Abstract
The three classes of enzymes which inactivate aminoglycosides and lead to bacterial resistance are reviewed. DNA hybridization studies have shown that different genes can encode aminoglycoside-modifying enzymes with identical resistance profiles. Comparisons of the amino acid sequences of 49 aminoglycoside-modifying enzymes have revealed new insights into the evolution and relatedness of these proteins. A preliminary assessment of the amino acids which may be important in binding aminoglycosides was obtained from these data and from the results of mutational analysis of several of the genes encoding aminoglycoside-modifying enzymes. Recent studies have demonstrated that aminoglycoside resistance can emerge as a result of alterations in the regulation of normally quiescent cellular genes or as a result of acquiring genes which may have originated from aminoglycoside-producing organisms or from other resistant organisms. Dissemination of these genes is aided by a variety of genetic elements including integrons, transposons, and broad-host-range plasmids. As knowledge of the molecular structure of these enzymes increases, progress can be made in our understanding of how resistance to new aminoglycosides emerges.
Full text
PDF










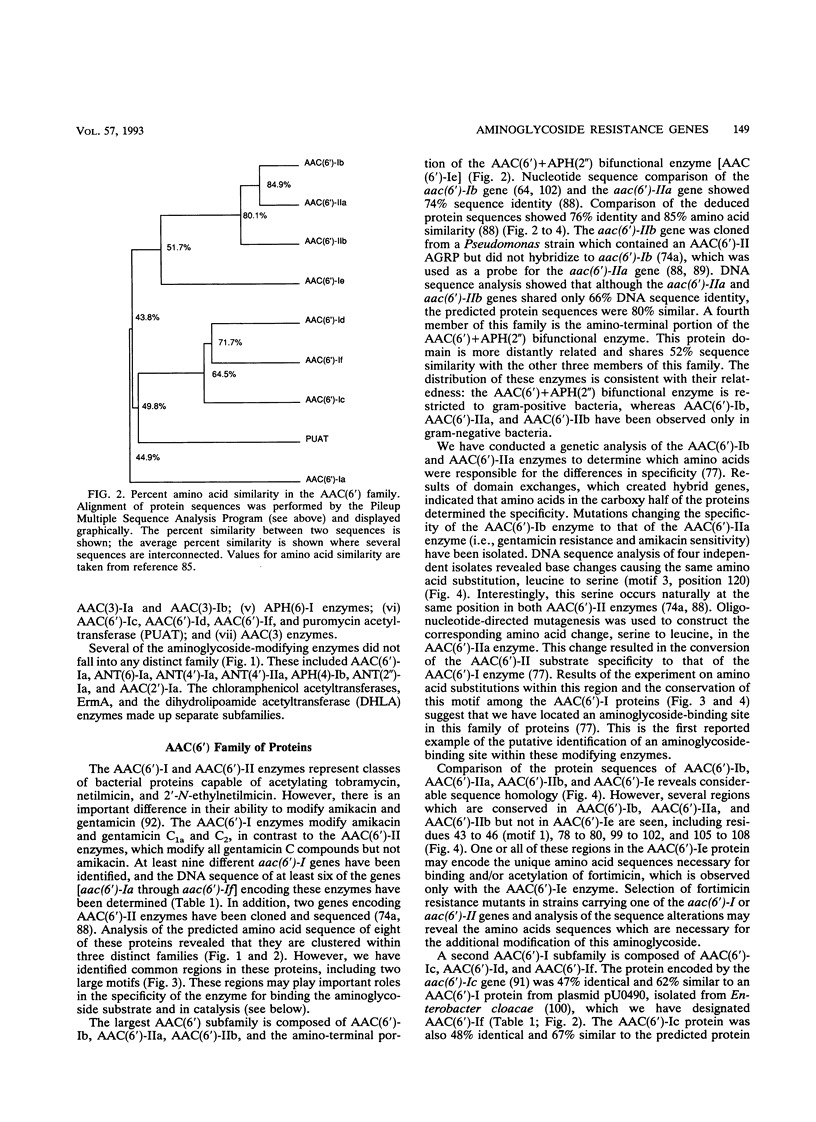














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allmansberger R., Bräu B., Piepersberg W. Genes for gentamicin-(3)-N-acetyl-transferases III and IV. II. Nucleotide sequences of three AAC(3)-III genes and evolutionary aspects. Mol Gen Genet. 1985;198(3):514–520. doi: 10.1007/BF00332949. [DOI] [PubMed] [Google Scholar]
- Barg N. L. Construction of a probe for the aminoglycoside 3-V-acetyltransferase gene and detection of the gene among endemic clinical isolates. Antimicrob Agents Chemother. 1988 Dec;32(12):1834–1838. doi: 10.1128/aac.32.12.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2276–2280. doi: 10.1073/pnas.70.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Berrard S., Brice A., Lottspeich F., Braun A., Barde Y. A., Mallet J. cDNA cloning and complete sequence of porcine choline acetyltransferase: in vitro translation of the corresponding RNA yields an active protein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9280–9284. doi: 10.1073/pnas.84.24.9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddlecome S., Haas M., Davies J., Miller G. H., Rane D. F., Daniels P. J. Enzymatic modification of aminoglycoside antibiotics: a new 3-N-acetylating enzyme from a Pseudomonas aeruginosa isolate. Antimicrob Agents Chemother. 1976 Jun;9(6):951–955. doi: 10.1128/aac.9.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez J., Davies J., Moreno F. Mutations in the aphA-2 gene of transposon Tn5 mapping within the regions highly conserved in aminoglycoside-phosphotransferases strongly reduce aminoglycoside resistance. Mol Microbiol. 1991 Jun;5(6):1511–1518. doi: 10.1111/j.1365-2958.1991.tb00798.x. [DOI] [PubMed] [Google Scholar]
- Brzezinska M., Benveniste R., Davies J., Daniels P. J., Weinstein J. Gentamicin resistance in strains of Pseudomonas aeruginosa mediated by enzymatic N-acetylation of the deoxystreptamine moiety. Biochemistry. 1972 Feb 29;11(5):761–765. doi: 10.1021/bi00755a013. [DOI] [PubMed] [Google Scholar]
- Bräu B., Piepersberg W. Purification and characterization of a plasmid-encoded aminoglycoside-(3)-N-acetyltransferase IV from Escherichia coli. FEBS Lett. 1985 Jun 3;185(1):43–46. doi: 10.1016/0014-5793(85)80737-7. [DOI] [PubMed] [Google Scholar]
- Bräu B., Pilz U., Piepersberg W. Genes for gentamicin-(3)-N-acetyltransferases III and IV: I. Nucleotide sequence of the AAC(3)-IV gene and possible involvement of an IS140 element in its expression. Mol Gen Genet. 1984;193(1):179–187. doi: 10.1007/BF00327434. [DOI] [PubMed] [Google Scholar]
- Cameron F. H., Groot Obbink D. J., Ackerman V. P., Hall R. M. Nucleotide sequence of the AAD(2'') aminoglycoside adenylyltransferase determinant aadB. Evolutionary relationship of this region with those surrounding aadA in R538-1 and dhfrII in R388. Nucleic Acids Res. 1986 Nov 11;14(21):8625–8635. doi: 10.1093/nar/14.21.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion H. M., Bennett P. M., Lewis D. A., Reeves D. S. Cloning and characterization of an AAC(6') gene from Serratia marcescens. J Antimicrob Chemother. 1988 Nov;22(5):587–596. doi: 10.1093/jac/22.5.587. [DOI] [PubMed] [Google Scholar]
- Chaslus-Dancla E., Glupcznski Y., Gerbaud G., Lagorce M., Lafont J. P., Courvalin P. Detection of apramycin resistant Enterobacteriaceae in hospital isolates. FEMS Microbiol Lett. 1989 Oct 15;52(3):261–265. doi: 10.1016/0378-1097(89)90208-5. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Carlier C. Resistance towards aminoglycoside-aminocyclitol antibiotics in bacteria. J Antimicrob Chemother. 1981 Jul;8 (Suppl A):57–69. doi: 10.1093/jac/8.suppl_a.57. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Davies J. Plasmid-medicated aminoglycoside phosphotransferase of broad substrate range that phosphorylates amikacin. Antimicrob Agents Chemother. 1977 Apr;11(4):619–624. doi: 10.1128/aac.11.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbey R. E. Leader peptidase. Mol Microbiol. 1991 Dec;5(12):2855–2860. doi: 10.1111/j.1365-2958.1991.tb01844.x. [DOI] [PubMed] [Google Scholar]
- Davies J., O'Connor S. Enzymatic modification of aminoglycoside antibiotics: 3-N-acetyltransferase with broad specificity that determines resistance to the novel aminoglycoside apramycin. Antimicrob Agents Chemother. 1978 Jul;14(1):69–72. doi: 10.1128/aac.14.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler J., Braun C., Ebert A., Piepersberg W. Gene cluster for streptomycin biosynthesis in Streptomyces griseus: analysis of a central region including the major resistance gene. Mol Gen Genet. 1987 Jun;208(1-2):204–210. doi: 10.1007/BF00330443. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J. Plasmid-determined resistance to antimicrobial drugs and toxic metal ions in bacteria. Microbiol Rev. 1983 Sep;47(3):361–409. doi: 10.1128/mr.47.3.361-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes R., Groisman E., Nelson E., Casadaban M., Lerner S. A. Isolation, characterization, and cloning of a plasmid-borne gene encoding a phosphotransferase that confers high-level amikacin resistance in enteric bacilli. Antimicrob Agents Chemother. 1988 Sep;32(9):1379–1384. doi: 10.1128/aac.32.9.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. S., Fitch W. M. Evolution of antibiotic resistance genes: the DNA sequence of a kanamycin resistance gene from Staphylococcus aureus. Mol Biol Evol. 1983 Dec;1(1):57–66. doi: 10.1093/oxfordjournals.molbev.a040298. [DOI] [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Gómez-Lus R., Rivera M. J., Bobey D., Martín C., Navarro M. Chromosomal origin of acetyltransferase AAC(6') specifying amikacin resistance in Serratia marcescens. Microbiologia. 1987 Oct;3(3):185–194. [PubMed] [Google Scholar]
- Hall R. M., Brookes D. E., Stokes H. W. Site-specific insertion of genes into integrons: role of the 59-base element and determination of the recombination cross-over point. Mol Microbiol. 1991 Aug;5(8):1941–1959. doi: 10.1111/j.1365-2958.1991.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Hawkey P. M., Constable H. K. Selection of netilmicin resistance, associated with increased 6' aminoglycoside acetyltransferase activity, in Serratia marcescens. J Antimicrob Chemother. 1988 May;21(5):535–544. doi: 10.1093/jac/21.5.535. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Shannon K. P. Resistance to apramycin in Escherichia coli isolated from animals: detection of a novel aminoglycoside-modifying enzyme. J Gen Microbiol. 1984 Mar;130(3):473–482. doi: 10.1099/00221287-130-3-473. [DOI] [PubMed] [Google Scholar]
- Heim U., Tietze E., Weschke W., Tschäpe H., Wobus U. Nucleotide sequence of a plasmid born streptothricin-acetyl-transferase gene (sat-1). Nucleic Acids Res. 1989 Sep 12;17(17):7103–7103. doi: 10.1093/nar/17.17.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel P., Werbitzky O., Distler J., Piepersberg W. A second streptomycin resistance gene from Streptomyces griseus codes for streptomycin-3"-phosphotransferase. Relationships between antibiotic and protein kinases. Arch Microbiol. 1988;150(2):184–192. doi: 10.1007/BF00425160. [DOI] [PubMed] [Google Scholar]
- Herbert C. J., Sarwar M., Ner S. S., Giles I. G., Akhtar M. Sequence and interspecies transfer of an aminoglycoside phosphotransferase gene (APH) of Bacillus circulans. Self-defence mechanism in antibiotic-producing organisms. Biochem J. 1986 Jan 15;233(2):383–393. doi: 10.1042/bj2330383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintermann G., Crameri R., Vögtli M., Hütter R. Streptomycin-sensitivity in Streptomyces glaucescens is due to deletions comprising the structural gene coding for a specific phosphotransferase. Mol Gen Genet. 1984;196(3):513–520. doi: 10.1007/BF00436201. [DOI] [PubMed] [Google Scholar]
- Hollingshead S., Vapnek D. Nucleotide sequence analysis of a gene encoding a streptomycin/spectinomycin adenylyltransferase. Plasmid. 1985 Jan;13(1):17–30. doi: 10.1016/0147-619x(85)90052-6. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Furuya K., Nishiyama M., Suzuki H., Beppu T. Nucleotide sequence of the streptothricin acetyltransferase gene from Streptomyces lavendulae and its expression in heterologous hosts. J Bacteriol. 1987 May;169(5):1929–1937. doi: 10.1128/jb.169.5.1929-1937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiko S., Nojiri C., Matsunaga K., Katsumata K., Satoh E., Nagaoka K. Nucleotide sequence of the ribostamycin phosphotransferase gene and of its control region in Streptomyces ribosidificus. Gene. 1988 Sep 7;68(2):285–296. doi: 10.1016/0378-1119(88)90031-5. [DOI] [PubMed] [Google Scholar]
- Ishikawa J., Hotta K. Nucleotide sequence and transcriptional start point of the kan gene encoding an aminoglycoside 3-N-acetyltransferase from Streptomyces griseus SS-1198PR. Gene. 1991 Dec 1;108(1):127–132. doi: 10.1016/0378-1119(91)90497-y. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Blaser M. J., Santanam P., Hächler H., Kayser F. H., Hare R. S., Miller G. H. Appearance of amikacin and tobramycin resistance due to 4'-aminoglycoside nucleotidyltransferase [ANT(4')-II] in gram-negative pathogens. Antimicrob Agents Chemother. 1990 Dec;34(12):2381–2386. doi: 10.1128/aac.34.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier Terán F., Alvarez M., Suárez J. E., Mendoza M. C. Characterization of two aminoglycoside-(3)-N-acetyltransferase genes and assay as epidemiological probes. J Antimicrob Chemother. 1991 Sep;28(3):333–346. doi: 10.1093/jac/28.3.333. [DOI] [PubMed] [Google Scholar]
- John J. F., Jr, McNeill W. F., Price K. E., Kresel P. A. Evidence for a chromosomal site specifying amikacin resistance in multiresistant Serratia marcescens. Antimicrob Agents Chemother. 1982 Apr;21(4):587–591. doi: 10.1128/aac.21.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocabiyik S., Perlin M. H. Altered substrate specificity by substitutions at Tyr218 in bacterial aminoglycoside 3'-phosphotransferase-II. FEMS Microbiol Lett. 1992 Jun 1;72(2):199–202. doi: 10.1016/0378-1097(92)90529-w. [DOI] [PubMed] [Google Scholar]
- Kocabiyik S., Perlin M. H. Site-specific mutations of conserved C-terminal residues in aminoglycoside 3'-phosphotransferase II: phenotypic and structural analysis of mutant enzymes. Biochem Biophys Res Commun. 1992 Jun 30;185(3):925–931. doi: 10.1016/0006-291x(92)91715-3. [DOI] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Bouvet P., Vieu J. F., Courvalin P. Dissemination of amikacin resistance gene aphA6 in Acinetobacter spp. Antimicrob Agents Chemother. 1990 Jun;34(6):1244–1248. doi: 10.1128/aac.34.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Courvalin P. Transferable amikacin resistance in Acinetobacter spp. due to a new type of 3'-aminoglycoside phosphotransferase. Antimicrob Agents Chemother. 1988 Jan;32(1):15–19. doi: 10.1128/aac.32.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Trieu-Cuot P., Courvalin P. Structural relationship between the genes encoding 3'-aminoglycoside phosphotransferases in Campylobacter and in gram-positive cocci. Ann Inst Pasteur Microbiol. 1985 Sep-Oct;136B(2):135–150. doi: 10.1016/s0769-2609(85)80040-5. [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Hopkins J. D., Syvanen M. Evolved neomycin phosphotransferase from an isolate of Klebsiella pneumoniae. Mol Microbiol. 1991 Aug;5(8):2039–2046. doi: 10.1111/j.1365-2958.1991.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Cleary P. P., Gerding D. N. More than one DNA sequence encodes the 2''-O-adenylyltransferase phenotype. Antimicrob Agents Chemother. 1987 Apr;31(4):667–670. doi: 10.1128/aac.31.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering A. M., White L. O., Reeves D. S. AAC(1): a new aminoglycoside-acetylating enzyme modifying the Cl aminogroup of apramycin. J Antimicrob Chemother. 1987 Dec;20(6):803–813. doi: 10.1093/jac/20.6.803. [DOI] [PubMed] [Google Scholar]
- López-Cabrera M., Pérez-González J. A., Heinzel P., Piepersberg W., Jiménez A. Isolation and nucleotide sequencing of an aminocyclitol acetyltransferase gene from Streptomyces rimosus forma paromomycinus. J Bacteriol. 1989 Jan;171(1):321–328. doi: 10.1128/jb.171.1.321-328.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel A., Moreau N., Capmau M. L., Soussy C. J., Duval J. 2"-O-phosphorylation of gentamicin components by a Staphylococcus aureus strain carrying a plasmid. Antimicrob Agents Chemother. 1977 Jul;12(1):26–30. doi: 10.1128/aac.12.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Jullien E., Courvalin P. Nucleotide sequence of Acinetobacter baumannii aphA-6 gene: evolutionary and functional implications of sequence homologies with nucleotide-binding proteins, kinases and other aminoglycoside-modifying enzymes. Mol Microbiol. 1988 Sep;2(5):615–625. doi: 10.1111/j.1365-2958.1988.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Matsuhashi Y., Yagisawa M., Kondo S., Takeuchi T., Umezawa H. Aminoglycoside 3'-phosphotransferases I and II in Pseudomonas aeruginosa. J Antibiot (Tokyo) 1975 Jun;28(6):442–447. doi: 10.7164/antibiotics.28.442. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Katakura Y., Imanaka T., Aiba S. Enzymatic and nucleotide sequence studies of a kanamycin-inactivating enzyme encoded by a plasmid from thermophilic bacilli in comparison with that encoded by plasmid pUB110. J Bacteriol. 1984 Oct;160(1):413–420. doi: 10.1128/jb.160.1.413-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazodier P., Cossart P., Giraud E., Gasser F. Completion of the nucleotide sequence of the central region of Tn5 confirms the presence of three resistance genes. Nucleic Acids Res. 1985 Jan 11;13(1):195–205. doi: 10.1093/nar/13.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. Nucleotide sequence of a spectinomycin adenyltransferase AAD(9) determinant from Staphylococcus aureus and its relationship to AAD(3") (9). Mol Gen Genet. 1985;200(1):33–39. doi: 10.1007/BF00383309. [DOI] [PubMed] [Google Scholar]
- Nobuta K., Tolmasky M. E., Crosa L. M., Crosa J. H. Sequencing and expression of the 6'-N-acetyltransferase gene of transposon Tn1331 from Klebsiella pneumoniae. J Bacteriol. 1988 Aug;170(8):3769–3773. doi: 10.1128/jb.170.8.3769-3773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Bissonnette L., Roy P. H. Precise insertion of antibiotic resistance determinants into Tn21-like transposons: nucleotide sequence of the OXA-1 beta-lactamase gene. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7378–7382. doi: 10.1073/pnas.84.21.7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounissi H., Derlot E., Carlier C., Courvalin P. Gene homogeneity for aminoglycoside-modifying enzymes in gram-positive cocci. Antimicrob Agents Chemother. 1990 Nov;34(11):2164–2168. doi: 10.1128/aac.34.11.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin M. H., Lerner S. A. High-level amikacin resistance in Escherichia coli due to phosphorylation and impaired aminoglycoside uptake. Antimicrob Agents Chemother. 1986 Feb;29(2):216–224. doi: 10.1128/aac.29.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin M. H., Lerner S. A. Localization of an amikacin 3'-phosphotransferase in Escherichia coli. J Bacteriol. 1981 Aug;147(2):320–325. doi: 10.1128/jb.147.2.320-325.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K. E., Kresel P. A., Farchione L. A., Siskin S. B., Karpow S. A. Epidemiological studies of aminoglycoside resistance in the U.S.A. J Antimicrob Chemother. 1981 Jul;8 (Suppl A):89–105. doi: 10.1093/jac/8.suppl_a.89. [DOI] [PubMed] [Google Scholar]
- Pérez-González J. A., López-Cabrera M., Pardo J. M., Jiménez A. Biochemical characterization of two cloned resistance determinants encoding a paromomycin acetyltransferase and a paromomycin phosphotransferase from Streptomyces rimosus forma paromomycinus. J Bacteriol. 1989 Jan;171(1):329–334. doi: 10.1128/jb.171.1.329-334.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather P. N., Mierzwa R., Hare R. S., Miller G. H., Shaw K. J. Cloning and DNA sequence analysis of an aac(3)-Vb gene from Serratia marcescens. Antimicrob Agents Chemother. 1992 Oct;36(10):2222–2227. doi: 10.1128/aac.36.10.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather P. N., Munayyer H., Mann P. A., Hare R. S., Miller G. H., Shaw K. J. Genetic analysis of bacterial acetyltransferases: identification of amino acids determining the specificities of the aminoglycoside 6'-N-acetyltransferase Ib and IIa proteins. J Bacteriol. 1992 May;174(10):3196–3203. doi: 10.1128/jb.174.10.3196-3203.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouch D. A., Byrne M. E., Kong Y. C., Skurray R. A. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: expression and nucleotide sequence analysis. J Gen Microbiol. 1987 Nov;133(11):3039–3052. doi: 10.1099/00221287-133-11-3039. [DOI] [PubMed] [Google Scholar]
- Salauze D., Davies J. Isolation and characterisation of an aminoglycoside phosphotransferase from neomycin-producing Micromonospora chalcea; comparison with that of Streptomyces fradiae and other producers of 4,6-disubstituted 2-deoxystreptamine antibiotics. J Antibiot (Tokyo) 1991 Dec;44(12):1432–1443. doi: 10.7164/antibiotics.44.1432. [DOI] [PubMed] [Google Scholar]
- Salauze D., Perez-Gonzalez J. A., Piepersberg W., Davies J. Characterisation of aminoglycoside acetyltransferase-encoding genes of neomycin-producing Micromonospora chalcea and Streptomyces fradiae. Gene. 1991 May 15;101(1):143–148. doi: 10.1016/0378-1119(91)90237-6. [DOI] [PubMed] [Google Scholar]
- Santanam P., Kayser F. H. Purification and characterization of an aminoglycoside inactivating enzyme from Staphylococcus epidermidis FK109 that nucleotidylates the 4'- and 4''-hydroxyl groups of the aminoglycoside antibiotics. J Antibiot (Tokyo) 1978 Apr;31(4):343–351. doi: 10.7164/antibiotics.31.343. [DOI] [PubMed] [Google Scholar]
- Schmidt F. R., Nücken E. J., Henschke R. B. Nucleotide sequence analysis of 2''-aminoglycoside nucleotidyl-transferase ANT(2'') from Tn4000: its relationship with AAD(3'') and impact on Tn21 evolution. Mol Microbiol. 1988 Nov;2(6):709–717. doi: 10.1111/j.1365-2958.1988.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Scholz P., Haring V., Wittmann-Liebold B., Ashman K., Bagdasarian M., Scherzinger E. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene. 1989 Feb 20;75(2):271–288. doi: 10.1016/0378-1119(89)90273-4. [DOI] [PubMed] [Google Scholar]
- Shaw K. J., Cramer C. A., Rizzo M., Mierzwa R., Gewain K., Miller G. H., Hare R. S. Isolation, characterization, and DNA sequence analysis of an AAC(6')-II gene from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989 Dec;33(12):2052–2062. doi: 10.1128/aac.33.12.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J., Hare R. S., Sabatelli F. J., Rizzo M., Cramer C. A., Naples L., Kocsi S., Munayyer H., Mann P., Miller G. H. Correlation between aminoglycoside resistance profiles and DNA hybridization of clinical isolates. Antimicrob Agents Chemother. 1991 Nov;35(11):2253–2261. doi: 10.1128/aac.35.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J., Rather P. N., Sabatelli F. J., Mann P., Munayyer H., Mierzwa R., Petrikkos G. L., Hare R. S., Miller G. H., Bennett P. Characterization of the chromosomal aac(6')-Ic gene from Serratia marcescens. Antimicrob Agents Chemother. 1992 Jul;36(7):1447–1455. doi: 10.1128/aac.36.7.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Kumada T., Hsieh W. C., Chung H. Y., Chong Y., Hare R. S., Miller G. H., Sabatelli F. J., Howard J. Comparison of aminoglycoside resistance patterns in Japan, Formosa, and Korea, Chile, and the United States. Antimicrob Agents Chemother. 1985 Aug;28(2):282–288. doi: 10.1128/aac.28.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes H. W., Hall R. M. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989 Dec;3(12):1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Sugihara H., Andrisani V., Salvaterra P. M. Drosophila choline acetyltransferase uses a non-AUG initiation codon and full length RNA is inefficiently translated. J Biol Chem. 1990 Dec 15;265(35):21714–21719. [PubMed] [Google Scholar]
- Tenover F. C., Elvrum P. M. Detection of two different kanamycin resistance genes in naturally occurring isolates of Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1988 Aug;32(8):1170–1173. doi: 10.1128/aac.32.8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Filpula D., Phillips K. L., Plorde J. J. Cloning and sequencing of a gene encoding an aminoglycoside 6'-N-acetyltransferase from an R factor of Citrobacter diversus. J Bacteriol. 1988 Jan;170(1):471–473. doi: 10.1128/jb.170.1.471-473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Gilbert T., O'Hara P. Nucleotide sequence of a novel kanamycin resistance gene, aphA-7, from Campylobacter jejuni and comparison to other kanamycin phosphotransferase genes. Plasmid. 1989 Jul;22(1):52–58. doi: 10.1016/0147-619x(89)90035-8. [DOI] [PubMed] [Google Scholar]
- Tenover F. C., Phillips K. L., Gilbert T., Lockhart P., O'Hara P. J., Plorde J. J. Development of a DNA probe from the deoxyribonucleotide sequence of a 3-N-aminoglycoside acetyltransferase [AAC(3)-I] resistance gene. Antimicrob Agents Chemother. 1989 Apr;33(4):551–559. doi: 10.1128/aac.33.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terán F. J., Suárez J. E., Mendoza M. C. Cloning, sequencing, and use as a molecular probe of a gene encoding an aminoglycoside 6'-N-acetyltransferase of broad substrate profile. Antimicrob Agents Chemother. 1991 Apr;35(4):714–719. doi: 10.1128/aac.35.4.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Gray G. S. Nucleotide sequence of a streptomycete aminoglycoside phosphotransferase gene and its relationship to phosphotransferases encoded by resistance plasmids. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5190–5194. doi: 10.1073/pnas.80.17.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran van Nhieu G., Collatz E. Primary structure of an aminoglycoside 6'-N-acetyltransferase AAC(6')-4, fused in vivo with the signal peptide of the Tn3-encoded beta-lactamase. J Bacteriol. 1987 Dec;169(12):5708–5714. doi: 10.1128/jb.169.12.5708-5714.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3'5"-aminoglycoside phosphotransferase type III. Gene. 1983 Sep;23(3):331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Gotoh A., Konno M. Purification and characterization of aminoglycoside-modifying enzymes from Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1984 Jun;25(6):754–759. doi: 10.1128/aac.25.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliegenthart J. S., Ketelaar-van Gaalen P. A., van de Klundert J. A. Nucleotide sequence of the aacC2 gene, a gentamicin resistance determinant involved in a hospital epidemic of multiply resistant members of the family Enterobacteriaceae. Antimicrob Agents Chemother. 1989 Aug;33(8):1153–1159. doi: 10.1128/aac.33.8.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliegenthart J. S., Ketelaar-van Gaalen P. A., van de Klundert J. A. Nucleotide sequence of the aacC3 gene, a gentamicin resistance determinant encoding aminoglycoside-(3)-N-acetyltransferase III expressed in Pseudomonas aeruginosa but not in Escherichia coli. Antimicrob Agents Chemother. 1991 May;35(5):892–897. doi: 10.1128/aac.35.5.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vögtli M., Hütter R. Characterisation of the hydroxystreptomycin phosphotransferase gene (sph) of Streptomyces glaucescens: nucleotide sequence and promoter analysis. Mol Gen Genet. 1987 Jun;208(1-2):195–203. doi: 10.1007/BF00330442. [DOI] [PubMed] [Google Scholar]
- Wohlleben W., Arnold W., Bissonnette L., Pelletier A., Tanguay A., Roy P. H., Gamboa G. C., Barry G. F., Aubert E., Davies J. On the evolution of Tn21-like multiresistance transposons: sequence analysis of the gene (aacC1) for gentamicin acetyltransferase-3-I(AAC(3)-I), another member of the Tn21-based expression cassette. Mol Gen Genet. 1989 Jun;217(2-3):202–208. doi: 10.1007/BF02464882. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Mitsuhashi S., Kobayashi F., Zenda H. A 2'-N-acetylating enzyme of aminoglycosides. J Antibiot (Tokyo) 1974 Jul;27(7):507–515. doi: 10.7164/antibiotics.27.507. [DOI] [PubMed] [Google Scholar]
- Yenofsky R. L., Fine M., Pellow J. W. A mutant neomycin phosphotransferase II gene reduces the resistance of transformants to antibiotic selection pressure. Proc Natl Acad Sci U S A. 1990 May;87(9):3435–3439. doi: 10.1073/pnas.87.9.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalacain M., González A., Guerrero M. C., Mattaliano R. J., Malpartida F., Jiménez A. Nucleotide sequence of the hygromycin B phosphotransferase gene from Streptomyces hygroscopicus. Nucleic Acids Res. 1986 Feb 25;14(4):1565–1581. doi: 10.1093/nar/14.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]










