Abstract
The risk of cardiovascular morbidity and mortality is increased in rheumatoid arthritis. The classical cardiovascular risk factors, including smoking, hypertension, dyslipidaemia, insulin resistance and diabetes mellitus, obesity and physical inactivity do not appear to explain the excess cardiovascular risk in rheumatoid arthritis, although they do contribute, albeit in a different way or to a lesser extent, to rheumatoid arthritis in comparison with the general population. A very important link between rheumatoid arthritis and cardiovascular disease is inflammation as it plays a key role in all stages of atherosclerosis: from endothelial dysfunction to plaque rupture and thrombosis. It also has an influence on and accentuates some traditional cardiovascular risk factors, such as dyslipidaemia, obesity and insulin resistance. To date, the exact pathophysiologic mechanism by which this relation between cardiovascular disease and rheumatoid arthritis can be explained is not completely clear. Cardiovascular risk management in rheumatoid arthritis is mandatory. Unfortunately, the way this should be done remains a point of discussion. In this review issues regarding cardiovascular risk in rheumatoid arthritis and its management will be addressed, according to evidence presented in the latest studies and our own experience-based opinion.
Keywords: cardiovascular risk, cardiovascular risk management, inflammation, rheumatoid arthritis
Introduction
The risk of cardiovascular (CV) disease and mortality is increased in rheumatoid arthritis (RA). A substantial number of observational studies varying in factors such as the cohort type, disease duration and length of follow up, has produced a wide range of incidence rates for CV events and mortality. Some studies show a more than doubled risk which is comparable to the CV risk in diabetes mellitus (DM) [del Rincon et al. 2001; Peters et al. 2009]. Other studies show a more modest increased risk [Gabriel et al. 1999; Goodson et al. 2005a]. However, when the results of the most relevant studies are pooled the risk of CV events and mortality seems to be approximately 50% higher in RA patients compared with the general population [Avina-Zubieta et al. 2008, 2012]. To date, the exact pathophysiologic mechanism by which this relation between CV disease and RA can be explained is not completely clear. So the most important questions regarding this problem remain: ‘How can this increased risk be explained?’, ‘When does it start?’, ‘How can we predict the CV risk in RA?’ and, most important, ‘How can we prevent CV morbidity and mortality in RA?’.
In this review we will answer these questions according to evidence presented in the latest studies and according to our own experience-based opinion.
What causes the excess CV risk?
To be able to decrease the excess CV risk in RA it is essential to know what causes this problem. One of the reasons why this puzzle has not yet been solved is that there are many pathophysiological mechanisms involved that influence each other. First of all, the traditional CV risk factors, which cause and predict CV morbidity and mortality in the general population but also in people with DM, seem to be equally or only slightly more prevalent in the RA population. Some risk factors, such as smoking, even play an important role in the onset of RA, however, traditional CV risk factors alone do not explain the higher CV risk in RA [Chung et al. 2012; Gonzalez et al. 2008]. At present, RA and atherosclerosis are both regarded as inflammatory-driven diseases and this appears to be the most important reason why these two diseases coincide [Sattar et al. 2003]. There is much evidence that endorses this hypothesis. For instance, the inflammatory and immunological processes that occur in atherosclerotic plaques are very similar to those in inflammatory synovitis and markers of inflammation, as C-reactive protein (CRP), predict CV disease not only in healthy men and women but also in RA patients [Goodson et al. 2005b; Pasceri and Willerson, 1999; Ridker et al. 1998]. Furthermore, indicators of more severe disease and more systemic inflammation in RA, such as decreased functional capability, the presence of extra-articular manifestations, longer disease duration and seropositivity, are also associated with higher CV risk [Gabriel, 2008; Goodson et al. 2002; Soderlin et al. 1998; Turesson et al. 2007].
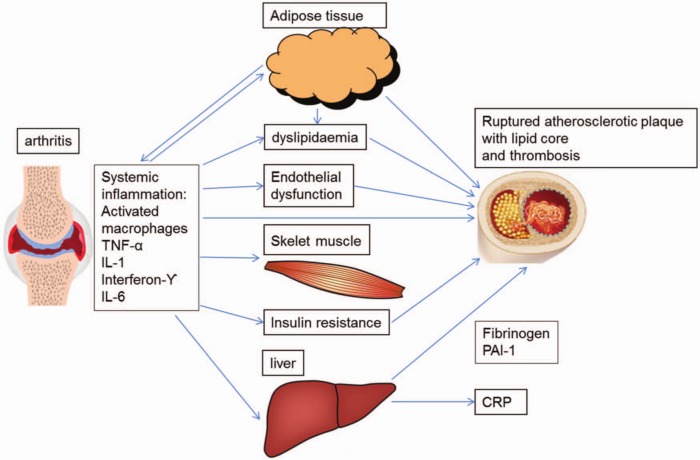
There are many ways by which RA-related inflammation may lead to atherosclerosis. Inflammation seems to play a key role in all stages of atherosclerosis: from endothelial dysfunction to plaque rupture and thrombosis [Libby, 2006]. Inflammation also has an influence on and accentuates some traditional CV risk factors, such as dyslipidaemia, obesity and insulin resistance (IR), as they behave differently in the RA population with regard to CV risk than in the general population [Gonzalez et al. 2008; Liao and Solomon, 2013; Sattar et al. 2003]. The link between synovitis and atherosclerosis is illustrated in Figure 1.
Figure 1.
An illustration of the pathways and cytokines by which synovitis can contribute to the formation of an atherosclerotic plaque and eventually CV events.
Another factor that may link atherosclerosis to RA is a common genetic background. An increasing number of studies report gene polymorphisms that are associated with a higher risk of CV risk in rheumatoid artritis [Cavagna et al. 2012]. Human leucocyte antigen shared epitope (HLA-DRB1) is a typical example of a gene that seems associated with CV mortality in RA [Farragher et al. 2008]. Together with the question of what causes the relationship between RA and atherosclerosis arises the question of when does CV risk increase? When genetics, traditional CV risk factors and autoimmune pathways are involved it could be possible that the CV risk is already increased at the time of diagnosis or even years before the first clinical symptoms appear. Actually, there is accumulating (circumstantial) evidence that CV risk is increased before the clinical onset of RA. There are studies that show that endothelial dysfunction and the first signs of atherosclerosis are already present in patients with recent-onset RA [Sodergren et al. 2010]. Most of the studies measuring carotid intima-media thickness (cIMT) in newly diagnosed RA patients versus controls found cIMT levels to be significantly elevated in the RA group and to be correlated with systemic inflammatory markers and disease severity markers [Kerola et al. 2012]. A meta-analysis looking at cIMT in established RA patients also showed statistically significant higher cIMT levels in RA versus controls but no relation with disease duration [van Sijl et al. 2012]. Also dyslipidaemia is already present in rheumatoid-factor-positive persons who later develop RA [Myasoedova et al. 2010; van de Stadt et al. 2012; van Halm et al. 2007]. One population-based cohort study reported an increased risk of coronary heart disease and myocardial infarction two years prior to RA diagnosis using the ACR 1987 criteria [Maradit-Kremers et al. 2005a]. There are some more recent studies that reported increased CV risk and mortality early in the RA disease course [Franklin et al. 2010; Holmqvist et al. 2010; Naz et al. 2008; Sodergren et al. 2010]. Altogether, it appears that CV risk already starts to increase as soon as the first signs of autoimmunity and inflammation appear which is often a few months to years prior to the actual RA diagnosis and increases further during disease progression as the inflammatory burden accumulates.
The role of traditional CV risk factors
Although the classical CV risk factors, including smoking, hypertension, dyslipidaemia, DM, obesity and inactivity do not seem to explain the excess CV risk in RA, they do contribute, albeit in a different way or to a lesser extent in RA in comparison with the general population. The following sections and Table 1 summarize and interpret data from recent studies on individual traditional CV risk factors and their contribution to CV risk in RA specifically.
Table 1.
Studies examining the prevalence of several traditional CV risk factors in RA patients.
| Study | Study design and population | CV risk factors Studied | Results |
|---|---|---|---|
| Park et al. [1999] | Case-control study with untreated, active RA patients | Lipid profile | RA patients significantly lower apoA-1 (128.5 versus 151.8 mg/dl), HDL-C (41.2 versus 54.9 mg/dl), higher Lp(a) (27.1 versus 18.0 mg/dl), apoB/apoA-1 (0.82 versus 0.67), TC/HDL-C (4.4 versus 3.4) and LDL-C/HDL-C (2.8 versus 1.9) ratios compared with controls (p ≤ 0.05). |
| Dessein et al. [2002] | Outpatient clinic-based case-control study of RA and OA patients | PA, DM, lipid profiles, insulin sensitivity | RA patients exercised more versus OA patients (χ2 = 3.9, p < 0.05). |
| DM more prevalent in RA (9) versus OA (1) patients (χ2 = 4.5, p < 0.05). | |||
| QUICKI 0.344 (0.332–0.355) and HDL-C 1.40 mmol/l (1.30–1.49) in RA patients versus 0.369 (0.356–0.383) and 1.68 mmol/l (1.50–1.85) in OA patients (p < 0.05). | |||
| Solomon et al. [2004] | Prospective longitudinal case-control study of RA women (the Nurses’ Health Study) | Smoking, hypertension, PA, BMI and DM | Except for past cigarette smoking (47.8% versus 38.0%) no significant differences in prevalence of CV risk factors between RA women and controls. |
| Choi and Seeger [2005] | Population-based case-control study of RA patients (NHANES III) | Lipid profile | Difference in HDL-C 2.5 (95% CI 0.8–4.9) mg/dl and apoA-1 4.5 (95% CI –0.8 to 9.8) mg/dl between RA and controls |
| Maradit-Kremers et al. [2005b] | Population-based cohort study of RA patients (Rochester Epidemiology Project) | Smoking, hypertension, dyslipidaemia, BMI and DM | Prevalence of former smokers 25.8%, current smokers 29.7% hypertension 51.7%, dyslipidaemia 49.4%, obesity 13.4% and DM 7.3% |
| Georgiadis et al. [2006] | Outpatient clinic-based case-control study of early RA patients | Lipid profile | Early RA patients significantly lower HDL-C (47.5±11.8 versus 51.1±7.4 mg/day), significantly higher TC (216.5±20.3 versus 190.4±33.9 mg/dl), LDL-C (141.6±42.3 versus 126.5±31.3 mg/dl), triglycerides (133.0±58.2 versus 97.1±28.3 mg/dl) and TC/HDL-C (4.9±1.3 versus 3.7±0.9), LDL-C/HDL-C (3.0±1.0 versus 2.5±0.8) ratios compared with controls. |
| Han et al. [2006] | Case-control study of RA, PsA, AS patients and healthy controls | Hypertension, hyperlipidemia, T2DM | Prevalence ratio of T2DM (1.4, 1.5, 1.2), hyperlipidemia (1.2, 1.2, 1.2), and hypertension (1.3, 1.3, 1.3) were higher in patients than controls. |
| Van Halm et al. [2007] | Longitudinal case-control study with blood samples of patients who later developed RA | Lipid profile | RA patients 4% higher TC, 9% lower HDL-C, 17% higher triglyceride and 6% higher apoB levels compared with controls (p ≤ 0.05). |
| Panoulas et al. [2007] | Cross-sectional outpatient clinic-based study of RA patients | Hypertension | Prevalence of hypertension 70.5%, of which 39.4 % undiagnosed. |
| Simard and Mittleman [2007] | Cross-sectional population-based cohort study (NHANES III) | T2DM | OR 1.1–1.5 between RA and DM not statistically significant |
| Mancuso et al. [2007] | Outpatient clinic-based case-control cohort study of RA patients | PA | Fewer RA patients met the recommendations for walking (32% versus 48%; p = 0.01) compared with general population. |
| Van den Berg et al. [2007] | Outpatient clinic-based cohort study compared with general Dutch population | PA | Proportions of RA patients meeting PA recommendation were similar. |
| The average number of minutes of PA per week was significantly lower in the RA population compared with the general population in the category 45–64 years (1836 versus 2199, p = 0.001) | |||
| Chung et al. [2008a] | Case-control cohort study of early and long-standing RA patients | Metabolic syndrome (modified WHO and NCEP III criteria) | Metabolic syndrome (WHO): long-standing RA 42%, early RA 31%, controls 11% (p < 0.001); |
| (NCEP III) long-standing RA 42%, early RA 30% and controls 22% (p = 0.03). | |||
| Gonzalez et al. [2008] | Longitudinal, population-based case-control study of RA subjects | Smoking, hypertension, dyslipidaemia, BMI and DM | Prevalence of smoking in RA patients 53% versus controls 43% (p < 0.001), prevalence of other CV risk factors similar in both groups |
| Rizzo et al. [2009] | Case-control study with early, untreated RA patients | Lipid profile | Higher triglycerides (1.8±0.5 versus 1.0±0.5mmol/L, p < 0.0001), lower HDL-C (1.2±0.2 versus 1.4±0.2 mmol/l, p = 0.0027) and lower LDL particle size (26±47 versus 281±9A, p < 0.0001) in RA patients than controls |
| Shahin et al. [2010] | Outpatient clinic-based case-control cohort study of early RA patients | Insulin resistance | Prevalence of IR higher in early RA patients with high disease activity than in patients with moderate disease activity and controls (p < 0.0001). |
| Solomon et al. [2010] | Longitudinal cohort of RA patients (CORRONA) | DM, hypertension, hyperlipidemia, current smoking | Prevalence of DM 7.1%, hypertension 29.8%, hyperlipidemia 9.2% and current smoking 16.1%. |
| Chung et al. [2012] | Case-control study of RA patients (ESCAPE RA and MESA cohort) | Hypertension, smoking, LDL, BMI and DM | Prevalence of hypertension in RA 57 % versus controls 42% (p = 0.001). |
| No significant differences in prevalence of other CV risk factors. |
AS, ankylosing spondylitis; BMI, body mass index; DM, diabetes mellitus; CV, cardiovascular; HDL-C, high-density lipoprotein cholesterol; IR, insulin resistance; LDL, low-density lipoprotein; LDL-C, low-density lipoprotein cholesterol; OA, osteoarthritis; OR, odds ratio; PA, physical activity; PsA, prostate-specific antigen; RA, rheumatoid arthritis; T2DM, type 2 diabetes mellitus; TC, total cholesterol.
Smoking
As already mentioned, cigarette smoking is classified not only as a predisposing factor for CV events but also for RA, particularly rheumatoid-factor-positive RA [Costenbader et al. 2006; Costenbader and Karlson, 2006; Heliovaara et al. 1993]. For rheumatologists this alone should be a trigger to advise every newly diagnosed RA patient who smokes to quit, apart from the notion that smoking probably also has an adverse influence on RA disease severity and prognosis [Saag et al. 1997].
Hypertension
Whether hypertension is more common in RA than in the general population has not been elucidated yet, because reports are contradictory [Liao and Solomon, 2013]. However, one of the most recent studies found a significant increased prevalence of hypertension in RA patients versus controls [Chung et al. 2012]. The reported prevalence of hypertension in RA varies substantially, depending on the study population, sample size and on the definition of hypertension used in the different studies [Panoulas et al. 2008b]. This does not mean that hypertension has no impact on CV risk in RA. Hypertension is a very common problem in the general as well as the RA population and is still underdiagnosed and undertreated, particularly in young RA patients [Panoulas et al. 2007]. There is no real evidence that the current disease-modifying antirheumatic drugs (DMARDs) have a beneficial effect on blood pressure. On the contrary, there are DMARDs and other drugs used for the treatment of RA that can induce high blood pressure, such as leflunomide, cyclosporine, glucocorticoids and the nonsteroidal anti-inflammatory drugs (NSAIDs) [Panoulas et al. 2008b; Van Doornum et al. 2006]. This means adequate blood pressure control and treatment could be of great influence to the CV risk in the RA population.
Dyslipidaemia
Abnormal cholesterol levels, particularly high levels of total cholesterol, triglycerides, low-density lipoprotein (LDL) and low levels of high-density lipoprotein (HDL) are strongly associated with CV disease. LDL particles can transport cholesterol in the arterial wall, which leads to atherosclerosis, while HDL particles are able to remove cholesterol from the arterial wall. Most studies which have investigated cholesterol levels in RA have found lower HDL levels and as a result an unfavourable atherogenic profile (total cholesterol/HDL cholesterol ratio) compared with healthy controls, even 10 years before the onset of RA [Choi and Seeger, 2005; Georgiadis et al. 2006; Myasoedova et al. 2010; Park et al. 1999; van de Stadt et al. 2012; van Halm et al. 2007; Yoo, 2004]. The data on total cholesterol, LDL and triglyceride levels are not so conclusive [Nurmohamed, 2007]. However, many studies demonstrate a very similar lipid pattern as seen in several other inflammatory conditions, indicating a profound influence of inflammation on lipid profile in RA [Khovidhunkit et al. 2000; Rizzo et al. 2009]. All in all, when LDL and HDL composition are also taken into account, the lipid profile in active RA tends to be highly proatherogenic [Hahn et al. 2008].
Diabetes mellitus and insulin resistance
There is a lot of data that points to an association between RA and IR, which is very likely caused by inflammation, as many studies showed a correlation between IR and disease activity or markers of inflammation such as CRP and erythrocyte sedimentation rate (ESR) [Chung et al. 2008a, 2008b; Dessein et al. 2002; Dessein and Joffe, 2006; Shahin et al. 2010; Svenson et al. 1987, 1988].
Several studies have investigated the prevalence of type 2 diabetes mellitus (DM2) in RA patients, expecting to find a positive association. However, apart from two studies, which found a modest significantly higher occurrence of DM2 in RA patients [Han et al. 2006; Solomon et al. 2010], all other studies found no significantly increased prevalence [Gabriel et al. 1999; Liao et al. 2009; Simard and Mittleman, 2007; Solomon et al. 2004]. This seems quite remarkable given the fact that IR often preceeds DM2 and indicates that further research is needed to clarify this issue. However, both IR and DM appear to be independent CV risk factors in patients with RA, as also seen in the general population [Chung et al. 2008a; del Rincon et al. 2005; Dessein et al. 2003; Maradit-Kremers et al. 2005b; Shahin et al. 2010].
Obesity
Obesity, specifically central or abdominal obesity, characterized by a large waist circumference, is a risk factor for CV disease in the general population [Mathieu et al. 2010]. The adipose tissue, especially abdominal visceral fat, may contribute to low-grade inflammation by producing pro-inflammatory cytokines such as tumour necrosis factor (TNF)-α and interleukin (IL)-6 [Despres and Lemieux, 2006].
Surprisingly, most studies reported a higher CV mortality in RA patients with a low body mass index (BMI) (<20 kg/m2) compared with patients with a higher BMI (≥30 kg/m2), although the prevalence of adiposity did not differ in RA compared with the general population [Chung et al. 2012; Escalante et al. 2005; Gabriel, 2008; Gonzalez et al. 2008; Kremers et al. 2004; Maradit-Kremers et al. 2005b; Rall and Roubenoff, 2004; Walsmith and Roubenoff, 2002]. This discrepancy can be explained by a phenomenon called rheumatoid cachexia, a condition characterized by decreased lean muscle mass and increased adiposity, which leads to an abnormal body composition in RA [Rall and Roubenoff, 2004; Summers et al. 2008; Walsmith and Roubenoff, 2002]. One study clearly demonstrated that RA patients had a different distribution of abdominal fat and especially visceral fat, despite no significant differences in BMI or waist circumference compared with non-RA controls [Giles et al. 2010]. Loss of lean muscle mass in RA seems associated with TNF-α [Roubenoff et al. 1994; Walsmith et al. 2004]. In conclusion, the chronic inflammatory state in RA seems to create an abnormal body composition characterized by muscle wasting and increasing visceral fat, whereby BMI can be perfectly normal and thereby less reliable when it comes to predicting CV risk.
Physical activity
RA patients tend to be less physically active due to disease symptoms, such as joint pain and stiffness and fear of aggravating their disease [Mancuso et al. 2007; Metsios et al. 2008; Roubenoff et al. 2002; van den Berg et al. 2007]. Exercise can prevent CV disease and it has a positive impact on all of the individual CV risk factors, such as adiposity, dyslipidaemia, IR and DM, hypertension and possibly even inflammation [Bensimhon et al. 2006; Blair et al. 1993; Kasapis and Thompson, 2005; Kelemen et al. 1990; Wallberg-Henriksson et al. 1998]. Physical inactivity has been associated with CV risk in RA [Metsios et al. 2009]. This could mean that stimulating RA patients to be more physically active could have even more effect on CV risk in RA patients than in healthy persons.
Cardiovascular risk scores: are they useful in RA?
Large longitudinal cohort studies performed in the 20th century have discovered which factors most adequately predict the risk for CV disease in the general population resulting in several CV risk estimation models, such as the Framingham Risk Score (FRS), the Reynolds Risk Score (RRS) and the Systematic Coronary Risk Evaluation (SCORE) [Conroy et al. 2003; D’Agostino et al. 2008; Ridker et al. 2007]. Although these CV risk assessment tools are based on the whole population, including people with chronic diseases, they often cannot be used for accurately predicting CV risk in these specific populations. A very good example of such a group is DM patients. They have a higher CV risk and therefore special risk scores, such as the UK Prospective Diabetes Study (UKPDS) score, have been developed [Stevens et al. 2001]. In the Netherlands, another way of calculating the excess CV risk of DM is being used. Instead of a separate risk model for DM, the SCORE risk model is adjusted by adding 15 years to the actual age of DM patients, because the excess CV risk in DM seems to match that of healthy persons 15 years older, the so-called ‘vascular age’ [Booth et al. 2006].
The same problem is true for RA patients: their vascular age also seems to be similar to that of healthy persons 10–15 years older and there are actually studies that have shown a CV risk that matches the CV risk of DM patients [Lindhardsen et al. 2011; Peters et al. 2009; Stamatelopoulos et al. 2009; van Halm et al. 2009].
The European League Against Rheumatism (EULAR) has set up guidelines with recommendations regarding CV risk management in RA and advised to add a multiplier of 1.5 to conventional risk assessment tools for estimating CV risk in RA patients, based on the evidence present at that time [Peters et al. 2010].
However, to date there is no evidence that this multiplier or any other CV risk estimation model or adjustment method accurately predicts CV risk in RA. Future research should therefore focus on developing accurate CV risk prediction models for the RA population.
Cardiovascular disease prevention in RA: what (not) to do?
Tight disease control
As stated above, CV risk in RA cannot be explained by traditional CV risk factors alone and is largely increased because of chronic systemic inflammation. Therefore, tight and sustained control of RA disease activity is necessary to effectively prevent CV disease development in RA. This starts by early recognition and diagnosis of RA followed by immediate aggressive treatment to diminish the grade of inflammation as quickly as possible to prevent damage in not only the joints but also the arteries. Although we do not know when the excess CV risk exactly starts to arise, it is likely at the same time signs of inflammation occur and it could even be sooner than that. The treatment goal should be remission, since even low-grade inflammation and especially cumulative (low-grade) inflammation can eventually cause atherosclerosis and CV events [Provan et al. 2011]. Indirectly, effective treatment can result in improved physical activity, subsequently leading to a decreased risk of obesity, diabetes, hypertension and in the end CV disease. Effective treatment of RA can therefore substantially reduce CV risk in RA, however some medications, such as corticosteroids and NSAIDs, often used in RA are known to enhance CV risk.
DMARDs
Methotrexate (MTX), the cornerstone DMARD in the treatment of RA, has been shown to reduce all-cause CV morbidity and mortality in RA and appears to have cardioprotective properties [Choi et al. 2002; Marks and Edwards, 2012; Prodanovich et al. 2005; van Halm et al. 2006; Westlake et al. 2010]. However, there are studies that found an association between MTX use and hyperhomocysteinemia, an independent CV risk factor, which could be explained by a depletion of folic acid levels [Haagsma et al. 1999; Hornung et al. 2004]. Hence, supplemental use of folic acid is advised to restore normal homocysteine levels [Morgan et al. 1998; Prodanovich et al. 2005]. Data on the effect of other DMARDs on CV morbidity and mortality are very scarce. Only two studies have looked at CV events and DMARD use and reported reductions in CV morbidity with sulfasalazine and leflunomide [Naranjo et al. 2008; van Halm et al. 2006]. There are, however, more studies that have looked at the effect of DMARDs on CV risk factors, such as DM, metabolic syndrome and dyslipidaemia. Hydroxychloroquine use in RA has been associated with improvement of lipid profiles, a reduced risk of developing DM and moreover exerts antithrombotic effects [Atzeni et al. 2010; Wasko et al. 2007]. Both leflunomide and cyclosporine can induce high blood pressure, hence are not DMARDs of first choice in patients with hypertension or increased CV risk [Kvien et al. 2002; Rozman et al. 2002].
Biologicals
TNF blockers are very effective drugs for RA and multiple other inflammatory diseases. Current available data show a positive but weaker effect of TNF blockers on CV disease occurrence, compared to MTX [Barnabe et al. 2011; Westlake et al. 2011]. Whether TNF blockers really exert a smaller CV risk reduction than MTX or the difference can be explained by other factors, such as study population and disease severity, is not clear yet. Altogether, it seems that TNF blockers have a positive influence on several CV risk factors, such as IR, HDL composition and endothelial function [Peters et al. 2012].
Tocilizumab (TCZ), an IL-6 inhibitor, also seems to have beneficial effects on endothelial function, despite the increase of total and LDL cholesterol. No data of the effect of TCZ on hard CV endpoints are available yet. The same counts for abatacept, a selective T-lymphocyte costimulation modulator and rituximab, a B-lymphocyte depleting agent. Since all of these medications are very effective at reducing inflammation, perhaps all of these biologicals exert CV risk reduction in RA patients who respond to treatment with these immunomodulating medication. However, this remains to be proven in the future.
Glucocorticosteroids
Glucocorticosteroids (GCs) are very powerful anti-inflammatory drugs and are commonly used as treatment for RA. They have strong anti-inflammatory properties which could mean that GCs have anti-atherosclerotic effects [Boers et al. 2003].
However, in the general population, therapeutic doses of oral GCs (≥7.5 mg/day) have been associated with increased CV disease and all-cause mortality [Souverein et al. 2004; Wei et al. 2004]. There are several population-based cohort studies that have looked at the effect of GC use at hard CV endpoints, such as myocardial infarction and cerebrovascular disease in RA patients with conflicting results [Avina-Zubieta et al. 2011, 2013; Ruyssen-Witrand et al. 2011]. Although data are not all congruent, GCs tend to have deleterious effects on several risk factors, as lipid profile, glucose tolerance, IR, blood pressure and abdominal obesity in RA [Avina-Zubieta et al. 2011, 2013; Dessein et al. 2004; Hafstrom et al. 2007; Panoulas et al. 2008a; Ruyssen-Witrand et al. 2011; Toms et al. 2008]. For now it remains unclear whether GC use in RA has a beneficial or detrimental effect on CV risk, since no randomized controlled trials of glucocorticoids versus placebo in RA patients have been conducted. In addition, confounding by indication probably plays an important role in the outcomes of studies that have looked at the effect of GCs on CV mortality and morbidity [Davis et al. 2005]. The effect of GCs on the CV risk probably depends on several factors, such as the population of RA patients, the circumstances and way it is used, the dosage and treatment duration [Davis et al. 2007; Mazzantini et al. 2010]. When GCs are used to treat highly active RA, during a short period as a ‘bridging therapy’, with an oral dosage as low as possible or via an intramuscular or intra-articular injection and preferably in patients who do not have DM or hypertension, the CV benefit/risk ratio could perhaps be positive. It is advisable to check blood pressure and glucose levels before start and during GC treatment, especially if the patient has DM or hypertension and GCs are prescribed for a longer period.
NSAIDs
NSAIDs are often used as pain medication in many rheumatic diseases, including RA. NSAIDs and also cyclooxygenase-2 inhibitors (COXIBs) are known to increase the risk for hypertension and myocardial infarction [Bolten, 2006; Farkouh and Greenberg, 2009; Kearney et al. 2006a; Morrison et al. 2007; Schjerning Olsen et al. 2011; Trelle et al. 2011; van der Linden et al. 2009]. Moreover, a population-based Danish case-control study found that the use of NSAIDs was associated with a twofold increased risk of venous thromboembolism (VTE) [Schmidt et al. 2011]. The different NSAIDs seem to have different effects on CV risk and especially naproxen seems less harmful than the rest of the NSAIDs [Kearney et al. 2006b; Solomon et al. 2008; Trelle et al. 2011]. Although there are not many studies that looked specifically at the effect of NSAIDs and COXIBs on CV risk in RA, one study showed no negative effects on CV mortality in patients with inflammatory polyarthritis [Goodson et al. 2009].
In summary, although evidence regarding the effects of NSAIDs on CV risk in RA is not conclusive, most data point to a negative influence, therefore the use of NSAIDs should be avoided if possible, at least in RA patients with known renal dysfunction, hypertension, heart failure or high CV risk. If NSAID use is unavoidable, blood pressure and renal function must be checked regularly and naproxen is probably the first choice. Furthermore, the combination of NSAIDs and aspirin is not advisable, since NSAIDs may impair the antiplatelet function of aspirin [Catella-Lawson et al. 2001; Hudson et al. 2005].
CV risk management
Although traditional CV risk factors may not explain the excess CV risk in RA, they do play an important role and should not be neglected when it comes to CV risk prevention. Since there are no CV risk assessment models for RA specifically, the national guidelines for CV risk management can best be used to determine CV risk and treatment, as advised by the EULAR guidelines for CV risk management in RA [Peters et al. 2010]. To adjust for the excess CV risk in RA, a multiplication factor of 1.5 is recommended in the presence of two of the following criteria: disease duration of more than 10 years, rheumatoid factor and/or anti-cyclic citrullinated peptide antibodies (anti-CCP) positivity or the presence of extra-articular manifestations. However, this multiplication factor needs validation, as there is indirect evidence that this multiplication factor improves CV risk estimation in RA. The same applies for the three above-mentioned criteria that are taken up in the EULAR recommendations to filter the RA patients that presumably have the highest CV risk. Although cumulative disease and therefore disease duration probably enhances CV risk, this does not mean CV risk treatment should only be started after 10 years of disease duration [Crowson and Gabriel, 2011]. Whether anti-CCP or rheumatoid factor positivity are real risk factors for CV disease or simply associated with CV disease because they are also associated with disease severity remains to be investigated. This means CV risk could be underestimated in RA patients who do not qualify to two of the three criteria. In the Dutch multidisciplinary guidelines for CV risk management RA has been recognized as an independent CV risk factor, equal to DM, and CV risk estimations are calculated using the SCORE formula, adjusted for data from Dutch studies. For both DM and RA patients, 15 years are added to the actual age of all patients to express the excess CV risk burden. However, this is also based on expert opinion and indirect evidence and, hence, needs to be validated.
When it comes to CV risk treatment in RA the first step is lifestyle adaptation. The two key messages for the rheumatologist to patients are to stop smoking and to get physically active [Stavropoulos-Kalinoglou et al. 2012]. The second step involves the determination of the CV risk profile, including at least assessment of blood pressure and lipid profile. On the basis of these and other easily accessible data (e.g. age, sex, family history of premature CV disease, etc.) and the aid of calculators such as Framingham and SCORE, the 10-year CV risk of a particular RA person can be calculated. Primary prevention involving treatment with statins and/or antihypertensives is only necessary if this 10-year CV risk is above a certain value. For instance, in the Netherlands this would be a 10-year risk of CV morbidity or mortality of 20% or more, based upon a Dutch version of the SCORE.
Unfortunately, thus far no intervention trials with statins or antihypertensives and CV disease prevention in RA have been published. Based on data from epidemiological studies and post hoc subgroup analyses of large, secondary CV prevention trials, the effects of statins on cholesterol levels in RA patients appear to be at least equivalent to the effects of statins in the general population [Rollefstad et al. 2012; Semb et al. 2011, 2012]. In contrast, one recently published population-based longitudinal study found that the effectiveness of statins varied in chronic diseases, including RA, and tended to be less effective than in the rest of the population [Sheng et al. 2012b]. There are, however, numerous other studies that show beneficial effects of statins and angiotensin-converting enzyme (ACE) inhibitors on CV risk in RA [Charles-Schoeman et al. 2007; Flammer et al. 2008; Hermann et al. 2005; Maki-Petaja et al. 2007; Sheng et al. 2012a; Van Doornum et al. 2004]. Actually, in RA the effects of cardioprotective agents might be more pronounced as the pleiotropic effects of statins, ACE inhibitors and angiotensin blockers include anti-inflammatory properties [Abud-Mendoza et al. 2003; Dagenais and Jamali, 2005; McCarey et al. 2004; Tikiz et al. 2005].
Randomized controlled intervention trials are necessary to assess the actual effect of statins, ACE inhibitors and other lifestyle intervention strategies on CV risk in RA.
Conclusion
RA patients are more susceptible to developing CV disease and therefore both tight disease control and also CV risk management in these patients is mandatory. An important issue is who is responsible: is it the rheumatologist or the primary care physician? Currently, this varies from country to country and even from practice to practice. Obviously, someone should take responsibility and this should be locally agreed upon.
Adequate CV risk management requires efforts from both the responsible doctor, whether this is the rheumatologist or the primary care physician, and the patient. A RA patient, who smokes, should quit smoking to decrease their CV risk substantially. However, it is up to the responsible doctor to point this out to the patient and to provide adequate support. This means periodically (annually) screening for CV risk factors, such as high blood pressure, blood glucose and cholesterol levels, but also to monitor physical activity, diet, waist/hip circumference and BMI. CV risk screening takes time, but is not difficult and can also be performed by adequately trained nurses. On the other hand, CV risk treatment and lifestyle advice takes a lot more time and effort and cannot be easily done by nurses practitioners, since it often involves prescribing medication. This requires innovative thinking and investing and is probably only feasible when rheumatologists work together with specialized nurses and other specialists, such as primary care physicians, internal doctors and cardiologists.
Since specific CV risk prediction models are not yet available for RA and the question remains which RA patients present the highest CV risk, it is advisable to start by screening all RA patients, preferably at an early stage of the disease and to treat them according to the existing national CV risk management guidelines. The enhanced CV risk in RA can be expressed by a multiplier 1.5, as advised by the EULAR guidelines. Meanwhile, research will have to continue to try to discover the exact pathophysiological mechanism behind the relation of atherosclerosis and rheumatic inflammatory diseases, so that adequate CV risk formulas can be developed for this group of patients. Moreover, large randomized controlled trials are needed to determine whether lifestyle interventions, statins and antihypertensives work as well or even better in rheumatic patients, but also to once and for all give clarity regarding the effects on CV risk of GCs, NSAIDs and other medications that are often prescribed to RA patients.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author declares that there are no conflicts of interest.
Contributor Information
Inge A.M. van den Oever, Jan van Breemen Research Institute Reade, Amsterdam, Netherlands and Department of Internal Medicine, VU University Medical Centre, Amsterdam, Netherlands
Alper M. van Sijl, Jan van Breemen Research Institute Reade, Amsterdam, Netherlands and Department of Internal Medicine, VU University Medical Centre, Amsterdam, Netherlands
Michael T. Nurmohamed, Departments of Internal Medicine and Rheumatology, VU University Medical Centre, PO Box 7057, de Boelelaan 1117, Amsterdam, Netherlands
References
- Abud-Mendoza C., de la Fuente H., Cuevas-Orta E., Baranda L., Cruz-Rizo J., Gonzalez-Amaro R. (2003) Therapy with statins in patients with refractory rheumatic diseases: a preliminary study. Lupus 12: 607–611 [DOI] [PubMed] [Google Scholar]
- Atzeni F., Turiel M., Caporali R., Cavagna L., Tomasoni L., Sitia S., et al. (2010) The effect of pharmacological therapy on the cardiovascular system of patients with systemic rheumatic diseases. Autoimmun Rev 9: 835–839 [DOI] [PubMed] [Google Scholar]
- Avina-Zubieta J., Abrahamowicz M., Choi H., Rahman M., Sylvestre M., Esdaile J., et al. (2011) Risk of cerebrovascular disease associated with the use of glucocorticoids in patients with incident rheumatoid arthritis: a population-based study. Ann Rheum Dis 70: 990–995 [DOI] [PubMed] [Google Scholar]
- Avina-Zubieta J., Abrahamowicz M., De Vera M., Choi H., Sayre E., Rahman M., et al. (2013) Immediate and past cumulative effects of oral glucocorticoids on the risk of acute myocardial infarction in rheumatoid arthritis: a population-based study. Rheumatology (Oxford) 52: 68–75 [DOI] [PubMed] [Google Scholar]
- Avina-Zubieta J., Choi H., Sadatsafavi M., Etminan M., Esdaile J., Lacaille D. (2008) Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 59: 1690–1697 [DOI] [PubMed] [Google Scholar]
- Avina-Zubieta J., Thomas J., Sadatsafavi M., Lehman A., Lacaille D. (2012) Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 71: 1524–1529 [DOI] [PubMed] [Google Scholar]
- Barnabe C., Martin B., Ghali W. (2011) Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 63: 522–529 [DOI] [PubMed] [Google Scholar]
- Bensimhon D., Kraus W., Donahue M. (2006) Obesity and physical activity: a review. Am Heart J 151: 598–603 [DOI] [PubMed] [Google Scholar]
- Blair S., Kohl H., Barlow C. (1993) Physical activity, physical fitness, and all-cause mortality in women: do women need to be active? J Am Coll Nutr 12: 368–371 [DOI] [PubMed] [Google Scholar]
- Boers M., Nurmohamed M., Doelman C., Lard L., Verhoeven A., Voskuyl A., et al. (2003) Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann Rheum Dis 62: 842–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolten W. (2006) Problem of the atherothrombotic potential of non-steroidal anti-inflammatory drugs. Ann Rheum Dis 65: 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth G., Kapral M., Fung K., Tu J. (2006) Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 368: 29–36 [DOI] [PubMed] [Google Scholar]
- Catella-Lawson F., Reilly M., Kapoor S., Cucchiara A., DeMarco S., Tournier B., et al. (2001) Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 345: 1809–1817 [DOI] [PubMed] [Google Scholar]
- Cavagna L., Boffini N., Cagnotto G., Inverardi F., Grosso V., Caporali R. (2012) Atherosclerosis and rheumatoid arthritis: more than a simple association. Mediators Inflamm 2012: 147354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles-Schoeman C., Khanna D., Furst D., McMahon M., Reddy S., Fogelman A., et al. (2007) Effects of high-dose atorvastatin on antiinflammatory properties of high density lipoprotein in patients with rheumatoid arthritis: a pilot study. J Rheumatol 34: 1459–1464 [PubMed] [Google Scholar]
- Choi H., Hernan M., Seeger J., Robins J., Wolfe F. (2002) Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet 359: 1173–1177 [DOI] [PubMed] [Google Scholar]
- Choi H., Seeger J. (2005) Lipid profiles among US elderly with untreated rheumatoid arthritis–the Third National Health and Nutrition Examination Survey. J Rheumatol 32: 2311–2316 [PubMed] [Google Scholar]
- Chung C., Giles J., Petri M., Szklo M., Post W., Blumenthal R., et al. (2012) Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum 41:535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C., Oeser A., Solus J., Avalos I., Gebretsadik T., Shintani A., et al. (2008a) Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis 196: 756–763 [DOI] [PubMed] [Google Scholar]
- Chung C., Oeser A., Solus J., Gebretsadik T., Shintani A., Avalos I., et al. (2008b) Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum 58: 2105–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy R., Pyorala K., Fitzgerald A., Sans S., Menotti A., De B.G., et al. (2003) Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 24: 987–1003 [DOI] [PubMed] [Google Scholar]
- Costenbader K., Feskanich D., Mandl L., Karlson E. (2006) Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med 119: 503–509 [DOI] [PubMed] [Google Scholar]
- Costenbader K., Karlson E. (2006) Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus 15: 737–745 [DOI] [PubMed] [Google Scholar]
- Crowson C., Gabriel S. (2011) Towards improving cardiovascular risk management in patients with rheumatoid arthritis: the need for accurate risk assessment. Ann Rheum Dis 70: 719–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais N., Jamali F. (2005) Protective effects of angiotensin II interruption: evidence for antiinflammatory actions. Pharmacotherapy 25: 1213–1229 [DOI] [PubMed] [Google Scholar]
- D’Agostino R., Sr, Vasan R., Pencina M., Wolf P., Cobain M., Massaro J., et al. (2008) General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117: 743–753 [DOI] [PubMed] [Google Scholar]
- Davis J., Maradit K., Crowson C., Nicola P., Ballman K., Therneau T., et al. (2007) Glucocorticoids and cardiovascular events in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 56: 820–830 [DOI] [PubMed] [Google Scholar]
- Davis J., Maradit-Kremers H., Gabriel S. (2005) Use of low-dose glucocorticoids and the risk of cardiovascular morbidity and mortality in rheumatoid arthritis: what is the true direction of effect? J Rheumatol 32: 1856–1862 [PubMed] [Google Scholar]
- del Rincon I., Freeman G., Haas R., O’Leary D., Escalante A. (2005) Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum 52: 3413–3423 [DOI] [PubMed] [Google Scholar]
- del Rincon I., Williams K., Stern M., Freeman G., Escalante A. (2001) High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 44: 2737–2745 [DOI] [PubMed] [Google Scholar]
- Despres J., Lemieux I. (2006) Abdominal obesity and metabolic syndrome. Nature 444: 881–887 [DOI] [PubMed] [Google Scholar]
- Dessein P., Joffe B.I. (2006) Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis Rheum 54: 2765–2775 [DOI] [PubMed] [Google Scholar]
- Dessein P., Joffe B., Stanwix A. (2003) Inflammation, insulin resistance, and aberrant lipid metabolism as cardiovascular risk factors in rheumatoid arthritis. J Rheumatol 30: 1403–1405 [PubMed] [Google Scholar]
- Dessein P., Joffe B., Stanwix A., Christian B., Veller M. (2004) Glucocorticoids and insulin sensitivity in rheumatoid arthritis. J Rheumatol 31: 867–874 [PubMed] [Google Scholar]
- Dessein P., Stanwix A., Joffe B. (2002) Cardiovascular risk in rheumatoid arthritis versus osteoarthritis: acute phase response related decreased insulin sensitivity and high-density lipoprotein cholesterol as well as clustering of metabolic syndrome features in rheumatoid arthritis. Arthritis Res 4: R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante A., Haas R., del Rincón I. (2005) Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med 165:1624–1629 [DOI] [PubMed] [Google Scholar]
- Farkouh M., Greenberg B. (2009) An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol 103: 1227–1237 [DOI] [PubMed] [Google Scholar]
- Farragher T., Goodson N., Naseem H., Silman A., Thomson W., Symmons D., et al. (2008) Association of the HLA-DRB1 gene with premature death, particularly from cardiovascular disease, in patients with rheumatoid arthritis and inflammatory polyarthritis 5. Arthritis Rheum 58: 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammer A., Sudano I., Hermann F., Gay S., Forster A., Neidhart M., et al. (2008) Angiotensin-converting enzyme inhibition improves vascular function in rheumatoid arthritis. Circulation 117: 2262–2269 [DOI] [PubMed] [Google Scholar]
- Franklin J., Farragher T., Lunt M., Camacho E., Bunn D., Marshall T., et al. (2010) Excess risk of hospital admission for cardiovascular disease within the first 7 years from onset of inflammatory polyarthritis. Ann Rheum Dis 69: 1660–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel S. (2008) Cardiovascular morbidity and mortality in rheumatoid arthritis. Am J Med 121: S9–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel S., Crowson C., O’Fallon W. (1999) Comorbidity in arthritis. J Rheumatol 26: 2475–2479 [PubMed] [Google Scholar]
- Georgiadis A., Papavasiliou E., Lourida E., Alamanos Y., Kostara C., Tselepis A., et al. (2006) Atherogenic lipid profile is a feature characteristic of patients with early rheumatoid arthritis: effect of early treatment - a prospective, controlled study. Arthritis Res Ther 8: R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles J., Allison M., Blumenthal R., Post W., Gelber A., Petri M., et al. (2010) Abdominal adiposity in rheumatoid arthritis: association with cardiometabolic risk factors and disease characteristics. Arthritis Rheum 62: 3173–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A., Maradit K., Crowson C., Ballman K., Roger V., Jacobsen S., et al. (2008) Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis 67: 64–69 [DOI] [PubMed] [Google Scholar]
- Goodson N., Marks J., Lunt M., Symmons D. (2005a) Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Ann Rheum Dis 64: 1595–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson N., Brookhart A., Symmons D., Silman A., Solomon D. (2009) Non-steroidal anti-inflammatory drug use does not appear to be associated with increased cardiovascular mortality in patients with inflammatory polyarthritis: results from a primary care based inception cohort of patients. Ann Rheum Dis 68: 367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson N., Symmons D., Scott D., Bunn D., Lunt M., Silman A. (2005b) Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year followup study of a primary care-based inception cohort. Arthritis Rheum 52: 2293–2299 [DOI] [PubMed] [Google Scholar]
- Goodson N., Wiles N., Lunt M., Barrett E., Silman A., Symmons D. (2002) Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum 46: 2010–2019 [DOI] [PubMed] [Google Scholar]
- Haagsma C., Blom H., van Riel P., van’t Hof M., Giesendorf B., van Oppenraaij-Emmerzaal D., et al. (1999) Influence of sulphasalazine, methotrexate, and the combination of both on plasma homocysteine concentrations in patients with rheumatoid arthritis. Ann Rheum Dis 58: 79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafstrom I., Rohani M., Deneberg S., Wornert M., Jogestrand T., Frostegard J. (2007) Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis - a randomized study. J Rheumatol 34: 1810–1816 [PubMed] [Google Scholar]
- Hahn B., Grossman J., Ansell B., Skaggs B., McMahon M. (2008) Altered lipoprotein metabolism in chronic inflammatory states: proinflammatory high-density lipoprotein and accelerated atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther 10: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Robinson D., Jr, Hackett M., Paramore L., Fraeman K., Bala M. (2006) Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 33: 2167–2172 [PubMed] [Google Scholar]
- Heliovaara M., Aho K., Aromaa A., Knekt P., Reunanen A. (1993) Smoking and risk of rheumatoid arthritis. J Rheumatol 20: 1830–1835 [PubMed] [Google Scholar]
- Hermann F., Forster A., Chenevard R., Enseleit F., Hurlimann D., Corti R., et al. (2005) Simvastatin improves endothelial function in patients with rheumatoid arthritis. J Am Coll Cardiol 45: 461–464 [DOI] [PubMed] [Google Scholar]
- Holmqvist M., Wedren S., Jacobsson L., Klareskog L., Nyberg F., Rantapaa-Dahlqvist S., et al. (2010) Rapid increase in myocardial infarction risk following diagnosis of rheumatoid arthritis amongst patients diagnosed between 1995 and 2006. J Intern Med 268: 578–585 [DOI] [PubMed] [Google Scholar]
- Hornung N., Ellingsen T., Stengaard-Pedersen K., Poulsen J. (2004) Folate, homocysteine, and cobalamin status in patients with rheumatoid arthritis treated with methotrexate, and the effect of low dose folic acid supplement. J Rheumatol 31: 2374–2381 [PubMed] [Google Scholar]
- Hudson M., Baron M., Rahme E., Pilote L. (2005) Ibuprofen may abrogate the benefits of aspirin when used for secondary prevention of myocardial infarction. J Rheumatol 32: 1589–1593 [PubMed] [Google Scholar]
- Kasapis C., Thompson P. (2005) The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol 45: 1563–1569 [DOI] [PubMed] [Google Scholar]
- Kearney P., Baigent C., Godwin J., Halls H., Emberson J., Patrono C. (2006a) Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 332: 1302–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney P., Baigent C., Godwin J., Halls H., Emberson J., Patrono C. (2006b) Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 332: 1302–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen M., Effron M., Valenti S., Stewart K. (1990) Exercise training combined with antihypertensive drug therapy. Effects on lipids, blood pressure, and left ventricular mass. JAMA 263: 2766–2771 [PubMed] [Google Scholar]
- Kerola A., Kauppi M., Kerola T., Nieminen T. (2012) How early in the course of rheumatoid arthritis does the excess cardiovascular risk appear? Ann Rheum Dis 71: 1606–1615 [DOI] [PubMed] [Google Scholar]
- Khovidhunkit W., Memon R., Feingold K., Grunfeld C. (2000) Infection and inflammation-induced proatherogenic changes of lipoproteins. J Infect Dis 181(Suppl. 3): S462–S472 [DOI] [PubMed] [Google Scholar]
- Kremers H., Nicola P., Crowson C., Ballman K., Gabriel S. (2004) Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum 50: 3450–3457 [DOI] [PubMed] [Google Scholar]
- Kvien T., Zeidler H., Hannonen P., Wollheim F., Forre O., Hafstrom I., et al. (2002) Long term efficacy and safety of cyclosporin versus parenteral gold in early rheumatoid arthritis: a three year study of radiographic progression, renal function, and arterial hypertension. Ann Rheum Dis 61: 511–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao K., Gunnarsson M., Kallberg H., Ding B., Plenge R., Padyukov L., et al. (2009) Specific association of type 1 diabetes mellitus with anti-cyclic citrullinated peptide-positive rheumatoid arthritis. Arthritis Rheum 60: 653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao K., Solomon D. (2013) Traditional cardiovascular risk factors, inflammation and cardiovascular risk in rheumatoid arthritis. Rheumatology (Oxford) 52: 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. (2006) Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 83:456S–460S [DOI] [PubMed] [Google Scholar]
- Libby P. (2008) Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am J Med 121: S21–S31 [DOI] [PubMed] [Google Scholar]
- Lindhardsen J., Ahlehoff O., Gislason G., Madsen O., Olesen J., Torp-Pedersen C., et al. (2011) The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis 70: 929–934 [DOI] [PubMed] [Google Scholar]
- Maki-Petaja K., Booth A., Hall F., Wallace S., Brown J., McEniery C., et al. (2007) Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol 50: 852–858 [DOI] [PubMed] [Google Scholar]
- Mancuso C., Rincon M., Sayles W., Paget S. (2007) Comparison of energy expenditure from lifestyle physical activities between patients with rheumatoid arthritis and healthy controls. Arthritis Rheum 57: 672–678 [DOI] [PubMed] [Google Scholar]
- Maradit-Kremers H., Crowson C., Nicola P., Ballman K., Roger V., Jacobsen S., et al. (2005a) Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 52: 402–411 [DOI] [PubMed] [Google Scholar]
- Maradit-Kremers H., Nicola P., Crowson C., Ballman K., Gabriel S. (2005b) Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum 52: 722–732 [DOI] [PubMed] [Google Scholar]
- Marks J., Edwards C. (2012) Protective effect of methotrexate in patients with rheumatoid arthritis and cardiovascular comorbidity. Ther Adv Musculoskelet Dis 4: 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu P., Lemieux I., Despres J. (2010) Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther 87: 407–416 [DOI] [PubMed] [Google Scholar]
- Mazzantini M., Talarico R., Doveri M., Consensi A., Cazzato M., Bazzichi L., et al. (2010) Incident comorbidity among patients with rheumatoid arthritis treated or not with low-dose glucocorticoids: a retrospective study. J Rheumatol 37: 2232–2236 [DOI] [PubMed] [Google Scholar]
- McCarey D., McInnes I., Madhok R., Hampson R., Scherbakov O., Ford I., et al. (2004) Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial Lancet 363: 2015–2021 [DOI] [PubMed] [Google Scholar]
- Metsios G., Stavropoulos-Kalinoglou A., Panoulas V., Wilson M., Nevill A., Koutedakis Y., et al. (2009) Association of physical inactivity with increased cardiovascular risk in patients with rheumatoid arthritis. Eur J Cardiovasc Prev Rehabil 16:188–194 [DOI] [PubMed] [Google Scholar]
- Metsios G., Stavropoulos-Kalinoglou A., Veldhuijzen van Zanten J., Treharne G., Panoulas V., Douglas K., et al. (2008) Rheumatoid arthritis, cardiovascular disease and physical exercise: a systematic review. Rheumatology (Oxford) 47: 239–248 [DOI] [PubMed] [Google Scholar]
- Morgan S., Baggott J., Lee J., Alarcon G. (1998) Folic acid supplementation prevents deficient blood folate levels and hyperhomocysteinemia during longterm, low dose methotrexate therapy for rheumatoid arthritis: implications for cardiovascular disease prevention. J Rheumatol 25: 441–446 [PubMed] [Google Scholar]
- Morrison A., Ramey D., van Adelsberg J., Watson D. (2007) Systematic review of trials of the effect of continued use of oral non-selective NSAIDs on blood pressure and hypertension. Curr Med Res Opin 23: 2395–2404 [DOI] [PubMed] [Google Scholar]
- Myasoedova E., Crowson C., Kremers H., Fitz-Gibbon P., Therneau T., Gabriel S. (2010) Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis 69: 1310–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo A., Sokka T., Descalzo M., Calvo-Alen J., Horslev-Petersen K., Luukkainen R., et al. (2008) Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 10: R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz S., Farragher T., Bunn D., Symmons D., Bruce I. (2008) The influence of age at symptom onset and length of followup on mortality in patients with recent-onset inflammatory polyarthritis. Arthritis Rheum 58: 985–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmohamed M. (2007) Atherogenic lipid profiles and its management in patients with rheumatoid arthritis. Vasc Health Risk Manag 3: 845–852 [PMC free article] [PubMed] [Google Scholar]
- Panoulas V., Douglas K., Milionis H., Stavropoulos-Kalinglou A., Nightingale P., Kita M., et al. (2007) Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 46: 1477–1482 [DOI] [PubMed] [Google Scholar]
- Panoulas V., Douglas K., Stavropoulos-Kalinoglou A., Metsios G., Nightingale P., Kita M., et al. (2008a) Long-term exposure to medium-dose glucocorticoid therapy associates with hypertension in patients with rheumatoid arthritis. Rheumatology (Oxford) 47: 72–75 [DOI] [PubMed] [Google Scholar]
- Panoulas V., Metsios G., Pace A., John H., Treharne G., Banks M., et al. (2008b) Hypertension in rheumatoid arthritis. Rheumatology (Oxford) 47: 1286–1298 [DOI] [PubMed] [Google Scholar]
- Park Y., Lee S., Lee W., Suh C., Lee C., Lee C., et al. (1999) Lipid profiles in untreated patients with rheumatoid arthritis. J Rheumatol 26: 1701–1704 [PubMed] [Google Scholar]
- Pasceri V., Willerson J. (1999) Homocysteine and coronary heart disease: a review of the current evidence. Semin Interv Cardiol 4: 121–128 [DOI] [PubMed] [Google Scholar]
- Peters M., Symmons D., McCarey D., Dijkmans B., Nicola P., Kvien T., et al. (2010) EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 69: 325–331 [DOI] [PubMed] [Google Scholar]
- Peters M., van Sijl A., Voskuyl A., Sattar N., Smulders Y., Nurmohamed M. (2012) The effects of tumor necrosis factor inhibitors on cardiovascular risk in rheumatoid arthritis. Curr Pharm Des 18: 1502–1511 [DOI] [PubMed] [Google Scholar]
- Peters M., van Halm V, Voskuyl A., Smulders Y., Boers M., Lems W., et al. (2009) Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum 61: 1571–1579 [DOI] [PubMed] [Google Scholar]
- Prodanovich S., Ma F., Taylor J., Pezon C., Fasihi T., Kirsner R. (2005) Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol 52: 262–267 [DOI] [PubMed] [Google Scholar]
- Provan S., Semb A., Hisdal J., Stranden E., Agewall S., Dagfinrud H., et al. (2011) Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: a cross-sectional comparative study. Ann Rheum Dis 70: 812–817 [DOI] [PubMed] [Google Scholar]
- Rall L., Roubenoff R. (2004) Rheumatoid cachexia: metabolic abnormalities, mechanisms and interventions. Rheumatology (Oxford) 43: 1219–1223 [DOI] [PubMed] [Google Scholar]
- Ridker P., Buring J., Rifai N., Cook N. (2007) Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 297: 611–619 [DOI] [PubMed] [Google Scholar]
- Ridker P., Buring J., Shih J., Matias M., Hennekens C. (1998) Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 98: 731–733 [DOI] [PubMed] [Google Scholar]
- Rizzo M., Spinas G., Cesur M., Ozbalkan Z., Rini G., Berneis K. (2009) Atherogenic lipoprotein phenotype and LDL size and subclasses in drug-naive patients with early rheumatoid arthritis. Atherosclerosis 207: 502–506 [DOI] [PubMed] [Google Scholar]
- Rollefstad S., Kvien T., Holme I., Eirheim A., Pedersen T., Semb A. (2012) Treatment to lipid targets in patients with inflammatory joint diseases in a preventive cardio-rheuma clinic. Ann Rheum Dis, in press [DOI] [PubMed] [Google Scholar]
- Roubenoff R., Roubenoff R., Cannon J., Kehayias J., Zhuang H., wson-Hughes B., et al. (1994) Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest 93: 2379–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubenoff R., Walsmith J., Lundgren N., Snydman L., Dolnikowski G., Roberts S. (2002) Low physical activity reduces total energy expenditure in women with rheumatoid arthritis: implications for dietary intake recommendations. Am J Clin Nutr 76: 774–779 [DOI] [PubMed] [Google Scholar]
- Rozman B., Praprotnik S., Logar D., Tomsic M., Hojnik M., Kos-Golja M., et al. (2002) Leflunomide and hypertension. Ann Rheum Dis 61: 567–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyssen-Witrand A., Fautrel B., Saraux A., Le L., X, Pham T. (2011) Cardiovascular risk induced by low-dose corticosteroids in rheumatoid arthritis: a systematic literature review. Joint Bone Spine 78: 23–30 [DOI] [PubMed] [Google Scholar]
- Saag K., Cerhan J., Kolluri S., Ohashi K., Hunninghake G., Schwartz D. (1997) Cigarette smoking and rheumatoid arthritis severity. Ann Rheum Dis 56: 463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N., McCarey D., Capell H., McInnes I. (2003) Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation 108: 2957–2963 [DOI] [PubMed] [Google Scholar]
- Schjerning Olsen A., Fosbol E., Lindhardsen J., Folke F., Charlot M., Selmer C., et al. (2011) Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: a nationwide cohort study. Circulation 123: 2226–2235 [DOI] [PubMed] [Google Scholar]
- Schmidt M., Christiansen C., Horvath-Puho E., Glynn R., Rothman K., Sorensen H. (2011) Non-steroidal anti-inflammatory drug use and risk of venous thromboembolism. J Thromb Haemost 9: 1326–1333 [DOI] [PubMed] [Google Scholar]
- Semb A., Holme I., Kvien T., Pedersen T. (2011) Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: an explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (Oxford) 50: 324–329 [DOI] [PubMed] [Google Scholar]
- Semb A., Kvien T., DeMicco D., Fayyad R., Wun C., LaRosa J., et al. (2012) Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum 64: 2836–2846 [DOI] [PubMed] [Google Scholar]
- Shahin D., Eltoraby E., Mesbah A., Houssen M. (2010) Insulin resistance in early untreated rheumatoid arthritis patients. Clin Biochem 43: 661–665 [DOI] [PubMed] [Google Scholar]
- Sheng X., Murphy M., Macdonald T., Wei L. (2012a) Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol 39: 32–40 [DOI] [PubMed] [Google Scholar]
- Sheng X., Murphy M., Macdonald T., Wei L. (2012b) The comparative effectiveness of statin therapy in selected chronic diseases compared with the remaining population. BMC Public Health 12: 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard J., Mittleman M. (2007) Prevalent rheumatoid arthritis and diabetes among NHANES III participants aged 60 and older. J Rheumatol 34: 469–473 [PubMed] [Google Scholar]
- Sodergren A., Karp K., Boman K., Eriksson C., Lundstrom E., Smedby T., et al. (2010) Atherosclerosis in early rheumatoid arthritis: very early endothelial activation and rapid progression of intima media thickness. Arthritis Res Ther 12: R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlin M., Nieminen P., Hakala M. (1998) Functional status predicts mortality in a community based rheumatoid arthritis population. J Rheumatol 25: 1895–1899 [PubMed] [Google Scholar]
- Solomon D., Curhan G., Rimm E., Cannuscio C., Karlson E. (2004) Cardiovascular risk factors in women with and without rheumatoid arthritis. Arthritis Rheum 50: 3444–3449 [DOI] [PubMed] [Google Scholar]
- Solomon D., Glynn R., Rothman K., Schneeweiss S., Setoguchi S., Mogun H., et al. (2008) Subgroup analyses to determine cardiovascular risk associated with nonsteroidal antiinflammatory drugs and coxibs in specific patient groups. Arthritis Rheum 59: 1097–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon D., Love T., Canning C., Schneeweiss S. (2010) Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis 69: 2114–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souverein P., Berard A., Van Staa T., Cooper C., Egberts A., Leufkens H., et al. (2004) Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart 90: 859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatelopoulos K., Kitas G., Papamichael C., Chryssohoou E., Kyrkou K., Georgiopoulos G., et al. (2009) Atherosclerosis in rheumatoid arthritis versus diabetes: a comparative study. Arterioscler Thromb Vasc Biol 29: 1702–1708 [DOI] [PubMed] [Google Scholar]
- Stavropoulos-Kalinoglou A., Metsios G., Veldhuijzen van Zanten J., Nightingale P., Kitas G., Koutedakis Y. (2012) Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis, in press. [DOI] [PubMed] [Google Scholar]
- Stevens R., Kothari V., Adler A., Stratton I. (2001) The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin Sci (Lond) 101: 671–679 [PubMed] [Google Scholar]
- Summers G., Deighton C., Rennie M., Booth A. (2008) Rheumatoid cachexia: a clinical perspective. Rheumatology (Oxford) 47: 1124–1131 [DOI] [PubMed] [Google Scholar]
- Svenson K., Lundqvist G., Wide L., Hallgren R. (1987) Impaired glucose handling in active rheumatoid arthritis: relationship to the secretion of insulin and counter-regulatory hormones. Metabolism 36: 940–943 [DOI] [PubMed] [Google Scholar]
- Svenson K., Pollare T., Lithell H., Hallgren R. (1988) Impaired glucose handling in active rheumatoid arthritis: relationship to peripheral insulin resistance. Metabolism 37: 125–130 [DOI] [PubMed] [Google Scholar]
- Tikiz C., Utuk O., Pirildar T., Bayturan O., Bayindir P., Taneli F., et al. (2005) Effects of Angiotensin-converting enzyme inhibition and statin treatment on inflammatory markers and endothelial functions in patients with longterm rheumatoid arthritis. J Rheumatol 32: 2095–2101 [PubMed] [Google Scholar]
- Toms T., Panoulas V., Douglas K., Griffiths H., Kitas G. (2008) Lack of association between glucocorticoid use and presence of the metabolic syndrome in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther 10: R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelle S., Reichenbach S., Wandel S., Hildebrand P., Tschannen B., Villiger P., et al. (2011) Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 342: c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesson C., McClelland R., Christianson T., Matteson E. (2007) Severe extra-articular disease manifestations are associated with an increased risk of first ever cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis 66: 70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de, Stadt L., van Sijl A., van Schaardenburg D., Nurmohamed M. (2012) Dyslipidaemia in patients with seropositive arthralgia predicts the development of arthritis. Ann Rheum Dis 71: 1915–1916 [DOI] [PubMed] [Google Scholar]
- van den Berg M., de Boor I, le Cessie S., Breedveld F., Vliet Vlieland T. (2007) Are patients with rheumatoid arthritis less physically active than the general population? J Clin Rheumatol 13: 181–186 [DOI] [PubMed] [Google Scholar]
- van der Linden M., van der Bij S., Welsing P., Kuipers E., Herings R. (2009) The balance between severe cardiovascular and gastrointestinal events among users of selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis 68: 668–673 [DOI] [PubMed] [Google Scholar]
- Van Doornum S., Jennings G., Wicks I. (2006) Reducing the cardiovascular disease burden in rheumatoid arthritis. Med J Aust 184: 287–290 [DOI] [PubMed] [Google Scholar]
- Van Doornum S., McColl G., Wicks I. (2004) Atorvastatin reduces arterial stiffness in patients with rheumatoid arthritis. Ann Rheum Dis 63: 1571–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Halm V., Nielen M., Nurmohamed M., van Schaardenburg D., Reesink H., Voskuyl A., et al. (2007) Lipids and inflammation: serial measurements of the lipid profile of blood donors who later developed rheumatoid arthritis. Ann Rheum Dis 66: 184–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Halm V., Nurmohamed M., Twisk J., Dijkmans B., Voskuyl A. (2006) Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther 8: R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Halm V., Peters M., Voskuyl A., Boers M., Lems W., Visser M., et al. (2009) Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: a cross-sectional study, the CARRE Investigation. Ann Rheum Dis 68: 1395–1400 [DOI] [PubMed] [Google Scholar]
- van Sijl A., van den Hurk K., Peters M., van Halm V., Nijpels G., Stehouwer C., et al. (2012) Different type of carotid arterial wall remodeling in rheumatoid arthritis compared with healthy subjects: a case-control study. J Rheumatol 39: 2261–2266 [DOI] [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Rincon J., Zierath J. (1998) Exercise in the management of non-insulin-dependent diabetes mellitus. Sports Med 25: 25–35 [DOI] [PubMed] [Google Scholar]
- Walsmith J., Abad L., Kehayias J., Roubenoff R. (2004) Tumor necrosis factor-alpha production is associated with less body cell mass in women with rheumatoid arthritis. J Rheumatol 31: 23–29 [PubMed] [Google Scholar]
- Walsmith J., Roubenoff R. (2002) Cachexia in rheumatoid arthritis. Int J Cardiol 85: 89–99 [DOI] [PubMed] [Google Scholar]
- Wasko M., Hubert H., Lingala V., Elliott J., Luggen M., Fries J., et al. (2007) Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA 298: 187–193 [DOI] [PubMed] [Google Scholar]
- Wei L., Macdonald T., Walker B. (2004) Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med 141: 764–770 [DOI] [PubMed] [Google Scholar]
- Westlake S., Colebatch A., Baird J., Curzen N., Kiely P., Quinn M., et al. (2011) Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 50: 518–531 [DOI] [PubMed] [Google Scholar]
- Westlake S., Colebatch A., Baird J., Kiely P., Quinn M., Choy E., et al. (2010) The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 49: 295–307 [DOI] [PubMed] [Google Scholar]
- Yoo W. (2004) Dyslipoproteinemia in patients with active rheumatoid arthritis: effects of disease activity, sex, and menopausal status on lipid profiles. J Rheumatol 31: 1746–1753 [PubMed] [Google Scholar]