R, Engelbrecht AM. Ischaemic preconditioning and TNF-alpha-mediated preconditioning is associated with a differential cPLA(2) translocation pattern in early ischaemia. Prostaglandins Leukotrienes and Essential Fatty Acids. 2008;78:403–413. doi: 10.1016/j.plefa.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 150.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 151.Cheng AS, Yau TM. Paracrine effects of cell transplantation: strategies to augment the efficacy of cell therapies. Seminars in thoracic and cardiovascular surgery. 2008;20:94–101. doi: 10.1053/j.semtcvs.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 152.Canfield SG, Sepac A, Sedlic F, Muravyeva MY, Bai XW, Bosnjak ZJ. Marked Hyperglycemia Attenuates Anesthetic Preconditioning in Human-induced Pluripotent Stem Cell-derived Cardiomyocytes. Anesthesiology. 2012;117:735–744. doi: 10.1097/ALN.0b013e3182655e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zeng XJ, Yu SP, Zhang L, Wei L. Neuroprotective effect of the endogenous neural peptide apelin in cultured mouse cortical neurons. Experimental Cell Research. 2010;316:1773–1783. doi: 10.1016/j.yexcr.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ito T, Itakura S, Todorov I, Rawson J, Asari S, Shintaku J, Nair I, Ferreri K, Kandeel F, Mullen Y. Mesenchymal Stem Cell and Islet Co-Transplantation Promotes Graft Revascularization and Function. Transplantation. 2010;89:1438–1445. doi: 10.1097/tp.0b013e3181db09c4. [DOI] [PubMed] [Google Scholar]
- 155.Oh JS, Ha Y, An SS, Khan M, Pennant WA, Kim HJ, Yoon DH, Lee M, Kim KN. Hypoxia-preconditioned adipose tissue-derived mesenchymal stem cell increase the survival and gene expression of engineered neural stem cells in a spinal cord injury model. Neuroscience Letters. 2010;472:215–219. doi: 10.1016/j.neulet.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 156.Kim HW, Haider HK, Jiang SJ, Ashraf M. Ischemic Preconditioning Augments Survival of Stem Cells via miR-210 Expression by Targeting Caspase-8-associated Protein 2. Journal of Biological Chemistry. 2009;284:33161–33168. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Noiseux N, Borie M, Desnoyers A, Menaouar A, Stevens LM, Mansour S, Danalache BA, Roy DC, Jankowski M, Gutkowska J. Preconditioning of stem cells by oxytocin to improve their therapeutic potential. Endocrinology. 2012;153:5361–72. doi: 10.1210/en.2012-1402. [DOI] [PubMed] [Google Scholar]
- 158.Chen TL, Wang JA, Shi H, Gui C, Luo RH, Xie XJ, Xiang MX, Zhang X, Cao J. Cyclosporin A pre-incubation attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Scandinavian Journal of Clinical & Laboratory Investigation. 2008;68:585–593. doi: 10.1080/00365510801918761. [DOI] [PubMed] [Google Scholar]


