Abstract
In most cholangiopathies, liver diseases of different etiologies in which the biliary epithelium is the primary target in the pathogenic sequence, the central mechanism involves inflammation. Inflammation, characterized by pleomorphic peribiliary infiltrate containing fibroblasts, macrophages, lymphocytes, as well as endothelial cells and pericytes, is associated to the emergence of “reactive cholangiocytes.” These biliary cells do not possess bile secretory functions, are in contiguity with terminal cholangioles, and are of a less-differentiated phenotype. They have acquired several mesenchymal properties, including motility and ability to secrete a vast number of proinflammatory chemo/cytokines and growth factors along with de novo expression of a rich receptor machinery. These functional properties enable reactive cholangiocytes to establish intimate contacts and to mutually exchange a variety of paracrine signals with the different mesenchymal cell types populating the portal infiltrate. The extensive crosstalk between the epithelial and mesenchymal compartments is the driver of liver repair mechanisms in cholangiopathies, ultimately evolving toward portal fibrosis. Herein, the authors first review the properties of the different cell types involved in their interaction, and then analyze the underlying molecular mechanisms as they relate to liver repair in cholangiopathies.
Keywords: Cholangiopathies, cholangiocytes, ductular reaction, myofibroblasts, endothelial cells
Cholangiopathies are a heterogeneous group of liver diseases caused by congenital, immune-mediated, toxic, infectious, or idiopatic insults to the biliary tree or from a failure in the secretory function of cholangiocytes. The central mechanism in most cholangiopathies is inflammation. The common features of cholangiopathies, including cholestasis, cholangiocyte proliferation, ductopenia, portal fibrosis, and carcinogenesis, are consequences of chronic inflammation and the reparative mechanisms triggered by the inflammation. The reader is referred to recent articles1,2 for discussions on cholangiopathies and on their main pathophysiologic mechanisms.
In addition to bile duct damage, most cholangiopathies are characterized by the presence of peribiliary and portal infiltrates containing fibroblasts, macrophages, endothelial cells, pericytes, and lymphocytes. This is the result of a highly orchestrated and dynamic process in which cholangiocytes and mesenchymal cells establish intimate contacts and mutually exchange a variety of signals (Fig. 1). Coordinated epithelial–mesenchymal interactions play a major role in biliary development, as well as in chronic cholangiopathies, where they modulate the reparative response. In addition, in the presence of chronic inflammation, the interactions between the damaged epithelium, mesenchymal cells, and the inflammatory infiltrate eventually promote the formation of biliary-type fibrosis, and ultimately determine the clinical progression of cholangiopathies.
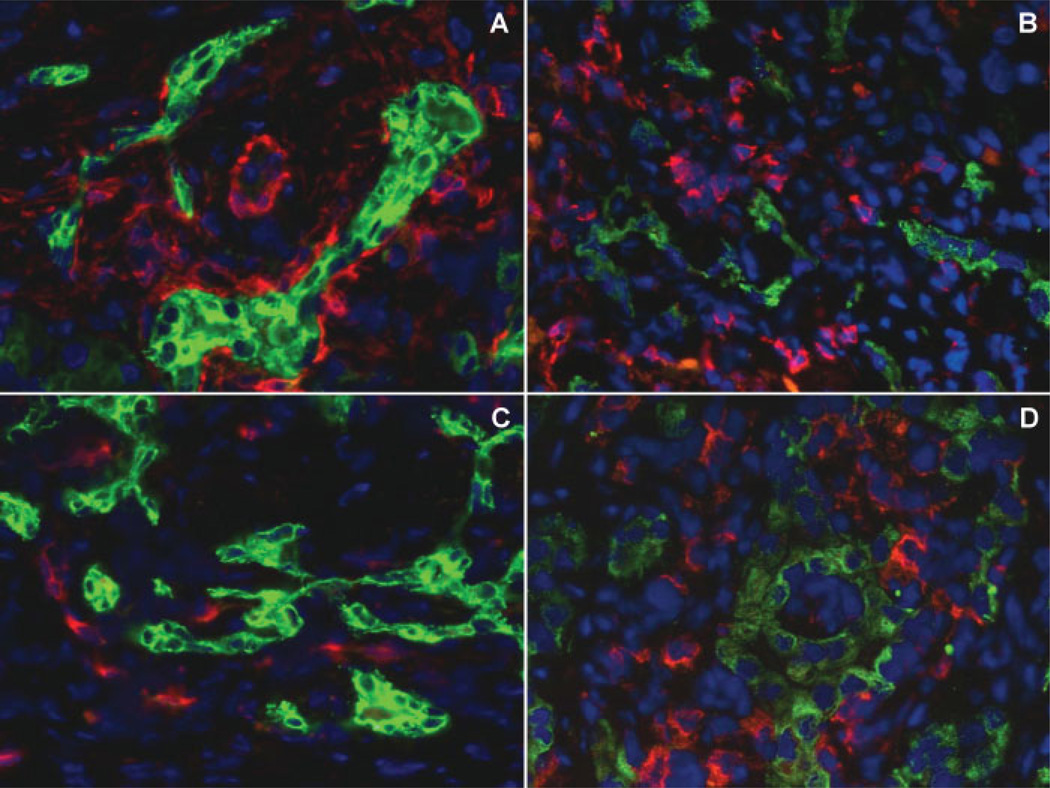
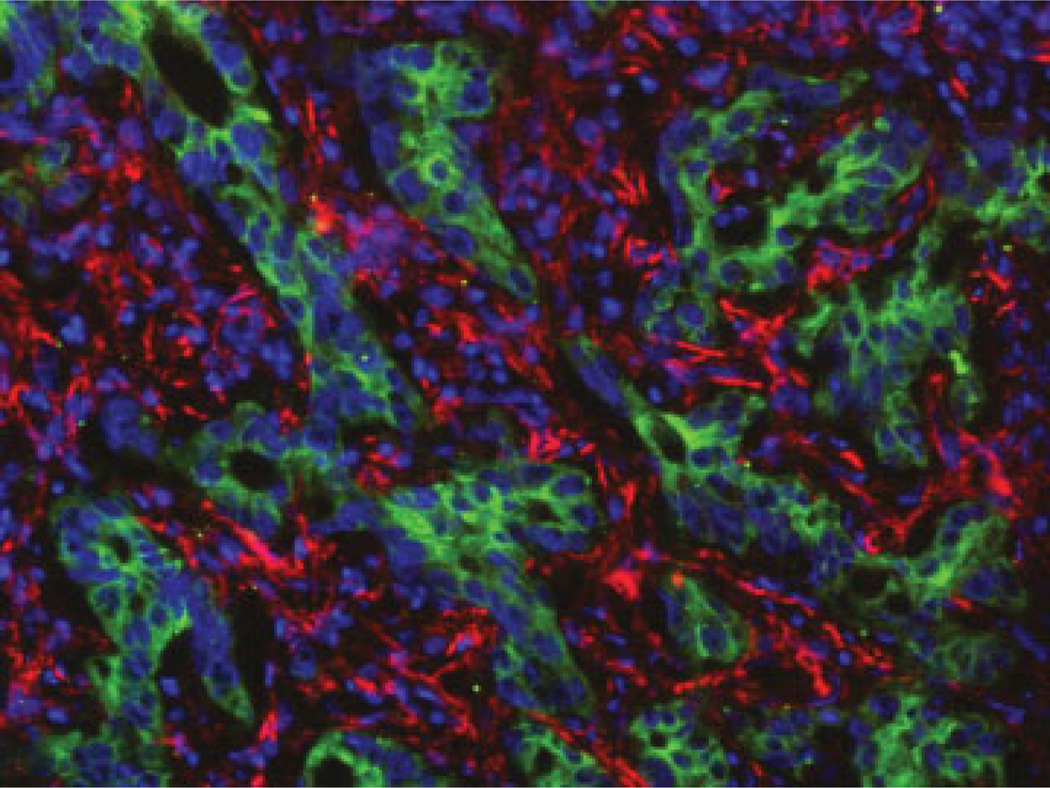
Figure 1.
Microanatomic relationships between reactive cholangiocytes and mesenchymal cell types in cholangiopathies. Dual-immunofluorescence of the biliary cell marker K19 (green fluorescence) with the myofibroblast marker α-SMA (red fluorescence, sample of biliary atresia, A), with the leucocyte marker CD45 (red fluorescence, biliary atresia, B), with the endothelial cell marker CD34 (red fluorescence, biliary atresia, C), and with the macrophage marker CD68 (red fluorescence, primary sclerosing cholangitis, D) shows that reactive cholangiocytes establish intimate contacts with multiple mesenchymal cell types. This represents the structural basis to mutually exchange a huge variety of paracrine signals. (Original magnification A–D: ×400)
The molecular mechanisms that regulate cell interactions within the portal spaces include paracrine and autocrine factors (cytokines, chemokines, proinflammatory mediators, and growth factors), their cognate membrane receptors, and several morphogenic pathways (Hedgehog, Wnt/β-catenin, and Notch). The extracellular matrix (ECM) provides an architectural scaffold along which biliary architecture is organized, as well as a reservoir for growth factors and matrix metalloproteinases (MMPs), and also signals to the epithelial cells through integrins.
CELL TYPES INVOLVED IN EPITHELIAL– MESENCHYMAL INTERACTIONS IN CHOLANGIOPATHIES
Multiple cell types are involved in response to liver damage in cholangiopathies. Epithelial cells cholangiocytes and “reactive.” cholangiocytes) interact with mesenchymal cells (hepatic stellate cells, portal fibroblasts, myofibroblasts, and fibrocytes), endothelial cells, macrophages, and lymphocytes. We will briefly review their properties as they relate to liver repair in cholangiopathies.
Cholangiocytes
Cholangiocytes, the epithelial cells that line the biliary tree, possess both absorptive and secretory properties. Secretory functions are mainly performed at the level of interlobular and major ducts that specifically express several different ion channels and transporters at the basolateral or apical domain. We refer to other reviews for discussions on the transport properties of cholangiocytes and their changes in cholestatic disorders.1,2 Other biologic properties, such as .“plasticity.” (the ability to acquire some phenotypic properties of mesenchymal cells), .“reactivity.” (the ability to participate in the inflammatory reaction in liver damage), and .“stemness.” (the propensity to behave as liver progenitor cells) are mostly restricted to the smaller bile ductules (terminal cholangioles and canals of Hering).
The functions of cholangiocytes are strongly influenced by proinflammatory mediators released in their microenvironment. For instance, interferon – γ (IFNγ) induces major histocompatibility complex class II antigens expression in biliary cells, affects the transport properties of the epithelium, and stimulates nitric oxide (NO) production by cholangiocytes,3 whereas interleukin- 6 (IL-6) and hepatocyte growth factor (HGF) potently stimulate cholangiocyte growth.4 Constitutive expression of stromal cell-derived factor-1 (SDF-1) by cholangiocytes facilitates the selective homing of CXCR4-positive inflammatory cells in close proximity to the biliary structures.5 Furthermore, cholangiocytes express several Toll-like receptors (TLRs) and produce specific immunomodulatory chemokines (IL-6, IL-8) that can influence both innate and adaptive immune cells,6 a fundamental defense mechanism against infectious agents.7
Reactive Cholangiocytes
Ductular reaction is a stereotyped response to biliary epithelial damage. It is defined by the marked expansion of the cholangiocyte compartment and by the de novo expression of (1) a variety of cytokines, chemokines, growth factors, and angiogenic factors; and (2) their cognate receptors. The dynamic interaction between mesenchymal cells, considered the effectors of fibrosis, and .“reactive cholangiocytes,.” considered the .“pacemaker of liver fibrosis.” is central to the development of portal fibrosis in cholangiopathies.8 Although devoid of bile secretory functions, reactive cholangiocytes are able to secrete several proinflammatory and chemotactic cytokines and growth factors that enable them to recruit inflammatory and mesenchymal cells.2 Reactive cholangiocytes activate myofibroblasts (MF) and stimulate angiogenesis by secreting vascular endothelial growth factor (VEGF),9,10 endothelin-1 (ET-1),11 platelet-derived growth factor-BB (PDGF-BB),12 transforming growth factor-β2 (TGF-β2),13 and connective tissue growth factor (CTGF).14 Reactive cholangiocytes also secrete NO,3 IL-6,4,15,16 IL-8,16 tumor necrosis factor-α (TNFα),15 IFNγ,17 monocyte chemotactic protein-1 (MCP-1)18 and cytokine-induced neutrophil chemoattractant.19 Notably, several of the above-mentioned factors are expressed by ductal plate cells in fetal life, reinforcing the concept that ductular reaction recapitulates liver ontogenesis.20 Reactive cholangiocytes also express integrins, a family of transmembrane heterodimeric cellular receptors that control cell–cell and cell– ECM interactions. For example, the inflammation-associated αvβ6 integrin is not expressed by the normal biliary epithelium, but it is upregulated in reactive bile ductules, and may promote fibrogenesis via activation of latent TGF-β1.21,22
Reactive cholangiocytes are believed to derive from a progenitor cell compartment situated in close proximity to the terminal cholangioles in the canals of Hering, although some data indicate that transdifferentiation from hepatocytes is also possible.23 The molecular mechanisms that activate reactive cholangiocytes require a finely coordinated process that recapitulates many features of liver development and is set in motion by inflammatory signals and changes in ECM composition. TNFα, TWEAK, TGF-β, HGF, VEGF, sonic Hedgehog (Hh), and Wingless (Wnt)/β -catenin signaling are among the key inducers of ductular reaction, unlocking the proliferative potential of the progenitor cell compartment.24–29 Recently, the role of Foxa1 and Foxa2 as regulators of IL-6 production has been elucidated.30 These important transcription factors act as terminators of bile duct development, by suppressing IL-6 production.30 It is possible that a decrease in Foxa1 and Foxa2 transcriptional activity acts as a triggering signal for the proliferation of reactive cholangiocytes.30,31
Endothelial Cells
Endothelial cells (ECs) are key players in several processes that mediate the progression of chronic liver diseases. ECs regulate vascular remodeling associated with the inflammatory production/respond to VEGF, PDGF, NO and other factors able to induce angiogenesis. As shown by Semela et al,32 ECs influence angiogenesis through a PDGF and ephrin-dependent mechanism. The extent of neo-angiogenesis has a pro-found impact on the rate of progression of chronic liver disease to cirrhosis.33 In cholangiopathies, a brisk angiogenesis takes place in close vicinity to the damaged bile ducts. An increased number of vascular structures in the inflamed portal tracts together with upregulation of VEGF, angiopoietin-1 (Ang-1) and -2 (Ang-2) on periportal hepatocytes, and ECs, was observed in primary biliary cirrhosis (PBC).34
Unlike PBC, cholangiocarcinoma (CCA) is characterized by a reduced number of vascular structures. An enhanced expression of thrombospondin-1 (TSP-1), an inhibitor of EC migration and adhesion by cancer-associated fibroblasts (CAF) as well as by cancer cells is a possible determinant of hypovascularization in CCA.35
Macrophages/Monocytes
Kupffer cells (KCs) represent ~80 to 90% of the resident macrophages in the liver and account for around 15% of the total liver cell population. In addition to their well-established immune functions, KCs are actively involved in the initiation of hepatocellular damage and fibrogenesis, and are one of the major sources of inflammatory mediators in the liver (cytokines, chemokines, superoxide, NO). The function of macrophages depends upon their phenotype. The “classically” activated, or M1 macrophages secrete large amounts of proinflammatory cytokines, including TGF-β1 and PDGF, potent activators of the hepatic stellate cells (HSC). On the contrary, the “alternatively” activated, M2 macrophages have a low inflammatory profile. The balance between M1 and M2 macrophages is one of the factors determining the severity of cholestastic injury.
In experimental obstructive cholestasis (bile duct ligation [BDL]) followed by endotoxinemia, a KC blockade by gadolinium results in a significant attenuation of inflammatory lesions.36 On the other hand, KCs are involved in the resolution of liver fibrosis, given their ability to degrade ECM components and secrete several MMPs,37 including MMP-9, MMP-12, and MMP- 13.38 Following restoration of bile flow, cholangiocyte apoptosis triggers recruitment of macrophages into the scarred portal tracts, where they clear apoptotic cholangiocytes via phagocytosis, and secrete several MMPs, resulting in remodeling of the fibrous septa and in the reversal of biliary fibrosis.39 Furthermore, secretion of IL-6 by KC, may have protective effects against cholestatic injury, thanks to the anti-apoptotic action of IL-6 on hepatocytes, and to IL-6 proliferative effect on cholangiocytes.40 Finally, macrophages are also a major source of several members of the TNFα family, including TWEAK, known for their ability to promote the expansion of liver progenitor cells41 and thereby facilitate liver repair.
KCs can directly influence cholangiocyte function. In fact, in PBC, liver-infiltrating mononuclear cells (monocytes/macrophages) enhance the proinflammatory activity of cholangiocytes in response to TLR stimulation.42 Cholangiocyte production of CX3CL1 (fractalkine), a chemokine able to regulate periductal lymphocytic infiltration, is induced by their CD40/ CD154-dependent interaction with macrophages.43
There is consensus that the KC compartment is constantly supplied by monocytes, circulating blood leukocytes that serve as precursors for tissue macrophages.44 MCP-1/CCL2 and its cognate receptor CCR2 is one of the main mechanisms regulating liver monocyte recruitment in experimental liver fibrosis. In fact, inactivation of MCP-1 results in a reduction of monocyte/macrophage infiltration and in a marked reduction in liver fibrosis in dimethylnitrosamine-treated rats.45 Monocytes expressing CCR2 (Gr1hi in mice, CD14+CD16- in humans) are considered precursors of macrophages and dendritic cells following inflammatory stimuli, whereas monocytes lacking CCR2 (Gr1lo in mice, CD14+CD16+ in humans) serve as steady-state precursors for tissue macrophages.46 In experimental liver fibrosis Gr1hi monocytes are recruited to the liver through a CCR2-mediated mechanism.47 Given their ability to release large amounts of proinflammatory cytokines, CD14+CD16+ monocytes are likely to be important for HSC activation.48
Hepatic Stellate Cells and Portal Fibroblasts
Hepatic stellate cells (HSCs) and portal fibroblasts (PFs) are the main resident mesenchymal cells in normal liver. HSCs are located in the subendothelial space of Disse. In their quiescent state, HSCs are phenotypically characterized by the storage of vitamin A, and by the expression of desmin, whereas they are negative for α-smooth muscle actin (α-SMA). HSCs are highly responsive to stimuli released during inflammation, such as oxidative stress and proinflammatory cytokines that promote their transdifferentiation into MFs. In the BDL rat model, bile ducts may stimulate chemotaxis of HSCs through a PDGF-BB-dependent mechanism.49 According to the original description by Schaffner et al, PFs are resident cells that unlike HSCs are located in portal tracts, in close vicinity to the interlobular bile ducts.50 Their phenotype is different from HSCs, being positive for fibulin-2, elastin, and the ecto-AT-Pase nucleoside triphosphate diphosphohydrolase-2 (NTPD2).51
Signals derived from cholangiocytes have a pro-found impact on the behavior of mesenchymal cells. TGF-β2,52 IL-6,15 and MCP-1,53 released by damaged cholangiocytes, induce proliferation and transdifferentiation of PFs into portal MFs. Dranoff et al have shown that PFs can regulate ductular reaction in a paracrine fashion via expression of NTPD2.54 MCP-1 produced by cholangiocytes stimulates PF activation, which in turn downregulates NTPD2, and stimulates ductular reaction through the activation of P2Y receptors.51 Similarly to HSCs, cholangiocyte-derived PDGF-BB may activate PFs into collagen-producing MFs.55
Myofibroblasts
Liver MFs are profibrogenic cells, derived from HSCs and PFs and, to a lesser extent, from bone marrow-derived mesenchymal stem cells56 through a process of transdifferentiation. Other cell sources in the portal tract, such as vascular smooth muscle cells from the hepatic artery and portal vein branches, may theoretically contribute to the histogenesis of portal MFs.51 Whether epithelial to mesenchymal transition (EMT) may contribute to the generation of liver MFs is still a matter of controversy57 and no conclusive evidence has been provided yet (see below).
In cholangiopathies, MFs are localized mainly in the expanded fibrotic tissue around the portal space, rather than at the interface between fibrotic septa and hepatocellular parenchyma.58 Transdifferentiation of HSCs into MFs is characterized phenotypically by the expression of α– SMA and functionally by a range of biologic properties including motility and contractility.59 Contractility is a well-established feature of MFs enabling them to control sinusoidal blood flow and therefore to contribute to the generation of portal hypertension.59 However, the defining feature of MFs is their ability to produce interstitial fibril-forming collagens (mainly type I and III collagens), leading to the formation of scar tissue in cirrhotic septa. In addition to fibrogenesis, MFs may also participate to the modulation of immune responses60,61 and to the regulation of angiogenesis, especially under conditions of hypoxia.62,63
MFs possess the capability to secrete several cytokines, chemokines, and other soluble factors, acting both in autocrine and paracrine manner.59,64 Among them, TGF-β1, PDGF-BB and -DD, VEGF, CTGF, MCP-1, angiotensin II, cannabinoids, reactive oxygen species, and CXCR3 ligands are responsible for the main biologic effects exerted by MFs. Whereas TGF-β is the most important fibrogenic cytokine responsible for the collagen production by activated HSC, PDGF-BB is the most potent chemoattractant for HSC/MF.65 At a transcriptional level, the downregulation of the peroxisome proliferator-activated receptor-γ (PPARγ), a nuclear receptor that inhibits the α1(I) collagen promoter activity, is a fundamental feature of the switch toward the MF phenotype.66
The functional properties of MFs are then modulated by multiple autocrine and paracrine interactions with cytokines, chemokines, and growth factors, produced by HSC themselves or by neighboring cell types, including cholangiocytes. In portal fibrogenesis, the crosstalk between reactive cholangiocytes and MF is supported by the high homology of agonists/receptor systems shared by both cell types, as shown in Fig. 2. HSCs also express CXCR4, the cognate receptor of SDF1/CXCL12, an endogenous ligand constitutionally expressed by the biliary epithelium.67 Other important signaling mechanisms regulating the crosstalk between reactive cholangiocytes and MFs are the Wnt and Hh pathways (see below).
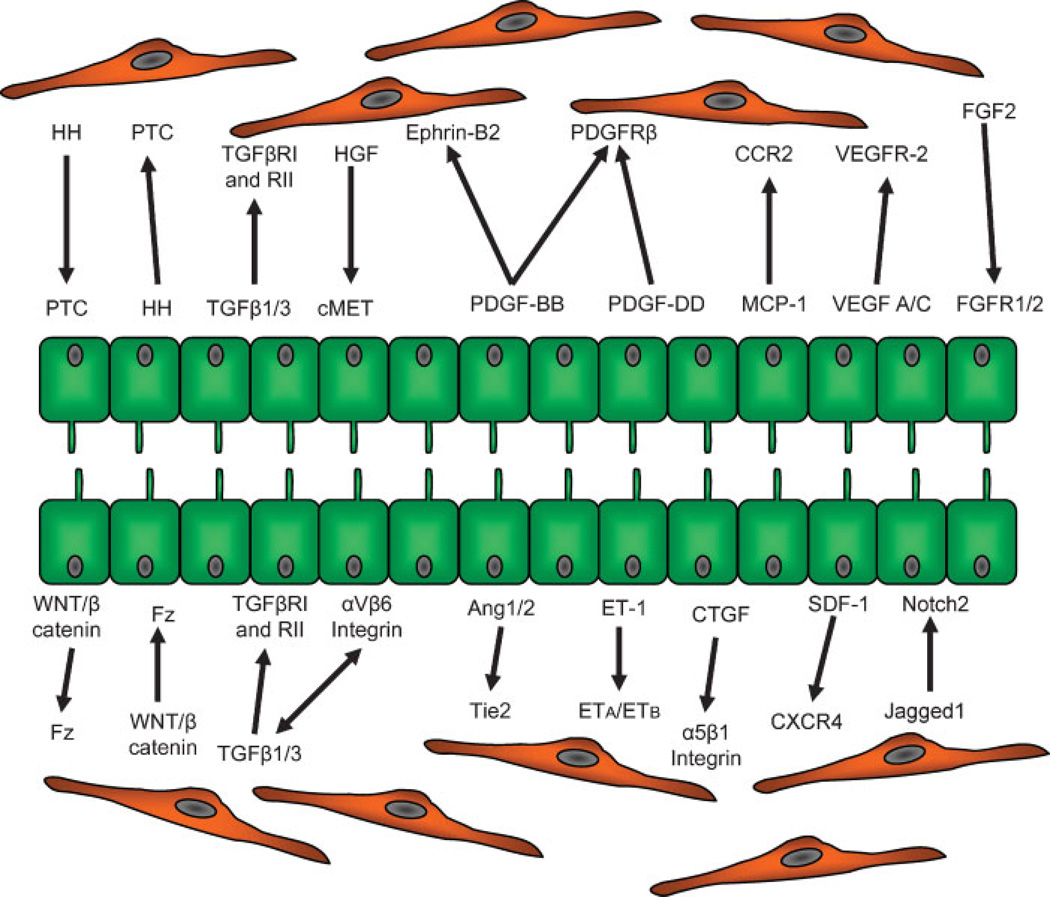
Figure 2.
Functional elements of the crosstalk between reactive cholangiocytes and hepatic stellate cells/myofibroblasts. A high homology of agonists/receptor systems is shared by reactive cholangiocytes and myofibroblasts that enable them to an extensive crosstalk, the molecular mechanism responsible for portal fibrogenesis. Biologic significance of the different systems involved in the crosstalk is summarized in Table 1.
Fibrocytes
Fibrocytes are bone marrow-derived mesenchymal cells that co-express both hematopoietic (CD45 and CD34) and fibroblasts (collagen type I and III, and fibronectin) phenotypic markers, and are able to secrete a variety of inflammatory cyto/chemokines, including IL-1β, IL-10, TNFα,MCP-1, PDGF-A, TGF-β1, monocyte colonystimulating factor, and macrophage inflammatory proteins.68,69 Fibrocytes are recruited from the peripheral blood, to the sites of tissue injury where they produce fibrillar matrix and eventually differentiate into or affect the MF.70 Recent studies have applied lineage tracing experiments to an experimental model of cholestasis with the aim to characterize the origin of fibrocyte-like cells. Experiments using GFP-tagged bone marrow cells in bile duct-ligated mice, have shown that fibrocytes appear to contribute to no more than 5 to 10% of all type I collagen-expressing cells. Around 15% of GFP-positive cells expressing α_SMA, recruited around the periductal area, were detectable at day 3, but decrease thereafter, suggesting that differentiation of bone marrow- derived cells into MFs occurs transiently in the early stage of periductal fibrosis.71
Mechanisms underlying fibrocyte recruitment to the liver are largely unknown. Fibrocytes express several chemokine receptors, including CXCR4.70 In lung fibrosis, CXCR4-SDF-1/CXCL12 axis plays an important role in fibrocyte trafficking, as seen in both experimental models and human diseases.72–75 Notably, SDF-1 strongly stimulates fibrocyte chemotaxis in vitro as well as in vivo.72 Given the strong expression of SDF-1 by reactive cholangiocytes, it is possible that the SDF-1/ CXCR4 axis is regulating the fibrocyte trafficking to the liver. Under TGF-β1 stimulation, cultured fibrocytes differentiate into α–SMA+/Desmin+ collagen-producing MFs.76 However, overall available data indicate that fibrocyte contribution to biliary fibrosis induced by BDL is likely minor.
Role of the Extracellular Matrix
The ECM modulates the interactions between epithelial cell and the stromal microenvironment. Signals derived from the ECM regulate differentiation, polarization, adhesion, migration, proliferation, and survival of surrounding cells.77 The ECM is formed by different structural components, including collagens, noncollagenous glycoproteins and proteoglycans, as well as by functional components, such as growth factors, cytokines, and MMPs, that are sequestered by ECM, and then released depending upon the needs. Several growth factors relevant for biliary remodeling, including TGF-β, are secreted as inactive precursors. Secreted TGF-β contains an amino-terminal sequence (the latency-associated peptide) that needs to be cleaved to allow activation of the cytokine. An additional protein, the latent TGF-β binding protein, links to the inactive cytokine before being secreted, and the complex is sequestered by ECM components (including fibronectin) thus functioning as reservoir. Activation of latent TGF-β is regulated, among others, by a class of transmembrane receptors mediating cell anchoring to distinct components of the ECM, the integrins. Integrins are expressed by several epithelial cell types, including cholangiocytes. Integrins are formed by different α–and β–subunits, which can variably assemble giving rise to at least 24 distinct heterodimers. Among them, α2β1 and αvβ6 are expressed by cholangiocytes, and are upregulated during tissue injury.78,79 The αvβ6 integrin, in particular, is involved in chronic wound healing processes leading to organ fibrosis.80 In addition to cholangiocytes, HSCs possess cell surface receptors enabling them to interact with other ECM components, such as galectins, by which they are activated.81,82
The role of the ECM in liver diseases is particularly complex; therefore, we will discuss only briefly some of the ECM changes that regulate epithelial– mesenchymal crosstalk in cholangiopathies. Changes in the ECM composition have been reported in different types of bile duct injury, in humans as well as in experimental models. Tenascin is an important component of the ECM during fetal development and oncogenesis.83 It possesses epidermal growth factor (EGF)- like repeats with high affinity for EGF receptor,84 and is able to induce proliferative and migratory activities. Tenascin expression is upregulated in cholestatic liver diseases.85–87 A transient expression of tenascin is found early after BDL in the rat, where it is restricted to the thin fibrotic areas characterized by brisk ductular reaction,87 indicating an involvement of tenascin in the modulation of reactive cholangiocytes/MFs interactions in the early phases of biliary fibrosis. In CCA, tenascin likely produced by stromal cells, is expressed in the intratumoral stroma as well as at the tumor–host interface.88
Heparan sulfate proteoglycans are structural components expressed both at the cell surface (syndecans, glypicans) and in the ECM (perlecans), which regulate several processes essential for liver repair, including cell adhesion to the ECM, cell–cell recognition and interactions. In chronic cholestasis a strong upregulation of syndecan-1, syndecan-3, and perlecan is found in reactive cholangiocytes as well as in HSCs.89 Glypican-3 may interfere with liver repair mechanisms by negatively regulating hepatocyte proliferation.90 Genome-wide association studies have identified the gene encoding for glypican-3 at chromosome 13q31, as a locus associated with primary sclerosing cholangitis (PSC). Silencing of glypican-6 induced a proinflammatory secretory profile in cholangiocyte cell lines.91
Yasoshima et al found that fibronectin expression is increased at the biliary cells basement membrane of PBC patients, and correlates with an accumulation of infiltrating lymphocytes into the biliary epithelial layer, that express integrin α4, a receptor of fibronectin. These findings suggest that integrin α4-fibronectin interactions at the basement membrane may promote epitheliotropism of lymphocytes in PBC.92 Alterations in matrix proteins composition of basement membrane are also observed in congenital hepatic fibrosis and Caroli disease, which are developmental cholangiopathies related to ductal plate malformations. They are characterized by cystic dilatation of aberrantly shaped bile ducts with exuberant portal fibrosis. In these conditions, laminin and type IV collagen, two main components of the basement membrane, are degraded along the bile duct profile.93
SIGNALING MECHANISMS REGULATING EPITHELIAL–MESENCHYMAL INTERACTIONS IN CHOLANGIOPATHIES
Following bile duct injury, several paracrine signals are mutually exchanged between the biliary epithelial and mesenchymal compartments. These signals may stimulate the mesenchymal compartment toward active fibrogenesis and angiogenesis, but also affect the cholangiocyte compartment resulting in ductular reaction and biliary remodeling. The molecular mechanisms underlying epithelial–mesenchymal interactions can be divided into two main categories: cytokines/growth factors and morphogenetic signaling pathways. Cellular and molecular mechanisms involved in the crosstalk, together with the corresponding biologic effects that are induced, are summarized in Table 1. Due to space limitations, some important signaling molecules, including bone morphogenetic proteins, will not be discussed.
Table 1.
Signaling Mechanisms Mediating Epithelial-Mesenchymal Interactions in Biliary Diseases
| Ligand | Receptor | Cell Source | Cell Target | Model/Disease | Biologic Effect |
|---|---|---|---|---|---|
| Hh | Patched | MF | BEC | BDL | BEC proliferation |
| BEC | EC | PBC | BEC EMT | ||
| MF | MF proliferation | ||||
| EC proliferation | |||||
| Wnt/β -catenin | Frizzled | BEC | BEC | BDL | BEC proliferation |
| HSC/MF | HSC | HSC activation | |||
| Jagged-1 | Notch2 | Portal mesenchymal cells | BEC | Development | Cell fate determination |
| Notch1 | AGS, BA | Ductal plate remodeling/ tubularization | |||
| Peripheral branching of biliary tree Ductular reaction | |||||
| HGF | Met | MF neutrophils | BEC | BDL | BEC proliferation |
| HSC | Inhibition of BEC EMT | ||||
| Inhibition of HSC activation | |||||
| PDGF-A/D | PDGFRα | BEC | HSC/PF | BDL | HSC activation |
| PDGFRβ | Pericyte | Up-regulation of Hh in HSC/BEC | |||
| BEC | Vascular tube formation/HSC and sinusoids | ||||
| TGF-β1/3 | TβRI | HSC/MF | HSC | BDL | HSC activation |
| TβR2 | EC | T cell | PBC, PSC | T cell activation | |
| KC | Cholangiocyte apoptosis? | ||||
| BEC | |||||
| VEGF-A/C | VEGFR1 | Ductal plate cells | Ductal plate cells | Development | Ductal plate remodeling |
| VEGFR2 | BEC | BEC | ADPKD | BEC/EC proliferation | |
| HSC | HSC | Caroli, CHF | Cyst growth | ||
| EC | Pericystic angiogenesis | ||||
| Pericytes | HSC activation | ||||
| Ang1/Ang2 | Tie2 | BEC | BEC | Development | Arterial neovasculogenesis |
| EC | ADPKD | Vascular remodeling | |||
| Pericytes | Caroli, CHF | BEC proliferation? | |||
| FGF-2 | FGFR1 | Monocytes | BEC | BA | Biliary susceptibility to EMT |
| FGFR2 | |||||
| SDF-1 | CXCR4 | Ductal plate cells | T cell | Development | Ductal plate remodeling |
| BEC | CCA cells | PBC, PSC | T cell recruitment | ||
| CAF | Mesenchymal stem cell | CCA | CCA cell invasiveness | ||
| Mesenchymal stem cell recruitment | |||||
| CTGF | α5β1 integrin | BEC | HSC | BDL | HSC activation |
| HSC/MF | CHF | ||||
| Mast cells | BA | ||||
| CCA cells | CCA | ||||
| CAF |
ADPKD, autosomal dominant polycystic kidney disease; AGS, Alagille syndrome; Ang, angiopoieitin; BA, biliary atresia; BEC, biliary epithelial cell; BDL, bile duct ligation; CAF, cancer-associated fibroblast; CCA, cholangiocarcinoma; CHF, congenital hepatic fibrosis; CTGF, connective tissue growth factor; EMT, epithelial mesenchymal transition; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; HGF, hepatocyte growth factor; Hh, Hedgehog; HSC, hepatic stellate cell; KC, Kupffer cell; MF, myofibroblast; PBC, primary biliary cirrhosis; PDGF, platelet-derived growth factor; PDGFR, platelet-derived growth factor receptor; PF, portal fibroblast; PSC, primary sclerosing cholangitis; SDF-1, stromal cell-derived factor 1; TGF-β, transforming growth factor-β, TβR, TGF-breceptor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; Wnt, Wingless.
Cytokines and Growth Factors
HEPATOCYTE GROWTH FACTOR (HGF)
HGF, or scatter factor, is a multifunctional protein, originally identified as a mitogen for hepatocytes, and then recognized as a potent growth factor for cholangiocytes as well.94 In BDL, HGF expression by periductal inflammatory and stromal cells (mainly MF and neutrophils) increases early after induction of obstructive cholestasis. HGF binds to the Met receptor expressed by reactive cholangiocytes and stimulates proliferation.4 When exposed to HGF, human cholangiocyte colonies cultured into collagen gels, develop irregular projections that invade the collagen and form anastomosing networks that resemble clusters of reactive cholangiocytes,95 suggesting that HGF production is one of the mechanisms by which mesenchymal cells promote the ductular reaction. On the other hand, studies in experimental liver fibrosis models raise the possibility that HGF may have an antifibrotic effect.96,97 In fact, HGF also antagonizes TGF-β -driven gene transcription of type I collagen in activated HSCs (see below) by promoting nuclear export of Smad-3 and its interaction with galectin-7, a factor belonging to a family of carbohydrate- binding proteins critical for HSC activation.98 In addition, it has been proposed that HGF attenuates liver fibrosis, by inhibiting cholangiocyte transition toward a mesenchymal phenotype.99 This interesting hypothesis will have to await a better understanding of the role of EMT in cholangiopathies57 (see below).
PLATELET-DERIVED GROWTH FACTOR (PDGF)
PDGF is a family of four polypeptide-chains (A, B, C, D) assembling in four homodimers (AA, BB, CC, DD) and heterodimer (AB). PDGF ligands exert their effects via binding to specific tyrosine kinase receptors, which are dimers of two different -α and -β subunits. Whereas PDGFRα binds to PDGF-A, -B, and -C, PDGFRβ is specific for PDGF-B and -D. PDGF is recognized as the most potent mitogen for HSCs. By stimulating HSC proliferation and migration, PDGF induces their transdifferentiation into MFs.100,101 PDGF-B is expressed by reactive cholangiocytes following BDL in rat, while its receptor PDGFRβ is restricted to periductular HSCs, and its expression increases over time, after induction of cholestasis.12 PDGF-B stimulates chemotaxis of HSCs toward bile ducts in the BDL rat,49 and proliferation of HSCs before their phenotypic conversion into MFs.102 PF conversion into portal MFs is also stimulated by PDGF-B.55 Downstream molecules of PDGF signaling in HSCs include phosphoinositide 3 (PI3) kinase and extracellular signal-regulated kinase-5 (ERK5), among others.103,104 Interestingly, PDGF-B-induced HSC chemotaxis is associated with multiple elongated peripheral protrusions that enable HSCs to detect localized concentration of PDGF and to transduce chemical gradient into mechanical forces driving migration.105 Available data support a central role for PDGF-B in biliary repair (Figs. 3A, 3B). PDGF-D is also emerging as a potent and physiologically relevant PDGF subunit in HSC activation, although its specific role in biliary fibrosis has not yet been investigated.106 In addition to fibrogenesis, PDGF is also involved in the regulation of hepatic vascular structure and function. In fact, PDGF-B induces HSCs to acquire an angiogenic phenotype similar to pericytes. PDGF-B promotes HSC-driven vascular tube formation in vitro and HSC coverage of sinusoids in vivo. PDGF-B-driven effects on pericytes are mediated by the multifunctional ephrin-B2 receptor tyrosine kinase.32
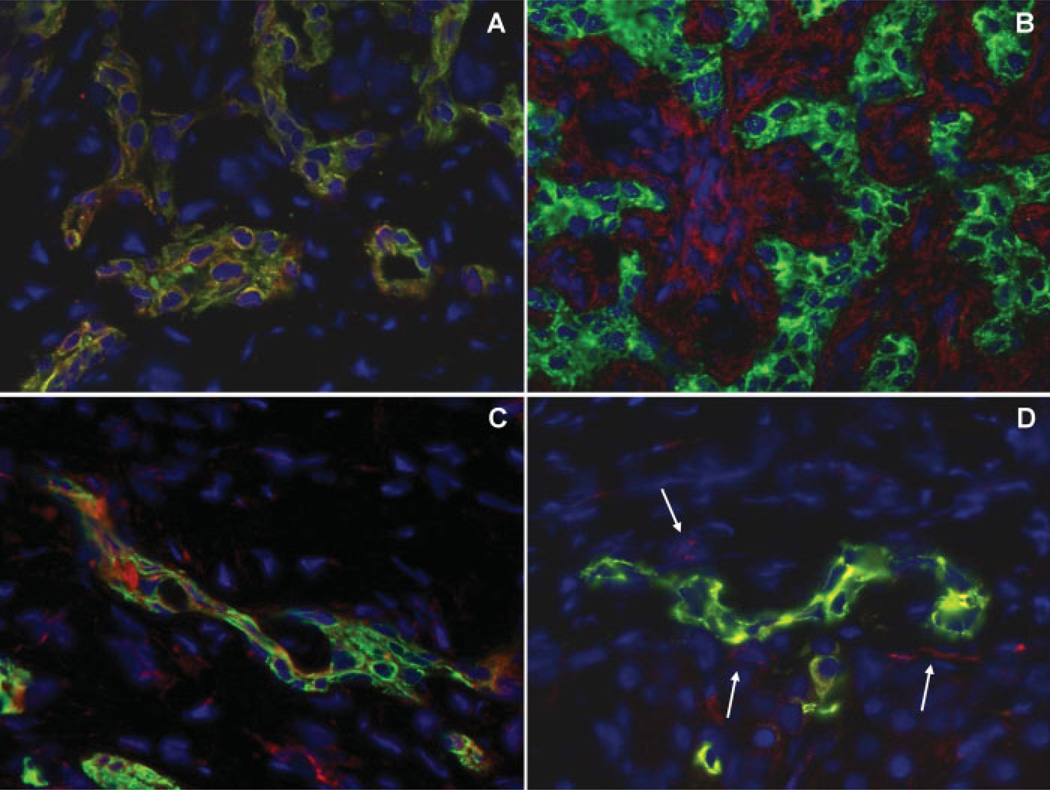
Figure 3.
Platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) are strongly expressed by reactive cholangiocytes and mediate paracrine communications with mesenchymal cells. Dual immunofluorescence of the biliary cell marker K19 (green fluorescence) with PDGF-B (red fluorescence, A) and with PDGFR-β (red fluorescence, B) shows strong expression of PDGF-B on reactive cholangiocytes (coincident staining, yellow fluorescence), while its cognate receptor PDGFR-β extensively decorates multiple mesenchymal cells in the portal tract, in proximity to K19-positive ductular structures. Reactive cholangiocytes stained by K19 (green fluorescence) also co-express VEGF (coincident staining, yellow fluorescence, C), that signals to VEGFR-2 expressed by fibroblasts and endothelial cells adjacent to reactive ductules (paracrine loop, arrows) and also by reactive cholangiocytes themselves (autocrine loop) (single staining, red fluorescence; coincident staining, yellow fluorescence, D). These findings are consistent with a central role played by PDGF and VEGF in biliary repair, as seen in these tissue samples derived from a patient with biliary atresia undergoing liver transplantation. (Original magnification A-D: ×400)
TRANSFORMING GROWTH FACTOR-β (TGF-β)
TGF-β exists in three different isoforms (-β1, -β2, -β3). Among them, TGF-β1 is the best characterized and is currently considered the most potent fibrogenic cytokine in the liver. A full description of the pleiotropic functions of TGF-β is beyond the scope of this review and we will briefly discuss some aspects relevant to epithelial–mesenchymal interactions in cholangiopathies. TGF-β is known to stimulate HSC activation and matrix production, including type I collagen. TGF-β also regulates the balance between MMPs and their inhibitors, modulates T-cell functions, and promotes liver cell apoptosis. TGFβ is secreted as latent complex that is .“stored.” by several ECM components, including fibronectin. After its activation, TGF-β 1 binds to the TGF-β type II receptor, then recruits TGF-β type I receptor, which in turn phosphorylates Smad proteins, leading to their nuclear translocation.107 Once delivered into the nucleus, Smad2 and Smad3 regulate the transcription of target genes, among which are COL1A1 and COL1A2 that encode for the α1 and α2 chains composing type I collagen. Transcriptional activity of Smad2 and Smad3 requires the transcriptional co-activators p300 and CBP and is negatively regulated by Smad7.108
Following liver injury, TGF-β1 production is strongly upregulated in multiple cell types (mainly HSCs, but even ECs and KCs) and stimulates HSC activation via both autocrine and paracrine loops. In cholestatic liver diseases, cholangiocytes themselves acquire the capability to produce both TGF-β1 and TGF-β2. Actually, after BDL, TGF-β2 expression appears to increase more than that of TGF-β1. The biologic effects of TGF-β2 are far less characterized than those of TGF-β1.109 Overall, the contribution of cholangiocytes to TGF-β production is less important with respect to other fibrogenic cytokines, such as PDGF-B, CTGF,14 and MCP-1.53 The effects of TGF-β on cholangiocytes and hepatic progenitor cells are not well known. One study suggested that HPC are less sensitive than hepatocytes to the proapoptotic effects of TGF-β, a mechanism that would favor their relative expansion in liver fibrosis. Our unpublished observations in cholangiocyte and hepatic progenitor cell lines suggest that TGF-β maintains an apoptotic effect (Strazzabosco, unpublished observations).
VASCULAR ENDOTHELIAL GROWTH FACTOR AND ANGIOPOIETINS
VEGF and angiopoietins are among the main regulators of vasculogenesis and angiogenesis during vascular development and remodeling. VEGF is a member of a family of related growth factors that includes VEGF-A, -B, -C, -D, and -E and placenta growth factor.110 VEGF can interact with three tyrosine kinase receptors, VEGFR-1 (Flt-1), VEGFR-2 (Flk-1/KDR), and VEGFR-3.110,111 The expression of VEGF is not restricted to vascular ECs. Cholangiocytes, HSCs, and ECs may express VEGF and its cognate receptors112 during biliary repair and remodeling. Angiopoietins, namely angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2), are a different family of vascular growth factors that act in concert with VEGF to promote the remodeling, maturation, and stabilization of blood vessels. Angiopoietins bind to the Tie-2 receptor, a tyrosine kinase expressed by ECs, together with VEGF receptors.111 Ang-1 and Ang-2 have opposite effects on Tie-2: whereas Ang-1 activates Tie-2 by inducing its tyrosine phosphorylation, Ang-2 antagonizes the Ang-1/Tie-2 binding. Therefore, the level of Tie-2 activation is determined by the relative balance between Ang-1 and Ang-2. On the other hand, Tie-1 is essential to maintain the structural integrity of the EC layer.112 VEGF and VEGF-receptors and angiopoietins are expressed by biliary cell progenitors (ductal plate cells) during development, when they have an important role in the regulation of liver arterial neovasculogenesis.113 In several forms of liver diseases, cholangiocytes regain the ability to produce VEGF (Figs. 3C, 3D). VEGF is secreted by cholangiocytes that also express VEGFR-2 and VEGFR-3. After BDL, both VEGF and its cognate receptors are upregulated in cholangiocytes, and stimulate proliferation through the MEK/ERK1/2 pathway.9,10,114,115 Ang-1 has a synergic effect with VEGF on cholangiocyte proliferation.10 VEGF-induced cholangiocyte proliferation may therefore represent an adaptive response to obstructive cholestasis.
We have recently shown that VEGF and Ang-1 are markedly upregulated in biliary microhamartomas and cysts of polycystic liver diseases,10 which are developmental cholangiopathies related to malformation of the ductal plate. These cholangiopathies are caused by mutations in one of two genes, PKD1 (85–90%) or PKD2 (10–15%) encoding for two primary cilia proteins, polycystin-1 and polycystin-2, respectively.116,117 Polycystins act as mechanoceptors and Ca2+ channels, able to sense changes in apical flow and are involved in epithelial cell proliferation, differentiation, and secretory processes. Cholangiocytes isolated and cultured from these cysts secrete VEGF and proliferate in response to VEGF indicating that VEGF is crucial for the progression of polycystic liver disease via autocrine stimulation of cholangiocyte proliferation and paracrine promotion of pericystic angiogenesis and fibrogenesis.10 In cholangiocytes of the cysts, there is crosstalk between the MEK/ERK1/2 pathway and the mTOR pathway increasing HIF1α and VEGF expression. The MEK/ ERK1/2 pathway is also involved in VEGFR-2 signaling and the proliferative effects of VEGF. In fact, administration of a competitive inhibitor of VEGFR-2 inhibits the growth of liver cysts in vivo, reduces the proliferative activity of the cystic epithelium, and the phosphorylation of ERK1/2.114 In addition to stimulating angiogenesis, VEGF may also contribute to liver fibrosis. In fact, VEGF, acting mainly through VEGFR- 2, stimulates proliferation of activated HSCs and increases their expression of α1 (I)-procollagen mRNA.118 The VEGF effects on HSCs can be driven by hypoxia. In fact, VEGF and to a lesser extent, Ang-1 are both hypoxia-dependent factors stimulating in autocrine and paracrine fashion, the migration and chemotaxis of human HSCs/MFs through the Ras/ERK pathway.63 The combination of these factors plays a significant role in the formation of the fibrovascular walls of the cysts in polycystic liver disease.119
STROMAL CELL-DERIVED FACTOR-1
SDF-1 (also termed CXCL12) is a cytokine with chemoattractant properties for monocytes, lymphocytes, hematopoietic stem cells, and B cell precursors.120,121 CXCR4 is the only known receptor for SDF-1.122 SDF- 1 is expressed by ductal plate cells during embryonic development (Fig. 4A)123 and its expression is then maintained in the normal biliary epithelium in the adult.5 SDF-1 is expressed also in reactive cholangiocytes; therefore, the mass of cells producing SDF-1 greatly increases during liver repair and facilitates recruitment and homing of CXCR4-positive cells (Fig. 4B). For example, SDF-1 is involved in the recruitment of CD34-positive hematopoietic stem cell to the liver. In immune-mediated cholangiopathies, such as PBC and PSC, SDF-1 is selectively upregulated in cholangiocytes, likely mediating the recruitment of CXCR4-positive infiltrating T lymphocytes around bile ducts.5 Recent data indicate that also HSC express functional CXCR4 receptor, and administration of SDF-1 stimulates HSC activation, proliferation, and production of collagen type I.67 Trafficking to the liver of CXCR4/CD34-positive hematopoietic stem cells is an additional SDF-1-dependent mechanism of liver repair.124 SDF-1 also is expressed by CAF, particularly at the invasive front, where it mediates paracrine communications with CXCR4- positive CCA cells, enhancing their invasive properties.125,126
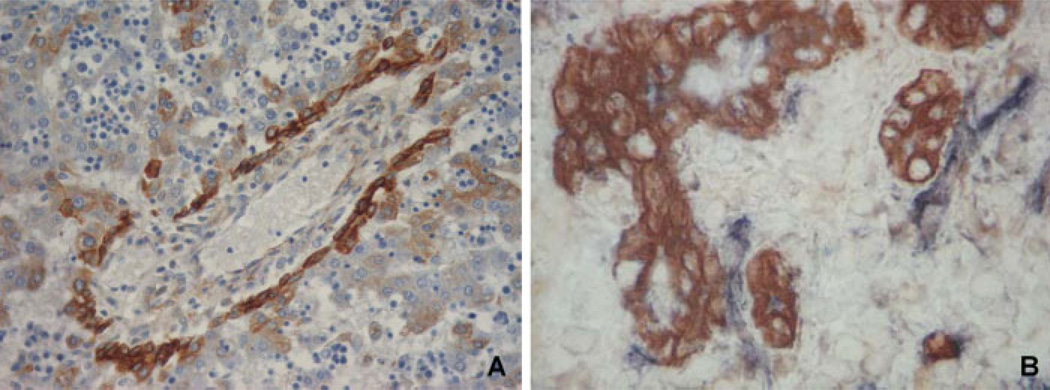
Figure 4.
SDF-1/CXCR4 crosstalk in biliary repair. Immunohistochemistry for SDF-1 shows its strong expression by ductal plate cells, starting from the earliest maturation ages when ductal plates are still organized as single layer cord, as seen in this sample obtained from abortive material at 16-gestation weeks (A, Original magnification ×200). Dual immunoperoxidase staining for SDF-1 (brown color) and CXCR4 (blue) in a tissue sample obtained from a patient with drug cholestatic injury, shows their expression is finely balanced in the epithelial and mesenchymal compartment (B, Original magnification ×400). Whereas SDF-1 is expressed by reactive cholangiocytes, CXCR4 is expressed by strictly adjacent mesenchymal cells. Given the brisk ductular expansion following biliary damage, reactive cholangiocyte-derived SDF-1 greatly increases and therefore facilitates recruitment and homing of CXCR4-positive cells, among which are fibrocytes.
CONNECTIVE TISSUE GROWTH FACTOR
CTGF (also known as CCN2) has PDGF-like chemotactic and mitogenic functions on fibroblasts.127 CTGF belongs to a family of early growth-responsive CCN genes (such as cyr61 and fisp-12 in mouse) that regulate cell proliferation and differentiation.128,129 CTGF expression is strongly upregulated by TGF-β 1.130 CTGF promotes proliferation and collagen production in HSCs.131 Proliferative effects induced by CFGF in HSCs are associated with transient induction of c-fos with activation of the ERK1/2 signal pathway.132 Reactive cholangiocytes are one of the main sources of CTGF in experimental BDL.14,133 Gene expression of CTGF in reactive cholangiocytes correlates with that of TGF-β 1.14 Recent studies suggest an important role of CTGF in cholangiopathies characterized by extensive fibrosis, such as biliary atresia (BA).134 CTGF is also likely to be one of the factors involved in the generation of the desmoplastic reaction in CCA. Because of its strong association with both tumor recurrence after surgical resection and overall survival,135 CTGF expression has been proposed as a prognostic marker in patients with intrahepatic CCA.
Morphogens
HEDGEHOG
Hedgehog signaling (Hh) is an important pathway involved in developmental processes, repair mechanisms, and cancer. In normal conditions, the ability to respond to Hh signals is a feature of stromal and progenitor cell populations, but not of mature epithelial cells. Hh ligands (such as sonic Hh) interact with patched (Ptc), a membrane receptor expressed by Hh-responsive cells, and prevent its inhibitory action on smoothened (Smo), the Ptc coreceptor. Following Smo activation, a series of intracellular events is triggered, leading to nuclear import and activation of downstream targets, including glioblastoma (Gli) transcription factors, and Hh-responsive genes (Ptc, Gli1, Gli2). Hh signaling is abolished by HIP (Hh-interacting protein), which interferes with the binding of Hh ligands to Ptc.
In HSC, Hh signaling activity is low due to the relatively high levels of HIP. In contrast, activated MFs show enhanced production of Hh ligands due to downregulation of HIP.136 Hh ligands released by MFs activate Hh signaling in adjacent Hh-responsive cells, such as ECs, reactive cholangiocytes, and liver progenitor cells.137 The mechanisms of Hh-dependent crosstalk between MFs and cholangiocytes have been studied using in vitro co-cultures. In this system, paracrine Hh signaling derived from MFs strongly affects cholangiocyte function, inducing expression of mesenchymal markers and motile properties. Vice-versa cholangiocyte- derived Hh ligands promote growth of MFs.24,138 In transgenic mice with increased Hh activity, BDL induces a parallel marked expansion of both MFs and reactive cholangiocytes.24,138 Expression of Hh ligands and Hh target genes by bile ductules and stromal cells was also reported in PBC.139
Recent evidence indicates that PDGF-B is a potent inducer of the Hh pathway after biliary obstruction. PDGF-B secreted by reactive cholangiocytes or infiltrating cells, would increase Hh production in HSC, and the paracrine loop would then promote the acquisition of EMT features by reactive cholangiocytes and MF.136,140 On one hand, in MFs, the Hh pathway enhances the proliferative effects of PDGF-B, through an AKT-dependent mechanism.136 On the other hand, in cultured cholangiocytes, PDGF-B induces Hh expression140 and also stabilizes the Hh-trancription factor Gli2 while repressing the Hh antagonist HIP.140
In summary, several data indicate that Hh signaling is one of the most important mediators of epithelial– mesenchymal crosstalk; this system is of particular therapeutic interest also because it is not active in normal liver epithelial cells.
WNT/β -CATENIN
Wnt/β-catenin signaling is a highly conserved pathway involved in the regulation of proliferation, differentiation, and polarity/migration of different cell types.141 In normal epithelia, β-catenin is bound to the cadherin complex to form the adherens junctions, where it helps to maintain the polarization of the epithelial sheet, clasping the actin cytoskeleton. In the absence of Wnt ligands (inactivate state), cytoplasmic β-catenin undergoes phosphorylation and subsequent proteosomal degradation. However, when Wnt binds to its receptor Frizzled (activated state), β-catenin phosphorylation is blocked; β-catenin can thus accumulate in the cytoplasm and translocate into the nucleus where it acts as transcription factor for TCF/LEF that regulates down-stream target-gene expression.
Wnt/β-catenin activation suppresses E-cadherin levels and induces expression of cyclin D1, a cell cycle protein promoting cell proliferation.141 In obstructive cholestasis (BDL), activation of the canonical Wnt/βcatenin pathway by Wnt3a and/or Wnt7b induces cholangiocyte proliferation through activation of cyclin D1.142 Cultured cholangiocytes show enhanced cell survival and increased proliferation under recombinant Wnt3a treatment.143 Wnt/β-catenin is also important in biliary differentiation. When added to explanted mouse embryos, Wnt3a induces a biliary phenotype with ductlike arrangement in the developing liver, while its suppression causes loss of architecture, proliferation, and increased apoptosis in hepatoblasts.144 In agreement with these data, activation of β-catenin signaling in hepatoblasts promotes bile duct morphogenesis.145
Wnt ligands and receptors are also expressed and functional in activated HSCs146,147 and are induced in experimental cholestasis, suggesting that the Wnt pathway is involved in the transdifferentiation of HSCs into MFs.148 Several cytokines relevant for HSC activation, including TGF-β, PDGF, and EGF, stimulate the expression of Wnt ligands. Thus, the Wnt/β-catenin pathway represents a common signaling pathway mediating both cholangiocyte proliferation and HSC activation in cholangiopathies. Wnt can also signal through βcatenin- independent pathways (noncanonical Wnt). In these pathways, Wnt 4, 5a, and 11 bind to the Frizzled receptors to activate Dishevelled, but the downstream signaling involve small GTPases and the C-Jun Nterminal kinase (JNK) instead of β-catenin. Both canonical and noncanonical Wnt pathways seem to be involved in the regulation of cell migration, and in maintaining a uniform orientation of cell division within an epithelial plane, a phenomenon known as plannar cell polarity.149 Recent evidence indicates that this role is relevant for developmental processes in the renal tubular epithelium, and that the disruption of both canonical and noncanonical Wnt pathways leads to cyst formation in the kidney.150 However, whether these pathways are involved in plannar cell polarity in biliary epithelium is currently unknown.
NOTCH
Notch signaling is a fundamental mechanism that regulates cell fate determination during the development of various tissues and organs.151 Through a process of .“lateral induction,.” Notch may stimulate cells to undergo a phenotypic switch; through a .“lateral inhibition,.” Notch may prevent further differentiation, and promote the maintenance of the original phenotype.152 Notch signaling can also delineate boundaries or establish niches necessary to maintain specific cell types.153 In adult tissue, Notch can influence cell fate determination by inhibiting organ-specific stem cells from further differentiation or by blocking the default differentiation pathway to favor alternative pathways.154 The Notch genes encode for four transmembrane receptors (Notch 1, 2, 3 and 4), which can interact with several ligands (Jagged-1, Jagged-2, Delta-like1, 3, and 4).155
The main feature that distinguishes Notch signaling from the other mechanisms discussed in this review is that Notch requires cell–cell contacts.156 Notch receptors expressed by .“receiver.” cells are activated by their binding with ligands, such as Jagged-1, expressed on adjacent .“transmitter.” cells. These features make Notch signaling particularly well suited for the fine-tuning of morphogenetic signals, typical of development, and of biliary remodeling. Activation of Notch receptors stimulates downstream signaling molecules, such as RBP-Jk and Hes-1, Hes5, and Hey-1. RBP-Jk is a transcription regulator common to all Notch receptors that is critically important for the regulation of biliary commitment.157 Hes-1 is a transcription factor belonging to the Hairy/ Enhancer-of Split family, crucial to trigger ductal plate remodeling and tubularization.158 Studies in mice have shown that during the neonatal period Jagged-1 is expressed in the portal mesenchyma, whereas Notch2 expression is observed in biliary epithelial cells adjacent to the Jagged-1-positive cells.159 Mutations in the genes encoding for Jagged-1160–162 cause Alagille syndrome (AGS), a cholangiopathy characterized by cholestasis, lack of intrahepatic bile ducts, and a wide range of extrahepatic manifestations.155,163,164
Notch also interacts with the Wnt, Hh, and TGFβ pathways at multiple levels.165,166 These are complex interactions that, for the most part, have not been explored in biliary disease and physiology. Their discussion is beyond the scope of the present review, but as our understading of paracrine signaling in biliary physiology progresses, their elucidation will become a priority.
In several tissues and organs, such as gastrointestinal epithelium,167 pancreas,168 mammary gland,169 bone marrow,170 central nervous system,171 skeleton,172 and dental epithelium,173 Notch signaling is involved in the maintenance of the stem cell niche. In this compartment, stem cell retention and expansion is regulated by direct interactions between stem cells and supporting cells, which entail juxtacrine activation of Notch signaling.174 In the liver, Notch signaling activation seems to be associated with biliary repair, rather than with maintaining the stem cell quiescence. In human liver diseases, both Notch2 and Jagged-1 appear to be expressed in reactive cholangiocytes.175 A recent study characterizing gene expression profiles in reactive cholangiocytes from a variety of liver disease, found increased expression of Jagged1, Jagged2, and Notch2 in reactive cholangiocytes from PBC, as compared with hepatitis C virus (HCV) hepatitis.176 In sharp contrast with BA, we have found that AGS is characterized by a marked reduction in reactive cholangiocytes and hepatic progenitor cells.177 This difference is likely related to a Notch-dependent block in cell fate determination upstream of HNF1β. Notably, differences in the pattern of ductular reaction between AGS and BA are associated with a different pattern and severity of liver fibrosis that is much more pronounced in BA.177 Data from our group show that liver repair and tubule formation is dramatically altered in mice with liver-specific RBPj-Jk defect exposed to cholestatic agents.178 These observations, strongly suggest that Notch may be an important modulator of liver repair in liver disease, a hypothesis that is worth investigating further.
The role of Notch signaling in HSCs and portal fibroblasts has not been systematically addressed yet. A recent article179 suggests, that along with laminin, SDF- 1, and Wnts, Jagged-1 expression by parenchymal cells continues to maintain the quiescent state of HSCs in the space of Disse, although the evidence for an involvement of Notch were only circumstantial. On the contrary, preliminary data from our group (Strazzabosco, unpublished) indicate that HSCs are activated following exposure to Jagged-1.
EPITHELIAL TO MESENCHYMAL TRANSITION (EMT) IN CHOLANGIOPATHIES
As discussed above, reactive cholangiocytes establish paracrine communications with mesenchymal cells to modulate the reparative process. Several authors have reported a direct correlation between the extent of ductular reaction and that of liver fibrosis. Cholangiocytes are also believed to participate in the generation of liver fibrosis by undergoing EMT, which is a process of cellular reprogramming whereby epithelial cells lose their original identity and acquire some of the phenotypic and functional features of mesenchymal cells. These include (1) the expression of fibroblast-specific markers (for example, the fibroblast specific protein-1 (FSP-1), as well as vimentin); (2) the ability to migrate, which requires loosening up the epithelial tight junctions and locally dismantling the basement membrane; and (3) the ability to produce ECM components such as collagen, fibronectin, elastin, and tenascin. EMT is a wellrecognized phenomenon in the kidney180 and in the lung,181 where it is thought to be involved in the pathogenesis of organ fibrosis. EMT leading to organ fibrosis has been recently defined as type 2 EMT to be distinguished from EMT occurring during development (type 1) or in cancer progression (type 3).182
Several studies suggest that EMT may also play a role in liver fibrosis.183,184 Functional markers of EMT have been detected in cholangiocytes in several liver diseases and cholangiopathies, as well as in culture. In a variety of human chronic liver diseases, including PBC and PSC, cholangiocytes lining small- and medium-sized bile ducts and reactive ductules show phenotypic markers of ongoing EMT (expression of FSP-1, and of phosphorylated forms of Smad2/3 in the nucleus, reduction of E-cadherin expression). It has been suggested that in BA, biliary EMT may be induced by biliary innate immunity response to double-stranded RNA genome of reovirus, and thereby contribute to the development of biliary fibrosis.184 Furthermore, in the polycystic kidney rat, an animal model of congenital hepatic fibrosis (CHF) and Caroli disease, cholangiocytes displaying mesenchymal features (flat-shaped morphology, reduced K19 expression and induced expression of vimentin, fibronectin, and collagen in response to TGF-β1) contribute to progressive portal fibrosis by producing ECM components, even though a full mesenchymal conversion characterized by the gain of immunoreactivity for α-SMA was not found.183 Features of EMT have also been observed in CCA, where EMT is induced in neoplastic cholangiocytes by TGF-β1/Snail activation and is associated with an enhanced invasive growth.185
Despite these observations, as pointed-out by Wells, the actual impact of EMT in liver diseases remains unclear and controversial.57 Morphologic studies with surrogate biomarkers revealing loss of epithelial cell polarity, acquisition of mesenchymal traits, and breakdown of the basement membrane, can only provide a suspicion, but do not prove the existence of a process that is dynamic. Furthermore, EMT biomarkers have several potential technical pitfalls and lack specificity. For example, the widely used FSP-1, originally proposed as an early marker of mesenchymal switch,186 is also expressed by leukocytes and macrophages.187 In vivo lineage-tracing experiments as performed by Taura using the model of hepatic fibrosis induced by CCl4188 is currently the best way to properly address the question. Unfortunately, such studies have not been performed yet in cholangiopathies.
The controversy on EMT and liver fibrosis is mainly a matter of definitions. Although, there is no demonstration that .“full.” transdifferentiation to the mesenchymal phenotype, marked by the acquisition of the α-SMA immunoreactivity, actually occurs in cholangiopathies, it is clear that reactive cholangiocytes express several morphologic and functional markers commonly associated with a mesenchymal phenotype. This is a fundamental property of reactive cholangiocytes and enables them to participate in biliary remodeling. In fact, to repair the epithelial wound, cholangiocytes need to reduce the strength of their tight junctions, to acquire motile properties, to be able to process and remodel the ECM, and to communicate with several other cell types. In other words, to help resolve the controversy, we propose that following bile duct injury, reactive cholangiocytes, undergo a “partial EMT” process that enable them to acquire several mesenchymal cell properties that are essential for the reparative process.
EPITHELIAL-MESENCHYMAL INTERACTIONS IN DEVELOPMENT
Epithelial–mesenchymal interactions play a crucial role during embryonic development. Signals exchanged between epithelial and mesenchymal cells induce the differentiation of the intrahepatic biliary epithelium and later coordinate morphogenesis of the bile ducts and that of the portal vasculature. In contrast to cholangiocytes lining the extrahepatic portion of the biliary tree that are directly formed from the endoderm, intrahepatic cholangiocytes derive from hepatoblasts. Biliary ontogenesis starts when the periportal hepatoblasts, in contact with the portal mesenchyme surrounding a portal vein branch, switch their phenotype and begin to organize into a single-layered cord of small flat epithelial cells, called ductal plates. These cells can be identified by their positivity to K19 and Sox9.
Studies fromzebrafish and mouse models157,189,190 indicate that Jagged-1, expressed by periportal mesenchymal cells, interacts with Notch-2 expressed by hepatoblasts promoting a phenotypic switch. Jagged-1 expression by periportal mesenchyme is considered a crucial step in differentiating the periportal leaflet of the duplicated plate, from its parenchymal layer. Only the former will generate the mature bile ducts. In fact, ductal plates are first duplicated by a second layer of cells over variably long segments of their perimeter, and then begin to assume the shape of a tubular structure in the process of being incorporated into the mesenchyma of the nascent portal space. Perturbations in the Jagged-1/Notch-2 interactions are clinically manifested in AGS, characterized by ductopenia resulting from a defect in the peripheral branching of the biliary tree,191 in absence of significant ductular reaction and activation of hepatic progenitor cells.177
During development, the periportal mesenchyme generates a portal to parenchymal gradient of TGF-β that stimulates hepatoblast differentiation.192 TGF-β2 and TGF-β3 are predominantly expressed in the periportal mesenchyme, in close vicinity to developing ductal plates.190 Cultured hepatoblasts switch toward a biliary phenotype under TGF-β treatment.190 TGF-β effects on ductal plates are mediated by the TGF-β receptor type II (TβRII). TβRII is transitorily expressed during ductal plate remodeling, being repressed once cholangiocyte differentiation has been achieved.190 Canonical Wnt/β-catenin also participates to bile duct development. Specific Wnt ligands, such as Wnt3a, induce biliary differentiation, characterized by the appearance of K19 positivity and generation of duct-like structures in mouse embryonic liver cell cultures.145 In particular, the role of Wnt/β-catenin is crucial for biliary commitment, by repressing the hepatocyte genetic program and by promoting in turn the ductal plate remodeling.146
The patterning of intrahepatic biliary tree develops in strict conjunction with hepatic arteriogenesis and is nourished by the peribiliary plexus originating from the hepatic artery. VEGF produced by the developing bile ducts is a likely signal linking ductal and arterial development in the liver. By secreting VEGF, which acts on EC and on their CD34-positive precursors, ductal plate cells promote arterial vasculogenesis. At the same time, Ang- 1, produced by hepatoblasts, induces artery maturation by recruiting α_SMA-positive mural pericytes to the nascent endothelial layer.113 A failure in the remodeling of the ductal plate results in excess of bile duct structures retaining a fetal configuration (ductal plate malformation), which are strictly associated with an abnormal ramification of the portal vasculature, featuring a .“pollard willow.” pattern.165 Interestingly, in cystic cholangiopathies related to ductal plate malformation, such as the liver phenotype of ADPKD and Caroli disease, the biliary epithelium retains an immature phenotype characterized by an upregulation of VEGF and angiopoietins. Therefore, angiogenic factor production by immature cholangiocytes may promote the aberrant vascularization around the cyst to provide its vascular supply, a mechanism reminiscent of fetal biologic behavior.10
EPITHELIAL-MESENCHYMAL INTERACTIONS IN BILIARY CANCER
Cholangiocarcinoma (CCA), an aggressive cancer with early invasiveness and limited opportunities for curative treatments, is characterized by an abundant desmoplastic reaction.193 This histopathologic feature, originally referred to as .“tumoral desmoplasia,.” has been recently redefined as .“tumor reactive stroma.” (Fig. 5), to underline the functional interactions between cancer cells and the cells populating the stromal microenvironment (fibroblasts, immune and inflammatory cells, fat cells, and blood vessel cells). Tumor reactive stroma plays a pivotal role in cancer invasiveness and progression.194,195 Cancer-associated fibroblasts (CAFs) provide cancer cells with proliferative and antiapoptotic signals, leading to cancer growth and metastatic spread. CAFs and cancer cells communicate using many of the abovedescribed mechanisms. Both cell types, in fact, secrete growth factors, chemokines, and proteases (TGF-β, IGF1, SDF-1, HGF, VEGF, NGF, WNT1, MCP1, MMPs), which favor tumor invasiveness, tumor cell survival, migration, and invasion. Recent data indicate that stromal-derived Hh signaling can support tumor growth, likely affecting biologic activities of different soluble factors in the tumor microenvironment, such as IGF1 and WNT.196 CAFs also communicate with ECs, promoting tumor neoangiogenesis. Recently, CAFs have been found to contribute also to the inflammatory reaction to the tumor in a NF-κB dependent manner.197 CAFs may derive from the recruitment of resident fibroblasts,198 from circulating mesenchymal progenitor cells,199 or from EMT of carcinoma cells.200,201 Among the molecular mechanisms able to mediate recruitment and phenotypic conversion of mesenchymal cells into CAF, are members of the TGF-β and PDGF families.194,195 Preliminary data from our laboratory indicate that PDGF-D secreted by cancer cells is able to recruit PDGFRβ-positive CAF in the tumor-reactive stroma of CCA (Fabris, Strazzabosco, unpublished).
Figure 5.
Abundant CAF enrichment in cholangiocarcinoma. Dual immunofluorescence for the cholangiocyte marker K19 (green fluorescence) and the CAF marker α-SMA (red fluorescence) in a tumoral sample of cholangiocarcinoma obtained from surgical resection. Extensive recruitment of CAF is observed among neoplastic bile ducts, leading to the formation of a rich tumor reactive stroma that represents a distinctive feature of CCA. This feature is relevant for cancer growth, given the ability of CAF to provide cancer cells with proliferative and antiapoptotic signals. (Original magnification ×200)
CONCLUSIONS
Epihelial–mesenchymal interactions are essential for the development and function of the biliary tree. These mechanisms are also at the basis of liver repair and biliary remodeling in cholangiopathies. In addition, the interactions between cancer cells and tumor-associated stroma influence the biologic behavior of CCA, including its invasiveness and poor prognosis.
In chronic biliary diseases, the inflammatory reaction to bile duct damage sets in motion a series of events leading to the secretion, by a variety of cells, of cytokines such as TNFα, IFNγ, IL-6, MCP-1, IL-8, IL-1, and later on TGF-β, CTGF, PDGF, etc. These cytokines, chemokines, and growth factors are produced not only by macrophages, lymphocytes, and leukocytes, but also by mesenchymal cells, ECs, and .“reactive cholangiocytes..” These are biliary cells with a less-differentiated phenotype that form structures devoid of a lumen, but in contiguity with cholangioles. Reactive cholangiocytes have no known bile secretory functions, but have acquired several mesenchymal cell properties, including enhanced motility, a phenomenon that can be called partial EMT. The role of cholangiocytes in biliary repair is actually central, and the epithelium is the pacemaker of the reparative process. Recent studies have clarified that biliary repair follows patterns reminiscent of biliary ontogenesis, and that several morphogenic signaling pathways (Hh, Wnt/β-catenin, and Notch) play an important and interdependent role, particularly in regulating epithelial/mesenchymal interactions.
The main manifestations of chronic inflammatory cholangiopathies (cholangiocyte proliferation, ductopenia, portal inflammation and fibrosis, angiogenesis, aberrant ductal development, neoplastic transformation) are ultimately the results of the reparative mechanisms. The flip-side of liver repair is the enhanced deposition of fibrous tissue in the portal tract. This is a common feature of chronic inflammatory cholangiopathies, where reactive cholangiocytes modulate the response of MFs, the effectors of liver fibrosis. As a result of the extensive crosstalk among epithelial, mesenchymal, endothelial, and inflammatory cells, the portal tract is transformed into a continuously expanding cell .“laboratory.” that gradually remodels the liver architecture forming portal– portal septa. It is still unclear whether reactive cholangiocytes directly induce an alteration of the mesenchymal cells or if the modifications in the ECM components to induce the phenotypic shift in the biliary epithelium and mesenchymal cells. Notwithstanding, when cholangiocytes undergo phenotypic and functional changes resulting in a reactive phenotype, they behave as important drivers of the repair mechanisms, thus contributing in a major way to the propagation of portal fibrosis and to the progression of inflammatory cholangiopathies. Therefore, combined targeting of both cholangiocyte and MF activation may offer a new therapeutic strategy to halt the progression of chronic cholangiopathies.
ACKNOWLEDGMENTS
The support of Telethon (grant #GGP09189), of Progetto di Ricerca Ateneo 2008 (grant #CPDA083217), of Associazione Scientifica Gastroenterologica di Treviso (ASGET, associazione di promozione sociale senza scopo di lucro), and of Federazione Amici Di Epatologia (FADE) to LF is gratefully acknowledged. The support of NIH DK079005 and of Yale University Liver Center (NIH DK34989) to MS is gratefully acknowledged. The support of Fondazione S. Martino, Bergamo to LF and MS is also gratefully acknowledged. The authors wish to thank Massimiliano Cadamuro, Ph.D. (Department of Surgical and Gastroenterological Sciences, University of Padua) for having done the micrographs and to Carlo Spirl03AF;, Ph.D. (Digestive Disease Section, Yale University School of Medicine) for helpful discussion.
ABBREVIATIONS
- ADPKD
autosomal dominant polycystic kidney disease
- AGS
Alagille syndrome
- Ang
angiopoieitin
- BA
biliary atresia
- BDL
bile duct ligation
- CAF
cancer-associated fibroblast
- CCA
cholangiocarcinoma
- CTGF
connective tissue growth factor
- EC
endothelial cell
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ERK
extracellular signal-regulated kinase
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- FSP-1
fibroblast specific protein-1
- HGF
hepatocyte growth factor
- Hh
Hedgehog
- HSC
hepatic stellate cell
- IFN
interferon
- IL
interleukin
- KC
Kupffer cell
- MCP-1
monocyte chemotactic protein-1
- MF
myofibroblast
- MMP
matrix metalloproteinase
- NO
nitric oxide
- NTPD2
ecto-AT-pase nucleoside triphosphate diphosphohydrolase-2
- PBC
primary biliary cirrhosis
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- PF
portal fibroblast
- PSC
primary sclerosing cholangitis
- SDF-1
stromal cell-derived factor 1
- TGF–β
transforming growth factor-β
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TSP-1
Thrombospondin-1
- TβR
TGF–βreceptor
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- Wnt
Wingless
- α–SMA
α -smooth muscle actin
References
- 1.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127(5):1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39(4) Suppl 2:S90–S102. doi: 10.1097/01.mcg.0000155549.29643.ad. [DOI] [PubMed] [Google Scholar]
- 3.Spirlì C, Fabris L, Duner E, et al. Cytokine-stimulated nitric oxide production inhibits adenylyl cyclase and cAMPdependent secretion in cholangiocytes. Gastroenterology. 2003;124(3):737–753. doi: 10.1053/gast.2003.50100. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Sakamoto T, Ezure T, et al. Interleukin-6, hepatocyte growth factor, and their receptors in biliary epithelial cells during a type I ductular reaction in mice: interactions between the periductal inflammatory and stromal cells and the biliary epithelium. Hepatology. 1998;28(5):1260–1268. doi: 10.1002/hep.510280514. [DOI] [PubMed] [Google Scholar]
- 5.Terada R, Yamamoto K, Hakoda T, et al. Stromal cell-derived factor-1 from biliary epithelial cells recruits CXCR4-positive cells: implications for inflammatory liver diseases. Lab Invest. 2003;83(5):665–672. doi: 10.1097/01.lab.0000067498.89585.06. [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama T, Komori A, Nakamura M, et al. Human intrahepatic biliary epithelial cells function in innate immunity by producing IL-6 and IL-8 via the TLR4-NFkappaB and -MAPK signaling pathways. Liver Int. 2006;26(4):467–476. doi: 10.1111/j.1478-3231.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- 7.Reynoso-Paz S, Coppel RL, Mackay IR, Bass NM, Ansari AA, Gershwin ME. The immunobiology of bile and biliary epithelium. Hepatology. 1999;30(2):351–357. doi: 10.1002/hep.510300218. [DOI] [PubMed] [Google Scholar]
- 8.Desmet VJ. Histopathology of chronic cholestasis and adult ductopenic syndrome. Clin Liver Dis. 1998;2(2):249–264. doi: 10.1016/s1089-3261(05)70006-4. viii. [DOI] [PubMed] [Google Scholar]
- 9.Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130(4):1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Fabris L, Cadamuro M, Fiorotto R, et al. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology. 2006;43(5):1001–1012. doi: 10.1002/hep.21143. [DOI] [PubMed] [Google Scholar]
- 11.Caligiuri A, Glaser S, Rodgers RE, et al. Endothelin-1 inhibits secretin-stimulated ductal secretion by interacting with ETA receptors on large cholangiocytes. Am J Physiol. 1998;275(4 Pt 1):G835–G846. doi: 10.1152/ajpgi.1998.275.4.G835. [DOI] [PubMed] [Google Scholar]
- 12.Grappone C, Pinzani M, Parola M, et al. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol. 1999;31(1):100–109. doi: 10.1016/s0168-8278(99)80169-x. [DOI] [PubMed] [Google Scholar]
- 13.George J, Roulot D, Koteliansky VE, Bissell DM. In vivo inhibition of rat stellate cell activation by soluble transforming growth factor beta type II receptor: a potential new therapy for hepatic fibrosis. Proc Natl Acad Sci U S A. 1999;96(22):12719–12724. doi: 10.1073/pnas.96.22.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedlaczek N, Jia JD, Bauer M, et al. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol. 2001;158(4):1239–1244. doi: 10.1016/S0002-9440(10)64074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasoshima M, Kono N, Sugawara H, Katayanagi K, Harada K, Nakanuma Y. Increased expression of interleukin-6 and tumor necrosis factor-alpha in pathologic biliary epithelial cells: in situ and culture study. Lab Invest. 1998;78(1):89–100. [PubMed] [Google Scholar]
- 16.Nichols MT, Gidey E, Matzakos T, et al. Secretion of cytokines and growth factors into autosomal dominant polycystic kidney disease liver cyst fluid. Hepatology. 2004;40(4):836–846. doi: 10.1002/hep.20401. [DOI] [PubMed] [Google Scholar]
- 17.Barnes BH, Tucker RM,Wehrmann F, Mack DG, Ueno Y, Mack CL. Cholangiocytes as immune modulators in rotavirus-induced murine biliary atresia. Liver Int. 2009;29(8):1253–1261. doi: 10.1111/j.1478-3231.2008.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marra F, DeFranco R, Grappone C, et al. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am J Pathol. 1998;152(2):423–430. [PMC free article] [PubMed] [Google Scholar]
- 19.Saito JM, Maher JJ. Bile duct ligation in rats induces biliary expression of cytokine-induced neutrophil chemoattractant. Gastroenterology. 2000;118(6):1157–1168. doi: 10.1016/s0016-5085(00)70369-6. [DOI] [PubMed] [Google Scholar]
- 20.Fabris L, Strazzabosco M, Crosby HA, et al. Characterization and isolation of ductular cells coexpressing neural cell adhesion molecule and Bcl-2 from primary cholangiopathies and ductal plate malformations. Am J Pathol. 2000;156(5):1599–1612. doi: 10.1016/S0002-9440(10)65032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popov Y, Patsenker E, Stickel F, et al. Integrin alphavbeta6 is a marker of the progression of biliary and portal liver fibrosis and a novel target for antifibrotic therapies. J Hepatol. 2008;48(3):453–464. doi: 10.1016/j.jhep.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D. Inhibition of integrin alphavbeta6 on cholangiocytes blocks transforming growth factor-beta activation and retards biliary fibrosis progression. Gastroenterology. 2008;135(2):660–670. doi: 10.1053/j.gastro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desmet VJ. The amazing universe of hepatic microstructure. Hepatology. 2009;50(2):333–344. doi: 10.1002/hep.23152. [DOI] [PubMed] [Google Scholar]
- 24.Omenetti A, Yang L, Li YX, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87(5):499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- 25.Ader T, Norel R, Levoci L, Rogler LE. Transcriptional profiling implicates TGFbeta/BMP and Notch signaling pathways in ductular differentiation of fetal murine hepatoblasts. Mech Dev. 2006;123(2):177–194. doi: 10.1016/j.mod.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45(5):1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 27.Michalopoulos GK, Bowen WC, Mulè K, Lopez-Talavera JC, Mars W. Hepatocytes undergo phenotypic transformation to biliary epithelium in organoid cultures. Hepatology. 2002;36(2):278–283. doi: 10.1053/jhep.2002.34858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47(1):288–295. doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]
- 29.Santoni-Rugiu E, Jelnes P, Thorgeirsson SS, Bisgaard HC. Progenitor cells in liver regeneration: molecular responses controlling their activation and expansion. APMIS. 2005;113(11–12):876–902. doi: 10.1111/j.1600-0463.2005.apm_386.x. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, White P, Tuteja G, Rubins N, Sackett S, Kaestner KH. Foxa1 and Foxa2 regulate bile duct development in mice. J Clin Invest. 2009;119(6):1537–1545. doi: 10.1172/JCI38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strazzabosco M. Foxa1 and Foxa2 regulate bile duct development in mice. J Hepatol. 2010;52(5):765–767. doi: 10.1016/j.jhep.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semela D, Das A, Langer D, Kang N, Leof E, Shah V. Platelet-derived growth factor signaling through ephrin-b2 regulates hepatic vascular structure and function. Gastroenterology. 2008;135(2):671–679. doi: 10.1053/j.gastro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50(3):604–620. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Medina J, Sanz-Cameno P, Garcίa-Buey L, Martίn-Vίlchez S, López-Cabrera M, Moreno-Otero R. Evidence of angiogenesis in primary biliary cirrhosis: an immunohistochemical descriptive study. J Hepatol. 2005;42(1):124–131. doi: 10.1016/j.jhep.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 35.Kawahara N, Ono M, Taguchi K, et al. Enhanced expression of thrombospondin-1 and hypovascularity in human cholangiocarcinoma. Hepatology. 1998;28(6):1512–1517. doi: 10.1002/hep.510280610. [DOI] [PubMed] [Google Scholar]
- 36.Abrahám S, Szabó A, Kaszaki J, et al. Kupffer cell blockade improves the endotoxin-induced microcirculatory inflammatory response in obstructive jaundice. Shock. 2008;30(1):69–74. doi: 10.1097/SHK.0b013e31815dceea. [DOI] [PubMed] [Google Scholar]
- 37.Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci U S A. 1996;93(9):3942–3946. doi: 10.1073/pnas.93.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fallowfield JA, Mizuno M, Kendall TJ, et al. Scarassociated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178(8):5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 39.Popov Y, Sverdlov DY, Bhaskar KR, et al. Macrophage-mediated phagocytosis of apoptotic cholangiocytes contributes to reversal of experimental biliary fibrosis. Am J Physiol Gastrointest Liver Physiol. 2010;298(3):G323–G334. doi: 10.1152/ajpgi.00394.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wuestefeld T, Klein C, Streetz KL, et al. Interleukin-6/ glycoprotein 130-dependent pathways are protective during liver regeneration. J Biol Chem. 2003;278(13):11281–11288. doi: 10.1074/jbc.M208470200. [DOI] [PubMed] [Google Scholar]
- 41.Jakubowski A, Ambrose C, Parr M, et al. TWEAK induces liver progenitor cell proliferation. J Clin Invest. 2005;115(9):2330–2340. doi: 10.1172/JCI23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimoda S, Harada K, Niiro H, et al. Biliary epithelial cells and primary biliary cirrhosis: the role of liver-infiltrating mononuclear cells. Hepatology. 2008;47(3):958–965. doi: 10.1002/hep.22102. [DOI] [PubMed] [Google Scholar]
- 43.Shimoda S, Harada K, Niiro H, et al. CX3CL1 (fractalkine): a signpost for biliary inflammation in primary biliary cirrhosis. Hepatology. 2010;51(2):567–575. doi: 10.1002/hep.23318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein I, Cornejo JC, Polakos NK, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110(12):4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imamura M, Ogawa T, Sasaguri Y, Chayama K, Ueno H. Suppression of macrophage infiltration inhibits activation of hepatic stellate cells and liver fibrogenesis in rats. Gastroenterology. 2005;128(1):138–146. doi: 10.1053/j.gastro.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117(1):185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karlmark KR, Weiskirchen R, Zimmermann HW, et al. Hepatic recruitment of the inflammatory Gr1+monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50(1):261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 48.Zimmermann HW, Seidler S, Nattermann J, et al. Functional contribution of elevated circulating and hepatic nonclassical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS ONE. 2010;5(6):e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinnman N, Hultcrantz R, Barbu V, et al. PDGF-mediated chemoattraction of hepatic stellate cells by bile duct segments in cholestatic liver injury. Lab Invest. 2000;80(5):697–707. doi: 10.1038/labinvest.3780073. [DOI] [PubMed] [Google Scholar]
- 50.Schaffner F, Barka T, Popper H. Hepatic mesenchymal cell reaction in liver disease. Exp Mol Pathol. 1963;31:419–441. doi: 10.1016/0014-4800(63)90020-0. [DOI] [PubMed] [Google Scholar]
- 51.Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology. 2010;51(4):1438–1444. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milani S, Herbst H, Schuppan D, Stein H, Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991;139(6):1221–1229. [PMC free article] [PubMed] [Google Scholar]
- 53.Kruglov EA, Nathanson RA, Nguyen T, Dranoff JA. Secretion of MCP-1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. Am J Physiol Gastrointest Liver Physiol. 2006;290(4):G765–G771. doi: 10.1152/ajpgi.00308.2005. [DOI] [PubMed] [Google Scholar]
- 54.Jhandier MN, Kruglov EA, Lavoie EG, Sévigny J, Dranoff JA. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J Biol Chem. 2005;280(24):22986–22992. doi: 10.1074/jbc.M412371200. [DOI] [PubMed] [Google Scholar]
- 55.Kinnman N, Francoz C, Barbu V, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83(2):163–173. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]
- 56.Russo FP, Alison MR, Bigger BW, et al. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130(6):1807–1821. doi: 10.1053/j.gastro.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 57.Wells RG. The epithelial-to-mesenchymal transition in liver fibrosis: here today, gone tomorrow? Hepatology. 2010;51(3):737–740. doi: 10.1002/hep.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36(2):200–209. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 59.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134(6):1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Viñas O, Bataller R, Sancho-Bru P, et al. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology. 2003;38(4):919–929. doi: 10.1053/jhep.2003.50392. [DOI] [PubMed] [Google Scholar]
- 61.Winau F, Hegasy G, Weiskirchen R, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26(1):117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 62.Ankoma-Sey V, Wang Y, Dai Z. Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology. 2000;31(1):141–148. doi: 10.1002/hep.510310122. [DOI] [PubMed] [Google Scholar]
- 63.Novo E, Cannito S, Zamara E, et al. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol. 2007;170(6):1942–1953. doi: 10.2353/ajpath.2007.060887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novo E, di Bonzo LV, Cannito S, Colombatto S, Parola M. Hepatic myofibroblasts: a heterogeneous population of multifunctional cells in liver fibrogenesis. Int J Biochem Cell Biol. 2009;41(11):2089–2093. doi: 10.1016/j.biocel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 65.Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21(3):397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 66.Yavrom S, Chen L, Xiong S, Wang J, Rippe RA, Tsukamoto H. Peroxisome proliferator-activated receptor gamma suppresses proximal alpha1(I) collagen promoter via inhibition of p300-facilitated NF-I binding to DNA in hepatic stellate cells. J Biol Chem. 2005;280(49):40650–40659. doi: 10.1074/jbc.M510094200. [DOI] [PubMed] [Google Scholar]
- 67.Hong F, Tuyama A, Lee TF, et al. Hepatic stellate cells express functional CXCR4: role in stromal cell-derived factor-1alpha-mediated stellate cell activation. Hepatology. 2009;49(6):2055–2067. doi: 10.1002/hep.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160(1):419–425. [PubMed] [Google Scholar]
- 69.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36(4):598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 70.Keeley EC, Mehrad B, Strieter RM. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of fibrotic disorders. Thromb Haemost. 2009;101(4):613–618. [PMC free article] [PubMed] [Google Scholar]
- 71.Asawa S, Saito T, Satoh A, et al. Participation of bone marrow cells in biliary fibrosis after bile duct ligation. J Gastroenterol Hepatol. 2007;22(11):2001–2008. doi: 10.1111/j.1440-1746.2006.04708.x. [DOI] [PubMed] [Google Scholar]
- 72.Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest. 2007;117(3):549–556. doi: 10.1172/JCI30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353(1):104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 75.Andersson-Sjöland A, de Alba CG, Nihlberg K, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40(10):2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 76.Kisseleva T, Uchinami H, Feirt N, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45(3):429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 77.Guo J, Friedman SL. Hepatic fibrogenesis. Semin Liver Dis. 2007;27(4):413–426. doi: 10.1055/s-2007-991517. [DOI] [PubMed] [Google Scholar]
- 78.Jafri M, Donnelly B, Allen S, et al. Cholangiocyte expression of alpha2beta1-integrin confers susceptibility to rotavirus-induced experimental biliary atresia. Am J Physiol Gastrointest Liver Physiol. 2008;295(1):G16–G26. doi: 10.1152/ajpgi.00442.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang B, Dolinski BM, Kikuchi N, et al. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46(5):1404–1412. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11(2):97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maeda N, Kawada N, Seki S, et al. Stimulation of proliferation of rat hepatic stellate cells by galectin-1 and galectin-3 through different intracellular signaling pathways. J Biol Chem. 2003;278(21):18938–18944. doi: 10.1074/jbc.M209673200. [DOI] [PubMed] [Google Scholar]
- 82.Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci U S A. 2006;103(13):5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986;47(1):131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- 84.Swindle CS, Tran KT, Johnson TD, et al. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol. 2001;154(2):459–468. doi: 10.1083/jcb.200103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Eyken P, Sciot R, Desmet VJ. Expression of the novel extracellular matrix component tenascin in normal and diseased human liver. An immunohistochemical study. J Hepatol. 1990;11(1):43–52. doi: 10.1016/0168-8278(90)90270-2. [DOI] [PubMed] [Google Scholar]
- 86.Van Eyken P, Geerts A, De Bleser P, et al. Localization and cellular source of the extracellular matrix protein tenascin in normal and fibrotic rat liver. Hepatology. 1992;15(5):909–916. doi: 10.1002/hep.1840150526. [DOI] [PubMed] [Google Scholar]
- 87.Miyazaki H, Van Eyken P, Roskams T, De Vos R, Desmet VJ. Transient expression of tenascin in experimentally induced cholestatic fibrosis in rat liver: an immunohistochemical study. J Hepatol. 1993;19(3):353–366. doi: 10.1016/s0168-8278(05)80543-4. [DOI] [PubMed] [Google Scholar]
- 88.Aishima S, Taguchi K, Terashi T, Matsuura S, Shimada M, Tsuneyoshi M. Tenascin expression at the invasive front is associated with poor prognosis in intrahepatic cholangiocarcinoma. Mod Pathol. 2003;16(10):1019–1027. doi: 10.1097/01.MP.0000086860.65672.73. [DOI] [PubMed] [Google Scholar]
- 89.Roskams T, Rosenbaum J, De Vos R, David G, Desmet V. Heparan sulfate proteoglycan expression in chronic cholestatic human liver diseases. Hepatology. 1996;24(3):524–532. doi: 10.1053/jhep.1996.v24.pm0008781318. [DOI] [PubMed] [Google Scholar]
- 90.Liu B, Paranjpe S, Bowen WC, et al. Investigation of the role of glypican 3 in liver regeneration and hepatocyte proliferation. Am J Pathol. 2009;175(2):717–724. doi: 10.2353/ajpath.2009.081129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karlsen TH, Franke A, Melum E, et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010;138(3):1102–1111. doi: 10.1053/j.gastro.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 92.Yasoshima M, Tsuneyama K, Harada K, Sasaki M, Gershwin ME, Nakanuma Y. Immunohistochemical analysis of cell-matrix adhesion molecules and their ligands in the portal tracts of primary biliary cirrhosis. J Pathol. 2000;190(1):93–99. doi: 10.1002/(SICI)1096-9896(200001)190:1<93::AID-PATH507>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 93.Yasoshima M, Sato Y, Furubo S, et al. Matrix proteins of basement membrane of intrahepatic bile ducts are degraded in congenital hepatic fibrosis and Caroli’s disease. J Pathol. 2009;217(3):442–451. doi: 10.1002/path.2472. [DOI] [PubMed] [Google Scholar]
- 94.Joplin R, Hishida T, Tsubouchi H, et al. Human intrahepatic biliary epithelial cells proliferate in vitro in response to human hepatocyte growth factor. J Clin Invest. 1992;90(4):1284–1289. doi: 10.1172/JCI115992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ishida Y, Smith S, Wallace L, et al. Ductular morphogenesis and functional polarization of normal human biliary epithelial cells in three-dimensional culture. J Hepatol. 2001;35(1):2–9. doi: 10.1016/s0168-8278(01)00078-2. [DOI] [PubMed] [Google Scholar]
- 96.Matsuda Y, Matsumoto K, Ichida T, Nakamura T. Hepatocyte growth factor suppresses the onset of liver cirrhosis and abrogates lethal hepatic dysfunction in rats. J Biochem. 1995;118(3):643–649. doi: 10.1093/oxfordjournals.jbchem.a124958. [DOI] [PubMed] [Google Scholar]
- 97.Yasuda H, Imai E, Shiota A, Fujise N, Morinaga T, Higashio K. Antifibrogenic effect of a deletion variant of hepatocyte growth factor on liver fibrosis in rats. Hepatology. 1996;24(3):636–642. doi: 10.1053/jhep.1996.v24.pm0008781336. [DOI] [PubMed] [Google Scholar]
- 98.Inagaki Y, Higashi K, Kushida M, et al. Hepatocyte growth factor suppresses profibrogenic signal transduction via nuclear export of Smad3 with galectin-7. Gastroenterology. 2008;134(4):1180–1190. doi: 10.1053/j.gastro.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 99.Xia JL, Dai C, Michalopoulos GK, Liu Y. Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol. 2006;168(5):1500–1512. doi: 10.2353/ajpath.2006.050747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pinzani M, Gesualdo L, Sabbah GM, Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989;84(6):1786–1793. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Friedman SL, Arthur MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989;84(6):1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kinnman N, Goria O, Wendum D, et al. Hepatic stellate cell proliferation is an early platelet-derived growth factor-mediated cellular event in rat cholestatic liver injury. Lab Invest. 2001;81(12):1709–1716. doi: 10.1038/labinvest.3780384. [DOI] [PubMed] [Google Scholar]
- 103.Lechuga CG, Hernández-Nazara ZH, Hernández E, et al. PI3K is involved in PDGF-beta receptor upregulation post- PDGF-BB treatment in mouse HSC. Am J Physiol Gastrointest Liver Physiol. 2006;291(6):G1051–G1061. doi: 10.1152/ajpgi.00058.2005. [DOI] [PubMed] [Google Scholar]
- 104.Rovida E, Navari N, Caligiuri A, Dello Sbarba P, Marra F. ERK5 differentially regulates PDGF-induced proliferation and migration of hepatic stellate cells. J Hepatol. 2008;48(1):107–115. doi: 10.1016/j.jhep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 105.Melton AC, Yee HF. Hepatic stellate cell protrusions couple platelet-derived growth factor-BB to chemotaxis. Hepatology. 2007;45(6):1446–1453. doi: 10.1002/hep.21606. [DOI] [PubMed] [Google Scholar]
- 106.Borkham-Kamphorst E, van Roeyen CR, Ostendorf T, Floege J, Gressner AM, Weiskirchen R. Pro-fibrogenic potential of PDGF-D in liver fibrosis. J Hepatol. 2007;46(6):1064–1074. doi: 10.1016/j.jhep.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 107.Liu C, Gaça MD, Swenson ES, Vellucci VF, Reiss M, Wells RG. Smads 2 and 3 are differentially activated by transforming growth factor-beta (TGF-beta) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-beta-independent. J Biol Chem. 2003;278(13):11721–11728. doi: 10.1074/jbc.M207728200. [DOI] [PubMed] [Google Scholar]
- 108.Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19(23):2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 109.Inagaki Y, Okazaki I. Emerging insights into transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56(2):284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roy H, Bhardwaj S, Ylä-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett. 2006;580(12):2879–2887. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 111.Morisada T, Kubota Y, Urano T, Suda T, Oike Y. Angiopoietins and angiopoietin-like proteins in angiogenesis. Endothelium. 2006;13(2):71–79. doi: 10.1080/10623320600697989. [DOI] [PubMed] [Google Scholar]
- 112.Gaudio E, Franchitto A, Pannarale L, et al. Cholangiocytes and blood supply. World J Gastroenterol. 2006;12(22):3546–3552. doi: 10.3748/wjg.v12.i22.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fabris L, Cadamuro M, Libbrecht L, et al. Epithelial expression of angiogenic growth factors modulate arterial vasculogenesis in human liver development. Hepatology. 2008;47(2):719–728. doi: 10.1002/hep.22015. [DOI] [PubMed] [Google Scholar]
- 114.Spirli C, Okolicsanyi S, Fiorotto R, et al. ERK1/2- dependent vascular endothelial growth factor signaling sustains cyst growth in polycystin-2 defective mice. Gastroenterology. 2010;138(1):360–371. doi: 10.1053/j.gastro.2009.09.005. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spirli C, Okolicsanyi S, Fiorotto R, et al. Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice. Hepatology. 2010;51(5):1778–1788. doi: 10.1002/hep.23511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13(9):2384–2398. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 117.Wilson PD. Polycystic kidney disease: new understanding in the pathogenesis. Int J Biochem Cell Biol. 2004;36(10):1868–1873. doi: 10.1016/j.biocel.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 118.Yoshiji H, Kuriyama S, Yoshii J, et al. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut. 2003;52(9):1347–1354. doi: 10.1136/gut.52.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brodsky KS, McWilliams RR, Amura CR, Barry NP, Doctor RB. Liver cyst cytokines promote endothelial cell proliferation and development. Exp Biol Med (Maywood) 2009;234(10):1155–1165. doi: 10.3181/0903-RM-112. [DOI] [PubMed] [Google Scholar]
- 120.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+progeprogenitors to peripheral blood. J Exp Med. 1997;185(1):111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bleul CC, Schultze JL, Springer TA. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J Exp Med. 1998;187(5):753–762. doi: 10.1084/jem.187.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393(6685):591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 123.Coulomb-Ľ Hermin A, Amara A, Schiff C, et al. Stromal cell-derived factor 1 (SDF-1) and antenatal human B cell lymphopoiesis: expression of SDF-1 by mesothelial cells and biliary ductal plate epithelial cells. Proc Natl Acad Sci U S A. 1999;96(15):8585–8590. doi: 10.1073/pnas.96.15.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kollet O, Shivtiel S, Chen YQ, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112(2):160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ohira S, Itatsu K, Sasaki M, et al. Local balance of transforming growth factor-beta1 secreted from cholangiocarcinoma cells and stromal-derived factor-1 secreted from stromal fibroblasts is a factor involved in invasion of cholangiocarcinoma. Pathol Int. 2006;56(7):381–389. doi: 10.1111/j.1440-1827.2006.01982.x. [DOI] [PubMed] [Google Scholar]
- 126.Ohira S, Sasaki M, Harada K, et al. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor-alpha and stromal-derived factor-1 released in stroma. Am J Pathol. 2006;168(4):1155–1168. doi: 10.2353/ajpath.2006.050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114(6):1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.ƠBrien TP, Yang GP, Sanders L, Lau LF. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol. 1990;10(7):3569–3577. doi: 10.1128/mcb.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ryseck RP, Macdonald-Bravo H, Mattéi MG, Bravo R. Structure, mapping, and expression of fisp-12, a growth factor-inducible gene encoding a secreted cysteine-rich protein. Cell Growth Differ. 1991;2(5):225–233. [PubMed] [Google Scholar]
- 130.Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993;4(6):637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Paradis V, Dargere D, Bonvoust F, Vidaud M, Segarini P, Bedossa P. Effects and regulation of connective tissue growth factor on hepatic stellate cells. Lab Invest. 2002;82(6):767–774. doi: 10.1097/01.lab.0000017365.18894.d3. [DOI] [PubMed] [Google Scholar]
- 132.Gao R, Ball DK, Perbal B, Brigstock DR. Connective tissue growth factor induces c-fos gene activation and cell proliferation through p44/42 MAP kinase in primary rat hepatic stellate cells. J Hepatol. 2004;40(3):431–438. doi: 10.1016/j.jhep.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 133.Paradis V, Dargere D, Vidaud M, et al. Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology. 1999;30(4):968–976. doi: 10.1002/hep.510300425. [DOI] [PubMed] [Google Scholar]
- 134.Kobayashi H, Hayashi N, Hayashi K, Yamataka A, Lane GJ, Miyano T. Connective tissue growth factor and progressive fibrosis in biliary atresia. Pediatr Surg Int. 2005;21(1):12–16. doi: 10.1007/s00383-004-1254-z. [DOI] [PubMed] [Google Scholar]
- 135.Gardini A, Corti B, Fiorentino M, et al. Expression of connective tissue growth factor is a prognostic marker for patients with intrahepatic cholangiocarcinoma. Dig Liver Dis. 2005;37(4):269–274. doi: 10.1016/j.dld.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 136.Yang L, Wang Y, Mao H, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48(1):98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Omenetti A, Diehl AM. The adventures of sonic hedgehog in development and repair. II. Sonic hedgehog and liver development, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G595–G598. doi: 10.1152/ajpgi.00543.2007. [DOI] [PubMed] [Google Scholar]
- 138.Omenetti A, Porrello A, Jung Y, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118(10):3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jung Y, McCall SJ, Li YX, Diehl AM. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology. 2007;45(5):1091–1096. doi: 10.1002/hep.21660. [DOI] [PubMed] [Google Scholar]
- 140.Omenetti A, Popov Y, Jung Y, et al. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut. 2008;57(9):1275–1282. doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
- 141.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 142.Sackett SD, Gao Y, Shin S, et al. Foxl1 promotes liver repair following cholestatic injury in mice. Lab Invest. 2009;89(12):1387–1396. doi: 10.1038/labinvest.2009.103. [DOI] [PubMed] [Google Scholar]
- 143.Monga SP, Monga HK, Tan X, Mulé K, Pediaditakis P, Michalopoulos GK. Beta-catenin antisense studies in embryonic liver cultures: role in proliferation, apoptosis, and lineage specification. Gastroenterology. 2003;124(1):202–216. doi: 10.1053/gast.2003.50000. [DOI] [PubMed] [Google Scholar]
- 144.Hussain SZ, Sneddon T, Tan X, Micsenyi A, Michalopoulos GK, Monga SP. Wnt impacts growth and differentiation in ex vivo liver development. Exp Cell Res. 2004;292(1):157–169. doi: 10.1016/j.yexcr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 145.Decaens T, Godard C, de Reyniés A, et al. Stabilization of beta-catenin affects mouse embryonic liver growth and hepatoblast fate. Hepatology. 2008;47(1):247–258. doi: 10.1002/hep.21952. [DOI] [PubMed] [Google Scholar]
- 146.Jiang F, Parsons CJ, Stefanovic B. Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J Hepatol. 2006;45(3):401–409. doi: 10.1016/j.jhep.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 147.Myung SJ, Yoon JH, Gwak GY, et al. Wnt signaling enhances the activation and survival of human hepatic stellate cells. FEBS Lett. 2007;581(16):2954–2958. doi: 10.1016/j.febslet.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 148.Cheng JH, She H, Han YP, et al. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G39–G49. doi: 10.1152/ajpgi.00263.2007. [DOI] [PubMed] [Google Scholar]
- 149.Montcouquiol M, Crenshaw EB, III, Kelley MW. Noncanonical Wnt signaling and neural polarity. Annu Rev Neurosci. 2006;29:363–386. doi: 10.1146/annurev.neuro.29.051605.112933. [DOI] [PubMed] [Google Scholar]
- 150.Lancaster MA, Gleeson JG. Cystic kidney disease: the role of Wnt signaling. Trends Mol Med. 2010;16(8):349– 360. doi: 10.1016/j.molmed.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131(5):965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 152.Cornell RA, Eisen JS. Notch in the pathway: the roles of Notch signaling in neural crest development. Semin Cell Dev Biol. 2005;16(6):663–672. doi: 10.1016/j.semcdb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 153.Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111(2):492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 154.Chiba S. Notch signaling in stem cell system. Stem Cells. 2006;24:2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- 155.Gridley T. Notch signaling and inherited disease syndromes. Hum Mol Genet. 2003;12(Spec No 1):R9–R13. doi: 10.1093/hmg/ddg052. [DOI] [PubMed] [Google Scholar]
- 156.Schweisguth F. Regulation of notch signaling activity. Curr Biol. 2004;14(3):R129–R138. [PubMed] [Google Scholar]
- 157.Zong Y, Panikkar A, Xu J, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136(10):1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lemaigre FP. Notch signaling in bile duct development: new insights raise new questions. Hepatology. 2008;48(2):358–360. doi: 10.1002/hep.22480. [DOI] [PubMed] [Google Scholar]
- 159.Tanimizu N, Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci. 2004;117(Pt 15):3165–3174. doi: 10.1242/jcs.01169. [DOI] [PubMed] [Google Scholar]
- 160.McDaniell R, Warthen DM, Sanchez-Lara PA, et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79(1):169–173. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16(3):243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 162.Oda T, Elkahloun AG, Pike BL, et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16(3):235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 163.Crosnier C, Attié-Bitach T, Encha-Razavi F, et al. JAGGED1 gene expression during human embryogenesis elucidates the wide phenotypic spectrum of Alagille syndrome. Hepatology. 2000;32(3):574–581. doi: 10.1053/jhep.2000.16600. [DOI] [PubMed] [Google Scholar]
- 164.Piccoli DA, Spinner NB. Alagille syndrome and the Jagged1 gene. Semin Liver Dis. 2001;21(4):525–534. doi: 10.1055/s-2001-19036. [DOI] [PubMed] [Google Scholar]
- 165.Desmet VJ. Ludwig symposium on biliary disorders—part I. Pathogenesis of ductal plate abnormalities. Mayo Clin Proc. 1998;73(1):80–89. doi: 10.4065/73.1.80. [DOI] [PubMed] [Google Scholar]
- 166.Katuri V, Tang Y, Li C, et al. Critical interactions between TGF-beta signaling/ELF, and E-cadherin/beta-catenin mediated tumor suppression. Oncogene. 2006;25(13):1871–1886. doi: 10.1038/sj.onc.1209211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brabletz S, Schmalhofer O, Brabletz T. Gastrointestinal stem cells in development and cancer. J Pathol. 2009;217(2):307–317. doi: 10.1002/path.2475. [DOI] [PubMed] [Google Scholar]
- 168.Fujikura J, Hosoda K, Iwakura H, et al. Notch/Rbp-j signaling prevents premature endocrine and ductal cell differentiation in the pancreas. Cell Metab. 2006;3(1):59–65. doi: 10.1016/j.cmet.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 169.Farnie G, Clarke RB. Mammary stem cells and breast cancer—role of Notch signalling. Stem Cell Rev. 2007;3(2):169–175. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- 170.Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111(2):492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 171.Alexson TO, Hitoshi S, Coles BL, Bernstein A, van der Kooy D. Notch signaling is required to maintain all neural stem cell populations—irrespective of spatial or temporal niche. Dev Neurosci. 2006;28(1–2):34–48. doi: 10.1159/000090751. [DOI] [PubMed] [Google Scholar]
- 172.Zanotti S, Canalis E. Notch and the skeleton. Mol Cell Biol. 2010;30(4):886–896. doi: 10.1128/MCB.01285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Felszeghy S, Suomalainen M, Thesleff I. Notch signalling is required for the survival of epithelial stem cells in the continuously growing mouse incisor. Differentiation. 2010;80:241–248. doi: 10.1016/j.diff.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 174.Dellatore SM, Garcia AS, Miller WM. Mimicking stem cell niches to increase stem cell expansion. Curr Opin Biotechnol. 2008;19(5):534–540. doi: 10.1016/j.copbio.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nijjar SS, Wallace L, Crosby HA, Hubscher SG, Strain AJ. Altered Notch ligand expression in human liver disease: further evidence for a role of the Notch signaling pathway in hepatic neovascularization and biliary ductular defects. Am J Pathol. 2002;160(5):1695–1703. doi: 10.1016/S0002-9440(10)61116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Spee B, Carpino G, Schotanus BA, et al. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 2010;59(2):247–257. doi: 10.1136/gut.2009.188367. [DOI] [PubMed] [Google Scholar]
- 177.Fabris L, Cadamuro M, Guido M, et al. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am J Pathol. 2007;171(2):641–653. doi: 10.2353/ajpath.2007.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fiorotto R, Spirli C, Scirpo R, et al. Progenitor cell activation and liver repair is altered in Notch2- and RBP-Jk-defective mice exposed to cholestatic injuries. J Hepatol. 2010;52:S45. [Google Scholar]
- 179.Sawitza I, Kordes C, Reister S, Häussinger D. The niche of stellate cells within rat liver. Hepatology. 2009;50(5):1617–1624. doi: 10.1002/hep.23184. [DOI] [PubMed] [Google Scholar]
- 180.Venkov CD, Link AJ, Jennings JL, et al. A proximal activator of transcription in epithelial-mesenchymal transition. J Clin Invest. 2007;117(2):482–491. doi: 10.1172/JCI29544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rhyu DY, Yang Y, Ha H, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16(3):667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 182.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sato Y, Harada K, Ozaki S, et al. Cholangiocytes with mesenchymal features contribute to progressive hepatic fibrosis of the polycystic kidney rat. Am J Pathol. 2007;171(6):1859–1871. doi: 10.2353/ajpath.2007.070337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Harada K, Sato Y, Ikeda H, et al. Epithelial-mesenchymal transition induced by biliary innate immunity contributes to the sclerosing cholangiopathy of biliary atresia. J Pathol. 2009;217(5):654–664. doi: 10.1002/path.2488. [DOI] [PubMed] [Google Scholar]
- 185.Sato Y, Harada K, Itatsu K, et al. Epithelial-mesenchymal transition induced by transforming growth factor-beta1/snail activation aggravates invasive growth of cholangiocarcinoma. Am J Pathol. 2010;177(1):141–152. doi: 10.2353/ajpath.2010.090747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Strutz F, Okada H, Lo CW, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130(2):393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Le Hir M, Hegyi I, Cueni-Loffing D, Loffing J, Kaissling B. Characterization of renal interstitial fibroblast-specific protein 1/S100A4-positive cells in healthy and inflamed rodent kidneys. Histochem Cell Biol. 2005;123(4–5):335–346. doi: 10.1007/s00418-005-0788-z. [DOI] [PubMed] [Google Scholar]
- 188.Taura K, Miura K, Iwaisako K, et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51(3):1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lorent K, Yeo SY, Oda T, et al. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development. 2004;131(22):5753–5766. doi: 10.1242/dev.01411. [DOI] [PubMed] [Google Scholar]
- 190.Antoniou A, Raynaud P, Cordi S, et al. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136(7):2325–2333. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Libbrecht L, Spinner NB, Moore EC, Cassiman D, Van Damme-Lombaerts R, Roskams T. Peripheral bile duct paucity and cholestasis in the liver of a patient with Alagille syndrome: further evidence supporting a lack of postnatal bile duct branching and elongation. Am J Surg Pathol. 2005;29(6):820–826. doi: 10.1097/01.pas.0000161325.36348.25. [DOI] [PubMed] [Google Scholar]
- 192.Clotman F, Jacquemin P, Plumb-Rudewiez N, et al. Control of liver cell fate decision by a gradient of TGF beta signaling modulated by Onecut transcription factors. Genes Dev. 2005;19(16):1849–1854. doi: 10.1101/gad.340305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48(1):308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4(11):839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 195.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455(7211):406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 197.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17(2):135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 198.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 199.Santamaria-Martίnez A, Barquinero J, Barbosa-Desongles A, et al. Identification of multipotent mesenchymal stromal cells in the reactive stroma of a prostate cancer xenograft by side population analysis. Exp Cell Res. 2009;315(17):3004–3013. doi: 10.1016/j.yexcr.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 200.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 201.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119(6):1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]