Abstract
Background
A maximal exercise respiratory exchange ratio (RERmax) ≥1.10 is commonly used as a criterion to determine if a “true” maximal oxygen uptake (VO2max) has been attained during maximal-effort exercise testing. Because RERmax is heavily influenced by CO2 production from acid buffering during maximal exercise, we postulated that dietary acid load, which affects acid-base regulation, might contribute to variability in RERmax.
Purpose
To determine if a habitual dietary intake that promotes systemic alkalinity results in higher RERmax during VO2max testing.
Methods
Sedentary men and women (47-63y, n=57) with no evidence of cardiovascular disease underwent maximal graded treadmill exercise tests. VO2max and RERmax were measured with indirect calorimetry. Habitual diet was assessed for its long-term effect on systemic acid-base status by performing nutrient analysis of food diaries and using this information to calculate the potential renal acid load (PRAL). Participants were grouped into tertiles based on PRAL.
Results
The lowest PRAL tertile (alkaline PRAL) had higher RERmax values (1.21±0.01, p≤0.05) than the middle tertile (1.17±0.01) and highest PRAL tertile (1.15±0.01). There were no significant differences (all p≥0.30) among PRAL tertiles for RER at submaximal exercise intensities of 70%, 80%, or 90% VO2max. After controlling for age, sex, VO2max, and maximal heart rate (HRmax), regression analysis demonstrated that 19% of the variability in RERmax was attributed to PRAL (r=−0.43, p=0.001). Unexpectedly, HRmax was lower (p≤0.05) in the low PRAL tertile (164±3 beats/min) versus the highest PRAL tertile (173±3 beats/min).
Conclusion
These results suggest that individuals on a diet that promotes systemic alkalinity may more easily achieve the RERmax criterion of ≥1.10 which might lead to false-positive conclusions about achieving maximal effort and VO2max during graded exercise testing.
Keywords: maximal oxygen uptake, stress test, renal acid load, alkaline diet, ash diet
Introduction
Maximal graded exercise tests with indirect calorimetry (GXTs) are commonly used to determine maximal oxygen uptake (VO2max) for purposes including general fitness assessment, performance testing of athletes, or in clinical settings, as a means for determining the severity of advanced heart failure. During these tests, respiratory exchange ratio during maximal effort exercise (RERmax) is typically measured to gain insights into the validity of the measured VO2max value. RERmax values of ≥1.10, along with other criteria, suggest that a “true” VO2max has been attained (23), although specific cut-points vary considerably among studies (12).
Unlike respiratory exchange ratios during rest or steady state exercise, RERmax is largely a function of non-metabolic carbon dioxide (CO2) production from bicarbonate buffering of hydrogen ions produced during maximal-intensity exercise (23). Numerous factors can affect the production of non-metabolic CO2 during progressive incremental exercise to exhaustion. These factors, which may also contribute to variability in RERmax, include the rate at which hydrogen ions are buffered by bicarbonate, the sensitivity of central and peripheral chemoreceptors that stimulate ventilation in response to pH perturbations, and the size of whole-body carbon dioxide stores (which affects the rate at which CO2 accumulates in blood). In light of these potential sources of variation in RERmax, it is conceivable that environmental and/or genetic factors can affect RERmax. However, to our knowledge, none has been identified.
Variation in the composition of common western diets can affect the pH of blood [by ∼0.03 pH units (8; 32)] and urine[(∼1.0 pH units (5)]. The physiology of these effects is complex and involves the mineral and protein content of the diet, intestinal absorption rates of specific nutrients, sulfur metabolism, and urinary acid excretion (21). However, in general, fruits and vegetables promote systemic alkalinity while grains, meats, and cheeses promote acidity (21). Not surprisingly, the pH altering qualities of various diets impact physiology and health. For example, alkaline-promoting diets appear to protect against osteoporosis (5; 24; 30) and decrease uric acid kidney stones formation (3). If diet composition can affect systemic pH, it seems plausible that it could also affect RERmax, which is largely determined by exercise-induced acidosis.
Although RERmax is a useful and objective measure for determining if a “true” VO2max was obtained during a GXT, it is not clear why some individuals are able to attain RERmax values of ≥1.10 long before reaching exhaustion while others may never reach values of ≥1.10 despite their greatest effort during a GXT (17). In light of the potential for habitual diet to affect systemic pH, and because it seems plausible that systemic pH could affect RERmax, it seems reasonable to propose that alkaline/acid promoting dietary qualities could contribute to this variability. Therefore the purpose of this study was to determine if the alkaline/acid promoting qualities of habitual dietary intake affect RERmax. More specifically, we hypothesized that alkaline-promoting diets are associated with greater RER values during maximal exercise.
Methods
Data were collected and analyzed from two intervention trials: One that was performed at Washington University School of Medicine (Phase 1 CALERIE study -Comprehensive Assessment of the Long-term Effects of Restricted Intake of Energy) and another that was started in August 2008 and is ongoing at both Saint Louis University and at Washington University School of Medicine (CREG Study - Calorie Restriction, Exercise, and Glucoregulation Study). Data for the present analysis included baseline data from both trials.
Subjects
All subjects were recruited from the St. Louis, Missouri metropolitan area. For both studies, participants underwent a medical evaluation which included a medical history, physical examination, fasting hematological assessment. Volunteers were excluded if they had a history or clinical evidence of diabetes, heart disease, stroke, recent malignancy, or other major diseases. Recent or current smokers, and physically active volunteers (i.e. performing vigorous exercise >2 d/wk and ≥20 min/d) were excluded. Women were required to be postmenopausal. For the CALERIE study, participants had to have a BMI in the 23.5 - 29.9 kg/m2 range and had to be 50 to 60 years of age. For the CREG study, BMI had to be 25.0 - 29.9 kg/m2 and age had to be 45 - 65 years. Written informed consent was obtained from all participants. The studies were reviewed and approved by the Washington University School of Medicine Human Research Protection Office and the Saint Louis University Institutional Review Board.
Graded Exercise Testing
Maximal effort incremental treadmill tests were performed with ECG monitoring (CALERIE study: Marquette Max 1, Marquette Electronics, Inc., Milwaukee, WI; CREG study: MedGraphics CardiO2, Medical Graphics Corportation, St. Paul, MN), blood pressure assessments, and indirect calorimetry (CALERIE study: True Max 2400, ParvoMedics, Salt Lake city, UT; CREG study: MedGraphics CardiO2, Medical Graphics Corportation, St. Paul, MN). Prior to each test, a 3-liter air syringe (Hans Rudolph, Inc., Shawnee, KS) was used to generate a series of flow profiles for calibrating the pneumotach flow meter on the indirect calorimeters. The carbon dioxide and oxygen analyzers were calibrated prior to each test with medical grade gases of known carbon dioxide and oxygen concentrations. The GXT was initiated at a speed determined during warm-up to increase HR to ∼70% of age-predicted maximal heart rate (HRmax) and a grade of 0%. Thereafter, the grade was increased by 2 percentage points every 2 minutes until the subject could no longer continue due to fatigue or medical complications. Tests that were terminated due to medical complications were excluded from the analysis for the current report. Peak VO2 was considered “true VO2 max” if two of the following criteria were met: 1) measured HRmax ≥ age-predicted HRmax minus 10 beats/min, 2) VO2 increased <150 ml/min during the last two stages of the test, and 3) RERmax was ≥ 1.10. HRmax was determined from at least five R-R intervals, as measured on an ECG that was printed during maximal exercise. Age predicted HRmax was calculated as 208 - 0.7 × age (yr) (28) and was used to calculate HRmax as a percentage of age-predicted HRmax. Ventilatory equivalent for CO2 production during maximal exercise (VE/VCO2max) was calculated as an index of ventilatory efficiency.
For tests performed as part of the CALERIE study, the coefficient of variation for outcomes from duplicate exercise test results performed 1-3 weeks apart were 3% for VO2max, 2% for RERmax, and 2% for HRmax. Reproducibility data were not available for repeat exercise tests performed as part of the CREG study; however, the same investigator (EPW) provided direct oversight for testing procedures and technician training for both studies and performed all routine maintenance on the indirect calorimeter equipment.
Submaximal RER values were determined by establishing a linear regression equation for each test/subject that described the relationship between oxygen uptake and RER. Metabolic data from early in the test, when RER values temporarily decline, and during a VO2 plateau, if present, were not used in the development of the regression equations. The subject-specific equations were then used to determine RER values at oxygen uptakes equivalent to 70%, 80%, and 90% of VO2max.
Dietary Assessment
Participants in the CALERIE study completed 7-day food diaries and those in the CREG study completed 3-day food diaries. The study dietitians met with participants prior to the diary recording period to give specific instructions on how to measure and record all food and liquids consumed. After the diary period, the study dietitians again met with the participant so that any ambiguities in the diary could be clarified. Computerized nutrient analysis was performed on the diaries by the study dietitians (CALERIE study: Nutrition Data System for Research, versions 4.05, 4.06, and 5.0, Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN; CREG study: Food Processor SQL, version 10, ESHA Research, Salem, OR).
The effect of habitual diet on systemic acid load was estimated by calculating potential renal acid load (PRAL) from the analyzed food diaries by using validated methods (2; 19; 20; 31). PRAL is highly correlated with net acid excretion (NAE) in urine (19; 20). Low (more negative) PR..AL corresponds with a low intake of acid equivalents (i.e. an alkaline diet) while high PRAL corresponds with a large acid-load. The equation used to calculate PRAL is as follows (21; 31):
Where P, K, Ca, and Mg are daily dietary intakes of phosphorus, potassium, calcium, and magnesium, respectively.
Plasma Electrolytes and Carbon Dioxide
After the subjects fasted overnight, venous blood was collected from a superficial arm vein into lithium heparin-containing tubes. Plasma was isolated by using centrifugation and analyzed for concentrations of sodium, potassium, chloride, and carbon dioxide (CO2) by the medical center's CLIA certified clinical laboratory.
Statistical Analysis
Comparisons of subject characteristics from the two studies (CALERIE and CREG studies) were performed by using independent t-tests. Subjects were grouped into tertiles according to PRAL and ANOVAs with protected F-tests (LSD) were used to compare means among PRAL tertile groups. Analysis of covariance was used for follow-up analysis, which included potential confounding factors as covariates. Stepwise multiple linear regression analysis and Pearson correlations were used to identify relationships between variables. Data are presented as means ± standard errors unless otherwise noted. Analyses were performed with PASW Statistics software (version 18.0.0). P-values of ≤0.05 were considered significant.
Results
Subjects
On average, the participants were in the middle of the targeted age ranges and were moderately overweight, according to BMI (Table 1). Approximately 2/3rds of the participants were women.
Table 1.
Subject characteristics.
| All Subjects N=57 | CALERIE Study N=43 | CREG Study N=14 | P-Value | |
|---|---|---|---|---|
|
|
||||
| Age, y | 55.8 ± 3.5 | 55.9 ± 3.3 | 55.5 ± 4.3 | 0.73 |
| Sex, % female | 67 | 63 | 79 | 0.28 |
| BMI, kg/m2 | 27.6 ± 2.0 | 27.4 ± 2.1 | 28.2 ± 1.8 | 0.22 |
| PRAL, mEq/day | 8.2 ± 17.3 | 6.6 ± 16.0 | 13.2 ± 20.6 | 0.21 |
| VO2max, L/min | 2.05 ± 0.54 | 2.07 ± 0.55 | 1.98 ± 0.53 | 0.59 |
| VO2max, ml/kg/min | 25.5 ± 4.7 | 26.0 ± 4.6 | 24.1 ± 5.0 | 0.19 |
| HRmax, beats/min | 169 ± 7 | 169 ± 12 | 171 ± 13 | 0.56 |
| HRmax, % of predicted HRmax | 100 ± 7 | 100 ± 7 | 101 ± 7 | 0.59 |
| Exercise test duration, min | 9.2 ± 1.5 | 9.1 ± 1.5 | 9.3 ± 1.7 | 0.72 |
Values are means ± SD except for sex data, which are presented as percentages. P-values are from independent t-tests comparing data from the CALERIE and CREG studies except for P-values for sex, which is from a Chi-square analysis. BMI, body mass index; PRAL, potential renal acid load of the diet (mEq/day) calculated based on nutrient analysis of 3- or 7-day food diaries; VO2max, maximal oxygen uptake; HRmax, maximal heart rate.
Metabolic Responses to Maximal Exercise
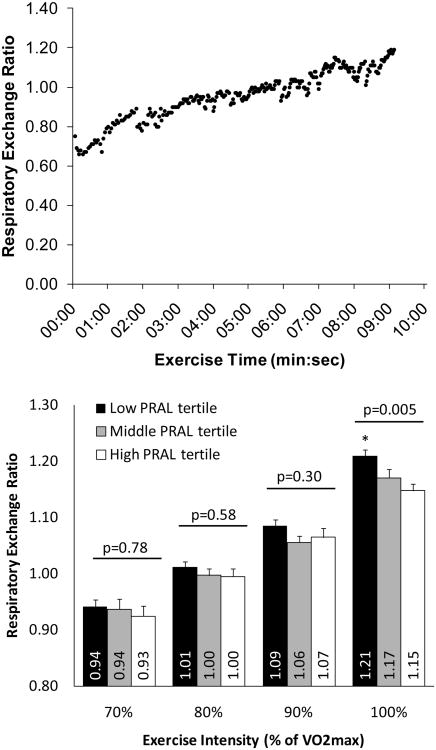
Based on normative data (26), VO2max values indicate that these subjects had below average cardiovascular fitness according to age and sex, as would be expected for individuals who are sedentary or perform low levels of physical activity (Table 1). Although the present study only included data that from tests that continued until volitional exhaustion/fatigue, some tests did not meet the criteria for “true” VO2max, as commonly occurs (10; 27). The percentage of GXTs that did not meet the criteria for “true” VO2max were 19% for the CALERIE study and 7% for the CREG study, which was not significantly different between groups (χ2 = 0.24, p = 0.63). A representative plot of the breath-by-breath increases in RER that occur with progressive intensity exercise is depicted in Figure 1.
Figure 1.
Respiratory exchange ratios during submaximal- and maximal-intensity exercise. The top panel depicts the breath-by-breath changes in respiratory exchange ratio during progressive-intensity exercise to exhaustion from a representative exercise test. The bottom panel depicts the mean (± SEM) respiratory exchange ratio values for subjects in each PRAL tertile during submaximal- and maximal-intensity exercise. P-values are from ANOVA tests comparing means among potential renal acid load (PRAL, mEq/day) tertiles. * p ≤ 0.05 versus the middle and high PRAL tertiles.
Dietary Potential Renal Acid Load
Average PRAL (Table 1) was greater than those reported for European adults (−4 to −7 mEq/d) (31) and slightly greater than those reported for postmenopausal women in the United States (2 mEq/d) (29), but similar to those for children in the United States (6-8 mEq/d)(19) and considerably lower than those for adolescent males (19 mEq/d) (19). Therefore, these data indicate that on average, our study participants consumed a diet resulting in a net systemic acid load as is common in the United States and is characteristic of diets containing large amounts of meat and grain (acid promoting foods) and low intakes of vegetables and fruits (alkaline promoting foods).
Relationships between potential renal acid load and metabolic outcomes
RERmax was significantly greater in the lower PRAL (alkaline) tertile than in the upper PRAL (acid) tertile and middle tertile (Figure 1). There were no differences among PRAL tertiles at any of the submaximal exercise intensities (i.e. 70%, 80%, or 90% of VO2max; Figure 1). HRmax was lower in the lowest PRAL tertile as compared to the highest PRAL tertile (Table 2). There were no differences among PRAL tertiles for VO2max or VE/VCO2 (Table 2). Plasma electrolytes and CO2 levels did not differ among PRAL tertiles (Table 3). The effect of PRAL on RERmax and HRmax remained significant after exclusion of data from the 9 subjects who did not meet the criteria for “true” VO2max (data not shown).
Table 2.
Maximal exercise metabolic data according to PRAL (mEq/day) tertiles.
| All Subjects (n=57) | Lower PRAL Tertile (n=19) | Middle PRAL Tertile (n=19) | Upper PRAL Tertile (n=19) | P-value | |
|---|---|---|---|---|---|
|
|
|||||
| PRAL, mEq/day | −8.2 (−29.8, 51.9) | −10.8 (−29.8, −1.1) | 8.2 (1.0, 15.1) | 27.1 (15.4, 51.9) | ------ |
| VO2max, L/min | 2.05 ± 0.07 | 1.95 ± 0.11 | 2.07 ± 0.13 | 2.13 ± 0.14 | 0.60 |
| VO2max, ml/kg/min | 25.5 ± 0.6 | 23.4 ± 0.9 | 26.1 ± 1.1 | 25.1 ± 1.3 | 0.82 |
| VE/VCO2max ratio | 26.1 ± 0.4 | 25.4 ± 0.8 | 26.3 ± 0.6 | 26.5 ± 0.7 | 0.52 |
| HRmax, beats/min | 169 ± 2 | 164 ± 3* | 171 ± 2 | 173 ± 3 | 0.04 |
| HRmax, % of predicted HRmax | 100 ± 1 | 97 ± 2* | 102 ± 1 | 102 ± 1 | 0.03 |
PRAL scores are given as means with ranges (min, max). Metabolic outcomes are means ± SE. P-value is from the ANOVA comparing outcomes across PRAL tertiles.
P ≤ 0.05 vs. the highest PRAL tertile. VE/VCO2max ratio, ventilatory equivalent for carbon dioxide production during maximal exercise. Heart rate maximum is presented as a percentage of age predicted HRmax (208-(0.7× age in yr).
Table 3.
Plasma electrolyte concentrations according to PRAL (mEq/day) tertiles.
| All Subjects (n=57) | Lower PRAL Tertile (n=19) | Middle PRAL Tertile (n=19) | Upper PRAL Tertile (n=19) | P-value | |
|---|---|---|---|---|---|
|
|
|||||
| Sodium, mmol/L | 140.5 ± 0.2 | 140.3 ± 0.3 | 140.7 ± 0.3 | 140.6 ± 0.4 | 0.73 |
| Potassium, mmol/L | 4.03 ± 0.04 | 4.07 ± 0.08 | 4.07 ± 0.08 | 3.95 ± 0.07 | 0.44 |
| Chloride, mmol/L | 104.4 ± 0.2 | 104.4 ± 0.4 | 104.6 ± 0.4 | 104.2 ± 0.4 | 0.77 |
| CO2, mmol/L | 26.4 ± 0.3 | 27.0 ± 0.4 | 26.3 ± 0.5 | 26.0 ± 0.5 | 0.37 |
Values are means ± SE. P-value is from the ANOVA comparing outcomes across PRAL tertiles.
In light of the possibility that the differences in HRmax among PRAL tertiles could have contributed to the differences in RERmax among tertiles, a covariate analysis was performed. RERmax remained significantly different among PRAL tertiles (p=0.001) after accounting for variation in HRmax among tertiles, although the differences in RERmax among groups became slightly larger (RERmax values: lowest tertile, 1.22±0.01; middle tertile, 1.17±0.01; highest tertile, 1.14±0.01). Furthermore, inclusion of VO2max, age, and height as possible confounders did not alter the findings.
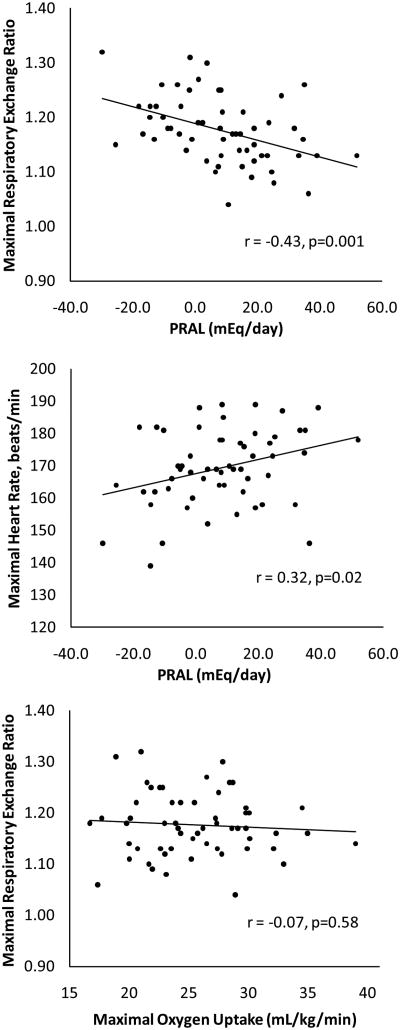
Correlation analysis indicated that lower (more alkaline) PRAL was associated with higher RERmax values (r = −0.43, p = 0.001; Figure 2). There were tendencies for higher RERmax values to correlate with lower intakes of protein (r = −0.25, p = 0.06) and phosphorous (r = −0.23, p = 0.08); none of the other nutrients used in the calculation of PRAL (i.e. dietary intakes of potassium, calcium, and magnesium) correlated with RERmax (all p ≥ 0.34). PRAL was not correlated with VO2max regardless of whether expressed in absolute terms (r = 0.20, p = 0.14) or relative to body weight (r = 0.04, p = 0.74). Furthermore, VE/VCO2max, as a marker of ventilatory efficiency, was not correlated with PRAL (r = 0.10, p = 0.48). However, a weak to moderate correlation was evident between lower PRAL and lower HRmax (Figure 2); this association remained significant (r = 0.33, p = 0.01) after accounting for age by expressing HRmax as a percentage of age-predicted HRmax. PRAL was not associated with plasma concentrations of sodium (r = 0.05, p = 0.73), potassium (r = −0.15, p = 0.27), chloride (r = −0.003, p = 0.98), or CO2 (r = −0.16, p = 0.23). There was no correlation between RERmax and VO2max (Figure 2), indicating that variation in RERmax could not be attributed to variation in cardiorespiratory fitness. After exclusion of data from the 9 subjects who did not meet the criteria for “true” VO2max, the correlations of PRAL with RERmax (r = −0.40, p = 0.004) and HRmax (r = 0.42, p = 0.003) remained significant.
Figure 2.
Association between potential renal acid load (PRAL, mEq/day) and maximal respiratory exchange ratio (top panel), PRAL and maximal heart rate (middle panel) and maximal respiratory exchange ratio and maximal oxygen uptake (mL/kg/min) (bottom panel). Correlation coefficient is from Pearson correlation analysis.
Using multiple linear regression analysis with RERmax as the dependent variable and PRAL, HRmax, sex, age, and VO2max (mL/kg/min) as independent variables, only PRAL (19% or the variance explained, p=0.001) and HRmax (7% of the variance explained, p=0.03) were related to RERmax:
No autocorrelations was present in the model (Durbin Watson Statistic = 1.66).
Discussion
The main finding of the present study is that diets resulting in a low systemic acid load (low PRAL) are associated with the attainment of a higher respiratory exchange ratio at the end of maximal-intensity treadmill exercise tests (∼1.20 vs. 1.14). As a result, individuals who habitually consume low PRAL diets might achieve the RER ≥ 1.10 criterion for a “true” VO2max at submaximal exercise intensities and VO2max would be underestimated if the test stopped when RER reached 1.10. Alternatively, individuals consuming an acid-promoting diet, which is common in the United States (19), would be less likely to achieve the true VO2max criterion of RER ≥ 1.10. In our study, all of the 19 participants with negative (alkaline) PRAL values reached an RER ≥ 1.10, while 34 of 38 participants (89%) with positive (acid) PRAL values achieved an RER ≥ 1.10, although these frequencies were not statistically different (p=0.34). It is conceivable diets that result in a greater acid load than those observed in the present study, or other conditions that increase systemic acid load (e.g. medications) might have more extreme effects on RERmax. Others have reported on the substantial heterogeneity of RERmax values from graded exercise tests (17), thereby bringing into question the use of RER ≥ 1.10 as a criterion for a valid or “true” VO2max. However, the factors that contribute to this variability in RERmax have been unknown. Our study demonstrates that habitual dietary patterns that influence systemic acid load account for 19% of the variability in RERmax. Further studies are needed to determine if the PRAL-related effects on RERmax are associated with alterations in pH, CO2 pressure, and bicarbonate levels in arterial blood.
PRAL, as a measure of dietary acid load, reflects the tendency for food to alter systemic pH, or the amount of acid that must be cleared or buffered in order to prevent pH changes. It is based on the absorption of specific nutrients and the capacity of these nutrients to produce anions and cations in circulation (18), but is not necessarily related to the acidity of the food ingested. For example, lemon juice is acidic (pH ∼ 2) outside of the body but it has a modestly low (alkaline) PRAL (-2.5 mEq/100 g) and therefore has an acid-load lowering effect on systemic pH (21). Furthermore, PRAL does not necessarily reflect systemic pH; rather, it reflects the physiologic burden to maintain the optimal systemic pH, with the kidney being the main organ responsible for long-term pH homeostasis (13). If the kidneys (and other pH control systems such as the bicarbonate buffering system) are able to maintain systemic pH in the face of a high systemic acid load, the urine will become acidic and optimal blood pH will be maintained. However, if the acid load exceeds the systemic capacity to excrete acid (for example, with compromised kidney function), systemic pH will decrease with an acid-promoting diet (11).
It is biologically plausible that a low PRAL diet would permit greater non-metabolic CO2 production during maximal exercise, resulting in a greater RERmax. In presence of a low systemic acid load (i.e. alkaline-promoting diet), circulating bicarbonate levels are elevated (7), thereby increasing bicarbonate availability for acid buffering during high-intensity, acid-producing exercise (13). This would allow for more H+ buffering and greater CO2 production during high-intensity exercise, thereby increasing maximal exercise VCO2 and RER (15). In support of this proposition, Peronnet et al. (16) demonstrated that during a ramp exercise test to exhaustion, bicarbonate infusion prevented the exercise-induced reductions in bicarbonate and pH and resulted in significantly higher RERmax values compared to a control condition (RERmax: 1.21 vs. 1.13). We did not measure circulating bicarbonate or blood pH in our preliminary study; although plasma CO2 content is partly reflective of plasma bicarbonate levels (9), we did not observe an association between PRAL and plasma CO2.
In contrast to maximal exercise RER values, RER values during submaximal exercise were not associated with PRAL. Bicarbonate buffering system activity is a major determinant of maximal exercise RER values; however, it has little or no influence on RER during submaximal exercise. Therefore, the finding that PRAL is associated with maximal but not submaximal exercise RER suggests dietary PRAL influences RERmax through effects on bicarbonate buffering.
An unexpected finding was that low-acid diets were associated with lower maximal heart rates. One possible explanation for this would be that, by chance, the subjects consuming a low PRAL diet did not give as much physical effort during maximal exercise. However, if this were the case, the RERmax differences among PRAL tertiles would be underestimated. Indeed, after accounting for differences in HRmax among PRAL tertiles, the differences in RERmax became slightly larger. Another explanation is that variations in PRAL might have altered plasma electrolyte concentrations, which could have cardiac effects; however, we saw no evidence of associations between PRAL and plasma electrolytes. Lastly, it is possible that diet-related alterations in blood pH had direct or indirect (for example, involving sympathetic nervous system activity) cardiac effects. At rest, acidosis has been shown to decrease contractility (14) and to either increase (6; 25) or decrease (1) heart rate. However, to the best of our knowledge, the effects of acid-base alterations on maximal exercise cardiac function have not been studied.
It cannot be determined, based on our study, whether the effects of PRAL on RERmax and HRmax are attributable to acute or chronic effects because we assessed habitual diet and these dietary patterns were presumably practiced by the participants for many years. In one respect, it seems likely that the effect of PRAL on RERmax would occur rapidly (i.e. in hours or days), as acute changes in diet have been shown to alter blood and urinary pH (5; 8). However, it is also possible that chronic exposure to mild acidosis/alkalosis has effects that develop over months, years, or decades. For example, acidosis in humans has clear effects on the growth hormone (GH)/insulin-like growth factor (IGF)-1 axis in humans (4). Because the GH/IGF-1 system has major effects on the heart (e.g. effects on cardiac growth and development and myocardial substrate metabolism and contractility) and has been implicated in clinically relevant cardiac dysfunction (reviewed in (22)), it is possible that chronic sub-clinical acidosis could affect cardiovascular function by altering long-term GH/IGF-1 function.
Because of the preliminary nature of this study, there are limitations. First, this was a cross-sectional study involving observation of habitual dietary intakes. Therefore, unidentified confounding factors might be responsible for some or all of the reported effects. To advance these preliminary cross-sectional findings, we are initiating an intervention study in which we are increasing PRAL (by using an isoenergetic diet rich in meats, cheeses and grains and low in fruits and vegetables) or decreasing PRAL (by using an isoenergetic diet rich in fruits and vegetable and low in meats, cheeses, and grains) to determine if 7 days of a low or high PRAL diet also alters RERmax. Another limitation is that we depended on estimates of dietary acid load from food diaries and nutrient analysis rather than direct measures of systemic pH or urinary measures of anion/cation content or pH. However, information from this study can be used to justify more advanced studies involving better measures of acid load and physiologic responses to exercise and randomization to controlled feeding interventions.
In conclusion, dietary qualities that result in a low systemic acid load (i.e. alkaline diets) are associated with the attainment of higher peak values for respiratory exchange ratio during maximal-intensity exercise testing. Such diets would typically be very rich in vegetables and fruits and low in meats, grains, and dairy. The implications of this finding are twofold. First, because maximal exercise RER ≥ 1.10 is commonly used as a criterion for determining whether a “true” VO2max has been attained during an exercise test, this finding brings into question the use of maximal RER as a true VO2max criterion. Secondly, although more preliminary, this finding also suggests that dietary acid load affects acid-base regulation during high-intensity exercise. In this context, future studies to investigate the possibility that dietary acid load affects physical performance during acidosis-inducing exercise are perhaps warranted.
Acknowledgments
Funding support was provided by NIH grants: U01 AG20487, DK080886, and AG00078.
Footnotes
Disclosures: Financial support for this study was provided by NIH grants: U01 AG20487, DK080886, and AG00078. The authors have not received financial or other support from companies, manufacturers, or outside organizations that could be considered conflicts of interest.
Conflict of Interest: The authors have not received financial or other support from companies, manufacturers, or outside organizations that could be considered conflicts of interest. The contents of the present report do not constitute endorsement of any products by the authors or ACSM.
Rererences
- 1.Aberra A, Komukai K, Howarth FC, Orchard CH. The effect of acidosis on the ECG of the rat heart. Exp Physiol. 2001;86:27–31. doi: 10.1113/eph8602051. [DOI] [PubMed] [Google Scholar]
- 2.Ausman LM, Oliver LM, Goldin BR, Woods MN, Gorbach SL, Dwyer JT. Estimated net acid excretion inversely correlates with urine pH in vegans, lacto-ovo vegetarians, and omnivores. J Ren Nutr. 2008;18:456–465. doi: 10.1053/j.jrn.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. 1988;66:140–146. doi: 10.1210/jcem-66-1-140. [DOI] [PubMed] [Google Scholar]
- 4.Brungger M, Hulter HN, Krapf R. Effect of chronic metabolic acidosis on the growth hormone/IGF-1 endocrine axis: new cause of growth hormone insensitivity in humans. Kidney Int. 1997;51:216–221. doi: 10.1038/ki.1997.26. [DOI] [PubMed] [Google Scholar]
- 5.Buclin T, Cosma M, Appenzeller M, Jacquet AF, Decosterd LA, Biollaz J, Burckhardt P. Diet acids and alkalis influence calcium retention in bone. Osteoporos Int. 2001;12:493–499. doi: 10.1007/s001980170095. [DOI] [PubMed] [Google Scholar]
- 6.Cristina M, de Hurtado C, Gende OA, Cingolani HE. Species differences in the chronotropic response to acid-base alterations. Arch Int Physiol Biochim. 1979;87:592–602. [PubMed] [Google Scholar]
- 7.Frassetto LA, Morris RC, Jr, Sebastian A. Dietary sodium chloride intake independently predicts the degree of hyperchloremic metabolic acidosis in healthy humans consuming a net acid-producing diet. Am J Physiol Renal Physiol. 2007;293:F521–F525. doi: 10.1152/ajprenal.00048.2007. [DOI] [PubMed] [Google Scholar]
- 8.Giannini S, Nobile M, Sartori L, Dalle CL, Ciuffreda M, Corro P, D'Angelo A, Calo L, Crepaldi G. Acute effects of moderate dietary protein restriction in patients with idiopathic hypercalciuria and calcium nephrolithiasis. Am J Clin Nutr. 1999;69:267–271. doi: 10.1093/ajcn/69.2.267. [DOI] [PubMed] [Google Scholar]
- 9.Heusel JW, Siggaard-Andersen O, Scott MG. Physiology and Distorders of Water, Electrolyte, and Acid-Base Metabolism. In: Burtes CA, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. Philadelphia: W.B. Saunders Co.; 1999. pp. 1095–1124. [Google Scholar]
- 10.Howley ET, Bassett DR, Jr, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27:1292–1301. [PubMed] [Google Scholar]
- 11.Leal VO, Delgado AG, Leite M, Jr, Mitch WE, Mafra D. Influence of renal function and diet on acid-base status in chronic kidney disease patients. J Ren Nutr. 2009;19:178–182. doi: 10.1053/j.jrn.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Midgley AW, McNaughton LR, Polman R, Marchant D. Criteria for determination of maximal oxygen uptake: a brief critique and recommendations for future research. Sports Med. 2007;37:1019–1028. doi: 10.2165/00007256-200737120-00002. [DOI] [PubMed] [Google Scholar]
- 13.Oh MS. New perspectives on acid-base balance. Semin Dial. 2000;13:212–219. doi: 10.1046/j.1525-139x.2000.00061.x. [DOI] [PubMed] [Google Scholar]
- 14.Orchard CH, Kentish JC. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990;258:C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- 15.Peronnet F, Aguilaniu B. Lactic acid buffering, nonmetabolic CO2 and exercise hyperventilation: a critical reappraisal. Respir Physiol Neurobiol. 2006;150:4–18. doi: 10.1016/j.resp.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Peronnet F, Meyer T, Aguilaniu B, Juneau CE, Faude O, Kindermann W. Bicarbonate infusion and pH clamp moderately reduce hyperventilation during ramp exercise in humans. J Appl Physiol. 2007;102:426–428. doi: 10.1152/japplphysiol.00559.2006. [DOI] [PubMed] [Google Scholar]
- 17.Poole DC, Wilkerson DP, Jones AM. Validity of criteria for establishing maximal O2 uptake during ramp exercise tests. Eur J Appl Physiol. 2008;102:403–410. doi: 10.1007/s00421-007-0596-3. [DOI] [PubMed] [Google Scholar]
- 18.Remer T. Influence of nutrition on acid-base balance--metabolic aspects. Eur J Nutr. 2001;40:214–220. doi: 10.1007/s394-001-8348-1. [DOI] [PubMed] [Google Scholar]
- 19.Remer T, Dimitriou T, Manz F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr. 2003;77:1255–1260. doi: 10.1093/ajcn/77.5.1255. [DOI] [PubMed] [Google Scholar]
- 20.Remer T, Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 1994;59:1356–1361. doi: 10.1093/ajcn/59.6.1356. [DOI] [PubMed] [Google Scholar]
- 21.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95:791–797. doi: 10.1016/S0002-8223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 22.Ren J, Samson WK, Sowers JR. Insulin-like growth factor I as a cardiac hormone: physiological and pathophysiological implications in heart disease. J Mol Cell Cardiol. 1999;31:2049–2061. doi: 10.1006/jmcc.1999.1036. [DOI] [PubMed] [Google Scholar]
- 23.Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol. 2004;287:R502–R516. doi: 10.1152/ajpregu.00114.2004. [DOI] [PubMed] [Google Scholar]
- 24.Sellmeyer DE, Stone KL, Sebastian A, Cummings SR. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Study of Osteoporotic Fractures Research Group. Am J Clin Nutr. 2001;73:118–122. doi: 10.1093/ajcn/73.1.118. [DOI] [PubMed] [Google Scholar]
- 25.Severi S, Cavalcanti S, Mancini E, Santoro A. Effect of electrolyte and pH changes on the sinus node pacemaking in humans. J Electrocardiol. 2002;35:115–124. doi: 10.1054/jelc.2002.31819. [DOI] [PubMed] [Google Scholar]
- 26.Shvartz E, Reibold RC. Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviat Space Environ Med. 1990;61:3–11. [PubMed] [Google Scholar]
- 27.Stachenfeld NS, Eskenazi M, Gleim GW, Coplan NL, Nicholas JA. Predictive accuracy of criteria used to assess maximal oxygen consumption. Am Heart J. 1992;123:922–925. doi: 10.1016/0002-8703(92)90697-t. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 29.Thorpe M, Mojtahedi MC, Chapman-Novakofski K, McAuley E, Evans EM. A positive association of lumbar spine bone mineral density with dietary protein is suppressed by a negative association with protein sulfur. J Nutr. 2008;138:80–85. doi: 10.1093/jn/138.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tucker KL, Hannan MT, Kiel DP. The acid-base hypothesis: diet and bone in the Framingham Osteoporosis Study. Eur J Nutr. 2001;40:231–237. doi: 10.1007/s394-001-8350-8. [DOI] [PubMed] [Google Scholar]
- 31.Welch AA, Mulligan A, Bingham SA, Khaw KT. Urine pH is an indicator of dietary acid-base load, fruit and vegetables and meat intakes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk population study. Br J Nutr. 2008;99:1335–1343. doi: 10.1017/S0007114507862350. [DOI] [PubMed] [Google Scholar]
- 32.Yancy WS, Jr, Olsen MK, Dudley T, Westman EC. Acid-base analysis of individuals following two weight loss diets. Eur J Clin Nutr. 2007;61:1416–1422. doi: 10.1038/sj.ejcn.1602661. [DOI] [PubMed] [Google Scholar]