Abstract
Immunosuppression-associated lymphoproliferative disorders can be related to primary as well as acquired immune disorders. Interferon gamma receptor (IFN-γR) deficiency is a rare primary immune disorder, characterized by increased susceptibility to mycobacterial infections. Here we report the first case of an Epstein Barr Virus (EBV) related B-cell lymphoma in a patient with complete IFN-γR1 deficiency.
The patient was a 20-year-old man with homozygous 22Cdel in IFNGR1 resulting in complete absence of IFN-γR1 surface expression and complete lack of responsiveness to IFN-γ in vitro. He had disseminated refractory Mycobacterium avium complex and Mycobacterium abscessus infections. At age 18 he presented with new spiking fever and weight loss that was due to an EBV-positive B-cell non-Hodgkin lymphoma. Two years later he died of progressive lymphoma.
IFN-γ plays an important role in tumor protection and rejection. Patients with IFN-γR deficiencies and other immune deficits predisposing to mycobacterial disease seem to have an increased risk of malignancies, especially those related to viral infections. As more of these patients survive their early infections, cancer awareness and tumor surveillance may need to become a more routine part of management.
Keywords: B-cell lymphoma, interferon gamma receptor deficiency
Introduction
Susceptibility to mycobacteria is caused by a set of rare primary immunodeficiencies characterized by marked predisposition to infections with poorly pathogenic mycobacteria, such as nontuberculous mycobacteria (NTM) and Bacillus Calmette-Guérin (1). In addition, patients are susceptible to Salmonella spp (2), Listeria monocytogenes (3), dimorphic yeasts (4, 5), and some viruses (6-8). So far, mutations in eight different genes have been associated with mycobacterial susceptibility: interferon gamma receptors (IFNGR) 1 and 2, Signal Transducer and Activator of Transcription (STAT) 1, the p40 subunit of IL-12 and IL-23 (IL-12B), the β1 chain shared by the IL-12 and IL-23 receptor (IL-12RB1), nuclear factor-κB-essential modulator (NEMO), interferon response factor 8 (IRF-8) (9), GATA2 (10), CYBB (11) and ISG15 (12). Most of these defects are associated with some degree of impaired IFN-γ production or activity.
The relationship between immunosuppression and the development of lymphoproliferative disorders is well known. The so-called immunosuppression associated lymphoproliferative disorders (IALDs) are usually B-cell derived and Epstein Barr Virus (EBV) related, behave aggressively, and predominantly occur in extranodal sites (13). IALDs can be related to acquired immune disorders, such as infections with Human Immunodeficiency Virus (HIV) (14, 15), following organ or bone marrow transplantation (16), associated with the use of immunosuppressive therapy (17), or associated with primary immune disorders.
The incidence of lymphoproliferative disorders in patients with primary immune disorders ranges from 0.7-18% (13). Primary immunodeficiencies that are most commonly linked to an increased risk of IALD are severe combined immunodeficiency (SCID), X-linked hyper-IgM syndrome due to CD40L deficiency, common variable immune deficiency, X-linked agammaglobulinemia due to BTK deficiency, X-linked lymphoproliferative syndrome (XLP) due to SAP deficiency, autoimmune lymphoproliferative syndrome (ALPS) due to Fas and FasL deficiency, ataxia telangiectasia and Wiskott-Aldrich Syndrome (17). In addition, the occurrence of Hodgkin lymphoma has recently been reported in 2 patients with Chronic Granulomatous Disease (CGD) (18). So far, no lymphoproliferative disorders have been described in patients with syndromes predominantly predisposing to mycobacterial infections. To our knowledge, this is the first report of disseminated non-Hodgkin B-cell lymphoma in a patient with complete IFN-γR1 deficiency.
Case description
The patient was a 20-year-old Pakistani man born in Norway to consanguineous parents. He had complete IFNγ-R1 deficiency (homozygous 22Cdel in IFNGR1), resulting in complete absence of IFN-γR1 surface expression and complete lack of responsiveness to IFN-γ in vitro (19). His brother had died of disseminated NTM infection at age 6 years. The patient was started on antimycobacterial therapy at the age of 18 months when he first presented with disseminated Mycobacterium avium complex (MAC), after which he did reasonably well for several years. At age thirteen new submandibular and axillary lymphadenopathy yielded M. abscessus from tissue and blood; linezolid was added to azithromycin, ethambutol, rifabutin, moxifloxacin and ertapenem. After two years he had anorexia, weight loss, growth retardation and lymphadenopathy. M. abscessus was recovered from blood, liver, lung, lymph nodes and skin. Tigecycline was added to azithromycin, ethambutol, rifabutin and moxifloxacin and IFN-α 3 million units subcutaneously three times weekly was added to try to bypass the defect in IFN-γ signaling. He remained culture positive for M. abscessus despite several courses of aggressive intravenous antimycobacterial therapy. At age 18 he presented with new hectic fever and weight loss. CT chest and abdomen showed new nodular masses with necrosis in the right lung and liver (Figure 1A, B), biopsies of which showed focal necrosis with atypical CD20+ and MUM-1+ lymphoid cells (Figure 1C, 2A). EBV was detected by hybridization in atypical lymphoid cells and clonal rearrangement of the IgH locus was present, all consistent with an aggressive B-cell lymphoma (Figure 2B). The EBV blood load was elevated at 4800 EBV genomes/106 mononuclear cell equivalents and CMV was elevated at 5200-7300 genomes/ml of whole blood. The patient declined chemotherapy in view of his persistent mycobacteremia, its likelihood of being exacerbated by chemotherapy, and his preference to return home. A short course of single agent rituximab was ineffective. He died at age 20 due to progressive disseminated non-Hodgkin lymphoma. An autopsy was not performed.
Figure 1.
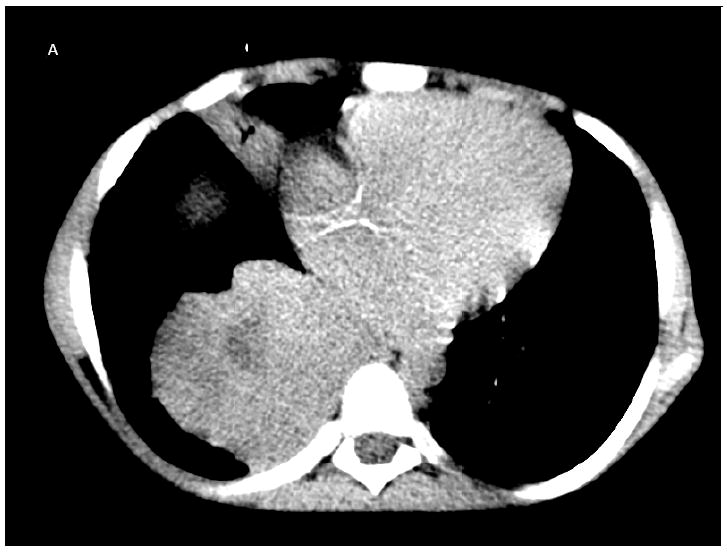
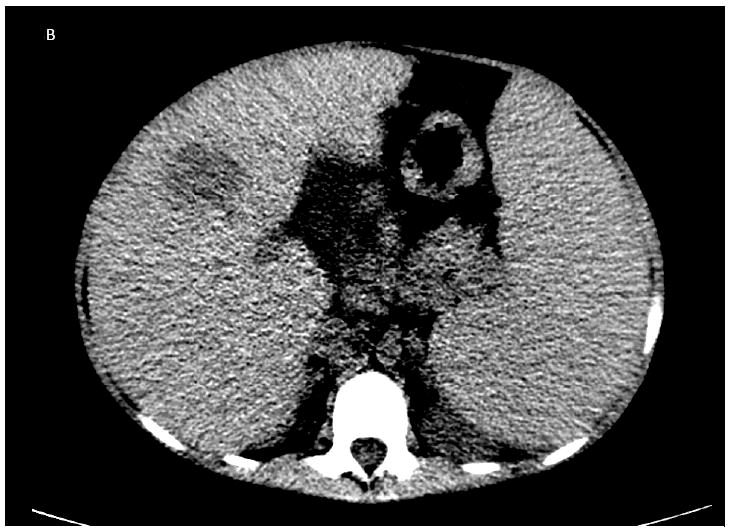
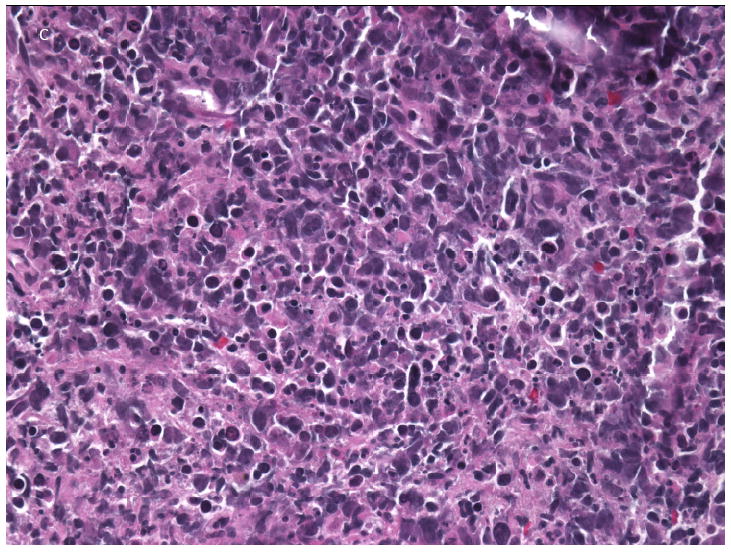
A: Non-contrast chest CT showing extensive consolidation in the right lower lobe with central hypodensity consistent with necrosis. B: Non-contrast abdominal CT showing a large necrotic lesion in the anterior right lobe. C: Liver core biopsy from the necrotic area with pleomorphic lymphoid infiltrate composed of large cells with necrotic debris and frequent apoptotic bodies.
Figure 2.
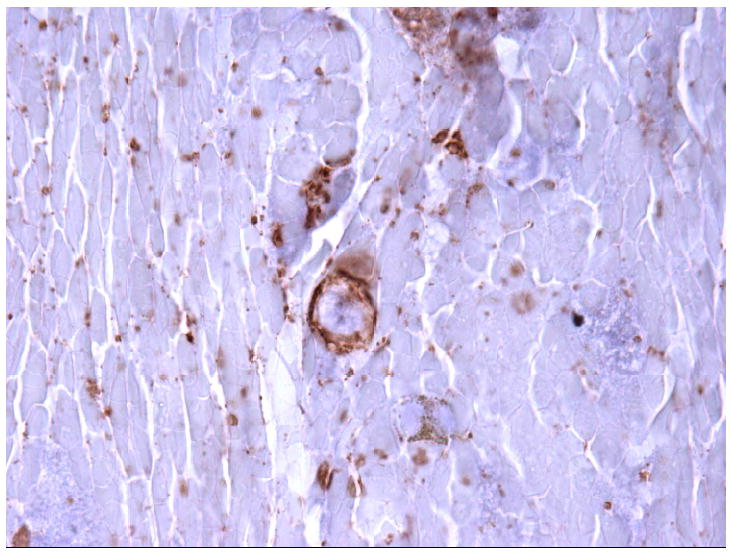
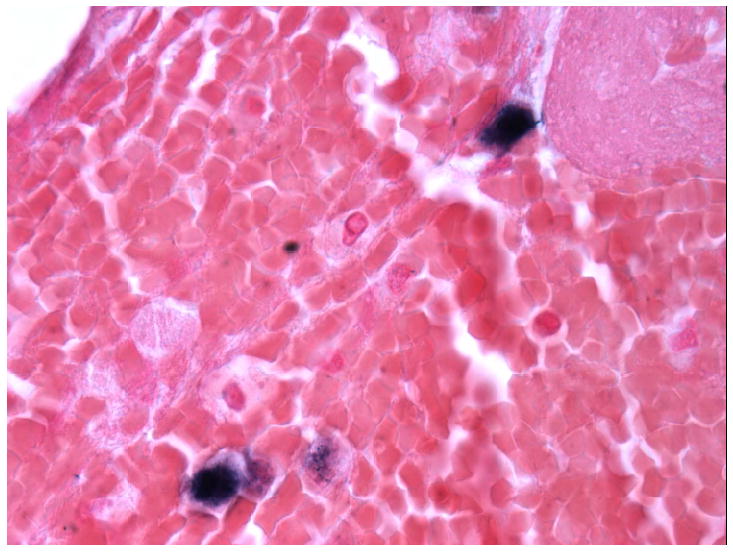
Stains of the fine needle aspirate biopsy of the necrotic liver lesion. A.
Immunohistochemistry for CD20+ cells, showing rare B cells. B. In situ hybridization for EBV, showing rare EBV+ cells consistent with EBV+ lymphoma. Images were taken using an Olympus Bx41 microscope, objective UPlanFI 40x/0.75 ∞/0.17, with an adaptor U-TV0.5xC using a digital camera Q-imaging Micropublisher 5.0 RTV. The images were captured using “Q-Capture Version 3.1” and imported into Adobe Photoshop 7.0.
Discussion
IFN-γ is an immunomodulatory cytokine that plays important roles in both innate and adaptive immune responses (20). It is also crucial for cancer immunoediting, a process by which the immune response modulates the immunogenicity of tumors (21). IFN-γ is known to accentuate the immunogenicity of tumors increasing their likelihood of immune recognition and rejection. Multiple in vitro and murine cancer models have shown that mice lacking IFN-γR1 and/or STAT1 have higher rates of methylcholanthrene (MCA)-induced tumors, which occur more rapidly and more frequently than in wild type mice (21). Injection of recombinant IL-12 was associated with reduced metastases and tumor growth and even tumor regression in mice with established tumors, but abolished by administration of neutralizing anti-IFN-γ antibodies, illustrating the crucial role for IL-12 induced IFN-γ in tumor protection (22)]. Further, mice lacking IFN-γ are at risk for developing spontaneous lymphomas (23).
In humans, the occurrence and outcome of different types of malignancies have been associated with acquired mutations affecting IFN-γ signaling or production (24-26). In some reports, up to 25% of certain human tumor cell lines have complete and irreversible unresponsiveness to IFN-γ, allowing them to escape immune activation, evade IFN-γ-induced MHC display, and elude rejection (27). Proposed mechanisms underlying the protective role of IFN-γ in tumor prevention and response include direct anti-proliferative and pro-apoptotic effects, up-regulation of MHC class I, inhibition of tumor angiogenesis, and activation of innate and adaptive immune responses against tumor invasion (28, 29). The antiproliferative and pro-apoptotic effects of IFN-γ are thought to be mediated through STAT1-dependent upregulation of genes regulating cell cycle inhibition and cellular apoptosis, respectively (28). In addition, natural killer (NK) cells have been associated with anti-tumor activities through IFN-γ production as well as through direct cytolytic activity via perforin (30)] and TRAIL (31).
Two malignancies have been reported in patients with complete IFN-γR deficiency: one child with complete IFN-γR1 deficiency developed human herpesvirus-8 associated Kaposi sarcoma (7); another child with complete IFN-γR2 deficiency developed fatal disseminated squamous cell carcinoma (SCC) of the skin (32). In a retrospective study of 141 patients with IL-12RB1 deficiency, one case of esophageal carcinoma was recently described (1). Interestingly, the occurrence of both SCC of the skin as well as esophageal carcinoma have been associated with human papilloma virus (HPV) infections (32). Mutations in GATA2, a critical regulator of stem cell integrity, have recently been found in patients with a novel inherited immunodeficiency, characterized by an increased susceptibility to mycobacterial, fungal and viral (especially HPV) infections as well as to myelodysplasia and malignancy (10). Besides a clear predisposition to myelodysplasia and leukemia, HPV related cancers such as vulvar and cervical carcinoma as well as Bowen’s disease of the vulva and EBV related leiomyosarcoma have been described in these patients.
Our patient’s lymphoma was associated with high blood loads of EBV and CMV, and EBV positivity of the tumor was confirmed by additional staining. It seems likely that the occurrence of malignancies in patients with IFN-γ receptor deficiencies and other hereditary immunodeficiencies is linked to specific viral infections, especially those of the herpes family. Patients with IFN-γ signaling disorders are at increased risk for developing severe viral infections, including herpes viruses (8).
This is the first report of non-Hodgkin lymphoma in a patient with IFN-γR1 deficiency. Since the first reports in 1996, almost 150 patients with either partial or complete IFN-γR1/2 deficiencies have been described (see (33) for an excellent review and (32, 34, 35) for a more recent update of total number of patients). In the US, the reported age-adjusted incidence rate of non-Hodgkin lymphoma is 1 per 5000 women and men per year (www.seer.cancer.gov). The fact that we have encountered one case of non-Hodgkin lymphoma out of a total of almost 150 patients with IFN-γR deficiencies (of which a substantial proportion is deceased), suggests that the incidence of non-Hodgkin lymphoma in patients with IFN-γR deficiencies may be higher than that of the general population, as would be predicted from the previous mouse and human studies. As more of these patients survive their early infections and enter the ages at which non-Hodgkin lymphoma is more common, tumor surveillance should become a more routine part of management. Because tumor prevention and rejection are so dependent on the integrity of immune cell IFN-γ signaling, bone marrow transplantation may correct both the infection predisposition and the propensity to lymphoid malignancies while leaving non-hematopoietic tissues still unable to respond to IFN-γ. Lymphoma is yet another risk for patients with predisposition to mycobacterial infections.
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest
References
- 1.de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J, et al. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 2010 Nov;89(6):381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorman SE, Picard C, Lammas D, Heyne K, van Dissel JT, Baretto R, et al. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 2004 Dec 11-17;364(9451):2113–21. doi: 10.1016/S0140-6736(04)17552-1. [DOI] [PubMed] [Google Scholar]
- 3.Roesler J, Kofink B, Wendisch J, Heyden S, Paul D, Friedrich W, et al. Listeria monocytogenes and recurrent mycobacterial infections in a child with complete interferon-gamma-receptor (IFNgammaR1) deficiency: mutational analysis and evaluation of therapeutic options. Exp Hematol. 1999 Sep;27(9):1368–74. doi: 10.1016/s0301-472x(99)00077-6. [DOI] [PubMed] [Google Scholar]
- 4.Zerbe CS, Holland SM. Disseminated histoplasmosis in persons with interferon-gamma receptor 1 deficiency. Clin Infect Dis. 2005 Aug 15;41(4):e38–41. doi: 10.1086/432120. [DOI] [PubMed] [Google Scholar]
- 5.Vinh DC, Masannat F, Dzioba RB, Galgiani JN, Holland SM. Refractory disseminated coccidioidomycosis and mycobacteriosis in interferon-gamma receptor 1 deficiency. Clin Infect Dis. 2009 Sep 15;49(6):e62–5. doi: 10.1086/605532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roesler J, Hedrich C, Laass MW, Heyne K, Rosen-Wolff A. Meningoencephalitis caused by varicella-zoster virus reactivation in a child with dominant partial interferon-gamma receptor-1 deficiency. Pediatr Infect Dis J. 2011 Mar;30(3):265–6. doi: 10.1097/INF.0b013e3181f6f78a. [DOI] [PubMed] [Google Scholar]
- 7.Camcioglu Y, Picard C, Lacoste V, Dupuis S, Akcakaya N, Cokura H, et al. HHV-8-associated Kaposi sarcoma in a child with IFNgammaR1 deficiency. J Pediatr. 2004 Apr;144(4):519–23. doi: 10.1016/j.jpeds.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Dorman SE, Uzel G, Roesler J, Bradley JS, Bastian J, Billman G, et al. Viral infections in interferon-gamma receptor deficiency. J Pediatr. 1999 Nov;135(5):640–3. doi: 10.1016/S0022-3476(99)70064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011 Jul 14;365(2):127–38. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011 Jun 13; doi: 10.1182/blood-2011-05-356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bustamante J, Arias AA, Vogt G, Picard C, Galicia LB, Prando C, et al. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol. 2011 Mar;12(3):213–21. doi: 10.1038/ni.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogunovic D, Byun M, Durfee LA, Abhyankar A, Sanal O, Mansouri D, et al. Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science. 2012 Sep 28;337(6102):1684–8. doi: 10.1126/science.1224026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oertel SH, Riess H. Immunosurveillance, immunodeficiency and lymphoproliferations. Recent Results Cancer Res. 2002;159:1–8. doi: 10.1007/978-3-642-56352-2_1. [DOI] [PubMed] [Google Scholar]
- 14.Bonnet F, Chene G. Evolving epidemiology of malignancies in HIV. Curr Opin Oncol. 2008 Sep;20(5):534–40. doi: 10.1097/CCO.0b013e32830a5080. [DOI] [PubMed] [Google Scholar]
- 15.Carbone A, Cesarman E, Spina M, Gloghini A, Schulz TF. HIV-associated lymphomas and gamma-herpesviruses. Blood. 2009 Feb 5;113(6):1213–24. doi: 10.1182/blood-2008-09-180315. [DOI] [PubMed] [Google Scholar]
- 16.Parker A, Bowles K, Bradley JA, Emery V, Featherstone C, Gupte G, et al. Diagnosis of post-transplant lymphoproliferative disorder in solid organ transplant recipients - BCSH and BTS Guidelines. Br J Haematol. 2010 Jun;149(5):675–92. doi: 10.1111/j.1365-2141.2010.08161.x. [DOI] [PubMed] [Google Scholar]
- 17.Tran H, Nourse J, Hall S, Green M, Griffiths L, Gandhi MK. Immunodeficiency-associated lymphomas. Blood Rev. 2008 Sep;22(5):261–81. doi: 10.1016/j.blre.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Lugo Reyes SO, Suarez F, Herbigneaux RM, Pacquement H, Reguerre Y, Riviere JP, et al. Hodgkin lymphoma in 2 children with chronic granulomatous disease. J Allergy Clin Immunol. 2011 Feb;127(2):543–4. e1–3. doi: 10.1016/j.jaci.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland SM, Dorman SE, Kwon A, Pitha-Rowe IF, Frucht DM, Gerstberger SM, et al. Abnormal regulation of interferon-gamma, interleukin-12, and tumor necrosis factor-alpha in human interferon-gamma receptor 1 deficiency. J Infect Dis. 1998 Oct;178(4):1095–104. doi: 10.1086/515670. [DOI] [PubMed] [Google Scholar]
- 20.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004 Feb;75(2):163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 21.Dunn GP, Ikeda H, Bruce AT, Koebel C, Uppaluri R, Bui J, et al. Interferon-gamma and cancer immunoediting. Immunol Res. 2005;32(1-3):231–45. doi: 10.1385/ir:32:1-3:231. [DOI] [PubMed] [Google Scholar]
- 22.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994 Aug 15;153(4):1697–706. [PubMed] [Google Scholar]
- 23.Street SE, Trapani JA, MacGregor D, Smyth MJ. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J Exp Med. 2002 Jul 1;196(1):129–34. doi: 10.1084/jem.20020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Critchley-Thorne RJ, Simons DL, Yan N, Miyahira AK, Dirbas FM, Johnson DL, et al. Impaired interferon signaling is a common immune defect in human cancer. Proc Natl Acad Sci U S A. 2009 Jun 2;106(22):9010–5. doi: 10.1073/pnas.0901329106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duncan TJ, Rolland P, Deen S, Scott IV, Liu DT, Spendlove I, et al. Loss of IFN gamma receptor is an independent prognostic factor in ovarian cancer. Clin Cancer Res. 2007 Jul 15;13(14):4139–45. doi: 10.1158/1078-0432.CCR-06-2833. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Tunon I, Ricote M, Ruiz AA, Fraile B, Paniagua R, Royuela M. Influence of IFN-gamma and its receptors in human breast cancer. BMC Cancer. 2007;7:158. doi: 10.1186/1471-2407-7-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998 Jun 23;95(13):7556–61. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002 Apr;13(2):95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y, Yang W, Qin C, Zhang L, Deng J, Liu S, et al. Responsiveness of stromal fibroblasts to IFN-gamma blocks tumor growth via angiostasis. J Immunol. 2009 Nov 15;183(10):6413–21. doi: 10.4049/jimmunol.0901073. [DOI] [PubMed] [Google Scholar]
- 30.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000 Sep 4;192(5):755–60. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001 Jan;7(1):94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 32.Toyoda H, Ido M, Nakanishi K, Nakano T, Kamiya H, Matsumine A, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon gamma receptor 2 (IFN gamma R2) deficiency. J Med Genet. 2010 Sep;47(9):631–4. doi: 10.1136/jmg.2009.072108. [DOI] [PubMed] [Google Scholar]
- 33.van de Vosse E, Hoeve MA, Ottenhoff TH. Human genetics of intracellular infectious diseases: molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect Dis. 2004 Dec;4(12):739–49. doi: 10.1016/S1473-3099(04)01203-4. [DOI] [PubMed] [Google Scholar]
- 34.Kong XF, Vogt G, Chapgier A, Lamaze C, Bustamante J, Prando C, et al. A novel form of cell type-specific partial IFN-gammaR1 deficiency caused by a germ line mutation of the IFNGR1 initiation codon. Hum Mol Genet. 2010 Feb 1;19(3):434–44. doi: 10.1093/hmg/ddp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sologuren I, Boisson-Dupuis S, Pestano J, Vincent QB, Fernandez-Perez L, Chapgier A, et al. Partial recessive IFN-gammaR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds. Hum Mol Genet. 2011 Apr 15;20(8):1509–23. doi: 10.1093/hmg/ddr029. [DOI] [PMC free article] [PubMed] [Google Scholar]


