Abstract
Background
New approaches are needed in the treatment of multi- and extensively drug resistant pulmonary tuberculosis (M/XDR-PTB). We evaluated the role of adjunctive surgical therapy in the treatment of M/XDR-PTB in the setting of DOTS-Plus implementation.
Methods
We conducted an observational cohort study consisting of M/XDR-PTB patients who underwent thoracic surgery at the National Tuberculosis Center in Tbilisi, Georgia between October 2008 and February 2011. Indications for surgery included presence of M/XDR-PTB, localized pulmonary disease, fit to undergo surgery, and either medical treatment failure or such extensive drug-resistance that failure was likely. Second-line anti-tuberculosis medical therapy was administered per WHO recommendations.
Results
80 patients (55 MDR, 25 XDR) with PTB underwent adjunctive thoracic surgery. Median age was 30 years and average duration of preoperative M/XDR-PTB medical therapy was 350 days. The following surgical procedures were performed: pneumonectomy (10%), lobectomy (51%), segmentectomy (33%), and thoracoplasty (6%). Mean postoperative follow up time was 372 days. Of 77 patients with evaluable outcomes, 64 (83%) had favorable outcomes including 90% of MDR and 68% of XDR-TB patients. There was no postoperative mortality; postoperative complications occurred in 7 patients (9%). Risk factors for poor treatment outcomes in univariate analysis included cavitary disease, XDR, increasing effective drugs received, positive preoperative sputum culture, and major postoperative surgical complication.
Conclusions
Patients with M/XDR-PTB undergoing adjunctive thoracic surgery had high rates of favorable outcomes, no surgical related mortality, and low rates of complications. Adjunctive surgery appears to play an important role in the treatment of select patients with M/XDR-PTB.
Keywords: tuberculosis, infection, thoracotomy
INTRODUCTION
The emergence of multidrug-resistant tuberculosis (MDR-TB; resistance to isoniazid and rifampicin) is a major global public health problem and barrier to improving TB control.(1) World Health Organization has estimated a worldwide prevalence of 660,000 cases of MDR-TB and 150,000 MDR-TB related deaths annually.(2) Especially alarming is the increasing prevalence of difficult to treat extensively drug-resistant tuberculosis (XDR-TB; resistance to isoniazid, rifampicin, a fluoroquinolone, and ≥1 injectable agent). Recent data from a multi-country study found that among 1278 patients with MDR-TB, approximately 7% had XDR-TB.(3) The increasing number of MDR- and XDR-TB cases portends an uncertain future in global TB control. Current M/XDR-TB treatment regimens are costly, lengthy, toxic, and are associated with poor treatment outcomes as compared to drug-susceptible disease.(1) A resurgence in TB drug development(4) has given reason for optimism; however, improved care for M/XDR-TB is needed now for the many patients living with drug-resistant TB.
A potentially beneficial adjunctive treatment for pulmonary M/XDR-TB patients is thoracic surgery.(5) The increasing prevalence of M/XDR-TB has been paralleled by increasing reports of surgical resections suggesting more frequent use of thoracic surgery in TB management. Additionally, two recent literature reviews found high rates of favorable outcomes among patients with pulmonary M/XDR-TB undergoing adjuvant surgical resection.(5),(6) More current data on the treatment outcomes of M/XDR-TB patients undergoing surgical resection in settings of DOTS plus implementation will help determine the optimal role of adjuvant surgery.
The country of Georgia is a high burdened M/XDR-TB country as designated by WHO.(7) Georgian National TB program data from 2011 found the prevalence of MDR-TB among newly diagnosed patients to be 10.8% and 31.4% among previously treated TB patients. The prevalence of XDR among those with MDR-TB was 6.4%. Georgia is one of few high burdened M/XDR-TB countries that routinely perform second-line drug (SLD) testing and with the assistance of the Green Light Committee implemented DOTS plus (treatment of MDR- and XDR-TB) in 2008. It is in this setting that we report on the experience of adjuvant surgical therapy for patients with pulmonary M/XDR-TB in Georgia.
PATIENTS AND METHODS
Setting
An observational cohort study design was utilized. The study population consisted of all patients with pulmonary M/XDR-TB who had thoracic surgery performed at the National Center for Tuberculosis and Lung Diseases (NCTBLD) in Tbilisi, Georgia, between October 2008 and February 2011. General criteria for surgical intervention included M/XDR-TB, failure of medical therapy (persistently sputum culture positive and/or positive preoperative sputum culture), a high likelihood of treatment failure or disease relapse, localized lesion, and sufficient pulmonary function to tolerate surgery. A high likelihood of treatment failure or disease relapse was determined by level of drug resistance, current treatment regimen, and presence of significant parenchymal lung damage (cavitary and/or destroyed lung). Contraindications for surgery included a FEV1 < 1000 ml, severe malnutrition, or patients at high risk for perioperative cardiovascular complications(8). The final decision to offer adjunctive surgery was reached by consensus opinion of the NCTBLD Drug Resistance Committee. Medical treatment regimens were individualized based on drug susceptibility (DST) results and guided by WHO criteria.(9) Regimens were designed to include ≥4 active drugs based on DST and included a fluoroquinolone and either kanamycin or capreomycin. Treatment was given by directly observed therapy (DOT). The NCTBLD and Emory University institutional review boards approved the study.
Laboratory
All cultures and DST were performed at the Georgian National Tuberculosis Reference laboratory. Sputum cultures were performed monthly until three consecutive negative sputum culture results and then every two to three months until treatment completion. Decontaminated sputum samples were inoculated into both Löwenstein-Jensen media and the BACTEC MGIT 960 broth culture system. For all M. tuberculosis positive sputum cultures, first-line and second-line DST were performed using the absolute and proportion concentration methods, respectively as previously described.(10) Pyrazinamide testing was performed using the MGIT960 liquid broth system.
Surgery
All patients were admitted to the hospital prior to surgery for counseling and evaluation. Preoperative evaluation included chest radiography and computed tomography for localizing the TB lesions, bronchoscopy for visualizing the airways and ruling out bronchial lesions, electrocardiogram, and spirometry assessing lung function. Laboratory analyses included a complete blood count, chemistry panel, and HIV serologic testing.
In regards to the surgical procedure, all patients received general anesthesia, were intubated with a double lumen endotracheal tube, and had a chest tube placed. Resections were approached through a posterolateral thoracotomy. The type of resection was based on the extent of the pulmonary lesion. Bronchial stump closure was performed using propylene sutures and stapled binding. No specific pre-emptive techniques were used to protect the bronchial stump. The chest tube was removed 24–48 hours after surgery. Patients received aggressive postoperative physiotherapy while in the hospital. Postoperative follow up was conducted at NCTBLD outpatient clinics.
Patients were expected to remain on anti-tuberculosis drugs for 12–24 months after surgery depending on when they achieved negative sputum; however some patients defaulted treatment and did not reenter care. We attempted to contact these patients approximately 12 months after default. For those who could be traced and returned to clinic, a follow up interview, chest radiography and sputum culture were performed. Final treatment outcomes were determined by WHO criteria.(9) A favorable outcome was defined as cure and/or treatment completion; a poor outcome was defined as treatment failure, death during treatment, and default (with the below exception). Patients who defaulted treatment but had no evidence of TB disease by history or imaging, and had a negative follow up sputum culture were considered to have a favorable outcome. Operative mortality was defined as any death occurring 1) within 30 days after surgery in or out of the hospital, or 2) after 30 days during the same hospitalization subsequent to the operation. (11)
Data Analysis
Data were collected and managed using a REDCap database.(12) Medical chart abstraction was performed to collect information on demographics, medical history, TB treatment history, chest imaging findings, surgical therapy, and TB treatment outcomes.
Statistical analysis was performed using SAS version 9.3. Differences in categorical variables were tested using the Chi-square test and for continuous variables a two-samples t-test was used. A p value <0.05 was considered significant.
RESULTS
Patient Characteristics
A total of 75 patients with pulmonary M/XDR-TB underwent adjuvant surgical resection (Table 1). The mean age was 30 years and 47 (63%) were male; 31% of patients were infected with hepatitis C, 8% had diabetes mellitus, and one person had HIV infection. There were high rates of tobacco (36%), alcohol (15%), and injection drug use (11%). Most patients had a BMI ≥18.5 kg/m2 (83%) and a FEV1 ≥ 2000 ml (91%).
Table 1.
Characteristics of 75 patients with pulmonary M/XDR-TB undergoing adjunctive surgery
| Demographics | N (% or range) |
|---|---|
| Mean age, years | 30 (15–54) |
| Male | 47 (63) |
| Any Comorbidity | 29 (39) |
| Diabetes | 6 (8) |
| Cardiovascular disease | 3 (4) |
| Hepatitis C | 23 (31) |
| HIV | 1 (1) |
| Current tobacco use | 27 (36) |
| Current alcohol use | 11 (15) |
| Intravenous drug use | 8 (11) |
| BMI (kg/m2) ≤ 18.5 | 13 (17) |
| FEV1 (ml) | |
| 1000–2000 | 7 (9) |
| 2000–3000 | 19 (25) |
| > 3000 | 49 (65) |
| Tuberculosis History and Treatment | |
| Case definition | |
| New | 30 (40) |
| Previously treated with FLDs | 35 (47) |
| Previously treated with SLDs | 10 (13) |
| MDR | 51 (68) |
| XDR | 24 (32) |
| Mean days of TB treatment before surgery | 500 (95–1220) |
| Mean days of M/XDR-TB treatment before surgery | 342 (95–693) |
| Median number of drugs with documented resistance | 6 (2–10) |
| Median number of drugs receiving for MDR/XDR-TB treatment | 6 (3–9) |
| Median known sensitive drugs receiving for MDR/XDR-TB treatment | 3 (0–5) |
| Positive pre-surgery sputum AFB smear | 12 (16) |
| Positive pre-surgery sputum AFB culture | 18 (27) |
| Radiological Results | |
| Multilobar disease | 37 (49) |
| Bilateral disease | 24 (32) |
| Cavitary disease | 71 (95) |
| Bilateral cavitary disease | 12 (16) |
| Surgical Indication | |
| Medical treatment failure | 22 (36) |
| High likelihood of treatment failure or disease relapse | 47 (63) |
| Massive Hemoptysis | 1 (1) |
| Hospital and Treatment Follow Up Time | |
| Mean total hospital days (IQR) | 32 (5–133) |
| Mean postoperative hospital days (IQR) | 21 (5–113) |
| Mean postoperative follow time in days (IQR) | 379 (38–885) |
BMI=body mass index; FEV=forced expiratory volume; FLDs=first line drugs; SLDs=second line drugs; MDR=multidrug resistance; XDR=extensively drug resistance; TB=tuberculosis; AFB=acid fast bacillus;
Most patients (60%) were retreatment TB cases who had been previously treated with either first (47%) or second-line drugs (13%); 32% of those undergoing surgical therapy had XDR-TB. Patients were receiving a median of 6 anti-tuberculosis drugs and had been receiving M/XDRTB treatment an average of 342 days prior to surgery. Almost all patients (95%) had cavitary disease on chest radiograph; 16% had bilateral cavity disease. Twenty seven percent had a positive pre-surgery sputum culture for M. tuberculosis. Those patients with a negative preoperative sputum culture had a prolonged period of infectiousness and delayed culture conversion as demonstrated by a mean time of sputum culture positivity after initiation of M/XDR-TB treatment of 200 days.
Surgery
The most common indication for surgery was a high likelihood of treatment failure or disease relapse (63%) followed by medical treatment failure (36%), and massive hemoptysis (1%) (Table 1). All patients had surgical resection performed including lobectomy (54%), segmentectomy (35%), and pneumonectomy (11%) (Table 2). Seven patients (9%) experienced a major or minor postoperative complication. Four patients (5%) developed a bronchopleural fistula while one patient each developed a postoperative empyema (1%) and hemorrhage (1%). There were no cases of operative mortality (within 30 days or during the hospitalization subsequent the operation). Minor complications occurred in one patient who had both a pneumothorax and postoperative surgical site wound infection. A subsequent surgical procedure was required in 3 (4%) patients; two had surgical bronchopleural fistula repair while another had a repeat thoracotomy for postoperative hemorrhage. The mean duration of postoperative hospital stay was 21 days.
Table 2.
Surgical procedures and postoperative surgical complications (n=75)
| Initial Surgery | N (%) |
|---|---|
| Pneumonectomy | 8 (11) |
| Lobectomy | 41 (54) |
| Segmentectomy | 26 (35) |
| Subsequent Surgical Procedures | 3 (4) |
| Fistula repair | 2 (3) |
| Repeat thoracotomy | 1 (1) |
| Surgical Complications | |
| Patients with any surgical complication | 7 (9) |
| Major complication | 6 (8) |
| Bronchopleural fistula | 4 (5) |
| Empyema | 1 (1) |
| Hemorrhage | 1 (1) |
| Minor Complication* | 1 (1) |
| Wound infection | 1 (1) |
| Pneumothorax | 1 (1) |
One patient had a postoperative wound infection and pneumothorax
Treatment Outcomes
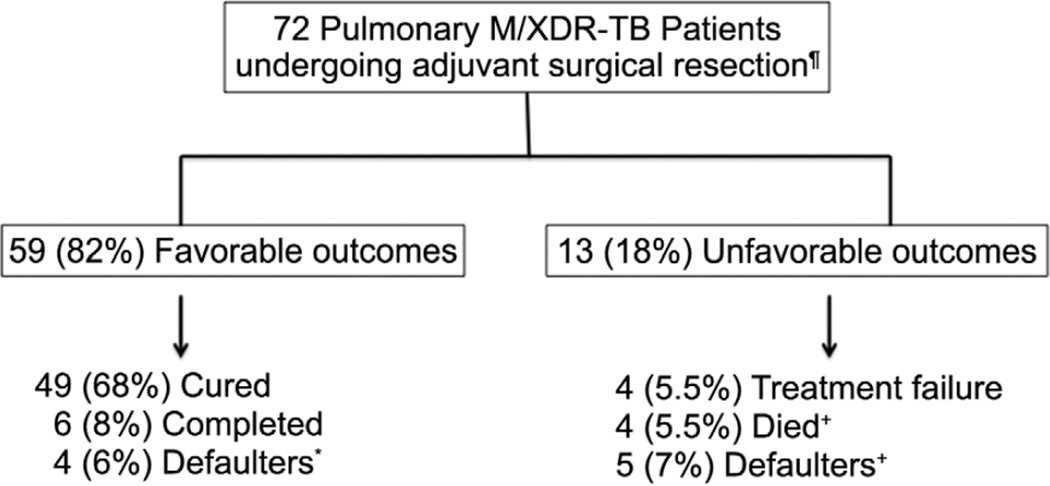
Treatment outcomes were available for 72 of 75 patients (Figure 1) (3 patients left Georgia after surgery). The mean follow up after surgery was 379 days. Favorable outcomes were achieved in 59 (82%) patients including 90% in those with MDR-TB and 67% in XDR-TB patients. Favorable outcomes in the 59 patients included 49 with cure (68%), 6 (8%) who completed treatment, and 4 (6%) patients who defaulted before completing treatment but upon further follow up had no evidence of active TB disease and appeared to be cured (no TB symptoms, normal chest radiography and negative sputum culture). The 4 defaulted patients with a favorable outcome received a mean 377 days (range 313–449) of M/XDR-TB treatment before surgery and 217 days (range 171–276) after surgery. Of the 13 (18%) patients with unfavorable outcomes, 4 (5.5%) died, 4 (5.5%) experienced treatment failure, and 5 (7%) defaulted treatment (2 patients remained sputum culture positive 12 months after default and 3 were lost to follow up). The four deaths occurred between 8–14 months after surgery and were related to progression of TB disease. Two of these deaths occurred in patients who defaulted from TB treatment. Immediate postoperative sputum culture conversion to negative occurred in all 19 patients with positive preoperative sputum cultures; however, 4 reverted their sputum culture to positive within four months and subsequently experienced treatment failure.
Figure 1. Treatment outcomes of pulmonary M/XDR-TB patients undergoing adjuvant surgical treatment and with final evaluable outcomes (n=72).
¶ 3 patients transferred treatment outside Georgia; final outcomes not available
+ All deaths due to tuberculosis
* Negative culture at default and normal CXR and negative sputum culture ≥12 months after default
++ 2 with positive sputum culture ≥12months after default; 3 lost to follow up
Risk Factors for a Poor Outcome
In univariate analysis, variables significantly associated with poor outcomes included factors related to extent and burden of TB disease, drug resistance, and surgery related complications. Patients with poor outcomes were more likely to have bilateral disease (62% vs. 27%, p =0.02) and bilateral cavitary disease (39% vs. 12%. P=0.02) as compared to those with favorable outcomes. Additionally, patients with poor outcomes had higher rates of XDR disease (62% vs. 27%, p=0.02) and positive preoperative sputum cultures (77% vs. 14%, p <0.001) than those with favorable outcomes. Patients with a poor outcome also received on average less known effective drugs based on DST (1.9 vs. 2.8, p=0.03) and were more likely to have a major postoperative complication (23% vs. 5%, p=0.03) compared to patients with favorable outcomes.
COMMENT
Utilizing a combination of individualized medical treatment for M/XDR-TB (based on WHO guidelines)(9) and adjunctive surgical therapy we attained a high rate of favorable treatment outcomes (82%) in a cohort of patients with chronic pulmonary M/XDR-TB. This included favorable outcomes in 90% of those with MDR-TB and 67% with XDR-TB. The high rate of favorable outcomes was achieved in patients with high levels of drug resistance, prior TB treatment, bilateral disease, and prolonged sputum culture positivity. The favorable outcomes among those undergoing adjunctive surgery were notably higher than those reported for the first cohort of patients treated for M/XDR-TB in Georgia (53%).(13) Our experience as well as others(5) suggest that adjunctive surgery be considered in selected patients with highly drug-resistant TB in an effort to improve treatment outcomes.
While the 2012 WHO Global TB report documents progress in TB control it also describes the response to MDR-TB as inadequate and slow. Only 30 of 107 countries reporting MDR-TB treatment outcomes achieved the target treatment success rate of ≥75%.(2) Additionally, an individual patient data meta-analysis of >9000 MDR-TB patients found a 54% treatment success rate.(14) XDR-TB treatment outcomes are worse with a 560 patient meta-analysis finding a favorable outcome rate of 44%.(15) New drugs have shown promising results in clinical trials(16, 17) and have led the FDA to approve bedaquiline for use in MDR-TB.(18) While bedaquiline and other drugs in development offer great promise it is unlikely they will reach all M/XDR-TB patients soon and there impact in enhancing cure rates remains to be determined. The suboptimal outcomes achieved with existing medical therapy stress the need to evaluate other adjunctive treatment options including surgical resection.
The purpose of surgery is to remove a large, focal burden of bacilli present in necrotic and nonviable lung tissue. By removing the main focus of infection, it may be possible to prevent further disease spread and allow medical therapy to work better. Two potential mechanisms by which the tuberculous cavity hinders effective medical therapy include reducing drug penetration and providing an environment conducive to drug resistance amplification.(19, 20) While no clinical data exists for second-line drugs, older studies found reduced cavitary penetration of isoniazid and rifampicin.(19) Studies finding worse outcomes among MDR-TB patients with severe cavitary disease compared to those without cavitary disease provide indirect evidence for poor second-line drug penetration into cavitary lesions.(21, 22) The concern of amplified drug resistance has been highlighted by recent data from the Preserved Effective Tuberculosis Treatment Study which found up to 20% of MDR-TB patients developed new and increasing phenotypic drug resistance during second-line drug treatment.(23) Based on reports from our group(24) and another(20) a major site of amplified drug resistance may be the tuberculous cavity. We found increasing drug resistance in 16% of M. tuberculosis isolates recovered from resected cavitary tissue as compared to sputum isolates in M/XDR-TB patients undergoing surgical resection.(24)
Our high rate of favorable outcomes (82%) is similar to those seen in other studies of M/XDRTB patients undergoing adjunctive surgery. In a recent review of 18 case series, totaling 964 highly drug-resistant pulmonary TB patients (895 MDR and 69 XDR) undergoing adjunctive surgical resection, the median rate of favorable outcomes was 89.5%.(5) Our cohort includes the second largest reported series of XDR-TB patients to undergo adjunctive surgical therapy(25) and only a few other studies report similar or higher level of documented drug resistance. (26–28) Our results highlight and support the potential benefit of surgery in patients with high levels of drug resistance and limited medical treatment options.
In addition to a high rate of favorable outcomes, low rates of postoperative surgical complications (9%) were observed and there was no perioperative mortality. Our complication rate was lower than most reports(5) and demonstrates surgery can be performed safely in carefully selected patients. Our most common complication was bronchopleural fistula (5%), which is the most frequently reported major post surgical complication in the literature.(19, 29) The issue of bronchial stump reinforcement as a means of decreasing rates of bronchopleural fistula remains an unresolved issue. While one study found that no bronchial reinforcement was related to an increased incidence of bronchopleural fistula,(30) others have noted a low incidence of bronchopleural fistula in patients with and without bronchial reinforcement.(25, 31, 32) We did not perform bronchial reinforcement and had rates of bronchopleural fistula similar to other studies. There was no operative mortality in our series and the four patients who died among our cohort were all late deaths (8–14 months after surgery) due to the progression of TB. Two patients that died left the hospital against medical advice and defaulted, highlighting the challenges in completing a full course of M/XDR-TB treatment.
Risk factors associated with poor outcomes in univariate analysis indicated more severe TB disease. Specifically, bilateral disease, increasing drug resistance, and persistent sputum culture positivity were all significantly associated with poor outcomes. While these factors were associated with a poor outcome many of these patients still had a favorable outcome. Unfortunately, there is very limited literature on risk factors for poor outcome among M/XDR-TB surgical patients. A study from Latvia found that less drug resistance was associated with favorable outcomes(28) and another study from Korea found low BMI, increasing drug resistance, and bilateral disease were associated with poor outcomes.(33) An issue with all reported M/XDR-TB surgery studies including ours is limited sample size, making it difficult to conduct a meaningful multivariate analysis evaluating risk factors for a poor outcome.
The criteria we used for selecting patients for adjunctive surgery were similar to those first proposed by Iseman(34) and subsequently adapted by most groups performing thoracic surgery in M/XDR-TB patients.(5) These include patients with M/XDR-TB who have a localized lesion amenable to resection, treatment failure despite aggressive medical treatment, and patients with a high risk of relapse. The WHO(9) and Partners in Health(35) support similar surgical indications for drug-resistant TB and stress the importance of specialized surgical facilities and experienced surgeons. All studies of adjunctive surgery for M/XDR-TB were performed at either hospitals affiliated with national TB centers or specialized thoracic surgery centers. Our study was conducted at the Georgian National Tuberculosis Center Thoracic Surgery Center and is one of the few to be conducted in a low to middle-income country.(5) The optimal timing of adjunctive surgery remains unknown and has not been adequately addressed in treatment guidelines or clinical studies.
A limitation of our study is the lack of a non-surgical control group, which prevented us from evaluating whether adjunctive surgery provides additional benefit in improving pulmonary M/XDR-TB treatment outcomes beyond medical treatment. To address the essential question of whether adjunctive surgery improves outcomes among patients with M/XDR-TB, a randomized controlled trial is needed. Given the challenges in conducting such a study, we suspect that it is unlikely to be performed. One alternative study design would be to use people who are recommended but refuse surgery as a control group. Another alternative is to evaluate the effect of adjunctive surgery on treatment outcomes in large cohorts of M/XDR-TB patients, some who undergo thoracic surgery. In a study of 380 M/XDR-TB patients in Georgia we found that after controlling for confounding risk factors adjunctive surgery significantly decreased the risk of a poor outcome (aOR 0.28, 95% CI 0.12–0.62).(13) Other comparison studies have shown similar results;(5) however, none of the studies including ours was designed specifically to assess the impact of surgery on outcomes and likely did not control for all confounders.
In summary, our experience in Georgia demonstrates that adjunctive thoracic surgery can be performed safely and along with appropriate medical treatment lead to high rates of favorable outcomes among patients with cavitary M/XDR-TB. In settings that have the proper surgical expertise and facilities, surgical resection should be considered in M/XDR-TB management. While further studies such as randomized controlled trials are needed to definitively address the benefit of adjunctive surgery, available evidence supports a beneficial role for surgical therapy among patients with pulmonary M/XDR-TB.
Table 3.
Comparison of risk factors for treatment outcomes
| Demographics | Poor Outcome n=13 number (%) |
Favorable Outcome n=59 number (%) |
P |
|---|---|---|---|
| Mean Age, years | 28 | 30 | 0.62 |
| Male | 10 (77) | 34 (57) | 0.20 |
| Hepatitis C | 4 (31) | 19 (32) | 0.92 |
| Current tobacco use | 4 (31) | 23 (39) | 0.58 |
| Current alcohol use | 1 (8) | 10 (17) | 0.40 |
| Intravenous drug use | 2 (15) | 6 (10) | 0.59 |
| BMI (kg/m2) ≤ 18.5 | 3 (23) | 10 (17) | 0.60 |
| FEV1 < 2000 ml | 1 (8) | 6 (10) | 0.78 |
| Radiological Results | |||
| Bilateral disease | 8 (62) | 16 (27) | 0.02 |
| Bilateral cavitary disease | 5 (39) | 7 (12) | 0.02 |
| Tuberculosis History and Treatment | |||
| Prior TB treatment | 10 (77) | 32 (54) | 0.13 |
| XDR vs. MDR | 8 (62) | 16 (27) | 0.02 |
| Mean days of M/XDR-TB treatment before surgery | 394 | 332 | 0.20 |
| Mean drugs with documented resistance | 7.1 | 5.8 | 0.04 |
| Mean drugs receiving for M/XDR-TB | 7.3 | 5.9 | 0.004 |
| Mean sensitive drugs receiving for M/XDR-TB | 1.9 | 2.8 | 0.03 |
| Receiving ≥ 3 effective drugs based on DST results | 4 (31) | 35 (59) | 0.06 |
| Positive preoperative sputum culture | 10 (77) | 8 (14) | <.0001 |
| Treatment failure indication for surgery | 7 (54) | 19 (32) | 0.14 |
| Major postoperative surgical complication | 3 (23) | 3 (5) | 0.03 |
BMI=body mass index; FEV=forced expiratory volume; TB=tuberculosis; XDR=extensively drug resistant; MDR=multidrug resistance; DST=drug susceptibility testing
Acknowledgements
This work was supported in part by the NIH Fogarty International Center [D43TW007124 and D43TW007124-06S1], the Atlanta Clinical and Translational Science Institute [NIH/NCATS UL1TR000454], and the Emory Global Health Institute. The funders had no role in any aspect of the study.
Abbreviations and Acronyms
- BMI
body mass index
- DST
drug susceptibility testing
- FEV
forced expiratory volume
- FDA
food and drug administration
- HIV
human immunodeficiency virus
- MDR
multidrug-resistant
- NCTBLD
National Center for Tuberculosis and Lung Diseases
- PTB
pulmonary tuberculosis
- TB
tuberculosis
- WHO
World Health Organization
- XDR
extensively drug-resistant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375(9728):1830–1843. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 2.WHO. WHO/HTM/20126. Geneva: World Health Organization; 2012. Global tuberculosis control: WHO report 2012. 2012. [Google Scholar]
- 3.Dalton T, Cegielski P, Akksilp S, et al. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet. 2012;380(9851):1406–1417. doi: 10.1016/S0140-6736(12)60734-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grosset JH, Singer TG, Bishai WR. New drugs for the treatment of tuberculosis: hope and reality. Int J Tuberc Lung Dis. 2012;16(8):1005–1014. doi: 10.5588/ijtld.12.0277. [DOI] [PubMed] [Google Scholar]
- 5.Kempker RR, Vashakidze S, Solomonia N, et al. Surgical treatment of drug-resistant tuberculosis. Lancet Infect Dis. 2012;12(2):157–166. doi: 10.1016/S1473-3099(11)70244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu HB, Jiang RH, Li L. Pulmonary resection for patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. J Antimicrob Chemother. 2011;66(8):1687–1695. doi: 10.1093/jac/dkr210. [DOI] [PubMed] [Google Scholar]
- 7.WHO. WHO/HTM/TB/20103. Geneva: World Health Organization; 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB2010 global report on surveillance and response. 2010. [Google Scholar]
- 8.BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001;56(2):89–108. doi: 10.1136/thorax.56.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Guidelines for the programmatic management of drug-resistant tuberculosis, 2011 Update. WHO/HTM/TB/20116. Geneva: World Health Organization; 2011. 2011. [PubMed] [Google Scholar]
- 10.Tukvadze N, Kempker RR, Kalandadze I, et al. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLoS One. 2012;7(2):e31563. doi: 10.1371/journal.pone.0031563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs JP, Mavroudis C, Jacobs ML, et al. What is operative mortality? Defining death in a surgical registry database: a report of the STS Congenital Database Taskforce and the Joint EACTS-STS Congenital Database Committee. Ann Thorac Surg. 2006;81(5):1937–1941. doi: 10.1016/j.athoracsur.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gegia M, Kalandadze I, Kempker RR, et al. Adjunctive surgery improves treatment outcomes among patients with multidrug-resistant and extensively drug-resistant tuberculosis. Int J Infect Dis. 2012;16(5):e391–e396. doi: 10.1016/j.ijid.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahuja SD, Ashkin D, Avendano M, et al. Multidrug Resistant Pulmonary Tuberculosis Treatment Regimens and Patient Outcomes: An Individual Patient Data Meta-analysis of 9,153 Patients. PLoS Med. 2012;9(8):e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson KR, Tierney DB, Jeon CY, et al. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51(1):6–14. doi: 10.1086/653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360(23):2397–2405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- 17.Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366(23):2151–2160. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- 18.FDA. [cited 2012 January 2, 2012];2012 Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm333695.htm.
- 19.Dartois V, Barry CE. Clinical pharmacology and lesion penetrating properties of second- and third-line antituberculous agents used in the management of multidrug-resistant (MDR) and extensively-drug resistant (XDR) tuberculosis. Current clinical pharmacology. 2010;5(2):96–114. doi: 10.2174/157488410791110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan G, Post FA, Moreira AL, et al. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun. 2003;71(12):7099–7108. doi: 10.1128/IAI.71.12.7099-7108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HR, Hwang SS, Kim HJ, et al. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2007;45(10):1290–1295. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 22.Torun T, Tahaoglu K, Ozmen I, et al. The role of surgery and fluoroquinolones in the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2007;11(9):979–985. [PubMed] [Google Scholar]
- 23.Dalton T, Diem L, Hartline D, Ershova J, Caoili J, Cegielski P, editors. Acquired resistance to second-line anti-tuberculosis drugs during MDR-TB treatment in PETTS study. 42nd Union World Conference on Lung Health; October 29, 2011; Lille, France. 2011. [Google Scholar]
- 24.Kempker RR, Rabin AS, Nikolaishvili K, et al. Additional Drug Resistance in Mycobacterium tuberculosis Isolates From Resected Cavities Among Patients With Multidrug-Resistant or Extensively Drug-Resistant Pulmonary Tuberculosis. Clin Infect Dis. 2011 doi: 10.1093/cid/cir904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang MW, Kim HK, Choi YS, et al. Surgical treatment for multidrug-resistant and extensive drug-resistant tuberculosis. Ann Thorac Surg. 2010;89(5):1597–1602. doi: 10.1016/j.athoracsur.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Pomerantz BJ, Cleveland JC, Jr, Olson HK, et al. Pulmonary resection for multi-drug resistant tuberculosis. J Thorac Cardiovasc Surg. 2001;121(3):448–453. doi: 10.1067/mtc.2001.112339. [DOI] [PubMed] [Google Scholar]
- 27.Somocurcio JG, Sotomayor A, Shin S, et al. Surgery for patients with drug-resistant tuberculosis: report of 121 cases receiving community-based treatment in Lima, Peru. Thorax. 2007;62(5):416–421. doi: 10.1136/thx.2005.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dravniece G, Cain KP, Holtz TH, et al. Adjunctive resectional lung surgery for extensively drug-resistant tuberculosis. Eur Respir J. 2009;34(1):180–183. doi: 10.1183/09031936.00047208. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan G, Post FA, Moreira AL, et al. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infection and immunity. 2003;71(12):7099–7108. doi: 10.1128/IAI.71.12.7099-7108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Lin H, Jiang G. Pulmonary resection in the treatment of multidrug-resistant tuberculosis: a retrospective study of 56 cases. Ann Thorac Surg. 2008;86(5):1640–1645. doi: 10.1016/j.athoracsur.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 31.Shiraishi Y, Katsuragi N, Kita H, et al. Aggressive surgical treatment of multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg. 2009;138(5):1180–1184. doi: 10.1016/j.jtcvs.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Park SK, Lee CM, Heu JP, et al. A retrospective study for the outcome of pulmonary resection in 49 patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2002;6(2):143–149. [PubMed] [Google Scholar]
- 33.Kim HJ, Kang CH, Kim YT, et al. Prognostic factors for surgical resection in patients with multidrug-resistant tuberculosis. Eur Respir J. 2006;28(3):576–580. doi: 10.1183/09031936.06.00023006. [DOI] [PubMed] [Google Scholar]
- 34.Iseman MD, Madsen L, Goble M, et al. Surgical intervention in the treatment of pulmonary disease caused by drug-resistant Mycobacterium tuberculosis. Am Rev Respir Dis. 1990;141(3):623–625. doi: 10.1164/ajrccm/141.3.623. [DOI] [PubMed] [Google Scholar]
- 35.Health Pi. Adjuvant Therapies and Strategies. The PIH Guide to the Medical Management of Multidrug-Resistant Tuberculosis United States Partners in Health. 2003:29–32. [Google Scholar]