Abstract
In Papua New Guinea (PNG), complex patterns of malaria commonly include single and mixed infections of Plasmodium falciparum, P. vivax, P. malariae, and P. ovale. Here, we assess recent epidemiologic characteristics of Plasmodium bloodstage infections in the Wosera region through four cross-sectional surveys (August 2001 to June 2003). Whereas previous studies performed here have relied on blood smear/light microscopy (LM) for diagnosing Plasmodium species infections, we introduce a newly developed, post-polymerase chain reaction (PCR), semiquantitative, ligase detection reaction-fluorescent microsphere assay (LDR-FMA). A direct comparison of the two methods for > 1,100 samples showed that diagnosis was concordant for > 80% of the analyses performed for P. falciparum (PF), P. vivax (PV), and P. malariae (PM). Greater sensitivity of the LDR-FMA accounted for 75% of the discordance between diagnoses. Based on LM, the prevalence of blood-stage PF, PV, and PM infections was found to be markedly reduced compared with an early 1990s survey. In addition, there were significant shifts in age distribution of infections, with PV becoming the most common parasite in children < 4 years of age. Consistent with previous studies, prevalence of all Plasmodium species infections increased significantly in samples analyzed by the PCR-based LDRFMA. This increase was most pronounced for PM, PO, and mixed infections and in adolescent (10–19 years) and adult age groups, suggesting that LM may lead to under-reported prevalence of less common Plasmodium species, infection complexity, and a skewed distribution of infections towards younger age groups. This study shows that the application of LDR-FMA diagnosis in large epidemiologic studies or malaria control interventions is feasible and may contribute novel insights regarding the epidemiology of malaria.
INTRODUCTION
Malaria is one of the primary reasons for health center use and admission in endemic areas of Papua New Guinea (PNG).1 We and others have conducted a number of epidemiologic surveys within PNG to improve our understanding of the relationships between malaria parasite infection and disease,2–16 anti-malarial drug and vaccine effectiveness,17–19 and human genetic polymorphisms.20–28 These studies have shown that all four Plasmodium species (P. falciparum [PF], P. vivax [PV], P. malariae [PM], and P. ovale [PO]) cause infection in PNG. Although PF is presently the predominant malaria parasite species, PV and PM were observed to be the majority malaria parasite species before the 1980s.6 The observed change in dominance is thought to have coincided with the development of chloroquine resistance by PF.5 PO is observed infrequently in blood smears but is common when diagnosis is performed using more sensitive polymerase chain reaction (PCR)-based techniques.12 Drug-resistant strains of PF are now distributed widely throughout the coastal lowlands and into highland regions in PNG.29–31 Additionally, a number of PNG-based studies provide evidence that mixed Plasmodium species infections are common and exhibit dynamic fluctuation.11–13,32,33
Prevention and control of human Plasmodium infections through new anti-malarial drugs, vaccines, and vector control strategies requires a thorough knowledge of parasite species and strain prevalence, distribution, and transmission. To evaluate initial and sustained impact of control efforts, it will be necessary to perform diagnosis in a timely manner on thousands of individuals at frequent intervals. To accomplish this, diagnostic strategies must be able to differentiate Plasmodium species with accuracy regardless of infection complexity, and with development of specific probes, should be capable of differentiating strains that vary according to drug susceptibility or antigenic variation. It is also important for diagnostic strategies to provide semi-quantitative assessment of Plasmodium species and strain infection levels. Finally, it would be most efficient if the same approaches used to evaluate human infections could also be used to evaluate the vectorial capacity of mosquito populations.
Here, we report on characteristics of Plasmodium bloodstage infections in the Wosera region of PNG using standard blood smear microscopy and molecular epidemiologic tools. Prevalence and parasite levels were evaluated after diagnosis by light microscopy (LM) and a semi-quantitative, Plasmodium species-specific, post-PCR/ ligase detection reaction–fluorescent microsphere-based detection assay (LDR-FMA).34 Continuous sample processing from DNA extraction through data analysis in a 96-well plate format avoids cumbersome aspects of previously used molecular diagnostic assays. The performance, versatility, and capacity of this DNA-based assay addresses many challenges confronting diagnosis in large-scale malaria control programs.
MATERIALS AND METHODS
Study population and blood sample collection
The study was conducted in the Wosera district of East Sepik Province.8 This region of PNG is characterized by perennial transmission of malaria, with an entomologic inoculation rate of ~30 bites/ person/yr.35,36 Between August 2001 and June 2003, we conducted a series of four cross-sectional surveys at 6-month intervals within 29 Wosera villages participating in collaborative research studies between Case Western Reserve University and the PNG Institute of Medical Research (PNGIMR). These surveys were performed during periods of the year characterized by different rainfall intensities.8 Based on the annually updated PNGIMR census, the combined population of the villages reached 13,000 at the completion of the surveys. The total number of participants in each survey was as follows: Survey A, N = 4,813 (August–November 2001); Survey B, N = 3,476 (April–June 2002); Survey C, N = 4,124 (August–November 2002); Survey D, N = 3,797 (April–June 2003). Demographic information and finger-prick blood samples were collected from each study volunteer. Blood samples were used to produce thick and thin blood smears, determine hemoglobin concentration, and enable human host and parasite DNA extraction. Informed consent was obtained from all adult participants and from the parents or legal guardians of minors. This project was approved by the PNG Medical Research Advisory Committee, the Institutional Review Board for Human Investigation at University Hospitals of Cleveland, and the International Centers for Tropical Disease Research Network/NIAID/NIH.
Blood smear examination
Thick/thin blood smears were prepared as described previously.8,37 Blood smears were stained with a 4% Giemsa solution and examined under oil immersion (×100). PNGIMR expert microscopists evaluated Plasmodium species-specific parasitemia while counting the number of microscope fields containing 200 leukocytes (population average leukocyte counts = 8,000/µL of blood8); parasite counts × 40 = parasites per microliter of blood.
DNA template preparation
DNA was extracted from whole blood (200 µL) using protocols recommended for the QIAamp 96 DNA Blood Kit (QIAGEN, Valencia, CA).
PCR amplification and Plasmodium species-specific LDR-FMA
All methods for PCR amplification of small sub-unit rRNA target sequences and Plasmodium species-specific detection by LDR-FMA were described in detail by McNamara and others.34 Species-specific fluorescence data were collected using Bio-Rad software, Bio-Plex Manager 3.0 (Bio-Rad Laboratories, Hercules, CA). To differentiate the negative from positive fluorescent signals, we analyzed 353 samples from a random sample of American Red Cross blood donors.38 All donors (18–55 years of age) were self-identified as African American, American Oriental, white American, or Hispanic American and had no history of malaria exposure. Median fluorescent intensity (MFI) LDR-FMA signals from these samples were normally distributed (PF: mean 110.9 ± 31.0 [SD], median 110, 99% quantile 187; PV: mean 86.5 ± 30.5, median 85, 99% quantile 158; PM: mean 94.1 ± 30.8, median 93, 99% quantile 173; PO: mean 84.7 ± 31.6, median 85, 99% quantile 163). Samples with MFIs greater than the 99% quantile values were determined to be positive for infection. Before data analysis of the PNG study volunteers, these species-specific background MFI values (99% quantile values) were subtracted from the LDR-FMA signals.
Statistical analyses and graphing
All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC) and graphed using GraphPad PRISM version 4.0 (GraphPad Software, San Diego, CA). Plasmodium species prevalence in parasite assemblages was determined as previously described.12,13
RESULTS
Description of the study population
During the four serial cross-sectional surveys (Table 1), we collected a total of 16,209 blood samples from 8,793 villagers (67.6% of the population). With the exception of children < 2 years of age, the demographic profile of survey participants was generally representative of Wosera village populations: 49.4% were men, 2.1% were < 2 years, 7.1% were 2.0–3.9 years, 10.4% were 4.0–6.9 years, 11.2% were 7.0–9.9 years, 24.1% were 10.0–19.9 years, 28.5% were 20.0–39.9 years, and 16.7% were ≥ 40 years of age. The age and sex distribution of participants was comparable among the four surveys. In all surveys, the majority of participants were asymptomatic. Only 2.6% (N = 426) of the survey participants had a parasitemia based on light microscopy (LM) and an axillary temperature of at least 37.5°C indicating clinical malaria.
Table 1.
Distribution of cross-sectional survey participants by age group, sex, and survey
| Survey A |
Survey B |
Survey C |
Survey D |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) |
Aug–Nov 2001 (dry) |
April–June 2002 (wet) |
Aug–Nov 2002 (dry) |
April–June 2003 (wet) |
||||||
| N | Percent | N | Percent | N | Percent | N | Percent | N | Percent | |
| <2 | 131 | 2.7% | 54 | 1.6% | 98 | 2.4% | 53 | 1.4% | 336 | 2.1% |
| 2–3 | 352 | 7.3% | 220 | 6.3% | 321 | 7.8% | 257 | 6.8% | 1,150 | 7.1% |
| 4–6 | 539 | 11.2% | 332 | 9.6% | 423 | 10.3% | 398 | 10.5% | 1,692 | 10.4% |
| 7–9 | 532 | 11.1% | 387 | 11.1% | 471 | 11.4% | 429 | 11.3% | 1,819 | 11.2% |
| 10–19 | 1,116 | 23.2% | 927 | 26.7% | 964 | 23.4% | 892 | 23.5% | 3,899 | 24.1% |
| 20–39 | 1,351 | 28.1% | 991 | 28.5% | 1,153 | 28.0% | 1,113 | 29.3% | 4,608 | 28.4% |
| ≥ 40 | 792 | 16.5% | 565 | 16.3% | 693 | 16.8% | 655 | 17.3% | 2,705 | 16.7% |
| All | 4,813 | 3,476 | 4,123 | 3,797 | 16,209 | |||||
| Sex | ||||||||||
| Male | 2,300 | 47.8% | 1,742 | 50.1% | 1,987 | 48.2% | 1,880 | 49.5% | 7,909 | 48.8% |
| Female | 2,513 | 52.2% | 1,734 | 49.9% | 2,136 | 51.8% | 1,917 | 50.5% | 8,300 | 51.2% |
Prevalence of single and mixed Plasmodium infections
Blood smear/LM results. Overall, 34.9% of all samples showed evidence of a blood-stage malaria infection by LM. The prevalence of each Plasmodium species was as follows: PF, 22.3%; PV, 10.4%; PM, 4.2%; PO, 0.2%. The majority of the blood-stage infections (94.1%) were composed of a single Plasmodium species, whereas 5.9% of the infections contained mixed Plasmodium species. The prevalence of infections differed significantly between surveys (Table 2) and among age groups (Figure 1A). Generally, the prevalence of different Plasmodium infections was lower in the second year of study (Surveys C and D). While the prevalence of PF infections did not vary significantly between seasons, PV increased and PM infections decreased in the dry season (Surveys A and C).
Table 2.
Prevalence of malaria infections (light microscopy) by survey, study year, and season
| Survey |
Year* |
Season† |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| A (year 1, dry) |
B (year 1, wet) |
C (year 2, dry) |
D (year 2, wet) |
All | 1 | 2 | Dry | Wet | |
| P. falciparum (%) | 23.9 | 23.7 | 19.5 | 22 | 22.3 | 23.8¶ | 20.7¶ | 21.8 | 22.8 |
| P. vivax (%) | 11.4 | 11.3 | 10.7 | 7.8 | 10.4 | 11.4¶ | 9.3¶ | 11.1¶ | 9.5¶ |
| P. malariae (%) | 4 | 5.4 | 3.4 | 4.2 | 4.2 | 4.6§ | 3.8§ | 3.7¶ | 4.8¶ |
| P. ovale (%) | 0.3 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2‡ | 0.1‡ |
| Any species (%) | 36.2 | 38 | 32.6 | 33.1 | 34.9 | 36.9¶ | 32.8¶ | 34.5 | 35.4 |
| Mixed infections (%)** | 8.9 | 6.5 | 4 | 3.2 | 5.9 | 7.9¶ | 3.6¶ | 6.7§ | 4.9§ |
P value references below pertain to statistical comparisons between years 1 and 2.
P value references below pertain to statistical comparisons between “dry” and “wet” seasons.
P < 0.05
P < 0.01
P < 0.001.
Proportion of infections with more than one Plasmodium species.
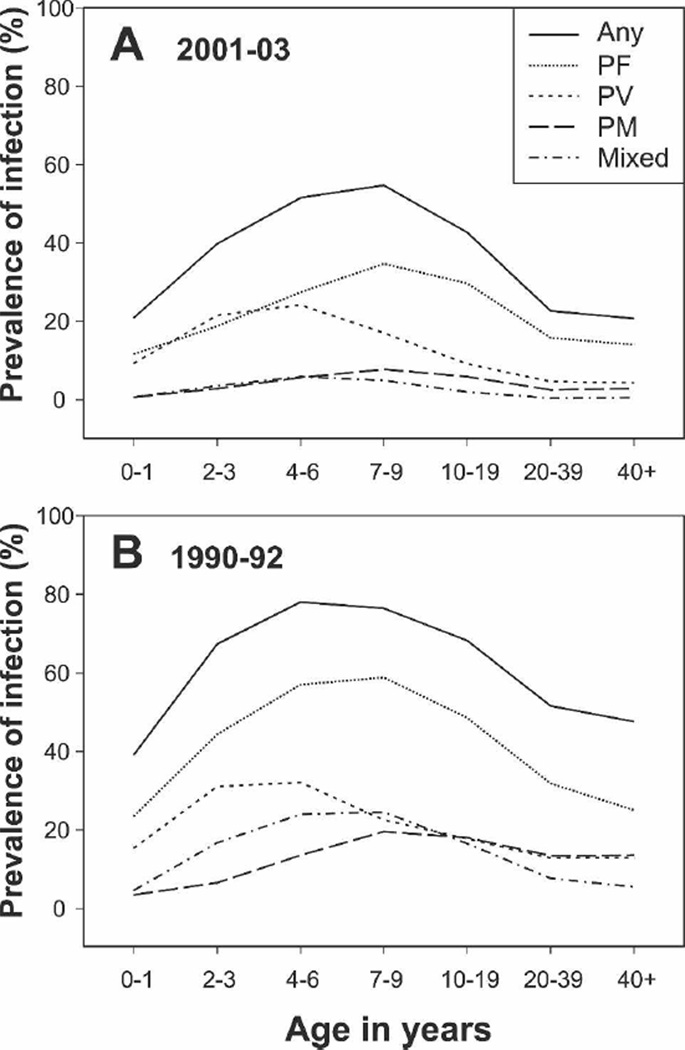
Figure 1.
Prevalence of Plasmodium species infections in different age groups. (A) 2001–2003 surveys (A–D combined). (B) 1990–1992 surveys.8 Any, infected with any Plasmodium species; PF, P. falciparum; PV, P. vivax; PM, P. malariae; Mixed, concurrent infection with more than one Plasmodium species.
Plasmodium infections were most frequent in the 7- to 9-and 4- to 6-year-old children (54.7%, CL: 52.4–57.0 and 51.5%, CL: 49.1–53.9, respectively), followed by adolescents (10–19 years: 42.7%, CL: 41.1–44.2), 2–3 year olds (39.7%, CL: 36.9–42.6), adults (20–39 years: 22.6%, CL: 21.4–23.8), infants (0–1 years: 20.8%, CL: 16.6–25.6), and those ≥ 40 years of age (20.7, CL: 19.1–22.2). PV reached peak prevalence in younger children (4–6 years: 24.2%, CL: 22.1–26.3) than PF (7–9 years: 34.7%, CL: 32.5–36.9) or PM (7–9 years: 7.7%, CL: 6.5–9.0). The age distribution of infections by individual species did not differ significantly between surveys.
Overall, the prevalence rates of Plasmodium infections were significantly lower in these surveys compared with those performed from 1990 to 19928 (Figure 1A versus B). Prevalence of PF was reduced from 39.6% to 22.2% (RR: 0.56, CL: 0.54–0.58, P < 0.001); PV from 18.3% to 10.4% (RR: 0.57 CL: 0.53–0.60, P < 0.001), and PM from 13.8% to 4.2% (RR: 0.31 CL: 0.28–0.34 P < 0.001). For PF, the reduced prevalence of infections was significantly greater in children 2–6 years of age (RR range: 0.42–0.49) than in older children (7–9 years) and adolescents (RR range: 0.59–0.61, P < 0.01), suggesting that the highest burden of PF infection has shifted into older age groups. A contrasting pattern was observed for PV, where reduced prevalence was significantly greater for adults (RR range: 0.33–0.36) than children 2–9 years of age (RR range: 0.69–0.75, P < 0.001).
Plasmodium species-specific LDR-FMA results
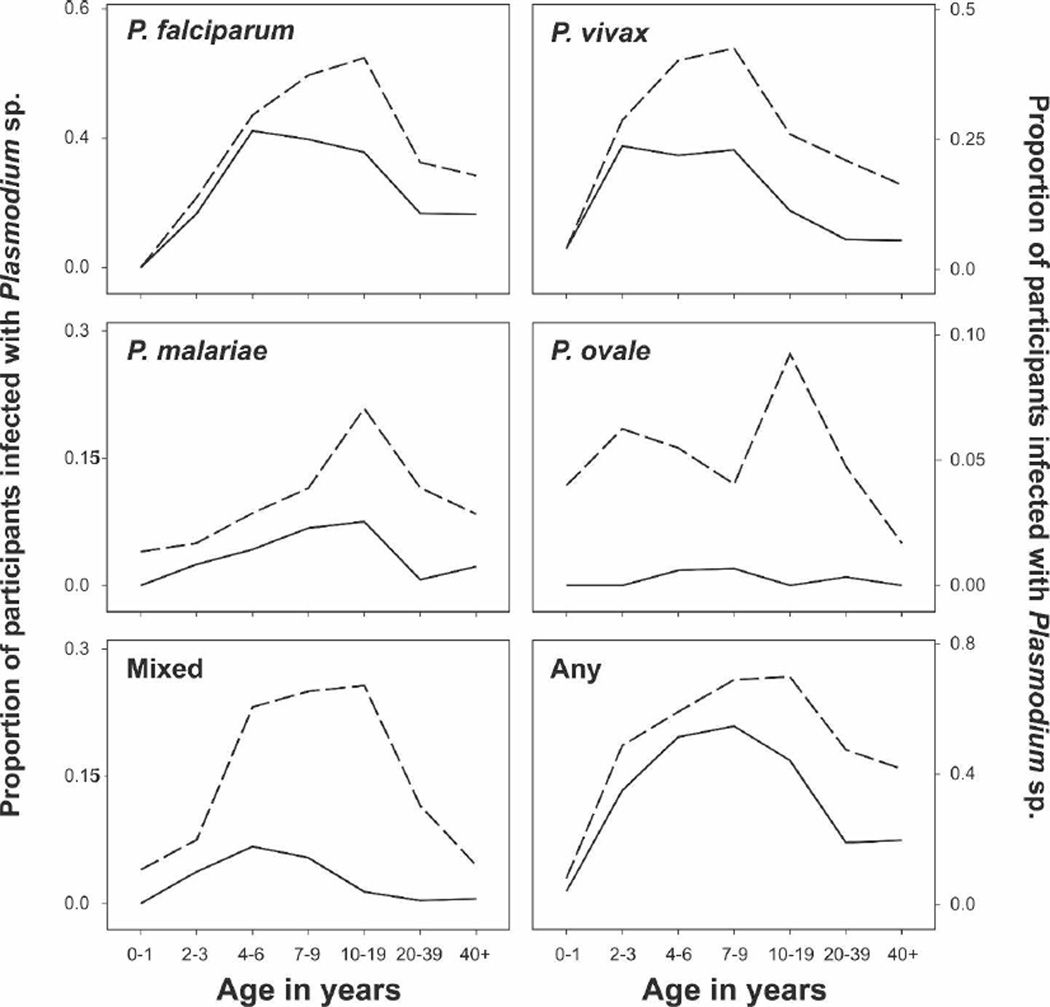
To evaluate diagnosis by the Plasmodium species–specific LDR-FMA, we selected a set (N = 1,182) of samples from the four serial cross-sectional surveys that were representative of the age, sex, and village composition of the Wosera. All samples included in this analysis had a corresponding blood smear evaluated by LM. By this assay, 55.7% of all samples showed evidence of a blood-stage malaria infection. In comparison with LM diagnosis, the prevalence of infection observed by LDR-FMA increased by 1.6-fold for PF (20.6% versus 32.9%, P < 0.001), 2.1-fold for PV (12.7% versus 27.1%, P < 0.001), 3.1-fold for PM (4.0% versus 12.4%, P < 0.001), and 22-fold for PO (0.25% versus 5.5%, P < 0.001), with the prevalence of mixed infections increasing from 2.4% to 16.8% (P < 0.001). Mixed species infections were thus present in 30.3% of all LDR-FMA–positive infections compared with only 6.8% of all LM-positive cases.
For all Plasmodium species, the increase in prevalence of infections by the LDR-FMA assay was proportionally larger in the adults and adolescents than in children (Figure 2). Consistent with this observation, the prevalence of infection peaked at a later age for LDR-FMA–detectable infections than when diagnosis was performed by LM (Figure 2). In all species except PV, where infections were most commonly found in children 7–9 years of age (42.6%), prevalence of infections peaked in older age groups (10–19 years: overall, 69.9%; PF, 48.6%; PM, 20.9%; PO, 9.3%; mixed infections, 25.7%).
Figure 2.
Age-specific prevalence of malaria infection as detected by LM and LDR-FMA assay. Solid line, LM; dashed line, LDR-FMA assay; Any, infected with any Plasmodium species; Mixed, concurrent infection with more than one Plasmodium species.
Parasite assemblage comparison
To determine whether there was a random distribution of Plasmodium species in the survey population, we analyzed parasite assemblages using the multiple-kind lottery model described previously.12,13 Analyses were performed on both blood smear and LDR-FMA data. Results of the LM analysis of the overall study population in Table 3 showed that single species infections were observed at higher frequencies than expected, and different mixed Plasmodium species infections were observed at frequencies that were equal to or lower than the expected frequencies. Overall, mixed infections were found in only 2.1% of all samples compared with the expected 3.6% (P < 0.001). In the LDR-FMA diagnostic data, a different pattern was found. Single species and double infections were observed at frequencies equal to or less than expected, whereas triple and quadruple infections assemblages (4.4% versus 1.8%, P < 0.001) and negative samples (44.3% versus 40.5%, P = 0.008) were more significantly common than expected. Mixed infections were most commonly found in older children (4–9 years) and adolescents, with 36.3% to 39.3% of all LDR-FMA–positive infections containing more than one species.
Table 3.
Plasmodium species assemblages by different diagnostic techniques
| Blood smear (n = 16,209) |
LDR-FMA (n = 1,182) |
|||
|---|---|---|---|---|
| Parasite assemblage | Observed | Expected | Observed | Expected |
| PF | 3,312 | 3,094.41 | 224 | 234.97 |
| PV | 1,404 | 1,249.16 | 174 | 177.82 |
| PM | 591 | 476.37 | 46 | 67.5 |
| PO | 19 | 18.68 | 15 | 27.87 |
| PF + PV | 231 | 358.02 | 72 | 87.23 |
| PF + PM | 54 | 136.53 | 32 | 33.11 |
| PF + PO | 3 | 5.35 | 13 | 13.67 |
| PV + PM | 29 | 55.12 | 17 | 25.06 |
| PV + PO | 6 | 2.16 | 9 | 10.35 |
| PM + PO | 0 | 0.82 | 4 | 3.93 |
| PF + PV + PM | 11 | 15.8 | 28 | 12.29 |
| PF + PV + PO | 0 | 0.62 | 5 | 5.08 |
| PF + PM + PO | 0 | 0.24 | 4 | 1.93 |
| PV + PM + PO | 0 | 0.1 | 4 | 1.46 |
| PF + PV + PM + PO | 0 | 0.03 | 11 | 0.72 |
| Non-infected | 10,549 | 10,796.59 | 524 | 479.01 |
Concordance between LM and LDR-FMA diagnosis
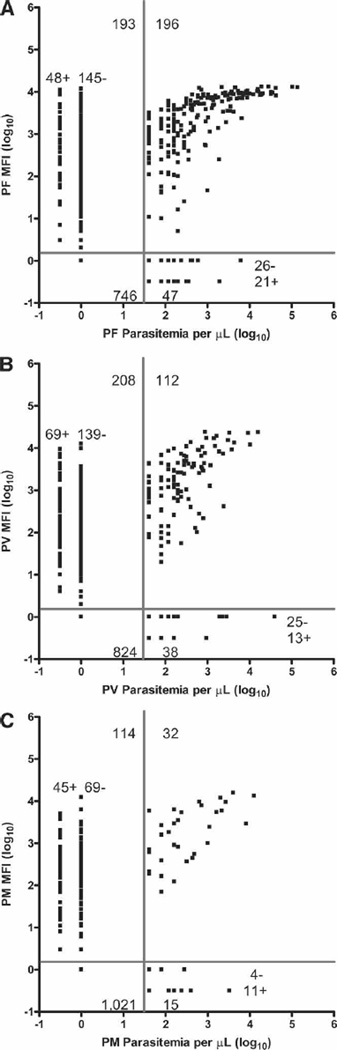
The above results indicate that diagnosis by LDR-FMA detected a higher prevalence of infection than LM. We therefore compared semi-quantitative outcomes in those 1,182 samples that were evaluated with both diagnostic methods (Figure 3A–C). In diagnosis of PF, 942 analyses were concordant (79.7%) between the two diagnostic techniques. Among the 240 (20.3%) discordant analyses, 193 samples were LDR-FMA positive and LM negative (LDR-FMA+:LM−), and 47 were LDR-FMA negative and LM positive (LDR-FMA−:LM+; LDR-FMA sensitivity = 0.81 and specificity = 0.79; Figure 3A). Where PF diagnosis was LDR-FMA+LM−, 48 samples were judged by LM as infected with at least one other Plasmodium species. For PF LDR-FMA−:LM+ discordance, 21 samples were identified by LDR-FMA as infected with at least one other Plasmodium species. Diagnosis of PV found that 936 analyses were concordant (79.2%), whereas 246 were discordant. Among the discordant analyses, 208 samples were LDR-FMA+LM−, and 38 were LDR-FMA−LM+ (LDR-FMA sensitivity = 0.75 and specificity = 0.80; Figure 3B). Where PV diagnosis was LDR-FMA+LM−, 69 samples were judged by microscopy as infected with at least one other Plasmodium species. For PV LDR-FMA−:LM+ discordance, 13 samples were identified by LDR-FMA as infected with at least one other Plasmodium species. Diagnosis of PM found that 1,053 analyses were concordant (89.1%), and 129 were discordant. Among discordant analyses, 114 samples were LDR-FMA+:LM− and 15 samples were LDR-FMA−:LM+ (LDR-FMA sensitivity = 0.68 and specificity = 0.90; Figure 3C). Where PM diagnosis was LDR-FMA+LM−, 45 samples were judged by LM as infected with at least one other Plasmodium species. For PM LDR-FMA−:LM+ discordance, 11 samples were identified by LDR-FMA as infected with at least one other Plasmodium species.
Figure 3.
Association between semi-quantitative blood-stage parasitemia and LDR-FMA fluorescent signal intensity. The association between species-specific Plasmodium fluorescent signal intensity and parasitemia was determined using blood smear LM for N = 1,182 paired samples. Quadrants delineated by the horizontal and vertical bars denote the limits of negative and positive values. Top left quadrant, LDR-FMA+:LM−; top right quadrant, LDR-FMA+:LM+; bottom left quadrant, LDR-FMA−:LM−; bottom right quadrant, LDR-FMA−:LM+. In the top left quadrant, LDR-FMA+:LM− samples designated as + showed evidence of one or more Plasmodium species in the blood smear other than the species detected by LDR-FMA; samples designated as – showed evidence of no other Plasmodium species. In the bottom right quadrant, LDR-FMA−:LM+ positive samples designated as + showed evidence of one or more Plasmodium species by LDR-FMA other than the species detected by microscopy; samples designated as – showed evidence of no other Plasmodium species. Panel A reports results for PF, panel B for PV, and panel C for PM.
The overall concordance between microscopy and LDR-FMA for the detection of all possible Plasmodium assemblage combinations was Kw = 0.48 (95% CL: 0.43, 0.53). Based on the weighted κ test,37 56.7% of the samples were 100% concordant, 88.2% of the samples were at least 75% concordant, and 97.6% of the samples were at least 50% concordant (Supplemental Table 1).
Correlation between LM parasitemia and LDR-FMA MFI
In contrast to our previous studies where semiquantitative data on blood-stage Plasmodium species infections was limited to LM, development of the LDR-FMA allowed semi-quantitative assessment of differences in speciesspecific, blood-stage infection levels by a molecular diagnostic assay.34 In this study, we found positive correlations between the LDR-FMA MFI and LM parasitemia for each of the Plasmodium species parasite infections (N = 1,182 paired samples; PF Pearson’s r = 0.65, 95% CI: 0.62–0.68, P < 0.0001, Figure 3A; PV Pearson’s r = 0.55, 95% CI: 0.51–0.59, P < 0.0001, Figure 3B; PM Pearson’s r = 0.47, 95% CI: 0.42– 0.51, P < 0.0001, Figure 3C).
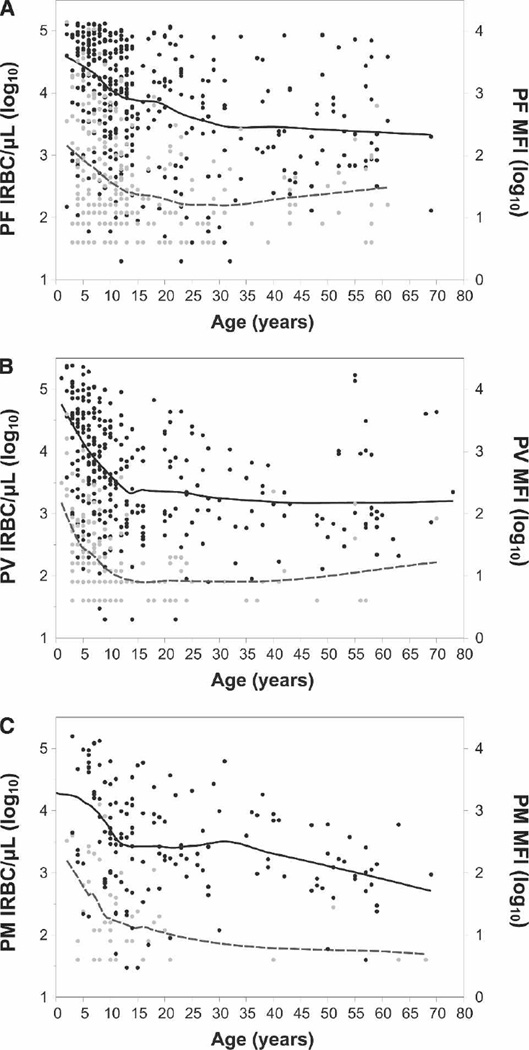
Correlation between LM parasitemia/LDR-FMA MFI and age
For samples where diagnosis was performed by both assays, we compared these semi-quantitative results for both techniques to the age of the study participants. As in earlier studies,8,13 parasitemia assessed by LM decreased significantly with age (PF Pearson’s r = −0.25, 95% CI: −0.34, −0.15, P < 0.0001, N = 389, Figure 4A; PV Pearson’s r = −0.26, 95% CI: −0.36, −0.16, P < 0.0001, N = 320, Figure 4B; PM Pearson’s r = −0.25, 95% CI: −0.39, −0.09, P = 0.0028, N = 146, Figure 4C). A parallel decrease with age was observed in the LDR-FMA data (PF Pearson’s r = −0.30, 95% CI: −0.39, −0.20, P < 0.0001, N = 389, Figure 4A; PV Pearson’s r = −0.26, 95% CI: −0.36, −0.16, P < 0.0001, N = 320, Figure 4B; PM Pearson’s r = −0.19, 95% CI: −0.34, −0.02, P = 0.0224, N = 146, Figure 4C).
Figure 4.
Age distribution of geometric mean blood-stage parasitemia determined by semi-quantitative blood smear microscopy and LDR-FMA. Blood smear parasitemia (infected red blood cells [IRBCs]/µL of blood) and median fluorescent signal intensity (MFI) for individuals who were positive for Plasmodium species infection by either LDR-FMA and/or LM. (A) P. falciparum. (B) P. vivax. (C) P. malariae. Black circles, LDR-FMA MFI values; gray circles, no. of IRBCs/µL; black solid line, geom. mean LDR-FMA MFI; gray dashed line, geom. mean parasitaemia/µL. Mean values estimated using a symmetric local regression (Loess) smoother with 1 degree of freedom.
DISCUSSION
LM, the mainstay of malaria diagnosis in epidemiologic studies, has bedeviled malariologists attempting to assess Plasmodium species infection prevalence in endemic communities for > 100 years.39,40 Recent studies provide a consistent reminder that LM exhibits limited sensitivity for detecting low level infections41–45 and is known to under-estimate significantly the frequency of mixed Plasmodium species infections.12,13,46 To address these shortcomings, a number of nucleic acid–based diagnostic assays have been developed recently that attempt to address the major challenges of diagnosing all four species of human malaria parasites in large epidemiologic studies.47–53 In previous studies, we characterized the prevalence and complexity of mixed Plasmodium species infections using both LM and more sensitive and specific PCR-based techniques in cross-sectional surveys from several different malaria-endemic regions in northern PNG.12,13,37 As in studies outside PNG, the prevalence of Plasmodium blood-stage infections of all types was found to be under-represented by LM compared with observations made by PCR-based methods.48–50,52,54–57 Here we expanded the scope of our surveys in the Wosera to include all 29 villages participating in PNGIMR malaria epidemiology studies. The four surveys summarized here were conducted at 6-month intervals over a 2-year period to monitor general seasonal or annual differences in the prevalence of Plasmodium species infections. More importantly, we took significant steps toward performing efficient large-scale molecular epidemiologic analysis of malaria infection in endemic communities.
Because PCR-based diagnosis of Plasmodium species provides a powerful approach for both species and strain assessment, we continued to refine methods for performing largescale epidemiologic studies.12,13,34,37 Approaches used here have improved efficiency of sample processing by using 96-well plate–based methods from DNA extraction through data processing and enhanced diagnosis by adding semiquantitative assessment capabilities comparable to real-time PCR-based methods.34 Details of the Plasmodium species LDR-FMA method were presented recently.34 Results showed that the LDR-FMA was consistently more sensitive than microscopy in detecting Plasmodium infected erythrocytes; numerous mixing experiments showed that the sequence specificity of the LDR-FMA probes constrains the possibility for false-positive diagnoses (only 1/33 of nucleotides was positionally identical among PF, PV, PM, and PO LDR probes).34,37 These assay performance characteristics must be considered in interpreting head-to-head comparisons between the LDR-FMA and LM data from the field-based samples evaluated here.
Data presented in Figure 3A–C showed that results for PF, PV, and PM were 80–90% concordant between the two diagnostic methods. Evaluation of the discrepancies between the diagnostic methods showed that LDR-FMA+:LM− outcomes accounted for 80–88% of the observed discordance and is most likely explained by greater sensitivity of the PCR-based assay. LDR-FMA−:LM+ outcomes accounting for 11–20% may have resulted from misdiagnosis, inhibition of PCR amplification of template DNA, or target (ssu rRNA) sequence variability.48,58
With enhanced capabilities of molecular diagnostic methods to perform semi-quantitative analysis of Plasmodium species infections, we anticipate that new elements of discordance will be observed, particularly in field studies performed in regions with complex malaria epidemiology. For example, whereas the correlation between LDR-FMA signal strength and the blood smear parasitemia improved with increasing numbers of infected erythrocytes identified by LM (common in concordance studies between microscopists43,59), we observed a wide range of LDR-FMA fluorescence for samples judged to contain a low number of infected erythrocytes by microscopy. Our results also showed that the range of fluorescent signals varied across 4 logs of fluorescent signal intensity for samples judged by microscopy to be negative. Numerous factors may have contributed to this wide range of LDR-FMA fluorescence associated with low-level parasitemia or for samples deemed negative by microscopy. Although inconsistent estimates have been reported, DNA persisting in circulating blood after parasites have been killed (24–144 hours)60,61 could contribute to high LDR-FMA signal strength in samples with low blood smear parasitemia. Additionally, if the number of PCR amplification cycles was too high, LDR-FMA signals could reach maximum strength for all dilution controls. However, LDR-FMA signal intensities correlating with species-specific controls throughout our studies34 would counter this possibility. Alternatively, because mixed Plasmodium species infections are common in PNG, it is possible that infection complexity may have influenced the accuracy of both species identification and speciesspecific enumeration of infected erythrocytes. This latter possibility consistently challenges comparisons between LM and molecular diagnostic tools.42,43
Overall comparisons between our study and previous malariometric surveys in the Wosera are limited to blood smear microscopy results because earlier studies did not include molecular diagnostic analyses. Comparing results from our study to the early 1990s surveys of Genton and others8 in 10 Wosera villages uncovers a significant reduction in the prevalence of Plasmodium species infections from 38% to 22.3% for PF, 19.7% to 10.4% for PV, and 15.7% to 4.2% for PM.8 It is potentially important to note that the prevalence of PM has been on a consistent decline since the mid-1980s.2–4,6,8 Explanations underlying this decrease in PM prevalence, beyond apparent susceptibility to malaria control measures, could include morphologic variations that may contribute to misdiagnosis.63 These changes in Plasmodium species prevalence in the Wosera have occurred in the absence of any sustained community-wide malaria control program between 1990 and 2003. During this time, however, the PNG Ministry of Health did change its anti-malarial treatment guidelines from chloroquine or amodiaquine monotherapy to a combination of sulphadoxine-pyrimethamine plus chloroquine or amodiaquine in 2000. Introducing this combination treatment has greatly improved the effectiveness of treatment of PF infections in the study area (Mueller and others, unpublished data). Although no coverage data are available, a gradual increase in the use of insecticide treated nets has also been observed over this region of PNG in recent years.
Despite the important reduction in prevalence of Plasmodium species infections, many features of malaria infection within the Wosera were similar between the early 1990s8 and 2001–2003 surveys reported here. PF and PV remain the predominant malaria species in this endemic community, although their relative importance in different age groups has changed. Detailed observations showed that PV was more prevalent than PF in children < 4 years of age. After 7 years of age, the prevalence of PV declined, and a shift to PF predominance was observed. The earlier age of peak prevalence of PV in both earlier8 and these later surveys suggests that immunity to PV is likely to be acquired at an earlier age than for PF. Although greatly reduced in prevalence, age distribution of PM remains notably similar to that observed by Genton and others.8 These changing patterns between prevalence of parasitemia and age seem to be unique for each species.
While LDR-FMA was only performed on a subset of the total 16,209 samples collected, our results show promise for providing significant new insights into the epidemiology of all four human Plasmodium parasite species. In particular, we show that PV, PM, and PO contribute to a greater burden of infection than appreciated by LM. Sub-patent infections of all species are most commonly found in adolescents and adults. The resulting shift in the age of peak prevalence and complexity of infection suggests that older age groups that have developed clinical immunity may contribute significantly more to the total parasite pool and transmission levels than previously thought. If these low-level infections are treated with indiscriminate use of anti-malarial drugs, this portion of the parasite population may encounter selection pressure favoring acquisition of anti-malarial drug resistance polymorphisms.
Finally, as in earlier studies conducted in PNG12,13 and elsewhere,46,63 mixed Plasmodium species infections were observed more frequently by PCR-based assays compared with LM diagnostic methods. While the apparent shortage of mixed infections by LM might suggest the possibility of crossspecies protection from infection, this interpretation is not supported by the LDR-FMA data. Observed patterns in both LM and LDR-FMA studies may be influenced by small-scale (village-to-village) heterogeneity in transmission found in our study population.64,65 The substantial difference in mixed species patterns observed by LM and LDR-FMA reinforces the importance of using a reliable PCR-based diagnostic assay in all studies on mixed Plasmodium species interactions.
Future analyses using LDR-FMA in comparison to blood smear microscopy will evaluate the distribution heterogeneity of Plasmodium species infections within endemic communities, Plasmodium strain (drug resistant and antigenic variants) prevalence and diversity, susceptibility to recurrence of Plasmodium species blood stage infections, and host genetic polymorphism associations with levels of infection and clinical malaria.
Supplementary Material
Acknowledgments
The authors thank W. E. Collins, C. L. King, C. H. King, R. K. Mehlotra, and E. L. Goldman for critical evaluation of this manuscript and B. Genton for allowing reference to original data. This study could not have been conducted without the participation of the Wosera residents and PNGIMR demography, field, data management, and microscopy units.
Financial support: This work was supported by NIAID/NIH Grants AI063135, AI46919, and AI52312.
Footnotes
Supplemental Table 1 and supplemental Figure 1 appear online at www.ajtmh.org.
REFERENCES
- 1.Papua New Guinea National Health Plan 2001–2010. Port Moresby: Papua New Guinea Ministry of Health; 2000. [Google Scholar]
- 2.Peters W. Studies on the epidemiology of malaria in New Guinea. 1. Holoendemic malaria—a clinical picture. Trans R Soc Trop Med Hyg. 1960;54:242–249. doi: 10.1016/0035-9203(60)90068-7. [DOI] [PubMed] [Google Scholar]
- 3.Peters W, Standfast HA. Studies on the epidemiology of malaria in New Guinea. 2. Holoendemic malaria—the entomological picture. Trans R Soc Trop Med Hyg. 1960;54:249–254. doi: 10.1016/0035-9203(60)90069-9. [DOI] [PubMed] [Google Scholar]
- 4.Desowitz RS, Saave JJ. The application of the haemagglutination test to a study of the immunity to malaria in protected and unprotected groups in Australian New Guinea. Bull WHO. 1965;32:149–159. [PMC free article] [PubMed] [Google Scholar]
- 5.Cattani JA, Tulloch JL, Vrbova H, Jolley D, Gibson FD, Moir JS, Heywood PF, Alpers MP, Stevenson A, Clancy R. The epidemiology of malaria in a population surrounding Madang, Papua New Guinea. Am J Trop Med Hyg. 1986;35:3–15. doi: 10.4269/ajtmh.1986.35.3. [DOI] [PubMed] [Google Scholar]
- 6.Desowitz RS, Spark RA. Malaria in the Maprik area of the Sepik region, Papua New Guinea: 1957–1984. Trans R Soc Trop Med Hyg. 1987;81:175–176. doi: 10.1016/0035-9203(87)90333-6. [DOI] [PubMed] [Google Scholar]
- 7.Cox MJ, Kum DE, Tavul L, Narara A, Raiko A, Baisor M, Alpers MP, Medley GF, Day KP. Dynamics of malaria para-sitaemia associated with febrile illness in children from a rural area of Madang, Papua New Guinea. Trans R Soc Trop Med Hyg. 1994;88:191–197. doi: 10.1016/0035-9203(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 8.Genton B, al-Yaman F, Beck HP, Hii J, Mellor S, Narara A, Gibson N, Smith T, Alpers MP. The epidemiology of malaria in the Wosera area, East Sepik Province, Papua New Guinea, in preparation for vaccine trials. I. Malariometric indices and immunity. Ann Trop Med Parasitol. 1995;89:359–376. doi: 10.1080/00034983.1995.11812965. [DOI] [PubMed] [Google Scholar]
- 9.Genton B, al-Yaman F, Beck HP, Hii J, Mellor S, Rare L, Ginny M, Smith T, Alpers MP. The epidemiology of malaria in the Wosera area, East Sepik Province, Papua New Guinea, in preparation for vaccine trials. II. Mortality and morbidity. Ann Trop Med Parasitol. 1995;89:377–390. doi: 10.1080/00034983.1995.11812966. [DOI] [PubMed] [Google Scholar]
- 10.Shankar AH, Genton B, Semba RD, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Tielsch JM, Alpers MP, West KP., Jr Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: A randomised trial. Lancet. 1999;354:203–209. doi: 10.1016/S0140-6736(98)08293-2. [DOI] [PubMed] [Google Scholar]
- 11.Bruce MC, Donnelly CA, Alpers MP, Galinski MR, Barnwell JW, Walliker D, Day KP. Cross-species interactions between malaria parasites in humans. Science. 2000;287:845–848. doi: 10.1126/science.287.5454.845. [DOI] [PubMed] [Google Scholar]
- 12.Mehlotra RK, Lorry K, Kastens W, Miller SM, Alpers MP, Bockarie M, Kazura JW, Zimmerman PA. Random distribution of mixed species malaria infections in Papua New Guinea. Am J Trop Med Hyg. 2000;62:225–231. doi: 10.4269/ajtmh.2000.62.225. [DOI] [PubMed] [Google Scholar]
- 13.Mehlotra RK, Kasehagen LJ, Baisor M, Lorry K, Kazura JW, Bockarie MJ, Zimmerman PA. Malaria infections are randomly distributed in diverse holoendemic areas of Papua New Guinea. Am J Trop Med Hyg. 2002;67:555–562. doi: 10.4269/ajtmh.2002.67.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller I, Bjorge S, Poigeno G, Kundi J, Tandrapah T, Riley ID, Reeder JC. The epidemiology of malaria in the Papua New Guinea highlands: 2. Eastern Highlands Province. P N G Med J. 2003;46:166–179. [PubMed] [Google Scholar]
- 15.Mueller I, Taime J, Ivivi R, Yala S, Bjorge S, Riley ID, Reeder JC. The epidemiology of malaria in the Papua New Guinea highlands: 1. Western Highlands Province. P N G Med J. 2003;46:16–31. [PubMed] [Google Scholar]
- 16.Mueller I, Namuigi P, Kundi J, Ivivi R, Tandrapah T, Bjorge S, Reeder JC. Epidemic malaria in the highlands of Papua New Guinea. Am J Trop Med Hyg. 2005;72:554–560. [PubMed] [Google Scholar]
- 17.Brabin BJ, Ginny M, Alpers M, Brabin L, Eggelte T, Van der Kaay HJ. Failure of chloroquine prophylaxis for falci-parum malaria in pregnant women in Madang, Papua New Guinea. Ann Trop Med Parasitol. 1990;84:1–9. doi: 10.1080/00034983.1990.11812428. [DOI] [PubMed] [Google Scholar]
- 18.al-Yaman F, Genton B, Mokela D, Narara A, Raiko A, Alpers MP. Resistance of Plasmodium falciparum malaria to amodiaquine, chloroquine and quinine in the Madang Province of Papua New Guinea. P N G Med J. 1996;39:16–22. [PubMed] [Google Scholar]
- 19.Genton B, Anders RF, Alpers MP, Reeder JC. The malaria vaccine development program in Papua New Guinea. Trends Parasitol. 2003;19:264–270. doi: 10.1016/s1471-4922(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 20.Booth PB, Tills D, Warlow A, Kopec AC, Mourant AE, Teesdale P, Hornabrook RW. Red cell antigen, serum protein and red cell enzyme polymorphisms in Karkar Islanders and inhabitants of the adjacent North Coast of New Guinea. Hum Hered. 1982;32:385–403. doi: 10.1159/000153328. [DOI] [PubMed] [Google Scholar]
- 21.Mourant AE, Tills D, Kopec AC, Warlow A, Teesdale P, Booth PB, Hornabrook RW. Red cell antigen, serum protein and red cell enzyme polymorphisms in Eastern Highlanders of New Guinea. Hum Hered. 1982;32:374–384. doi: 10.1159/000153327. [DOI] [PubMed] [Google Scholar]
- 22.Brabin L, Brabin BJ. Malaria and glucose 6-phosphate dehydrogenase deficiency in populations with high and low spleen rates in Madang, Papua New Guinea. Hum Hered. 1990;40:15–21. doi: 10.1159/000153896. [DOI] [PubMed] [Google Scholar]
- 23.Genton B, al-Yaman F, Mgone CS, Alexander N, Paniu MM, Alpers MP, Mokela D. Ovalocytosis and cerebral malaria. Nature. 1995;378:564–565. doi: 10.1038/378564a0. [DOI] [PubMed] [Google Scholar]
- 24.Allen SJ, O’Donnell A, Alexander ND, Mgone CS, Peto TE, Clegg JB, Alpers MP, Weatherall DJ. Prevention of cerebral malaria in children in Papua New Guinea by southeast Asian ovalocytosis band 3. Am J Trop Med Hyg. 1999;60:1056–1060. doi: 10.4269/ajtmh.1999.60.1056. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman PA, Woolley I, Masinde GL, Miller SM, McNamara DT, Hazlett F, Mgone CS, Alpers MP, Genton B, Boatin BA, Kazura JW. Emergence of Fy*A(null) in a Plasmodium vivax-endemic region of Papua New Guinea. Proc Natl Acad Sci USA. 1999;96:13973–13977. doi: 10.1073/pnas.96.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel SS, Mehlotra RK, Kastens W, Mgone CS, Kazura JW, Zimmerman PA. The association of the glycophorin C exon 3 deletion with ovalocytosis and malaria susceptibility in the Wosera, Papua New Guinea. Blood. 2001;98:3489–3491. doi: 10.1182/blood.v98.12.3489. [DOI] [PubMed] [Google Scholar]
- 27.Maier AG, Duraisingh MT, Reeder JC, Patel SS, Kazura JW, Zimmerman PA, Cowman AF. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat Med. 2003;9:87–92. doi: 10.1038/nm807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel SS, King CL, Mgone CS, Kazura JW, Zimmerman PA. Glycophorin C (Gerbich antigen blood group) and band 3 polymorphisms in two malaria holoendemic regions of Papua New Guinea. Am J Hematol. 2004;75:1–5. doi: 10.1002/ajh.10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.al-Yaman F, Genton B, Mokela D, Narara A, Raiko A, Alpers MP. Resistance of Plasmodium falciparum malaria to amodiaquine, chloroquine and quinine in the Madang Province of Papua New Guinea, 1990–1993. P N G Med J. 1996;39:16–22. [PubMed] [Google Scholar]
- 30.Muller I, Bockarie M, Alpers M, Smith T. The epidemiology of malaria in Papua New Guinea. Trends Parasitol. 2003;19:253–259. doi: 10.1016/s1471-4922(03)00091-6. [DOI] [PubMed] [Google Scholar]
- 31.Mehlotra RK, Mattera G, Bhatia K, Reeder JC, Stoneking M, Zimmerman PA. Insight into the early spread of chloro-quine-resistant Plasmodium falciparum infections in Papua New Guinea. J Infect Dis. 2005;192:2174–2179. doi: 10.1086/497694. [DOI] [PubMed] [Google Scholar]
- 32.Bruce MC, Galinski MR, Barnwell JW, Donnelly CA, Walmsley M, Alpers MP, Walliker D, Day KP. Genetic diversity and dynamics of plasmodium falciparum and P. vivax populations in multiply infected children with asymptomatic malaria infections in Papua New Guinea. Parasitology. 2000;121:257–272. doi: 10.1017/s0031182099006356. [DOI] [PubMed] [Google Scholar]
- 33.Mueller I, Taime J, Ibam E, Kundi J, Lagog M, Bockarie M, Reeder JC. Complex patterns of malaria epidemiology in the highlands region of Papua New Guinea. P N G Med J. 2002;45:200–205. [PubMed] [Google Scholar]
- 34.McNamara DT, Kasehagen LJ, Grimberg BT, Cole-Tobian J, Collins WE, Zimmerman PA. Diagnosing infection levels of four human malaria parasite species by a polymerase chain reaction/ligase detection reaction fluorescent microsphere-based assay. Am J Trop Med Hyg. 2006;74:413–421. [PMC free article] [PubMed] [Google Scholar]
- 35.Hii JL, Smith T, Mai A, Mellor S, Lewis D, Alexander N, Alpers MP. Spatial and temporal variation in abundance of Anopheles (Diptera:Culicidae) in a malaria endemic area in Papua New Guinea. J Med Entomol. 1997;34:193–205. doi: 10.1093/jmedent/34.2.193. [DOI] [PubMed] [Google Scholar]
- 36.Hii JL, Smith T, Mai A, Ibam E, Alpers MP. Comparison between anopheline mosquitoes (Diptera: Culicidae) caught using different methods in a malaria endemic area of Papua New Guinea. Bull Entomol Res. 2000;90:211–219. doi: 10.1017/s000748530000033x. [DOI] [PubMed] [Google Scholar]
- 37.McNamara DT, Thomson JM, Kasehagen LJ, Zimmerman PA. Development of a multiplex PCR-ligase detection reaction assay for diagnosis of infection by the four parasite species causing malaria in humans. J Clin Microbiol. 2004;42:2403–2410. doi: 10.1128/JCM.42.6.2403-2410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmerman PA, Buckler-White A, Alkhatib G, Spalding T, Kubofcik J, Combadiere C, Weissman D, Cohen O, Rubbert A, Lam G, Vaccarezza M, Kennedy PE, Kumaraswami V, Giorgi JV, Detels R, Hunter J, Chopek M, Berger EA, Fauci AS, Nutman TB, Murphy PM. Inherited resistance to HIV-1 conferred by an inactivating mutation in CC Chemokine Receptor 5: Studies in populations with contrasting clinical phenotypes, defined racial background, and quantified risk. Mol Med. 1997;3:23–36. [PMC free article] [PubMed] [Google Scholar]
- 39.Ross R. The measurement of malaria (section 31) In: Ross R, editor. The Prevention of Malaria. New York: E.P. Dutton & Company; 1910. pp. 217–246. [Google Scholar]
- 40.Knowles R, White RS. Studies in the parasitology of malaria. Indian Medical Research Memoirs. 1930;18:436. [Google Scholar]
- 41.WHO/MAL/2000.1091 New Perspectives in Malaria Diagnosis. Geneva: World Health Organization; 2000. [Google Scholar]
- 42.Ohrt C, Purnomo, Sutamihardja MA, Tang D, Kain KC. Impact of microscopy error on estimates of protective efficacy in malaria-prevention trials. J Infect Dis. 2002;186:540–546. doi: 10.1086/341938. [DOI] [PubMed] [Google Scholar]
- 43.McKenzie FE, Sirichaisinthop J, Miller RS, Gasser RA, Jr, Wong- srichanalai C. Dependence of malaria detection and species diagnosis by microscopy on parasite density. Am J Trop Med Hyg. 2003;69:372–376. [PMC free article] [PubMed] [Google Scholar]
- 44.O’Meara WP, McKenzie FE, Magill AJ, Forney JR, Permpanich B, Lucas C, Gasser RA, Jr, Wongsrichanalai C. Sources of variability in determining malaria parasite density by microscopy. Am J Trop Med Hyg. 2005;73:593–598. [PMC free article] [PubMed] [Google Scholar]
- 45.Prudhomme O’meara W, Remich S, Ogutu B, Lucas M, Mtalib R, Obare P, Oloo F, Onoka C, Osoga J, Ohrt C, McKenzie FE. Systematic comparison of two methods to measure parasite density from malaria blood smears. Parasitol Res. 2006 doi: 10.1007/s00436-006-0135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends Parasitol. 2004;20:233–240. doi: 10.1016/j.pt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Lee MA, Tan CH, Aw LT, Tang CS, Singh M, Lee SH, Chia HP, Yap EP. Real-time fluorescence-based PCR for detection of malaria parasites. J Clin Microbiol. 2002;40:4343–4345. doi: 10.1128/JCM.40.11.4343-4345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perandin F, Manca N, Calderaro A, Piccolo G, Galati L, Ricci L, Medici MC, Arcangeletti MC, Snounou G, Dettori G, Chezzi C. Development of a real-time PCR assay for detection of Plasmodium falciparum, Plasmodium vivax, and Plasmodium ovale for routine clinical diagnosis. J Clin Microbiol 42. 2004:1214–1219. doi: 10.1128/JCM.42.3.1214-1219.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rougemont M, Van Saanen M, Sahli R, Hinrikson HP, Bille J, Jaton K. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol. 2004;42:5636–5643. doi: 10.1128/JCM.42.12.5636-5643.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mangold KA, Manson RU, Koay ES, Stephens L, Regner M, Thomson RB, Jr, Peterson LR, Kaul KL. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–2440. doi: 10.1128/JCM.43.5.2435-2440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider P, Wolters L, Schoone G, Schallig H, Sillekens P, Hermsen R, Sauerwein R. Real-time nucleic acid sequencebased amplification is more convenient than real-time PCR for quantification of Plasmodium falciparum . J Clin Mi-crobiol. 2005;43:402–405. doi: 10.1128/JCM.43.1.402-405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swan H, Sloan L, Muyombwe A, Chavalitshewinkoon-Petmitr P, Krudsood S, Leowattana W, Wilairatana P, Looareesuwan S, Rosenblatt J. Evaluation of a real-time polymerase chain reaction assay for the diagnosis of malaria in patients from Thailand. Am J Trop Med Hyg. 2005;73:850–854. [PubMed] [Google Scholar]
- 53.Elsayed S, Plewes K, Church D, Chow B, Zhang K. Use of molecular beacon probes for real-time PCR detection of Plas-modium falciparum and other plasmodium species in peripheral blood specimens. J Clin Microbiol. 2006;44:622–624. doi: 10.1128/JCM.44.2.622-624.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58:283–292. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- 55.Postigo M, Mendoza-Leon A, Perez HA. Malaria diagnosis by the polymerase chain reaction: a field study in southeastern Venezuela. Trans R Soc Trop Med Hyg. 1998;92:509–511. doi: 10.1016/s0035-9203(98)90893-8. [DOI] [PubMed] [Google Scholar]
- 56.May J, Mockenhaupt FP, Ademowo OG, Falusi AG, Olumese PE, Bienzle U, Meyer CG. High rate of mixed and sub-patent malarial infections in southwest Nigeria. Am J Trop Med Hyg. 1999;61:339–343. doi: 10.4269/ajtmh.1999.61.339. [DOI] [PubMed] [Google Scholar]
- 57.Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA. A genus- and species-specific nested poly-merase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg. 1999;60:687–692. doi: 10.4269/ajtmh.1999.60.687. [DOI] [PubMed] [Google Scholar]
- 58.Incardona S, Chy S, Chiv L, Nhem S, Sem R, Hewitt S, Doung S, Mercereau-Puijalon O, Fandeur T. Large sequence heterogeneity of the small subunit ribosomal RNA gene of Plas-modium ovale in cambodia. Am J Trop Med Hyg. 2005;72:719–724. [PubMed] [Google Scholar]
- 59.Coleman RE, Maneechai N, Rachaphaew N, Kumpitak C, Miller RS, Soyseng V, Thimasarn K, Sattabongkot J. Comparison of field and expert laboratory microscopy for active surveillance for asymptomatic Plasmodium falciparum and Plas-modium vivax in western Thailand. Am J Trop Med Hyg. 2002;67:141–144. doi: 10.4269/ajtmh.2002.67.141. [DOI] [PubMed] [Google Scholar]
- 60.Kain KC, Kyle DE, Wongsrichanalai C, Brown AE, Webster HK, Vanijanonta S, Looareesuwan S. Qualitative and semi-quantitative polymerase chain reaction to predict Plasmodium falciparum treatment failure. J Infect Dis. 1994;170:1626–1630. doi: 10.1093/infdis/170.6.1626. [DOI] [PubMed] [Google Scholar]
- 61.Jarra W, Snounou G. Only viable parasites are detectec by PCR following clearance of rodent malarial infections by drug treatment or immune responses. Infect Immun. 1998;66:3783–3787. doi: 10.1128/iai.66.8.3783-3787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawamoto F, Win TT, Mizuno S, Lin K, Kyaw O, Tantulart IS, Mason DP, Kimura M, Wongsrichanalai C. Unusual plasmodium malariae-like parasites in southeast Asia. J Parasitol. 2002;88:350–357. doi: 10.1645/0022-3395(2002)088[0350:UPMLPI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 63.Snounou G, White NJ. The co-existence of Plasmodium: sidelights from falciparum and vivax malaria in Thailand. Trends Parasitol. 2004;20:333–339. doi: 10.1016/j.pt.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Hii JL, Smith T, Vounatsou P, Alexander N, Mai A, Ibam E, Alpers MP. Area effects of bednet use in a malariaendemic area in Papua New Guinea. Trans R Soc Trop Med Hyg. 2001;95:7–13. doi: 10.1016/s0035-9203(01)90315-3. [DOI] [PubMed] [Google Scholar]
- 65.Smith T, Hii JL, Genton B, Muller I, Booth M, Gibson N, Narara A, Alpers MP. Associations of peak shifts in age- prevalence for human malarias with bednet coverage. Trans R Soc Trop Med Hyg. 2001;95:1–6. doi: 10.1016/s0035-9203(01)90314-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.