Abstract
Microtubules (MTs) are essential for neuronal morphogenesis in the developing brain. The MT cytoskeleton provides physical support to shape the fine structure of neuronal processes. MT-based motors play important roles in nucleokinesis, process formation and retraction. Regulation of MT stability downstream of extracellular cues is proposed to be critical for axonogenesis. Axons and dendrites exhibit different patterns of MT organization, underlying the divergent functions of these processes. Centrosomal positioning has drawn the attention of researchers because it is a major clue to understanding neuronal MT organization. In this review, we focus on how recent advances in live imaging have revealed the dynamics of MT organization and centrosome positioning during neural development.
Keywords: microtubules, polarity, centrosome, axon, migration, neuron
2. Introduction
Neuronal migration and polarization are key activities in brain morphogenesis, and both rely on microtubule (MT) function [1–8]. MTs have intrinsic polarity based on the asymmetry of the αβ-tubulin heterodimer. MTs exhibit two distinct ends: a slow-growing minus end at which α-tubulin subunits are exposed, and a fast-growing plus end at which β-tubulin subunits are exposed [9,10]. MT network polarity within a cellular process affects not only its dynamic nature but also directed transport along MTs [3,6,9,10]. Formation of cytoplasmic MTs is initiated by binding of αβ-tubulin heterodimers to the γ-tubulin ring complex on the surface of an MT organizing centre such as the centrosome [9,10]. MT elongation through addition of tubulin heterodimers to the plus end forms a polarized cytoskeleton. Forming fibres undergo cycles of growth and shortening, a behaviour known as dynamic instability [11,12]. Neurons form large cellular protrusions such as leading processes, axons and dendrites, which function in neuronal migration and circuit formation. These processes contain an MT cytoskeleton, and dynamic changes in MTs underlie their extension and retraction [13–20]. Furthermore, the MT cytoskeleton is critical to maintain integrity of neuronal processes in the developing brain [21]. The highly polarized MT structure provides tracks for MT-based motors to enable directional movement of intracellular cargos within processes [22]. The minus-end-directed dynein motor complex plays a pivotal role in nucleokinesis in migrating neurons [23,24]. Kinesin super family proteins (KIFs), most of which are plus-end-directed motors, show multiple effects on MT dynamics and neuronal morphogenesis [22]. For example, kinesin-2 (KIF3) reportedly polarizes the Par3 complex leading to axon specification [25,26], whereas kinesin-1 (KIF5) promotes axon formation and elongation via transporting cargos such as membrane vesicles and the CRMP2–tubulin complex [27–29]. The mitotic MT-associated motor proteins kinesin-5 (Eg5, KIF11) and kinesin-12 (KIF15) negatively regulate short MT transport, limiting both axonal growth and neuronal migration [30–32]. Kinesin-6 (CHO1, MKLP1, KIF23) and kinesin-12 (HKLP2, KIF15) reportedly regulate MT organization in axons and dendrites [33]. Other kinesin family members, such as kinesin-8 (Kip3) and kinesin-13 (MCAK), are known to control dynamic instability by promoting MT catastrophe [34,35].
Defects in MT-related genes cause human diseases ranging from severe brain malformations to mental disorders [36–41]. Point mutations in genes encoding tubulin α- or β-subunits alter MT dynamics, and cause aberrant neurogenesis, migration and circuit formation [42–44]. The LIS1 gene encodes a key regulator of the dynein complex [45–48]. Heterozygous mutations in LIS1 or alterations in its normal dosage cause a range of developmental abnormalities, the most severe of which is lissencephaly (also known as smooth brain) [49,50]. Mutations in the X-linked doublecortin (DCX) gene, which encodes an unconventional MT-associated protein, underlie double cortex syndrome in humans [51–55]. The MT lattice-binding protein Tau is implicated in human intellectual disability and neurodegenerative tauopathies, among which are Alzheimer's disease, frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) [56,57].
3. Microtubule function in the neuronal migration
Neuronal migration is central to proper neuronal alignment in the developing brain and requires orchestrated activity of cellular components, including cytoplasmic MTs [1,4,23]. During the development of cerebral cortex, three modes of migration have been described for excitatory neurons that are born in deep layers of brain, and then migrate radially towards the brain surface [2,4]. Those modes include somal translocation, multipolar migration and glial-guided radial migration or locomotion. Radial migration itself consists of three sequential steps: (i) leading process extension, (ii) nucleokinesis (i.e. nuclear translocation into the leading process) and (iii) retraction of the posterior end of the cell. In migrating neurons, the centrosome of migrating neurons is located in front of the nucleus (a phenomenon called N–C coupling). This configuration is considered crucial for nucleokinesis, as an N–C coupling is perturbed in migration-defective neurons [58–63].
3.1. Limitations of the dynein-based nucleokinesis model
Based on the observation that the nucleus of migrating neurons is surrounded by a centrosome-derived MT cage [64], researchers have proposed a dynein-based nucleokinesis model [23]. According to this model, nucleokinesis consists of two steps: (i) centrosome uncoupling from the nucleus and advancement into a proximal ‘swelling’ in the leading process, and (ii) translocation of the nucleus to restore N–C coupling (figure 1a). The minus-end-directed motor, dynein, provides pull forces but cannot push the nucleus in this configuration. Importantly, centrosome-derived MTs orient their minus ends towards the centrosome and their plus ends towards the periphery. Thus, to explain this type of nucleokinesis by a dynein motor-based driving force, one must assume that forward movement of the centrosome always precedes nucleokinesis.
Figure 1.
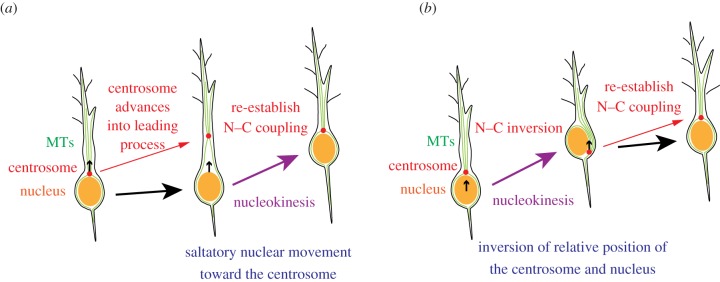
Potential function of MTs in nucleokinesis. (a) The dynein-dependent nucleokinesis model. In migrating neurons, the centrosome is positioned in front of the nucleus (N–C coupling). Cytoplasmic MTs derived from the centrosome surround the nucleus, and after the leading process elongates, the centrosome uncouples from the nucleus and advances into the leading process. Saltatory forward movement of the nucleus occurs using a minus-end-directed dynein motor along cytoplasmic MTs. (b) Occasionally, nucleokinesis occurs prior to centrosome movement, inverting the relative position of the centrosome and nucleus (N–C inversion). Centrosome positioning in front of the nucleus is then recaptured. This type of nucleokinesis cannot be explained by dynein-dependent pulling of the nucleus along cytoplasmic MTs. Small black arrows within the cells indicate the moving direction of centrosome or nucleus.
However, transient overtaking of the centrosome by a translocating nucleus has been observed in many types of migrating neurons (N–C inversion; figure 1b). Umeshima et al. [65] showed that nucleokinesis in migrating cerebellar granule cells is independent of centrosomal positioning. Distel et al., report that the translocating nucleus is not consistently led by the centrosome in migrating zebrafish tegmental hindbrain neurons [66]. Furthermore, we observed similar inversion of relative positions of the leading centrosome and following nucleus in locomoting neocortical neurons in the developing mouse cerebrum [67]. In these cases, the migration cycle consists of two steps: (i) forward nuclear translocation into the leading process associated with overtaking of the centrosome, and (ii) re-establishment of the centrosome position in front of nucleus (figure 1b). Because the dynein-based nucleokinesis model cannot account for nuclear overtaking of the centrosome, a non-MT-based motor probably functions in this type of nucleokinesis.
3.2. Function of the actomyosin system in nucleokinesis
To explain mechanisms underlying the N–C inversion, the actomyosin system is an attractive candidate as a force generator [4,24]. Intracellular localization of actin filaments changes dynamically during nucleokinesis. Some investigators propose that a concentrated actomyosin system in front of a translocating nucleus pulls the nucleus [68], whereas others posit that actomyosin squeezes a translocating nucleus from the rear [69–71]. It is also plausible that cooperative force generation by dynein and actomyosin is used in nucleokinesis.
4. Microtubule regulation of neuronal polarization
Formation of functionally and structurally differentiated compartments such as axons and dendrites is a characteristic feature of neurons. Axons and dendrites emerge during a process known as neuronal polarization [26,72–74]. Each compartment contains distinct and highly organized MT-based networks whose roles in neuronal polarization have been investigated in various studies [2,3,5,6].
4.1. Function of microtubules in the spatial regulation of polarization signals
Axonogenesis, the extension of a single immature process and its differentiation into an axon, is the initial morphological event that defines neuronal polarization. Axonogenesis depends on extracellular cues that initiate signalling cascade leading to asymmetric distribution of components, including the polarity regulator, Par complex and phosphatidylinositol-triphosphate [25,26,75–77]. During the polarization process, MTs serve as tracks for translocation of polarity-regulating molecules. The plus-end-directed motor, kinesin-1, regulates spatially restricted specification of axons via its polarized transport activity [27,29]. CRMP-2, which binds to αβ-tubulin heterodimers, facilitates axon formation by transporting those heterodimers to the distal part of the axon via interaction with the kinesin-1 motor [28,78]. Local inactivation of GSK-3β by polarity signals reportedly regulates axonogenesis by controlling CRMP-2 affinity to tubulin [79]. The kinesin-3 motor protein GAKIN/KIF13B promotes axon specification by transport of phosphatidylinositol-triphosphate, which is also important for spatial restriction of axonogenesis signals [80]. These observations support the notion that plus-end-directed kinesin motors mobilize polarity signals in an emerging axon.
4.2. Regulation of microtubule stability in axon formation
Axons are typically thin, elongated processes that contain MTs with distinct organization and dynamics [3,5,6]. Altered MT stability underlies the dynamic nature of growth/retraction processes, as well as MT-based motor function. In axonogenesis, multiple protein kinases probably regulate MT remodelling in response to extracellular cues [62,63,81–85]. Kishi et al. [82] first found that SAD kinases phosphorylate Tau-1 S262, which is highly phosphorylated in dendrites but not in axons. Double knockout of genes encoding SAD-A and SAD-B kinases impairs axonogenesis, suggesting that initial axon formation is regulated by local inactivation of these kinases. LKB1 reportedly phosphorylates SAD kinases, and LKB1 loss of function promotes axonogenesis defects [83,84]. Others reported that local MT stabilization downstream of SAD kinases is important for axon specification in hippocampal neurons [86]. MARKs/Par-1, a kinase downstream of the aPKC/Par complex, phosphorylates DCX and reduces its MT-binding affinity [1,62,63,87,88]. The DLK–JNK pathway is implicated in axonogenesis via phosphorylation of the MT regulators DCX, MAP1B, MAP2 and SCG10/stathmin-2 [81,85,89,90], whereas others reported that local inactivation of stathmin/Op18 downstream of the DOCK7-Rac pathway regulates MT stabilization in axons [91]. Taken together, these observations suggest that regulation of MT stability by extracellular polarization signals is a key step in axonogenesis.
4.3. The role of centrosome translocation in axonogenesis
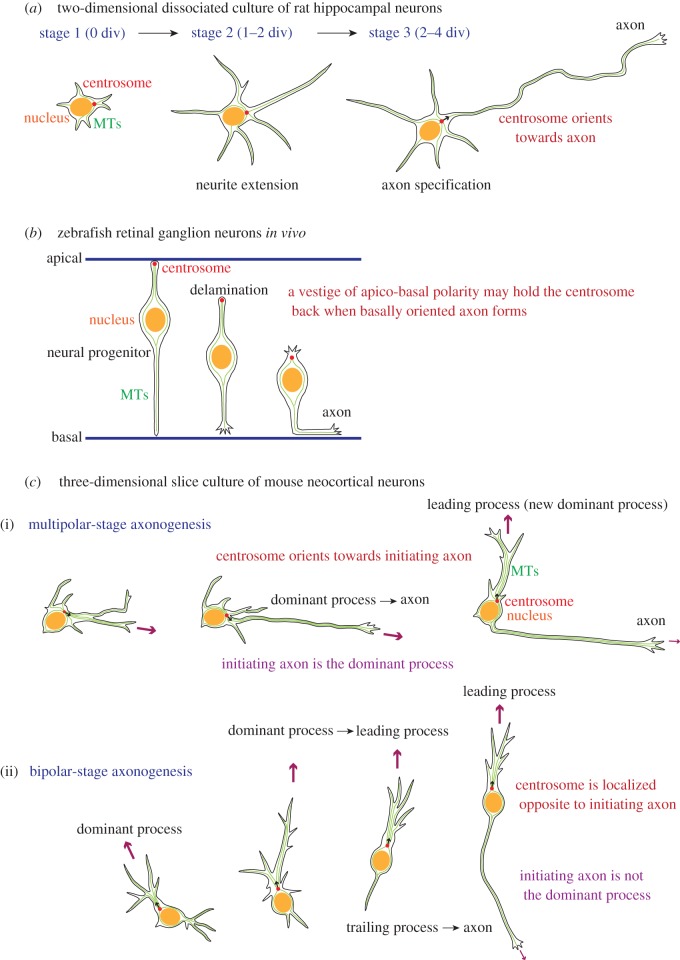
Centrosome translocation is observed during polarization of several types of neurons, but the significance of the dynamic nature of centrosomal positioning is unclear. Lefcort & Bentley [92] observed cytoskeletal organization of grasshopper pioneer neurons during axonogenesis and found that the axonal growth cone emerged preferentially at the opposite side of the cleavage point after the final cell division. The position of the centrosome after cell division corresponded to the axon emergence site, suggesting that centrosomal positioning determines axon orientation. Similar correlations of centrosome localization to the axon formation site have been observed during the early phase of axonogenesis in several types of neurons (figure 2a) [93–95]. These studies support the idea that centrosomal positioning plays an instructive role in determining axon orientation. However, there is a different conclusion in a recent manuscript from Dotti's laboratory reporting that localization of N-cadherin rather than centrosomal positioning specifies the first asymmetry in developing neurons [96].
Figure 2.
Centrosome positioning during neuronal polarization. (a) Rat hippocampal neurons cultured in vitro first extend multiple neurites (stage 2; 1–2 days in vitro (div)), and then one process differentiates into an axon (stage 3; 2–4 div). After axon specification, only the axon continues to extend, whereas other processes remain dynamic but short. In that case, the extending axon is likely to be the dominant process and attracts the centrosome (small black arrow). (b) In zebrafish retinal neural progenitors, the centrosome locates near the apical surface. After delamination, differentiated retinal ganglion neurons retain their bipolar shape along the apicobasal axis, and the centrosome stays on the apical side of the trailing process. The centrosome remains in the apical domain when an axon forms by extension of a basally oriented process, suggesting that a trace of apicobasal polarity may tether the centrosome. (c) Slice-cultured mouse neocortical excitatory neurons exhibit two modes of axonogenesis. (i) During multipolar (MP)-stage axonogenesis, an axon forms by extension of a dominant growing process (large purple arrows) targeted by the centrosome (small black arrows). (ii) Bipolar (BP)-stage axonogenesis is observed in neurons migrating radially towards the brain surface. An axon forms by extension of a thin trailing process from the rear of the cell, whereas the centrosome is located in front of the nucleus. In this case, the leading process (large purple arrow) rather than the initiating axon (small purple arrow) is likely to be the dominant process towards which the centrosome orients.
By contrast, using zebrafish retinal ganglion cells, Zolessi et al. [97] observed a form of axonogenesis during which an axon emerges from the basal side of the cell body after a cell delaminates from the apical domain of the neuroepithelium/ventricular zone (figure 2b). In this case, the centrosome remained in the apical cytoplasm opposite the site of axon formation. Distel et al. [66] also observed the formation of thin axon fibres from the basal side of migrating tegmental hindbrain neurons in zebrafish. Forward extension of axon was initiated from the basal side of soma, whereas the centrosome was located apically in the trailing process.
Recently, we found that mouse neocortical excitatory neurons show two distinct modes of axonogenesis (figure 2c) [67]. The morphology of migrating neocortical neurons changes from multipolar to bipolar as cells migrate towards the brain surface [2,4]. Interestingly, we observed that centrosomes in these neurons tend to move towards the most dominant growing process. Multipolar migrating neurons form an axon by extending a dominant process towards which the centrosome orients. In bipolar locomoting neurons, the axon extends from the rear opposite the dominant leading process. During this mode of axonogenesis, the centrosome remained at the base of the leading process and did not target the initiating axon. Because we found that the centrosome in migrating neurons tends to move towards the most actively extending process, we concluded that centrosome positioning reflects relative protrusive activities of processes and that, in these cases, centrosome translocation during axonogenesis is likely to be a passive rather than an instructive event in orienting the axon.
5. Microtubule organization in axons and dendrites
MT polarity within neurons affects not only process morphology but also motor protein-mediated transport, both of which have a profound effect on neuronal function [3,5–8]. Early electron microscopy studies using the in situ MT hook assay revealed that axonal MTs orient their plus ends towards the distal tip [8,98,99]. In analysing rat hippocampal neuron polarization in in vitro cultures, Baas et al. [100,101] further showed that MT polarity in neuronal processes dynamically changes as processes differentiate (figure 3a). In nascent neuronal processes, most MT plus ends are distally oriented. After dendritic processes mature, bidirectional MT alignment is observed, whereas MT polarity in the extended long axon remains mostly in a distal plus orientation [101]. By contrast, MT plus ends in the trailing axon of migrating granule cells in the developing cerebellum show mixed polarity, whereas MTs are uniformly aligned towards the growing tip of the leading process [102].
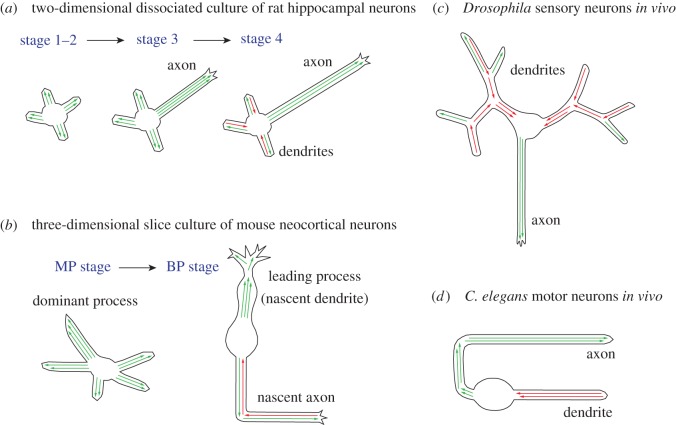
Figure 3.
MT organization in axons and dendrites. (a) In the nascent process of in vitro-cultured rodent hippocampal neurons during polarization (stage 1–2), MT polarity is mostly plus-end-distal (green arrows). MTs are aligned in this manner in axons (stage 3–4). Minus-end-distal MTs (red arrows) increase in differentiating dendrites after stage 4. (b) Distal-oriented MT growth is predominantly observed in processes of MP-stage mouse neocortical neurons. The dominant process probably contains a greater number of MTs than do other processes and attracts the centrosome. Growing MTs are enriched in the leading process of BP-stage neurons, whereas bidirectional movements of MT plus ends are observed in the trailing axon. (c) Drosophila DA neurons exhibit highly branched sensory dendrites and a projecting axon. MTs are uniformly aligned in a plus-end-distal manner in the axon. MT polarity in dendrite shafts near the cell soma is mostly minus-end-distal, whereas short branches contain more plus-end-distal MTs. (d) In C. elegans motor neurons, polarity orientation of axonal MTs is mostly plus-end-distal. In dendrites, minus-end-distal alignment of MTs is predominant.
Progress in live imaging technology now allows analysis of the orientation of growing MT plus ends in living neurons. Stepanova et al. [103] first used EB3-EGFP to specifically label growing MTs plus ends in rodent hippocampal neurons cultured in vitro. They observed alignment of plus-end-distal MTs in axons and mixed-polarity MTs in dendrites is in agreement with results reported earlier using the MT hook assay [100]. Several groups have applied live imaging of MT plus ends to monitor MT polarity in neurons migrating in vivo. Tsai et al. [59] first reported that MT plus ends in the leading process of locomoting neurons point primarily towards the tip of the process. Recently, we further showed that the trailing axon of locomoting neurons in mouse embryonic cerebral slices contains mixed polarity MTs (figure 3b) [67]. These observations are consistent with observations of MT organization made in cerebellar granule cells in vivo, but not in hippocampal neurons in vitro [100,102]. Taken together, these results suggest that MTs show unique organization in two structural components of migrating neurons—namely, a major leading process in which organization is relatively uniform and a thin trailing axon in which MTs display mixed polarity.
MT organization patterns in Drosophila peripheral nervous system neurons differ from those seen in vertebrate neurons [7]. In Drosophila dendritic arborization (DA) neurons, MTs orient plus ends distally in the axon, whereas most growing MTs in dendrites orient plus ends proximally (figure 3c) [104]. In Caenorhabditis elegans sensory and motor neurons, most axonal MTs grow towards the distal tip, whereas retrograde MT growth towards the cell body predominates in dendrites (figure 3d) [105,106]. Thus, MT organization patterns in nematode neurons resemble those seen in insect neurons.
In Drosophila DA neurons, formation of uniformly oriented MTs in axons depends on dynein function [107], and morphogenesis of dendritic branches requires MT-based transport of Rab5-endosomes by dynein and kinesin [108]. Longer dendrites reportedly contain more retrogradely growing MT plus ends than do short branches, and MT organizing centres on Golgi outposts function in nucleation of MT polarity [109]. Overall, these findings suggest that an interplay between MTs, motor proteins and membrane organelles is critical for MT organization and neuronal process formation.
In mammalian hippocampal neurons, Golgi outposts are localized at branching points in dendrites [110], yet MT orientation nucleated from Golgi outposts has not been analysed. In slice-cultured mouse neocortical neurons, we observed generation of retrogradely growing MTs after retraction of the process tip [67], suggesting that MT severing and/or catastrophe near the tip of processes contributes to nucleation of mixed-polarity MTs [111].
Interestingly, cytoplasmic MTs can be organized independently of the centrosome in fly neurons [112,113]. It has also been demonstrated in rodent hippocampal neurons in vitro that once a nascent axon forms, a functional centrosome is dispensable for further axon extension [114]. These findings suggest that non-centrosomal MT nucleation functions to organize neuronal cytoplasmic MTs. In addition, transport of short MTs also participates in MT organization [33,115]. Taken together, multiple mechanisms probably regulate complex neuronal MT polarity. Further analyses of MT polarity in different neuronal subtypes and in diverse environments are needed to properly understand MT function in neuronal morphogenesis in vivo.
6. Concluding remarks
MTs, which are essential for cellular polarization and migration, exhibit unique structural and physical properties. Modulation of their structure is important for differentiation of immature processes into axons or dendrites. MTs serve as tracks for directed transport and as transducers of force generated by molecular motors, which control neuronal morphology and function. The importance of MT structure is highlighted by identification of mutations in tubulin genes and genes encoding MT-related proteins in patients showing brain defects or disorders. MT polarity within structural components of migrating neurons and polarized neurons has recently been described, yet how this organization is controlled requires further study. The centrosome, the main cellular MT organizing centre, displays dynamic behaviour during neuronal polarization and migration. Comparison of multiple cellular systems is currently promoting re-evaluation of its instructive function.
Acknowledgements
We thank Namiko Noguchi for her excellent illustrations. We also thank Orly Reiner for valuable comments.
Funding statement
A.S. was supported by JSPS KAKENHI (21890096, 23500410), MEXT KAKENHI (23113507), the Nitto Foundation and the Daiko Foundation. T.S. was supported in part by the Israel Science Foundation (grant no. 47/10), Minerva foundation with funding from the Federal German Ministry for Education and Research, a grant from the Chief Scientist Office at the Israeli Ministry of Health, under the frame of ERA-Net NEURON (DISCover, IMOS 3-00000-6785), the Fritz-Thyseen Foundation (grant no. Az. 10.11.2.161), the Benoziyo Center for Neurological diseases, the Kekst Family Center for Medical Genetics and the David and Fela Shapell Family Center for Genetic Disorders Research.
References
- 1.Reiner O, Sapir T. 2009. Polarity regulation in migrating neurons in the cortex. Mol. Neurobiol. 40, 1–14 (doi:10.1007/s12035-009-8065-0) [DOI] [PubMed] [Google Scholar]
- 2.Barnes AP, Polleux F. 2009. Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 32, 347–381 (doi:10.1146/annurev.neuro.31.060407.125536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conde C, Cáceres A. 2009. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 10, 319–332 (doi:10.1038/nrn2631) [DOI] [PubMed] [Google Scholar]
- 4.Marín O, Valiente M, Ge X, Tsai LH. 2010. Guiding neuronal cell migrations. Cold Spring Harb. Perspect. Biol. 2, a001834 (doi:10.1101/cshperspect.a001834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuijpers M, Hoogenraad CC. 2011. Centrosomes, microtubules and neuronal development. Mol. Cell. Neurosci. 48, 349–358 (doi:10.1016/j.mcn.2011.05.004) [DOI] [PubMed] [Google Scholar]
- 6.Stiess M, Bradke F. 2011. Neuronal polarization: the cytoskeleton leads the way. Dev. Neurobiol. 71, 430–444 (doi:10.1002/dneu.20849) [DOI] [PubMed] [Google Scholar]
- 7.Rolls MM. 2011. Neuronal polarity in Drosophila: sorting out axons and dendrites. Dev. Neurobiol. 71, 419–429 (doi:10.1002/dneu.20836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baas PW, Lin S. 2011. Hooks and comets: the story of microtubule polarity orientation in the neuron. Dev. Neurobiol. 71, 403–418 (doi:10.1002/dneu.20818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang K, Akhmanova A. 2011. Microtubule tip-interacting proteins: a view from both ends. Curr. Opin. Cell Biol. 23, 94–101 (doi:10.1016/j.ceb.2010.08.008) [DOI] [PubMed] [Google Scholar]
- 10.de Forges H, Bouissou A, Perez F. 2012. Interplay between microtubule dynamics and intracellular organization. Int. J. Biochem. Cell Biol. 44, 266–274 (doi:10.1016/j.biocel.2011.11.009) [DOI] [PubMed] [Google Scholar]
- 11.Mitchison T, Kirschner M. 1984. Dynamic instability of microtubule growth. Nature 312, 237–242 (doi:10.1038/312237a0) [DOI] [PubMed] [Google Scholar]
- 12.Bowne-Anderson H, Zanic M, Kauer M, Howard J. 2013. Microtubule dynamic instability: a new model with coupled GTP hydrolysis and multistep catastrophe. Bioessays 35, 452–461 (doi:10.1002/bies.201200131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyser KM. 1964. Early differentiation of motor neuroblasts in the chick embryo as studied by electron microscopy. I. General aspects. Dev. Biol. 10, 433–466 (doi:10.1016/0012-1606(64)90054-5) [DOI] [PubMed] [Google Scholar]
- 14.Lyser KM. 1968. Early differentiation of motor neuroblasts in the chick embryo as studied by electron microscopy. II. Microtubules and neurofilaments. Dev. Biol. 17, 117–142 (doi:10.1016/0012-1606(68)90057-2) [DOI] [PubMed] [Google Scholar]
- 15.Tennyson VM. 1965. Electron microscopic study of the developing neuroblast of the dorsal root ganglion of the rabbit embryo. J. Comp. Neurol. 124, 267–317 (doi:10.1002/cne.901240302) [DOI] [PubMed] [Google Scholar]
- 16.Tennyson VM. 1970. The fine structure of the axon and growth cone of the dorsal root neuroblast of the rabbit embryo. J. Cell Biol. 44, 62–79 (doi:10.1083/jcb.44.1.62) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada KM, Spooner BS, Wessells NK. 1970. Axon growth: roles of microfilaments and microtubules. Proc. Natl Acad. Sci. USA 66, 1206–1212 (doi:10.1073/pnas.66.4.1206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada KM, Spooner BS, Wessells NK. 1971. Ultrastructure and function of growth cones and axons of cultured nerve cells. J. Cell Biol. 49, 614–635 (doi:10.1083/jcb.49.3.614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon F. 1980. Neuroblastoma cells recapitulate their detailed neurite morphologies after reversible microtubule disassembly. Cell 21, 333–338 (doi:10.1016/0092-8674(80)90469-9) [DOI] [PubMed] [Google Scholar]
- 20.Suter DM, Miller KE. 2011. The emerging role of forces in axonal elongation. Prog. Neurobiol. 94, 91–101 (doi:10.1016/j.pneurobio.2011.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakakibara A, Horwitz AF. 2006. Mechanism of polarized protrusion formation on neuronal precursors migrating in the developing chicken cerebellum. J. Cell Sci. 119, 3583–3592 (doi:10.1242/jcs.03080) [DOI] [PubMed] [Google Scholar]
- 22.Hirokawa N, Niwa S, Tanaka Y. 2010. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68, 610–638 (doi:10.1016/j.neuron.2010.09.039) [DOI] [PubMed] [Google Scholar]
- 23.Tsai LH, Gleeson JG. 2005. Nucleokinesis in neuronal migration. Neuron 46, 383–388 (doi:10.1016/j.neuron.2005.04.013) [DOI] [PubMed] [Google Scholar]
- 24.Vallee RB, Seale GE, Tsai J-W. 2009. Emerging roles for myosin II and cytoplasmic dynein in migrating neurons and growth cones. Trends Cell Biol. 19, 347–355 (doi:10.1016/j.tcb.2009.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura T, Kato K, Yamaguchi T, Fukata Y, Ohno S, Kaibuchi K. 2004. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat. Cell Biol. 6, 328–334 (doi:10.1038/ncb1118) [DOI] [PubMed] [Google Scholar]
- 26.Arimura N, Kaibuchi K. 2007. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 8, 194–205 (doi:10.1038/nrn2056) [DOI] [PubMed] [Google Scholar]
- 27.Nakata T, Hirokawa N. 2003. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J. Cell Biol. 162, 1045–1055 (doi:10.1083/jcb.200302175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura T, Arimura N, Fukata Y, Watanabe H, Iwamatsu A, Kaibuchi K. 2005. Tubulin and CRMP-2 complex is transported via kinesin-1. J. Neurochem. 93, 1371–1382 (doi:10.1111/j.1471-4159.2005.03063.x) [DOI] [PubMed] [Google Scholar]
- 29.Jacobson C, Schnapp B, Banker GA. 2006. A change in the selective translocation of the kinesin-1 motor domain marks the initial specification of the axon. Neuron 49, 797–804 (doi:10.1016/j.neuron.2006.02.005) [DOI] [PubMed] [Google Scholar]
- 30.Myers KA, Baas PW. 2007. Kinesin-5 regulates the growth of the axon by acting as a brake on its microtubule array. J. Cell Biol. 178, 1081–1091 (doi:10.1083/jcb.200702074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M, Nadar VC, Kozielski F, Kozlowska M, Yu W, Baas PW. 2010. Kinesin-12, a mitotic microtubule-associated motor protein, impacts axonal growth, navigation, and branching. J. Neurosci. 30, 14 896–14 906 (doi:10.1523/jneurosci.3739-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falnikar A, Tole S, Baas PW. 2011. Kinesin-5, a mitotic microtubule-associated motor protein, modulates neuronal migration. Mol. Biol. Cell 22, 1561–1574 (doi:10.1091/mbc.E10-11-0905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin S, Liu M, Mozgova OI, Yu W, Baas PW. 2012. Mitotic motors coregulate microtubule patterns in axons and dendrites. J. Neurosci. 32, 14 033–14 049 (doi:10.1523/jneurosci.3070-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner MK, Zanic M, Gell C, Bormuth V, Howard J. 2011. Depolymerizing kinesins Kip3 and MCAK shape cellular microtubule architecture by differential control of catastrophe. Cell 147, 1092–1103 (doi:10.1016/j.cell.2011.10.037) [DOI] [PubMed] [Google Scholar]
- 35.Gardner MK, Zanic M, Howard J. 2013. Microtubule catastrophe and rescue. Curr. Opin. Cell Biol. 25, 14–22 (doi:10.1016/j.ceb.2012.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta A, Tsai LH, Wynshaw-Boris A. 2002. Life is a journey: a genetic look at neocortical development. Nat. Rev. Genet. 3, 342–355 (doi:10.1038/nrg799) [DOI] [PubMed] [Google Scholar]
- 37.Jaglin XH, Chelly J. 2009. Tubulin-related cortical dysgenesis: microtubule dysfunction underlying neuronal migration defects. Trends Genet. 25, 555–566 (doi:10.1016/j.tig.2009.10.003) [DOI] [PubMed] [Google Scholar]
- 38.Wynshaw-Boris A, Pramparo T, Youn YH, Hirotsune S. 2010. Lissencephaly: mechanistic insights from animal models and potential therapeutic strategies. Semin. Cell Dev. Biol. 21, 823–830 (doi:10.1016/j.semcdb.2010.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manzini MC, Walsh CA. 2011. What disorders of cortical development tell us about the cortex: one plus one does not always make two. Curr. Opin. Genet. Dev. 21, 333–339 (doi:10.1016/j.gde.2011.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tischfield MA, Cederquist GY, Gupta ML, Jr, Engle EC. 2011. Phenotypic spectrum of the tubulin-related disorders and functional implications of disease-causing mutations. Curr. Opin. Genet. Dev. 21, 286–294 (doi:10.1016/j.gde.2011.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiner O. 2013. LIS1 and DCX: implications for brain development and human disease in relation to microtubules. Scientifica 2013, 393975 (doi:10.1155/2013/393975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keays DA, et al. 2007. Mutations in α-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell 128, 45–57 (doi:10.1016/j.cell.2006.12.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaglin XH, et al. 2009. Mutations in β-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat. Genet. 41, 746–752 (doi:10.1038/ng.380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tischfield MA, et al. 2010. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell 140, 74–87 (doi:10.1016/j.cell.2009.12.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith DS, Niethammer M, Ayala R, Zhou Y, Gambello MJ, Wynshaw-Boris A, Tsai LH. 2000. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat. Cell Biol. 2, 767–775 (doi:10.1038/35041000) [DOI] [PubMed] [Google Scholar]
- 46.Faulkner NE, Dujardin DL, Tai CY, Vaughan KT, O'Connell CB, Wang YI, Vallee RB. 2000. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat. Cell Biol. 2, 784–791 (doi:10.1038/35041020) [DOI] [PubMed] [Google Scholar]
- 47.Sasaki S, Shionoya A, Ishida M, Gambello MJ, Yingling J, Wynshaw-Boris A, Hirotsune S. 2000. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron 28, 681–696 (doi:10.1016/S0896-6273(00)00146-X) [DOI] [PubMed] [Google Scholar]
- 48.Kerjan G, Gleeson JG. 2007. Genetic mechanisms underlying abnormal neuronal migration in classical lissencephaly. Trends Genet. 23, 623–630 (doi:10.1016/j.tig.2007.09.003) [DOI] [PubMed] [Google Scholar]
- 49.Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, Caskey CT, Ledbetter DH. 1993. Isolation of a Miller–Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature 364, 717–721 (doi:10.1038/364717a0) [DOI] [PubMed] [Google Scholar]
- 50.Bi W, et al. 2009. Increased LIS1 expression affects human and mouse brain development. Nat. Genet. 41, 168–177 (doi:10.1038/ng.302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.des Portes V, et al. 1998. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell 92, 51–61 (doi:10.1016/S0092-8674(00)80898-3) [DOI] [PubMed] [Google Scholar]
- 52.Gleeson JG, et al. 1998. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell 92, 63–72 (doi:10.1016/S0092-8674(00)80899-5) [DOI] [PubMed] [Google Scholar]
- 53.Francis F, et al. 1999. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 23, 247–256 (doi:10.1016/S0896-6273(00)80777-1) [DOI] [PubMed] [Google Scholar]
- 54.Gleeson JG, Lin PT, Flanagan LA, Walsh CA. 1999. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23, 257–271 (doi:10.1016/S0896-6273(00)80778-3) [DOI] [PubMed] [Google Scholar]
- 55.Horesh D, Sapir T, Francis F, Wolf SG, Caspi M, Elbaum M, Chelly J, Reiner O. 1999. Doublecortin: a stabilizer of microtubules. Hum. Mol. Genet. 8, 1599–1610 (doi:10.1093/hmg/8.9.1599) [DOI] [PubMed] [Google Scholar]
- 56.Sapir T, Frotscher M, Levy T, Mandelkow EM, Reiner O. 2011. Tau's role in the developing brain: implications for intellectual disability. Hum. Mol. Genet. 21, 1681–1692 (doi:10.1093/hmg/ddr603) [DOI] [PubMed] [Google Scholar]
- 57.Niblock M, Gallo JM. 2012. Tau alternative splicing in familial and sporadic tauopathies. Biochem. Soc. Trans. 40, 677–680 (doi:10.1042/bst20120091) [DOI] [PubMed] [Google Scholar]
- 58.Tanaka T, Serneo FF, Higgins C, Gambello MJ, Wynshaw-Boris A, Gleeson JG. 2004. Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J. Cell Biol. 165, 709–721 (doi:10.1083/jcb.200309025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsai JW, Bremner KH, Vallee RB. 2007. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat. Neurosci. 10, 970–979 (doi:10.1038/nn1934) [DOI] [PubMed] [Google Scholar]
- 60.Asada N, Sanada K, Fukada Y. 2007. LKB1 regulates neuronal migration and neuronal differentiation in the developing neocortex through centrosomal positioning. J. Neurosci. 27, 11 769–11 775 (doi:10.1523/jneurosci.1938-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asada N, Sanada K. 2010. LKB1-mediated spatial control of GSK3β and adenomatous polyposis coli contributes to centrosomal forward movement and neuronal migration in the developing neocortex. J. Neurosci. 30, 8852–8865 (doi:10.1523/jneurosci.6140-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sapir T, Sapoznik S, Levy T, Finkelshtein D, Shmueli A, Timm T, Mandelkow EM, Reiner O. 2008. Accurate balance of the polarity kinase MARK2/Par-1 is required for proper cortical neuronal migration. J. Neurosci. 28, 5710–5720 (doi:10.1523/jneurosci.0911-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sapir T, Shmueli A, Levy T, Timm T, Elbaum M, Mandelkow EM, Reiner O. 2008. Antagonistic effects of doublecortin and MARK2/Par-1 in the developing cerebral cortex. J. Neurosci. 28, 13 008–13 013 (doi:10.1523/jneurosci.2363-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rivas RJ, Hatten ME. 1995. Motility and cytoskeletal organization of migrating cerebellar granule neurons. J. Neurosci. 15, 981–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Umeshima H, Hirano T, Kengaku M. 2007. Microtubule-based nuclear movement occurs independently of centrosome positioning in migrating neurons. Proc. Natl Acad. Sci. USA 104, 16 182–16 187 (doi:10.1073/pnas.0708047104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Distel M, Hocking JC, Volkmann K, Köster RW. 2010. The centrosome neither persistently leads migration nor determines the site of axonogenesis in migrating neurons in vivo. J. Cell Biol. 191, 875–890 (doi:10.1083/jcb.201004154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakakibara A, Sato T, Ando R, Noguchi N, Masaoka M, Miyata T. In press Dynamics of centrosome translocation and microtubule organization in neocortical neurons during distinct modes of polarization. Cereb. Cortex. (doi:10.1093/cercor/bhs411) [DOI] [PubMed] [Google Scholar]
- 68.Solecki DJ, Trivedi N, Govek EE, Kerekes RA, Gleason SS, Hatten ME. 2009. Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and soma during CNS glial-guided neuronal migration. Neuron 63, 63–80 (doi:10.1016/j.neuron.2009.05.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bellion A, Baudoin JP, Alvarez C, Bornens M, Metin C. 2005. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J. Neurosci. 25, 5691–5699 (doi:10.1523/jneurosci.1030-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schaar BT, McConnell SK. 2005. Cytoskeletal coordination during neuronal migration. Proc. Natl Acad. Sci. USA 102, 13 652–13 657 (doi:10.1073/pnas.0506008102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martini FJ, Valdeolmillos M. 2010. Actomyosin contraction at the cell rear drives nuclear translocation in migrating cortical interneurons. J. Neurosci. 30, 8660–8670 (doi:10.1523/jneurosci.1962-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dotti CG, Sullivan CA, Banker GA. 1988. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 8, 1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cáceres A, Ye B, Dotti CG. 2012. Neuronal polarity: demarcation, growth and commitment. Curr. Opin. Cell Biol. 24, 547–553 (doi:10.1016/j.ceb.2012.05.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hatanaka Y, Yamauchi K, Murakami F. 2012. Formation of axon-dendrite polarity in situ: initiation of axons from polarized and non-polarized cells. Dev. Growth Differ. 54, 398–407 (doi:10.1111/j.1440-169X.2012.01344.x) [DOI] [PubMed] [Google Scholar]
- 75.Shi SH, Jan LY, Jan YN. 2003. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 112, 63–75 (doi:10.1016/S0092-8674(02)01249-7) [DOI] [PubMed] [Google Scholar]
- 76.Ménager C, Arimura N, Fukata Y, Kaibuchi K. 2004. PIP3 is involved in neuronal polarization and axon formation. J. Neurochem. 89, 109–118 (doi:10.1046/j.1471-4159.2004.02302.x) [DOI] [PubMed] [Google Scholar]
- 77.Schwamborn JC, Püschel AW. 2004. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat. Neurosci. 7, 923–929 (doi:10.1038/nn1295) [DOI] [PubMed] [Google Scholar]
- 78.Fukata Y, et al. 2002. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat. Cell Biol. 4, 583–591 (doi:10.1038/ncb825) [DOI] [PubMed] [Google Scholar]
- 79.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. 2005. GSK-3β regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 120, 137–149 (doi:10.1016/j.cell.2004.11.012) [DOI] [PubMed] [Google Scholar]
- 80.Horiguchi K, Hanada T, Fukui Y, Chishti AH. 2006. Transport of PIP3 by GAKIN, a kinesin-3 family protein, regulates neuronal cell polarity. J. Cell Biol. 174, 425–436 (doi:10.1083/jcb.200604031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gdalyahu A, Ghosh I, Levy T, Sapir T, Sapoznik S, Fishler Y, Azoulai D, Reiner O. 2004. DCX, a new mediator of the JNK pathway. EMBO J. 23, 823–832 (doi:10.1038/sj.emboj.7600079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kishi M, Pan YA, Crump JG, Sanes JR. 2005. Mammalian SAD kinases are required for neuronal polarization. Science 30, 929–932 (doi:10.1126/science.1107403) [DOI] [PubMed] [Google Scholar]
- 83.Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. 2007. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell 129, 549–563 (doi:10.1016/j.cell.2007.03.025) [DOI] [PubMed] [Google Scholar]
- 84.Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. 2007. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell 129, 565–577 (doi:10.1016/j.cell.2007.04.012) [DOI] [PubMed] [Google Scholar]
- 85.Hirai S, Banba Y, Satake T, Ohno S. 2011. Axon formation in neocortical neurons depends on stage-specific regulation of microtubule stability by the dual leucine zipper kinase-c-Jun N-terminal kinase pathway. J. Neurosci. 31, 6468–6480 (doi:10.1523/jneurosci.5038-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Witte H, Neukirchen D, Bradke F. 2008. Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 180, 619–632 (doi:10.1083/jcb.200707042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schaar BT, Kinoshita K, McConnell SK. 2004. Doublecortin microtubule affinity is regulated by a balance of kinase and phosphatase activity at the leading edge of migrating neurons. Neuron 41, 203–213 (doi:10.1016/S0896-6273(03)00843-2) [DOI] [PubMed] [Google Scholar]
- 88.Chen YM, Wang QJ, Hu HS, Yu PC, Zhu J, Drewes G, Piwnica-Worms H, Luo ZG. 2006. Microtubule affinity-regulating kinase 2 functions downstream of the PAR-3/PAR-6/atypical PKC complex in regulating hippocampal neuronal polarity. Proc. Natl Acad. Sci. USA 103, 8534–8539 (doi:10.1073/pnas.0509955103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eto K, Kawauchi T, Osawa M, Tabata H, Nakajima K. 2010. Role of dual leucine zipper-bearing kinase (DLK/MUK/ZPK) in axonal growth. Neurosci. Res. 66, 37–45 (doi:10.1016/j.neures.2009.09.1708) [DOI] [PubMed] [Google Scholar]
- 90.Westerlund N, et al. 2011. Phosphorylation of SCG10/stathmin-2 determines multipolar stage exit and neuronal migration rate. Nat. Neurosci. 14, 305–313 (doi:10.1038/nn.2755) [DOI] [PubMed] [Google Scholar]
- 91.Watabe-Uchida M, John KA, Janas JA, Newey SE, Van Aelst L. 2006. The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18. Neuron 51, 727–739 (doi:10.1016/j.neuron.2006.07.020) [DOI] [PubMed] [Google Scholar]
- 92.Lefcort F, Bentley D. 1989. Organization of cytoskeletal elements and organelles preceding growth cone emergence from an identified neuron in situ. J. Cell Biol. 108, 1737–1749 (doi:10.1083/jcb.108.5.1737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zmuda JF, Rivas RJ. 1998. The Golgi apparatus and the centrosome are localized to the sites of newly emerging axons in cerebellar granule neurons in vitro. Cell Motil. Cytoskeleton 41, 18–38 (doi:10.1002/(SICI)1097-0169(1998)41:1<18::AID-CM2>3.0.CO;2-B) [DOI] [PubMed] [Google Scholar]
- 94.de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG. 2005. Centrosome localization determines neuronal polarity. Nature 436, 704–708 (doi:10.1038/nature03811) [DOI] [PubMed] [Google Scholar]
- 95.de Anda FC, Meletis K, Ge X, Rei D, Tsai L-H. 2010. Centrosome motility is essential for initial axon formation in the neocortex. J. Neurosci. 30, 10 391–10 406 (doi:10.1523/jneurosci.0381-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gärtner A, Fornasiero EF, Munck S, Vennekens K, Seuntjens E, Huttner WB, Valtorta F, Dotti CG. 2012. N-cadherin specifies first asymmetry in developing neurons. EMBO J. 31, 1893–1903 (doi:10.1038/emboj.2012.41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zolessi FR, Poggi L, Wilkinson CJ, Chien CB, Harris WA. 2006. Polarization and orientation of retinal ganglion cells in vivo. Neural Dev. 1, 2 (doi:10.1186/1749-8104-1-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burton PR, Paige JL. 1981. Polarity of axoplasmic microtubules in the olfactory nerve of the frog. Proc. Natl Acad. Sci. USA 78, 3269–3273 (doi:10.1073/pnas.78.5.3269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heidemann SR, Landers JM, Hamborg MA. 1981. Polarity orientation of axonal microtubules. J. Cell Biol. 91, 661–665 (doi:10.1083/jcb.91.3.661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baas PW, Deitch JS, Black MM, Banker GA. 1988. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl Acad. Sci. USA 85, 8335–8339 (doi:10.1073/pnas.85.21.8335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baas PW, Black MM, Banker GA. 1989. Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. J. Cell Biol. 109, 3085–3094 (doi:10.1083/jcb.109.6.3085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rakic P, Knyihar-Csillik E, Csillik B. 1996. Polarity of microtubule assemblies during neuronal cell migration. Proc. Natl Acad. Sci. USA 93, 9218–9222 (doi:10.1073/pnas.93.17.9218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stepanova T, et al. 2003. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J. Neurosci. 23, 2655–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rolls MM, Satoh D, Clyne PJ, Henner AL, Uemura T, Doe CQ. 2007. Polarity and intracellular compartmentalization of Drosophila neurons. Neural Dev. 2, 7 (doi:10.1186/1749-8104-2-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maniar TA, Kaplan M, Wang GJ, Shen K, Wei L, Shaw JE, Koushika SP, Bargmann CI. 2012. UNC-33 (CRMP) and ankyrin organize microtubules and localize kinesin to polarize axon-dendrite sorting. Nat. Neurosci. 15, 48–56 (doi:10.1038/nn.2970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goodwin PR, Sasaki JM, Juo P. 2012. Cyclin-dependent kinase 5 regulates the polarized trafficking of neuropeptide-containing dense-core vesicles in Caenorhabditis elegans motor neurons. J. Neurosci. 32, 8158–8172 (doi:10.1523/jneurosci.0251-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng Y, Wildonger J, Ye B, Zhang Y, Kita A, Younger SH, Zimmerman S, Jan LY, Jan YN. 2008. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat. Cell Biol. 10, 1172–1180 (doi:10.1038/ncb1777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Satoh D, Sato D, Tsuyama T, Saito M, Ohkura H, Rolls MM, Ishikawa F, Uemura T. 2008. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat. Cell Biol. 10, 1164–1171 (doi:10.1038/ncb1776) [DOI] [PubMed] [Google Scholar]
- 109.Ori-McKenney KM, Jan LY, Jan YN. 2012. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 76, 921–930 (doi:10.1016/j.neuron.2012.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. 2005. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron 48, 757–771 (doi:10.1016/j.neuron.2005.11.005) [DOI] [PubMed] [Google Scholar]
- 111.Roll-Mecak A, Vale RD. 2006. Making more microtubules by severing: a common theme of noncentrosomal microtubule arrays? J. Cell Biol. 175, 849–851 (doi:10.1083/jcb.200611149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW. 2006. Flies without centrioles. Cell 125, 1375–1386 (doi:10.1016/j.cell.2006.05.025) [DOI] [PubMed] [Google Scholar]
- 113.Nguyen MM, Stone MC, Rolls MM. 2011. Microtubules are organized independently of the centrosome in Drosophila neurons. Neural Dev. 6, 38 (doi:10.1186/1749-8104-6-38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stiess M, Maghelli N, Kapitein LC, Gomis-Rüth S, Wilsch-Bräuninger M, Hoogenraad CC, Tolić-Nørrelykke IM, Bradke F. 2010. Axon extension occurs independently of centrosomal microtubule nucleation. Science 327, 704–707 (doi:10.1126/science.1182179) [DOI] [PubMed] [Google Scholar]
- 115.Yu W, Centonze VE, Ahmad FJ, Baas PW. 1993. Microtubule nucleation and release from the neuronal centrosome. J. Cell Biol. 122, 349–359 (doi:10.1083/jcb.122.2.349) [DOI] [PMC free article] [PubMed] [Google Scholar]