Abstract
The epipolythiodiketopiperazine (ETP) alkaloids are a highly complex class of natural products with potent anticancer activity. Herein, we report the application of a flexible and scalable synthesis, allowing the construction of dozens of ETP derivatives. The evaluation of these compounds against cancer cell lines in culture allows for the first expansive structure–activity relationship (SAR) to be defined for monomeric and dimeric ETP-containing natural products and their synthetic cognates. Many ETP derivatives demonstrate potent anticancer activity across a broad range of cancer cell lines, and kill cancer cellsviainduction of apoptosis. Several traits thatbode well for the translational potential of the ETP class of natural products includeconcise and efficient synthetic access, potent induction of apoptotic cell death, activity against a wide range of cancer types, and a broad tolerance for modifications at multiple sitesthat should facilitate small-molecule drug development, mechanistic studies, and evaluation in vivo.
Introduction
Epipolythiodiketopiperazine (ETP)1 alkaloids constitute a large (ca. 120 members) and diverse family of biologically active secondary metabolites produced by a number of filamentous fungi including those from the Chaetomium, Leptosphaeria, Aspergillus, Verticillium, Penicillium, and Pithomyces genera. These small-molecule natural products are characterized by the incorporation of an intramolecular polysulfide bridge at the α,α'-positions of a cyclo-dipeptide (or diketopiperazine – DKP) (Fig. 1). Although mono-, di-, tri-, and tetrasulfide members are naturally occurring, the disulfides are most prevalent.2 These mycotoxins, containing one ortwo ETP rings, exhibit a wealth of structural diversity1c and display a fascinating spectrum of biological activities1a,3 including antibacterial,4 anticancer,3,5 antiviral,6 antiparasitic, antifungal,7 antimalarial, immunosuppressive, immunomodulatory,1b,5a,8 phytotoxic,9nematicidal,10antiplatelet,11and anti-inflammatory effects.1f
Fig. 1.
Representative thiodiketopiperazines.
As a consequence of the intriguing biological activities3 and the structural diversity1cthat surrounds the central ETP motif, access to greater quantities of the alkaloids and their analogs is desired; a considerable number of synthetic efforts have thus been directed toward the synthesis of the ETP core1b,12 and ETP-containing naturally occurring alkaloids.1f,13,14Due to the synthetic challenges posed by their complex molecular architecture, however, structure–activity relationship (SAR) studies of ETP-containing derivatives are still limited and warrant further exploration.1a,3Various ETP alkaloids have been assessed in a diverse array of biological tests, but the non-uniformity of these studies precludes comparative analysis and the inference of meaningful conclusions. As a result, although the polysulfide warhead has been noted to be a critical pharmacophore (vide infra), little is known about the influence of the number of sulfur atoms, the stereochemical configuration of the sulfurated centers, or the dimerization state of the carbocyclic structure on the bioactivity of these alkaloids. Investigation of the impact of each of these structural features is crucial to elucidating the mode of action of these compounds, to designing highly potentstructures with suitable physicochemical and biopharmaceutical properties, and to their translation in vivo in clinical applications (e.g., biological probes and chemotherapeutic agents).
Several studies have unequivocally demonstrated that the polysulfide bridge plays a key role and is oftensufficient for bioactivity.1a–bIndeed, the chemical ablation of the sulfur bridge in naturally occurring alkaloids (e.g., chaetocin A (4),13f gliotoxin (1)15, sporidesmin16) or the synthesis of analogs devoid of sulfur at the α-positions of the cyclo-dipeptide17a results in biologically inactive compounds. Similarly, reductive S-methylation of the epidithiodiketopiperazine motif in a number of natural products (e.g., gliotoxin (1),1b,5c,n,8a chaetocin A (4),18 scabrosin,19 sporidesmin,16,20 T988 B,21bionectin C (8),4and chetracin D22) unfailingly results in a dramatic loss of biological activity. Furthermore, a general pattern between the degree of sulfuration and the intensity of the biological response has not been clearly established.1a–b,3,10,18,21–23 It is ambiguous whether these molecules go through a common mode of action or are converted into a similar active species. Interestingly, epimonothiodiketopiperazine metabolites have been reported to be at least one order of magnitude less active than their congeners.1b,19,24,25
At least three pathways of toxicity have been proposed in the literature1a–c,f,5a for the ETP-containing products: (1) generation of deleterious reactive oxygen species (ROS) (e.g., superoxide radical anion, hydroxyl radical, hydrogen peroxide) by redox cycling5f,k–m,26 and induction of significant oxidative stress, DNA strand cleavage, and apoptosis; (2) covalent conjugation and inhibition of cellular proteins by forming mixed disulfides5a,11,17,20,26b or catalytic formation of intramolecular disulfide bonds between cysteine residues on proteins27; and (3) disruption of the global tertiary structure of proteins containing a Zn2+-binding cysteine-histidine rich protein domain via a zinc ion ejection mechanism.17,28
The epidithiodiketopiperazine class of natural products appears to have considerable anticancer potential,3,5 but definitive conclusions are difficult to draw as an array of these compounds has never been tested against a wide variety of cancer cell lines. The verticillins5g,i,k,n and the chaetocins5d,f,h,l–m,26d,29 both exhibit cytotoxic activity against cancer cell lines in culture and efficacy in in vivo tumor models. The true translational potential of the ETP-containing alkaloids, however, will only be defined after a larger number of more detailed cell culture and in vivo studies.
Recently, we reported the enantioselective total synthesis of dimeric epidithiodiketopiperazines (+)-12,12'-dideoxyverticillin A (3) and (+)-chaetocin A (4) as well as higher-order polysulfides (+)-chaetocin C (5) and (+)-12,12'-dideoxychetracin A (6) relying on a bioinspired approach.14a,bSimilarly, the development of our Lewis acid-promoted C3-nucleophilic substitution14c,30 culminated in the concise enantioselective total synthesis of (+)-gliocladin B (7)31and (+)-12-deoxybionectin A (10).4Thesesynthetic methodologiesoffer an opportunity to rapidly create multiple derivatives for evaluation against cancer cell lines in culture; many of these compounds cannot be created by simple and direct chemical modifications of the (scarce or sensitive) parent natural mycotoxins.
With the ultimate goal of identifying ETP derivatives worthy of evaluation in vivo, and to aid in chemotherapeutic development through potent but simplified analogs, a large set of structurally diverse natural and synthetic ETP alkaloids were synthesized. Sixty natural alkaloids and their derivatives (Fig. 2) were tested for their ability to induce cell death in two human cancer cell lines, enabling the derivation of a comprehensive SAR. A selection of 25 sulfurated alkaloids with(sub)nanomolar potencies was further evaluated for activityagainst threeadditional humancancer cell lines, allowing for a broad assessment of anticancer effects. Representative compounds from the monomer and dimer classes were shown to induce caspase-dependent apoptosis. The potent and broad anticancer activity of ETP-containing alkaloids suggests that they have considerable translational potential.
Fig 2.
Molecules synthesized and evaluated for anticancer activity in this study.
Design and synthesis of ETP alkaloids
Overview of synthetic goals
Sulfur atoms have long been recognized as essential to the anticancer activity of ETP natural products; however, a more refined understanding of structure–activity relationships has been lacking. To identify how this and other structural elements within the hexahydropyrroloindoline and diketopiperazine substructures are critical to the anticancer activity, multiple derivatives of both monomeric and dimeric ETP alkaloids were synthesized and evaluated. The general strategy for derivatives was to construct sets of compounds that varied in the substituents about the hexahydropyrroloindolinecarboskeleton and the nature, extent, and configuration of sulfuration. The compounds produced through these synthetic efforts are depicted in Fig. 2.
Design and synthesis of sarcosine-derived monomeric ETP derivatives
In an effort to formulatea SAR for homodimeric tryptophan-derived ETP alkaloids such as chaetocin A (4) and verticillin A (2), various derivatives of thisalkaloid class were synthesized and evaluated. Specifically, compounds that were differentially substituted at the C3-quaternary stereogenic center were constructed and then elaborated with different types of sulfur motifs. Relying on the versatility of the different synthetic methodologies developed in our group en route to the total synthesis of several naturally occurring monomeric and dimeric ETP alkaloids,14 compounds 3–7, 10, and14–67 were concisely and efficiently accessed as described in Schemes 1–3 or according to experimental procedures previously reported by our group.14a–c,32
Scheme 1.
Sarcosine-derived monomeric epidithiodiketopiperazines: modulation of the C3-substituent. Reagents and conditions: (a) AgBF4, DTBMP, EtNO2, 23 °C, 1 h, 90%; (b) N-TIPS-pyrrole, AgBF4, DTBMP, EtNO2, 0 °C, 1 h, 72%; (c) Anisole, AgBF4, DTBMP, EtNO2, 0 °C, 1 h, 99%; (d) 5-Br-N-TIPS-indole, AgBF4, DTBMP, EtNO2, 0 °C, 1 h; 83%; (e) H2, Pd/C, NEt3, MeOH–EtOAc (2:3 v/v), 23 °C, 8 h; Et3N•3HF, 23 °C, 13 h, quant.; (f) n-Bu4NMnO4, CH2Cl2, 23 °C, 2 h, 25–52%; (g) H2S, TFA–EtNO2 (2:3 v/v), 0 to 23 °C, 4 h; O2, EtOAc, 23 °C, 47–80%; TIPS = triisopropylsilyl; DTBMP = 2,6-di-tert-butyl-4-methylpyridine; TFA = trifluoroacetic acid.
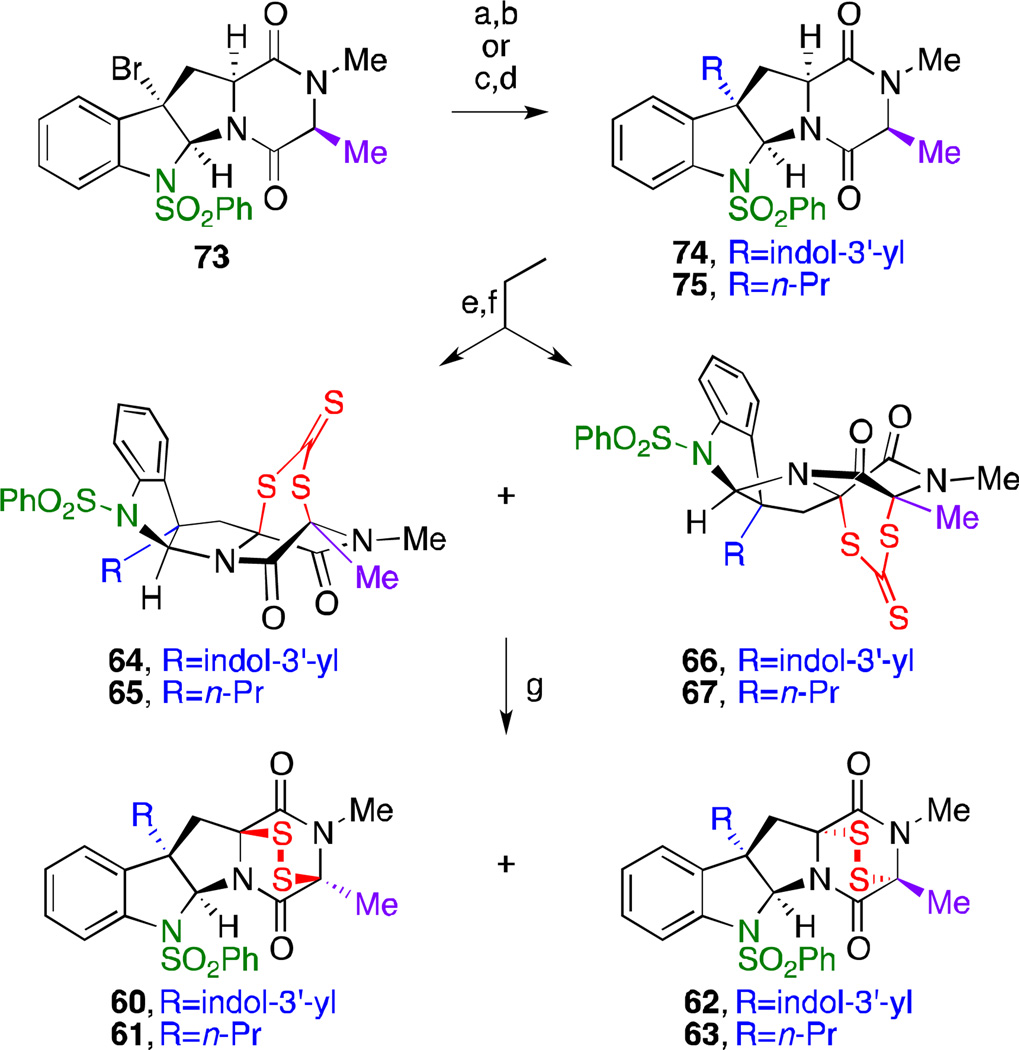
Scheme 3.
Alanine-derived monomeric polythiodiketopiperazines: modu-lation of the C3-substituent. Reagents and conditions: (a) 5-Br-N-TIPS-indole, AgBF4, DTBMP, EtNO2, 0 °C, 1 h, 67%; (b) H2, Pd/C, NEt3, MeOH–EtOAc (2:3 v/v), 23 °C, 8 h; Et3N•3HF, 23 °C, 13 h, quant.; (c) AllylSnBu3, AIBN, PhH, 80 °C, 5 h, 61%; (d) H2, Pd/C (5 mol %), CH2Cl2, 2 h, 100%; (e) Pyr2AgMnO4, Pyr, 23 °C, 4 h, 37% (R=indol-3’-yl), 68% (R=n-Pr); (f) K2CS3, TFA–CH2Cl2 (1:2 v/v), 23 °C, 2.5 h, 63%, 5:1 dr (64:66), 52% (65), 15% (67); (g) ethanolamine, acetone, 23°C, 45 min; KI3, Pyr, CH2Cl2, 23°C, 48%, 5:1 dr (60:62), 70% (61), 78% (63); TIPS = triisopropyl-silyl;DTBMP = 2,6-di-tert-butyl-4-methyl-pyridine; AIBN = α,α′-azoisobutyronitrile;Pyr2AgMnO4 = bis(pyridine) silver(I) permanganate; Pyr = pyridine; TFA = trifluoroacetic acid.
endo-Tetracyclic bromide 54, prepared from sarcosine l-tryptophan cyclo-dipeptide,14c was used to access epidithiodiketopiperazines bearing different C3-substituents (Scheme 1). Electrophilic activation using silver(I) tetrafluoroborate in nitroethane and trapping of the transient tertiary benzylic carbocation with the desired nucleophile (i.e., fluoride, N-TIPS-pyrrole,33 anisole, 5-Br-N-TIPS-indole) afforded the C3-substituted endo-tetracycles 59 and 68–70 in high yields and excellent levels of regio- and stereoselection.14c Dihydroxylation of 59 and 68–70 at the C11-methine and C15-methylene positions was achieved with tetra-n-butylammonium permanganate (n-Bu4NMnO4, 4 equiv) in dichloromethane to provide the corresponding diols in moderate to good yields as single diastereomers. The direct double cis-thiolation was accomplished in a single step and in good to high yields (47–80%) by exposure of the bis-hemiaminals to trifluoroacetic acid (TFA) in hydrogen sulfide-saturated dichloromethane solution followed by mild aerobic oxidation to access the bridgehead disulfides as β-epimers 26, 30–33 andα-epimers 34–35.34Interestingly, the diastereoselectivities are consistent with the steric bias imposed by the C3-substituents {β:α ratio= 2:1 (C3-F);4:1 (C3-Br);> 5:1 (C3-pyrrol-3'-yl); > 7:1 (C3-indol-3'-yl); > 10:1 (C3-p-MeOPh)}.
As shown in Scheme 2, we also prepared a set of 27 compounds with a modified sulfur motif within the DKP core using the versatile diol 56 as a strategic point of divergence. Relying on our unified and general solution to epidi-, epitri-, and epitetrathiodiketopiperazines14b and the innate reactivity differences between the C11 and C15 hemiaminals, chemo- and stereoselective thiolation of diol 56 by treatment with TFA in hydrogen sulfide-saturated dichloromethane solution at 0 °C generated the corresponding thiohemiaminal 48 in 90% yield and in a highly diastereoselective fashion (>10:1 dr). Masking of both alcohol and thiol groups as isobutyrates and photoinduced reductive removal of the benzenesulfonyl group gave 51. The desired degree of sulfidation was eventually accomplished by hydrazinolysis, chemoselective S-sulfenylation with chloro(triphenylmethane) sulfane or disulfane followed by hafnium triflate-mediated cyclization to afford (+)-12-deoxybionectin A (10) and its epitrithiodiketopiperazine congener 29 in 65 and 47% yield (3-steps), respectively. A similar two-step approach was employed to access benzenesulfonyl-protected epitri- and epitetrathiodiketopiperazines 27 and 28 in 42% and 44% yield, respectively. Ultimately, reduction of the bridgehead disulfide with NaBH4 followed by in situ S-methylation afforded (+)-gliocladin B (7)14c,31 and bis(methylthioether) 39 in high yields.
Scheme 2.
Sarcosine-derived monomeric polythiodiketopiperazines: modulation of the sulfur bridge. Reagents and conditions: (a) N2H4, THF, 0 °C, 1 h; Ph3CSCl, NEt3, THF, 0 °C, 90 min, 81% (2-steps); (b) N2H4, THF, 0 °C, 1 h; Ph3CSSCl, Hünig’s base, THF, 0 °C, 25 min; (c) Hf(OTf)4, MeCN, 23 °C, 15 min, n=0: 80%, n=1: 47% (3-steps); (d) H2S, TFA–CH2Cl2 (1:9 v/v), 0 to 23 °C, 2 h, 90%, >10:1 dr; (e) Ph3CSSmCl, Hünig’s base, THF, 0 °C, 25 min; Hf(OTf)4, MeCN, 23 °C, 50 min, m=1: 42% (2-steps), m=2: 44% (2-steps); (f) MeI, NaBH4, Pyr, THF, MeOH, 23 °C, 45 min, 80%; (g) BnSH, TFA–EtNO2 (2:3 v/v), 23 °C, 3 h, 80%, 17:3dr; (h) Boc2O, DMAP, MeCN, 23 °C, 3 h, 69%; (i) H2S, TFA–EtNO2 (3:4 v/v), 0 to 23 °C, 2 h; O2, EtOAc, 23 °C, 77%, >7:1 dr; (j) Boc2O, DMAP, CH2Cl2, 23 °C, 7 h, 81%; (k) NaBH4, THF, MeOH, 23 °C, 2 h; MOMCl, NEt3, 23 °C, 5 h, 73%; (l) TFA, CH2Cl2, 0 to 23 °C, 3 h, 81–91%; (m) hν (350 nm), 1,4-dimethoxynaphthalene, ascorbic acid, sodium ascorbate, H2O–MeCN (1:4 v/v), 25 °C, 2.5 h, 82%; (n) NaBH4, THF, MeOH, 23 °C, 80 min; MEMCl, NEt3, 23 °C, 12 h, 80% (42) and 19% (47); (o) NaBH4, THF, MeOH, 23 °C, 45 min; (p) TCDI, CH2Cl2, 23 °C, 22 h, 34% (2-steps); (q) CDI, CH2Cl2, 23 °C, 24 h, 8% (2-steps); (r) CH2I2, NaBH4, Pyr, THF, MeOH, 0 to 23 °C, 1 h, 46%; (s) P(OEt)3, THF, 23 °C, 6 h, 63%; (t) AcCl, Pyr, CH2Cl2, 23 °C, 4 h, 63% (2-steps); (u) MeSCl, Pyr, CH2Cl2, 0 to 23 °C, 2 h, 49% (2-steps); (v) (MeS)2, THF, 23 °C, 19 h, 41% (2-steps); TFA = trifluoroacetic acid; Pyr = pyridine; Boc2O = di-tert-butyl dicarbonate; DMAP = 4-(dimethylamino)pyridine; TCDI =1,1’-thiocarbonyldiimidazole; CDI =1,1’-carbonyldiimidazole;MOMCl = chloromethyl methyl ether; MEMCl = 2-methoxyethoxymethyl chloride.
(+)-Gliocladin C (52)31 and several C11-hydroxylated (57–58) and C11,C12-dehydrogenated (53) intermediates were prepared following the procedures previously reported for the synthesis ofthis atypical non-thiolated triketopiperazine.14c
Exposure of hemiaminal 56 to benzyl mercaptan and TFA in nitroethane resulted in the formation of the corresponding bis(benzylthioether) (C15β:C15α = 5.7:1) in 80% yield (single diastereomer, C15β). Further derivatization of the indole nitrogen with a t-butoxycarbonyl group gave 43 in 69% yield. After masking the indole substituent of the key ETP intermediate 26, the bridgehead disulfide was reduced with NaBH4 and S-methoxymethylated in a single flask. Subsequent t-butoxycarbonylremoval with TFA in dichloromethane afforded bis(thioether) 40 in 66% yield over two steps. A similar strategy including the photoinduced reductive removal of the N1-benzenesulfonyl group provided 41 in 55% over three steps. Reduction of the sulfur bridge of ETP 24 with NaBH4 in a mixture of THF and methanol and in situ trapping of the resulting thiolates with 2-methoxyethoxy-methyl chloride (MEMCl) led to thioether 47 and bis(thioether) 42 in 19% and 80% yield, respectively.
Further modifications to the sulfur bridge1b,12c,25 were accomplished by treatment of the corresponding dithiol (obtained from NaBH4reduction of ETP 26) with 1,1'-thiocarbonyldiimidazole (TCDI) or 1,1'-carbonyldiimidazole (CDI) to afford di- and trithiocarbonates 36 and 37, respectively. Similarly, thioacetal 38 was accessed directly by double alkylation using diiodomethane. Desulfurization of epidithiodiketopiperazine 26 was realized by treatment with triethylphosphite in THF to give epimonosulfide 25 in 63% yield.35 The sulfur atoms were also capped with the S-acetyl and S-methylsulfane functional groupings to afford compounds that are potentially more labile under intracellular conditions. After reduction of the sulfur bridge of epidisulfide 26, its treatment with an excess of acetyl chloride, methanesulfenyl chloride, or dimethyldisulfide afforded compounds 44, 45 and 46, respectively, in good yields.
Design and synthesis of N-methylalanine-derived monomeric ETP derivatives
We were also interested in investigating the effect of a different substituent at the C15 position of monomers. With this objective in mind, we prepared monomeric ETP-containing products derived from N-methyl-l-alanine l-tryptophan cyclo-dipeptide (Scheme 3). Synthesis of these derivatives commenced with endo-tetracyclic bromide 73.14a Tertiary benzylic bromide 73 also proved to be an excellent substrate for the desired regio- and stereoselective Friedel–Crafts-type coupling14c with 5-bromo-1-triisopropylsilylindole (67% over 2 steps) to afford C3-indolyl tetracycle 74. Allylation of the C3-tertiary benzylic halide using allyltributylstannane under radical conditions36 followed by hydrogenation of the terminal olefin afforded C3-n-propyl tetracycle 75. These two C3-substituted tetracyclic monomers were subsequently subject to hydroxylation conditions using bis(pyridine) silver(I) permanganate (Pyr2AgMnO4) in pyridine. Treatment of the resultant diols with potassium trithiocarbonate and TFA in dichloromethane resulted in rapid formation of the desired monomeric dithiepanethiones 64 and 66 in 63% yield as a 5:1 isomeric mixture, as well as 65 and 67 in 52% and 17% yield, respectively.14a Finally, exposure of these compounds to ethanolamine in acetone followed by oxidative workup using potassium triiodide yielded the corresponding epidithiodiketopiperazine analogs 60–63.
Design and synthesis of dimeric ETP derivatives
The synthesis of the homodimeric DKP and ETP derivatives (Schemes S1 and S2†) have been reported previously in the literature en route to the syntheses of (+)-12,12'-dideoxyverticillin A (3)14a as well as (+)-chaetocins A (4) and C (5) and (+)-12,12'-dideoxychetracin A (6).14b The diacetate forms of these epidi- and epitrithiodiketopiperazines (15–16) were also synthesized and evaluated.32 In order to explore the effects of N1-sulfonylation on their bioactivity, a variety of related derivatives (14, 18–19, 21–23) possessing the sulfonyl group were prepared analogously.32
Anticancer activity and structure–activity relationship studies
All 60 synthesized compounds (Fig. 2) were screened for their ability to induce death in two human cancer cell lines: U-937 (leukemic monocyte lymphoma) and HeLa (cervical cancer). Compounds that demonstrated anticancer activity at 1 µM or below were retested in triplicate at a range of compound concentrations to generate logistical dose-response curvesfrom which IC50 values were derived.32These results are presented in Table 1 and discussed below.
Table 1.
Assessment of the 60 ETPs and DKPs from Fig. 2 for anticancer activity against U-937 (hystiocytic lymphoma) and HeLa (cervical carcinoma) human cancer cell lines after a 72-hour exposure.a
 |
72-hour IC50 values (in nM) as determined by MTS (U-937) and SRB (HeLa).32 Error is standard deviation of the mean, n ≥ 3. Cmpd = compound DKP = diketopiperazine ETP = epipolythiodiketopiperazine IC50 = half maximal inhibitory concentration MTS = 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H–tetrazolium SRB = sulforhodamine B.
Carboskeleton
In both U-937 and HeLa cells, the homodimers are the most potent compounds {IC50 (U-937) ≥ 0.18 nM; IC50 (HeLa) ≥ 0.09 nM}, with the N1,N1'-benzenesulfonylated analog (14) of (+)-12,12'-dideoxyverticillin A (3) showing the best activity {IC50 (U-937): 0.18 nM; IC50 (HeLa): 0.09 nM}. Monomeric ETP derivatives also show satisfactory activity in both HeLa (IC50 ≥ 5.9 nM) and U-937 (IC50 ≥ 2.8 nM) human cancer cell lines. Within the N1-benzenesulfonyl monomeric class, various aromatic substituents (indol-3'-yl 26, N-Boc-indol-3'-yl 24, pyrrol-3'-yl 32, p-MeO-phenyl 33) are well tolerated at the C3-position and their IC50’s are of the same order of magnitude {IC50 (U-937): 2.8–14.8 nM; IC50 (HeLa): 22–75 nM}. Although halide substitution at C3 (bromide 30, fluorides 31 and 35) results in intermediate activity, C3-fluorinated epidithiodiketopiperazines do not follow the trend we observe with related ETP analogs in this SAR study. It appears that the steric environment of the C3 position is crucial for biological activity: n-alkyl groups at that position (n-propyl analogs 61 and 65) lead to substantially lower potencies than more sterically hindered (hetero)aryl andhalide substituentsorthe C3' quaternary carbon of a second monomeric subunit. The superior activity of the dimers over (hetero)arylated monomers further supports the role of steric crowding at C3.
The difference in activity between the monomers and the dimers was also observed by Numata et al. through biological evaluation (growth inhibition in murine P388 leukemia cell line) of the leptosins, a subset of fungal metabolites including structurally diverse dimeric and monomeric ETP natural products.23 Dimers containing two sulfur bridge groups are one order of magnitude more potent than monomeric C3-(3'-indolyl) analogs and 2 to 3 orders of magnitude more potent than heterodimers bearing a single sulfur bridge. This non-linear increase of biological activity between mono- and dimeric ETP natural products has also been observed in other families (e.g., chetracin,22 gliocladine,10leptosin,5f,23averticillin A (2)4) hinting at the possibility of a synergistic effect. It is unclear, however, if pharmacokinetic properties are playing a significant role.
Comparing homodimers (+)-12,12'-dideoxyverticillin A (3), (+)-chaetocin A (4), (+)-chaetocin C (5), and (+)-12,12'-dideoxychetracin A (6) head-to-head reveals that the chaetocin-type ETP derivatives are more potent than their non-C15-hydroxylated counterparts {IC50 (U-937): 0.75–1.3 nM vs. 15.5 nM; IC50 (HeLa): 5.6–6.9 nM vs. 7.2 nM}. Acetylation of the 17,17'-hydroxyl groups (15–16) also results in a reduction of potency (5.8- to 12.9-fold for U-937; 2.0- to 11.1-fold for HeLa). Additionally, methyl substitution at C15 in monomeric alkaloids (Trp–Ala cyclo-dipeptides 60 vs. 26 and 64 vs. 36) only affects the potency of the compoundsmoderately. In general, the differencein potency between the different types of substituents atC15 is minimal, although it is sensitive to variations of the steric environment. This suggests that this position could be amenable to additional modifications, especially to optimize the pharmacokinetic parameters during drug development.
Our SAR studies also indicate that substitution at N1 and N1' with benzenesulfonyl (14) or trifluoroacetyl (17) groups does enhance the anticancer activity of the alkaloids, although the magnitude of this effect is variable. In the case of a benzenesulfonyl group (14), the potency is dramatically increased {2 orders of magnitude more potent than the corresponding secondary aniline (+)-12,12'-dideoxyverticillin A (3)}. The trifluoroacetamide at N1 and N1' (17vs.16) plays only a minimal role in the anticancer activity, with slightly lower IC50 values (1.1-fold for U-937; 2.5-fold for HeLa) for these derivatives. For the monomeric ETP-containing analogs, the N1-benzenesulfonyl substitution generally amplifies the anticancer effect in U-937 cell line (epidisulfide: 26vs. 10; epitrisulfide: 27 vs. 29). The N1 group confers significantly higher chemical stability but also likely affects the pharmacodynamic properties of these ETPs.
Influence of the nature and degree of sulfuration
While prior studies have demonstrated that having no sulfur atoms {naturally occurring (+)-gliocladin C (52) and synthetic analogs: opened DKP (55),non-oxidized α-positions (21, 54, 59), α-hydroxylated (22–23, 56–58), α,β-unsaturated (53)} results in a complete loss of biological activity, our studies go further and demonstrate that sulfuration at only the tryptophan Cα-position is not sufficient for potent activity {bis(trisulfanes) (19 and 20), C11-thioesters (50–51), C11-thioether (49), and C11-thiols (47–48)}. This clearly reinforces the necessity of sulfur atoms at the α and α' positions and the importance of the sulfide bridge or sulfur containing bridge for anticancer activity. This is in accordance with the observation made by Numata in the leptosin class, where monomeric and dimeric ETP alkaloids are 2 to 4 orders of magnitude more potent than heterodimeric metabolites devoid of sulfur bridge.23 Both amino acid Cα positions appear torequire sulfuration by separate sulfur atoms, although the epimonosulfide (25) is not completely inactive.
Sulfur derivatives possessing non-labile alkyl groups {S-methylthioethers (7 and 39), S-(methoxymethyl)thioethers (40–41), S-(2-ethoxyethoxymethyl)thioether (42 and 47), S-benzylthioether (43)} did not display any anticancer activity (IC50> 10 µM). Remarkably, thioacetal 38 retains moderate cytotoxic activity.Interestingly, the degree of sulfuration of the polysulfide bridge {dimers: (+)-chaetocin A (4) vs. (+)-chaetocin C (5) vs. (+)-12,12'-dideoxychetracin A (6) or 15vs. 16; monomers: 10 vs. 29, 26vs. 27vs. 28} has no substantial impact on this cell death induction and the IC50 values are within the margin of error of each other. It is ambiguous whether these molecules go through a common mode of action or are converted into a similar active species. The different order of polysulfides may have similar biological activity if the rate determining step for their mode of action is after the conversion to a common active intermediate; however, if the rate determining step is the conversion of the bridging polysulfide into this putative species, then one may expect a difference in activity.
In addition to ETP-containing compounds, we found that several monomeric or dimeric derivatives possessing modifications directly on the sulfur bridge serve as competent anticancer agents; IC50 values are<10-fold less potent compared to the parents. These include thioacetate (44), dithiocarbonate (37), trithiocarbonates (18, 36, 64), and alkyl disulfides (45–46). The methyl disulfides would readily be converted to the thiols through reduction or nucleophilic displacement. The data are highly suggestive of a mode of action that involves a common intermediate. In the presence of a reducing cytoplasmic environment combined with the presence of enzymes – hydrolases, carboxylesterases, and lipases37 – it is reasonable to believe that the methyl disulfides, thioacetates, and thiocarbonates play the role of prodrugs.38 They may be converted to their corresponding epidisulfide pharmacophores,1a,26c which are potentially actively concentrated within the cell via a glutathione-dependent uptake mechanism.39,40
Furthermore, the relative stereochemistry of the ETP system seems to be critical since compounds with the sulfur bridge on the α-face of the DKP (34, 62–63, 66–67) are completely inactive.
Efficacy of ETP alkaloids across multiple cancer cell lines
In order to further probe the potential of ETP alkaloids as anticancer agents, 25 derivatives, selected among the most potentfrom the primary screening (Table 1), were tested in cultureagainsta panel of three supplementary human cancer cell lines representing three additional tumor histologies (H460, lung carcinoma; 786-O, renal carcinoma; MCF-7, breast carcinoma).32As shown in Table 2, the ETPs retain similar patterns of potency across all of the cell lines. Some compounds, such as 14, retain higher levels of activity in all of the cell lines as compared to others, such as 17. Generally, however, U-937 and HeLa are slightly more sensitive to the ETPs than the other three cell lines. In particular,thenon-adherent lymphoma cell line U-937 showsthe highest susceptibility to the ETP class, as evidenced by the generally high potency of both monomers and dimers against this cell line.
Table 2.
Assessment of the top 25 compounds for cytotoxicity in five human cell lines {U-937 (hystiocytic lymphoma), HeLa (cervical carcinoma), H460 (lung carcinoma), 786-O (renal carcinoma), and MCF-7 (breast carcinoma)} after a 72-hour exposure.a
 |
72-hour IC50 values (in nM) as determined by MTS (U-937) and SRB (HeLa, H460, 786-O, and MCF-7).32 Error is standard deviation of the mean, n ≥ 3 Cmpd = compound IC50 = half maximal inhibitory concentration MTS = 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H–tetrazolium SRB = sulforhodamine B.
The polysulfide dimers (+)-12,12'-dideoxyverticillin A (3), (+)-chaetocins A (4) and C (5), (+)-12,12'-dideoxychetracin A (6), 14, and bisdithiepanethione 18 are the most active compounds across the board. In the case of the N1,N1'-benzenesulfonylated analog of (+)-12,12'-dideoxyverticillin A (14), the potency is dramatically increased (2 orders of magnitude more potent than the parent natural product 3 on the five tested cell lines). Our SAR studies show that the number of sulfur atoms in the bridge for homodimeric ETP derivatives has no significant effect.
Some additional trends are apparent from the data derived from this extended panel of cell lines. First, the degree of sulfuration has a larger impact in some of the adherent cell lines tested (H460, 786-O, and MCF-7), especially in the case of 26–28. This set of compounds shows a 2-fold decrease in activity with each additional sulfur atom in the polysulfide bridge. However, the overall potency of these compounds is still reasonable (i.e., IC50< 300 nM). Interestingly, it appears that lack of substitution at N1 (10 and 29) mitigates this effect. This trend is also not observed within the dimers (4–6), which is again consistent with the lack of substitution at N1. Monomeric ETP derivativeswith weaker activity in our initial assays (31, 60, and 64) were generally less potent in these additional cancer cell lines. Thethree prodrugs of the epidisulfide bridge (bisthioacetate 44, trithiocarbonate 36, dithiocarbonate 37) display lower activity than the corresponding epidisulfide 26; however, they all showconsistently lower IC50 values than the parent epidisulfide 10 (up to 6.7-fold more potent). There were several derivatives which displayed drastically different activity across cell lines; C3-pyrrolyl 32 and C3-p-methoxyphenyl 33 epidisulfides displayed a consistent potency (4-fold change in activity, as compared to 6- to 40-fold changes for otherderivatives) in addition to good relative potency in H460 cells, especially when compared to the activity of structurally similar derivatives.
Hemolytic activity of ETP alkaloids
Compounds that induce death in a range of diverse cancer cell lines with single-digit nanomolar potency are valuable, and an emerging application of such compounds is as antibody-drug conjugates (ADC).41 ADCs use a covalently-bound antibody to selectively target a potent cytotoxin to a tumor site, as exemplified by Seattle Genetics’ brentuximab vedotin (an ADC with monomethyl auristatin E) that was recently approved by the FDA for the treatment of Hodgkin’s lymphoma and anaplastic large cell lymphoma.41Some of the compounds described herein possess traits that suggest potential as ADCs, including ready synthetic access, detailed knowledge of sites that can be modified without loss of activity, and significant potency across a broad panel of cancer cell types. Lack of hemolytic activity is an additional prerequisite for success in the ADC arena, as any hemolysis would be a non-starter for an intravenous drug. Thus the 25 derivatives listed in Table 2 were evaluated for their ability to induce hemolysis in human erythrocytes at concentrations well above their anticancer IC50 values.As shown in Figure S332, the compounds show no hemolytic activity. The concentrations at which hemolytic activity was assessed (1 and 10 µM) are, in some cases,over 1000-foldhigher than the IC50 values for cancer cells, indicating significant selectivity.
ETP alkaloids induce caspase-3 dependent apoptotic cell death
Two of the most active compounds from the monomer (26 and 33) and dimer (5 and 14) classes were further examined for their ability to induce caspase-dependent apoptotic pathway on U-937 cells. Apoptosis is a form of programmed cell death (type I) where cysteine proteases (caspases) are activated and cleave a host of cellular substrates, ultimately resulting in well-defined morphological changes in the cells leading to death. These changes include membrane blebbing, chromatin condensation, and externalization of phosphatidylserine in the plasma membrane.42
The induction of apoptosis was first evaluated by the level of phosphatidylserine externalization (detected by FITC-conjugated Annexin V (AnnV)) that occurs prior to the disruption of cell membrane integrity (detected by propidium iodide (PI)).32 The progression of cells through the AnnV+/PI- quadrant (lower right, Fig.3a) demonstrates the ability of both monomeric and dimeric ETP-containing derivatives to induce apoptosis.
Fig. 3.
ETP derivatives induce caspase-dependent apoptotic cell death.32 a) Analysis of phosphatidylserine exposure and propidium iodide inclusion at 24 hours in U-937 cells. Compounds were tested at 100-times their 72-hour IC50 values [14 (20 nM), 5 (75 nM), 26 (250 nM), and 33(500 nM)]. STSwas used at 50 nM as a positive control for apoptosis. b) Western blot analysis of Pro-C3 and PARP-1 cleavage at 24 hours in U-937 cells using β-actin as loading control. Compounds were tested as above, with the exception of STS (100 nM); C3 = caspase-3; ETP = epipolythiodiketopiperazine; FITC = fluorescein isothiocyanate; IC50 = half maximal inhibitory concentration;PARP = poly(ADP-ribose) polymerase 1; Pro-C3 = procaspase-3; STS = staurosporine.
Another marker of apoptosis is the cleavage patterns of various intracellular proteins. Caspase-3, one of the key apoptotic executioner caspases, is activated from its low-activity zymogen (procaspase-3) at an early stage of apoptosis. This activation can be visualized by Western blot (Fig. 3b) by the cleavage of procaspase-3 (35 kDa) to caspase-3 (12 and 17 kDa). Caspase-3 in turn cleaves one of its cellular substrates, PARP-1.
Treating cells with the four ETP derivatives, followed by Western blotting for procaspase-3/caspase-3 and PARP-1 reveals that all these compounds induce cleavage of procaspase-3 and PARP-1. Combined, the data in Fig.3 indicate that these ETP derivatives induce caspase-dependent apoptotic cell death rather than necrosis.
Conclusions
The implementation of a highly modular and generally applicable strategy for the synthesis of ETP-containing alkaloids has enabled the compilation of 60 derivatives of this class of natural products. This expansive collection of compounds was instrumental in the development of a comprehensive SAR profile. These verticillin- and chaetocin-related compounds were investigated for antitumor activity using five human cancer cell lines. Four sites were targeted for derivatization in this study:N1, C3, C17, and C11/C15 (Fig.4).
Fig. 4.
Summary of ETP structure–activity relationship.
Human cancer cell lines were most responsive to variations in functionalization at the C3 and C11/C15 centers while displaying a more modest response to modification at the N1 and C17 positions. In particular, it was found that the compounds were highlypotent only if the diketopiperazines were sulfurated at theC11/C15 sites in a manner consistent with a species capable of being converted to a β-epidisulfide in the biological milieu. Furthermore, the anticancer potencies of this collection of compounds correlate positively with the steric environment at the C3 position, rendering the dimeric ETP alkaloids the most potent with (sub)nanomolar IC50’s against a range of human cancer cell lines. The muted sensitivity of these cell lines to variations in N1 and C17 substitution make these ideal sites for compound optimization. Finally, despite their attenuated activity, the lower molecular-weight monomers may prove to have more optimal pharmacokinetic properties and provide further avenues for molecular modification in the optimization and development of small-molecule drugs.
Notwithstanding the considerable attention it has gained from biologists and chemists, the exact modes of action by which this class of compounds operates have yet to be fully determined. Three mechanistic pathways have been extensively discussed in the literature;1a–c,f,5ahowever, the role of these processes in toxicity is equivocal and many findings can be contradictory.26b,43The large collection of compounds described herein will enable a more thorough evaluation of the specific biological targets of the ETP-containing alkaloids, and novel synthetic dimer 14, in particular, has remarkable potency. Importantly, these compounds are efficiently prepared through concise synthetic routes that affordsubstantial chemical and structural diversity. The implementation of this synthetic discovery platform provides a unique opportunity to study important biological and physiological processes, to validate biological hypotheses, and to discover very promising small-molecules.44 Additionally, the ETPs would be excellent candidates for ADC therapies,41 a strategy which requires highly potent compounds and selectively delivers drug to a cellular target.
Cell culture MTS and SRB cytotoxicity assays reveal potent and wide-ranging anticancer activity for 25 natural and unnatural ETP derivatives in the (sub)nanomolar range against a broad variety of cancer types and demonstrate a broad structure–activity relationship:eight sarcosine-derived monomers and five homodimers exhibited potency in the single digit nanomolar range, while chaetocins A (4) and C (5) as well as verticillin-related compound 14displayed subnanomolar IC50 values. In addition to their activity against cancer cells, the ETPs are not active against human erythrocytes.These compounds are attractive candidates for further studies investigating mode ofaction and exploring translational potential.
Supplementary Material
Acknowledgements
This work is supported by National Institutes of Health, National Institute of General Medical Sciences (GM089732 to M.M.). M.M. is a Camille Dreyfus Teacher-Scholar. J.K. acknowledges a National Defense Science and Engineering Graduate Fellowship. K.C.M. is a National Science Foundation predoctoral fellow and a Robert C. and Carolyn J. Springborn graduate fellow.
Footnotes
Electronic Supplementary Information (ESI) available: Details for all biological assays as well as experimental procedures, spectroscopic data, and copies of 1H, 13C, and 19F NMR spectra. See DOI: 10.1039/b000000x/
Notes and references
- 1.For reviews on epipolythiodiketopiperazines, see: Jordan TW, Cordiner SJ. Trends Pharmacol. Sci. 2011;8:144.;Waring P, Eichner RD, Müllbacher A. Med. Res. Rev. 2011;8:499. doi: 10.1002/med.2610080404.;Gardiner DM, Waring P, Howlett BJ. Microbiology. 2011;151:1021. doi: 10.1099/mic.0.27847-0.;Patron NJ, Waller RF, Cozijnsen AJ, Straney DC, Gardiner DM, Nierman WC, Howlett BJ. BMC Evol. Biol. 2011;7:174. doi: 10.1186/1471-2148-7-174.;Huang R, Zhou X, Xu T, Yang X, Liu Y. Chem. Biodiv. 2011;7:2809. doi: 10.1002/cbdv.200900211.;Iwasa E, Hamashima Y, Sodeoka M. Isr. J. Chem. 2011;51:420.
- 2.For reviews about pharmacologically active sulfur-containing compounds, see: Řezanka T, Sobotka M, Spížek J, Sigler K. Anti-Infect. Agents Med. Chem. 2012;5:187.;Jiang C-S, Müller WEG, Schröder HC, Guo Y-W. Chem. Rev. 2012;112:2179. doi: 10.1021/cr200173z.
- 3.Jiang C-S, Guo Y-W. Mini Rev. Med. Chem. 2011;11:728. doi: 10.2174/138955711796355276. [DOI] [PubMed] [Google Scholar]
- 4.Zheng C-J, Kim C-J, Bae KS, Kim Y-H, Kim W-G. J. Nat. Prod. 2006;69:1816. doi: 10.1021/np060348t. [DOI] [PubMed] [Google Scholar]
- 5.(a) Waring P, Beaver J. Gen. Pharmac. 2012;27:1311. doi: 10.1016/s0306-3623(96)00083-3. [DOI] [PubMed] [Google Scholar]; (b) Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, Cornell-Kennon S, Lee J, Wang B, Wang J, Memmert K, Naegeli H-U, Petersen F, Eck MJ, Bair KW, Wood AW, Livingston DM. Cancer Cell. 2012;6:33. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]; (c) Vigushin DM, Mirsaidi N, Brooke G, Sun C, Pace P, Inman L, Moody CJ, Coombes RC. Med. Oncol. 2012;21:21. doi: 10.1385/MO:21:1:21. [DOI] [PubMed] [Google Scholar]; (d) Greiner D, Bonaldi T, Eskeland R, Roemer E, Imhof A. Nat. Chem. Biol. 2012;1:143. doi: 10.1038/nchembio721. [DOI] [PubMed] [Google Scholar]; (e) Yanagihara M, Sasaki-Takahashi N, Sugahara T, Yamamoto S, Shinomi M, Yamashita I, Hayashida M, Yamanoha B, Numata A, Yamori T, Andoh T. Cancer Sci. 2012;96:816. doi: 10.1111/j.1349-7006.2005.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Isham CR, Tibodeau JD, Jin W, Xu R, Timm MM, Bible KC. Blood. 2012;109:2579. doi: 10.1182/blood-2006-07-027326. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chen Y, Guo H, Du Z, Liu X-Z, Che Y, Ye X. Cell Prolif. 2012;42:838. doi: 10.1111/j.1365-2184.2009.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Lee Y-M, Lim J-H, Yoon H, Chun Y-S, Park J-W. Hepatology. 2012;53:171. doi: 10.1002/hep.24010. [DOI] [PubMed] [Google Scholar]; (i) Liu F, Liu Q, Yang D, Bollag WB, Robertson K, Wu P, Liu K. Cancer Res. 2012;71:6807. doi: 10.1158/0008-5472.CAN-11-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Yano K, Horinaka M, Yoshida T, Yasuda T, Taniguchi H, Goda AE, Wakada M, Yoshikawa S, Nakamura T, Kawauchi A, Miki T, Sakai T. Int. J. Oncol. 2012;38:365. doi: 10.3892/ijo.2010.874. [DOI] [PubMed] [Google Scholar]; (k) Zhang N, Chen Y, Jiang R, Li E, Chen X, Xi Z, Guo Y, Liu X, Zhou Y, Che Y, Jiang X. Autophagy. 2012;7:598. doi: 10.4161/auto.7.6.15103. [DOI] [PubMed] [Google Scholar]; (l) Chaib H, Nebbioso A, Prebet T, Castellano R, Garbit S, Restouin A, Vey N, Altucci L, Collette Y. Leukemia. 2012;26:662. doi: 10.1038/leu.2011.271. [DOI] [PubMed] [Google Scholar]; (m) Isham CR, Tibodeau JD, Bossou AR, Merchan JR, Bible KC. Br. J. Cancer. 2012;106:314. doi: 10.1038/bjc.2011.522. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Takahashi M, Takemoto Y, Shimazu T, Kawasaki H, Tachibana M, Shinkai Y, Takagi M, Shin-ya K, Igarashi Y, Ito A, Yoshida M. J. Antiobiot. 2012;65:263. doi: 10.1038/ja.2012.6. [DOI] [PubMed] [Google Scholar]
- 6.(a) Rightsel WA, Schneider HG, Sloan BJ, Graf PR, Miller FA, Bartz QR, Ehrlich J, Dixon GJ. Nature. 1968;204:1333. doi: 10.1038/2041333b0. [DOI] [PubMed] [Google Scholar]; Miller PA, Milstrey KP, Trown PW. Science. 1968;159:431. doi: 10.1126/science.159.3813.431. [DOI] [PubMed] [Google Scholar]
- 7.(a) Coleman JJ, Ghosh S, Okoli I, Mylonakis E. PLoS ONE. 2011;6:e25321. doi: 10.1371/journal.pone.0025321. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Speth C, Kupfahl C, Pfaller K, Hagleitner M, Deutinger M, Würzner R, Mohsenipour I, Lass-Flörl C, Rambach G. Mol. Immunol. 2011;48:2122. doi: 10.1016/j.molimm.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 8.(a) Müllbacher A, Waring P, Tiwari-Palni U, Eichner RD. Molec. Immunol. 2005;23:231. doi: 10.1016/0161-5890(86)90047-7. [DOI] [PubMed] [Google Scholar]; (b) Pahl HL, Krauss B, Schulze-Osthoff K, Decker T, Traenckner EB-M, Vogt M, Myers C, Parks T, Waring P, Mühlbacher A, Czernilofsky AP, Baeuerle PA. J. Exp. Med. 2005;183:1829. doi: 10.1084/jem.183.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nishida S, Yoshida LS, Shimoyama T, Nunoi H, Kobayashi T, Tsunawaki S. Infect. Immun. 2005;73:235. doi: 10.1128/IAI.73.1.235-244.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soledade M, Pedras C, Séguin-Swartz G, Abrams SR. Phytochem. 1990;29:777. [Google Scholar]
- 10.Dong J-Y, He H-P, Shen Y-M, Zhang K-Q. J. Nat. Prod. 2005;68:1510. doi: 10.1021/np0502241. [DOI] [PubMed] [Google Scholar]
- 11.Bertling A, Niemann S, Uekötter A, Fegeler W, Lass-Flörl C, von Eiff C, Kehrel BE. Thromb. Haemost. 2010;104:270. doi: 10.1160/TH09-11-0769. [DOI] [PubMed] [Google Scholar]
- 12.For approaches to epipolythiodiketopiperazines, see: Trown PW. Biochem. Biophys. Res. Commun. 2012;33:402. doi: 10.1016/0006-291x(68)90585-8.;Hino T, Sato T. Tetrahedron Lett. 2012;12:3127.;Poisel H, Schmidt U. Chem. Ber. 2012;104:1714.;Poisel H, Schmidt U. Chem. Ber. 1. 2012;105:625. doi: 10.1002/cber.19721050228.;Öhler E, Tataruch F, Schmidt U. Chem. Ber. 2012;106:396. doi: 10.1002/cber.19731060205.;Ottenheijm HCJ, Herscheid JDM, Kerkhoff GPC, Spande TF. J. Org. Chem. 2012;41:3433. doi: 10.1021/jo00883a024.;Coffen DL, Katonak DA, Nelson NR, Sancilio FD. J. Org. Chem. 2012;42:948. doi: 10.1021/jo00426a004.;Herscheid JDM, Nivard RJF, Tijhuis MW, Scholten HPH, Ottenheijm HCJ. J. Org. Chem. 2012;45:1885.;Williams RM, Armstrong RW, Maruyama LK, Dung J-S, Anderson OP. J. Am. Chem. Soc. 2012;107:3246.;Moody CJ, Slawin AMZ, Willows D. Org. Biomol. Chem. 2012;1:2716. doi: 10.1039/b305698h.;Aliev AE, Hilton ST, Motherwell WB, Selwood DL. Tetrahedron Lett. 2012;47:2387.;Overman LE, Sato T. Org. Lett. 2012;9:5267. doi: 10.1021/ol702518t.;Polaske NW, Dubey R, Nichol GS, Olenyuk B. Tetrahedron: Asym. 2012;20:2742. doi: 10.1016/j.tetasy.2009.10.037.;Ruff BM, Zhong S, Nieger M, Bräse S. Org. Biomol. Chem. 2012;10:935. doi: 10.1039/c2ob06663g.;Nicolaou KC, Giguère D, Totokotsopoulos S, Sun Y-P. Angew. Chem. Int. Ed. 2012;51:728. doi: 10.1002/anie.201107623.
- 13.For selected epidithiodiketopiperazine total syntheses, see: Kishi Y, Fukuyama T, Nakatsuka S. J. Am. Chem. Soc. 2011;95:6492. doi: 10.1021/ja00800a078.;Kishi Y, Nakatsuka S, Fukuyama T, Havel M. J. Am. Chem. Soc. 2011;95:6493. doi: 10.1021/ja00800a079.;Fukuyama T, Kishi Y. J. Am. Chem. Soc. 2011;98:6723. doi: 10.1021/ja00437a063.;Williams RM, Rastetter WH. J. Org. Chem. 2011;45:2625.;Miknis GF, Williams RM. J. Am. Chem. Soc. 2011;115:536.;Iwasa E, Hamashima Y, Fujishiro S, Higuchi E, Ito A, Yoshida M, Sodeoka M. J. Am. Chem. Soc. 2011;132:4078. doi: 10.1021/ja101280p.;DeLorbe JE, Jabri SY, Mennen SM, Overman LE, Zhang F-L. J. Am. Chem. Soc. 2011;133:6549. doi: 10.1021/ja201789v.;Nicolaou KC, Totokotsopoulos S, Giguère D, Sun Y-P, Sarlah D. J. Am. Chem. Soc. 2011;133:8150. doi: 10.1021/ja2032635.;Codelli JA, Puchlopek ALA, Reisman SE. J. Am. Chem. Soc. 2011;134:2012. 1930. doi: 10.1021/ja209354e.
- 14.For our synthetic strategies relevant to epipolythiodiketopiperazines, see: Kim J, Ashenhurst JA, Movassaghi M. Science. 2012;324:238. doi: 10.1126/science.1170777.;Kim J, Movassaghi M. J. Am. Chem. Soc. 2012;132:14376. doi: 10.1021/ja106869s.;Boyer N, Movassaghi M. Chem. Sci. 2012;3:1798. doi: 10.1039/C2SC20270K.
- 15.Mason JW, Kidd JG. J. Immunol. 1951;66:99. [PubMed] [Google Scholar]
- 16.Middleton MC. Biochem. Pharmacol. 1974;23:1811. doi: 10.1016/0006-2952(74)90210-x. [DOI] [PubMed] [Google Scholar]
- 17.(a) Block KM, Wang H, Szabó LZ, Polaske NW, Henchey LK, Dubey R, Kushal S, László CF, Makhoul J, Song Z, Meuillet EJ, Olenyuk BZ. J. Am. Chem. Soc. 2011;131:18078. doi: 10.1021/ja807601b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kushal S, Wang H, László CF, Szábo LZ, Olenyuk BZ. Biopolymers. 2011;95:8. doi: 10.1002/bip.21550. [DOI] [PubMed] [Google Scholar]
- 18.Saito T, Suzuki Y, Koyama K, Natori S, Iitaka Y, Kinoshita T. Chem. Pharm. Bull. 1988;36:1942. [Google Scholar]
- 19.Chai CLL, Elix JA, Huleatt PB, Waring P. Bioorg. Med. Chem. 2004;12:5991. doi: 10.1016/j.bmc.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan U, Bala A, Jao S-c, Starke DW, Jordan TW, Mieyal JJ. Biochemistry. 2006;45:8978. doi: 10.1021/bi060440o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Y, Blunt JW, Cole ALJ, Munro MHG. J. Nat. Prod. 2004;67:2090. doi: 10.1021/np030326w. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Li D, Luan Y, Gu Q, Zhu T. J. Nat. Prod. 2012;75:920. doi: 10.1021/np3000443. [DOI] [PubMed] [Google Scholar]
- 23.(a) Takahashi C, Numata A, Ito Y, Matsumura E, Araki H, Iwaki H, Kushida K. J. Chem. Soc., Perkin Trans 1. 1994:1859. [Google Scholar]; (b) Takahashi C, Numata A, Matsumura E, Minoura K, Eto H, Shingu T, Ito T, Hasegawa T. J. Antibiot. 2004;47:1994. 1242. doi: 10.7164/antibiotics.47.1242. [DOI] [PubMed] [Google Scholar]; (c) Takahashi C, Takai Y, Kimura Y, Numata A, Shigematsu N, Tanaka H. Phytochem. 2004;38:155. doi: 10.1016/0031-9422(94)00582-e. [DOI] [PubMed] [Google Scholar]; (d) Takahashi C, Minoura K, Yamada T, Numata A, Kushida K, Shingu T, Hagishita S, Nakai H, Sato T, Harada H. Tetrahedron. 2004;51:3483. [Google Scholar]; (e) Yamada T, Iwamoto C, Yamagaki N, Yamanouchi T, Minoura K, Yamori T, Uehara Y, Andoh T, Umemura K, Numata A. Tetrahedron. 2004;58:479. [Google Scholar]; (f) Yamada T, Iwamoto C, Yamagaki N, Yamanouchi T, Minoura K, Hagishita S, Numata A. Heterocycles. 2004;63:641. [Google Scholar]
- 24.Soledade M, Pedras C, Abrams SR, Séguin-Swartz G. Tetrahedron Lett. 1988;29:3471. [Google Scholar]
- 25.Murdock KC. J. Med. Chem. 1974;17:827. doi: 10.1021/jm00254a010. [DOI] [PubMed] [Google Scholar]
- 26.(a) Munday R. J. Appl. Toxicol. 2009;7:17. doi: 10.1002/jat.2550070105. [DOI] [PubMed] [Google Scholar]; (b) Waring P, Sjaarda A, Lin QH. Biochem. Pharmacol. 2009;49:1195. doi: 10.1016/0006-2952(95)00039-3. [DOI] [PubMed] [Google Scholar]; (c) Chai CLL, Waring P. Redox Rep. 2009;5:257. doi: 10.1179/135100000101535799. [DOI] [PubMed] [Google Scholar]; (d) Tibodeau JD, Benson LM, Isham CR, Owen WG, Bible KC. Antiox. Redox Signal. 2009;11:1097. doi: 10.1089/ars.2008.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurne AM, Chai CLL, Waring P. J. Biol. Chem. 2000;275:25202. doi: 10.1074/jbc.M002278200. [DOI] [PubMed] [Google Scholar]
- 28.Cook KM, Hilton ST, Mecinović J, Motherwell WB, Figg WD, Schofield CJ. J. Biol. Chem. 2009;284:26831. doi: 10.1074/jbc.M109.009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.(a) Teng Y, Iuchi K, Iwasa E, Fujishiro S, Hamashima Y, Dodo K, Sodeoka M. Bioorg. Med. Chem. Lett. 2012;20:5085. doi: 10.1016/j.bmcl.2010.07.032. [DOI] [PubMed] [Google Scholar]; (b) Sodeoka M, Dodo K, Teng Y, Iuchi K, Hamashima Y, Iwasa E, Fujishiro S. Pure Appl. Chem. 2012;84:1369. [Google Scholar]
- 30.Kim J, Movassaghi M. J. Am. Chem. Soc. 2011;133:14940. doi: 10.1021/ja206743v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usami Y, Yamaguchi J, Numata A. Heterocycles. 2004;63:1123. [Google Scholar]
- 32.See the ESI† for details.
- 33.Beck EM, Grimster NP, Hatley R, Gaunt MJ. J. Am. Chem. Soc. 2006;128:2528. doi: 10.1021/ja058141u. [DOI] [PubMed] [Google Scholar]
- 34.The relative stereochemistry of the α-epimers 26, 30–33 of the epidisulfide bridges has been confirmed by key NOESY cross-peaks on the corresponding bis(thiomethylether).See the ESI† for details. This derivatized compound was prepared in a single step using the methodology developed to access (+)-gliocladin B (7, see reference 14c).
- 35.Cherblanc F, Lo Y-P, De Gussem E, Alcazar-Fuoli L, Bignell E, He Y, Chapman-Rothe N, Bultinck P, Herrebout WA, Brown R, Rzepa HS, Fuchter MJ. Chem.–Eur. J. 2011;17:11868. doi: 10.1002/chem.201101129. [DOI] [PubMed] [Google Scholar]
- 36.Depew KM, Marsden SP, Zatorska D, Zatorski A, Bornmann WG, Danishefsky SJ. J. Am. Chem. Soc. 1999;121:11953. [Google Scholar]
- 37.Kroutil W, Stämpfli AA, Dahinden R, Jörg M, Müller U, Pachlatko JP. Tetrahedron. 2002;58:2589. [Google Scholar]
- 38.(a) Fink CA, Carlson JE, McTaggart PA, Qiao Y, Webb R, Chatelain R, Jeng AY, Trapani AJ. J. Med. Chem. 39:3158. doi: 10.1021/jm960323z. [DOI] [PubMed] [Google Scholar]; (b) Testa B, Mayer JM. Hydrolysis in Drug and Prodrug Metabolism. Wiley-VCH: Weinheim; 2008. [Google Scholar]; (c) Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Järvinen T, Savolainen J. Nature Rev. Drug Disc. 7:255. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 39.Bernardo PH, Brasch N, Chai CCL, Waring P. J. Biol. Chem. 2003;278:46549. doi: 10.1074/jbc.M304825200. [DOI] [PubMed] [Google Scholar]
- 40.Sevier CS, Kaiser CA. Nature Rev. Mol. Cell Biol. 2002;3:836. doi: 10.1038/nrm954. [DOI] [PubMed] [Google Scholar]
- 41.(a) Flygare JA, Pillow TH, Aristoff P. Chem. Biol. Drug Des. 2012;81:113. doi: 10.1111/cbdd.12085. [DOI] [PubMed] [Google Scholar]; (b) Teicher BA, Doroshow JH. New Engl. J. Med. 2012;367:1847. doi: 10.1056/NEJMe1211736. [DOI] [PubMed] [Google Scholar]
- 42.(a) Krysko DV, Berghe TV, D’Herde K, Vandenabeele P. Methods. 2011;44:205. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]; (b) Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G. Nat. Rev. Drug Disc. 2011;10:221. doi: 10.1038/nrd3373. [DOI] [PubMed] [Google Scholar]
- 43.Waring P, Mamchak A, Khan T, Sjaarda A, Sutton P. Cell Death Diff. 1995;2:201. [PubMed] [Google Scholar]
- 44.Szpilman AM, Carreira EM. Angew. Chem. Int. Ed. 2010;49:9592. doi: 10.1002/anie.200904761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.