Abstract
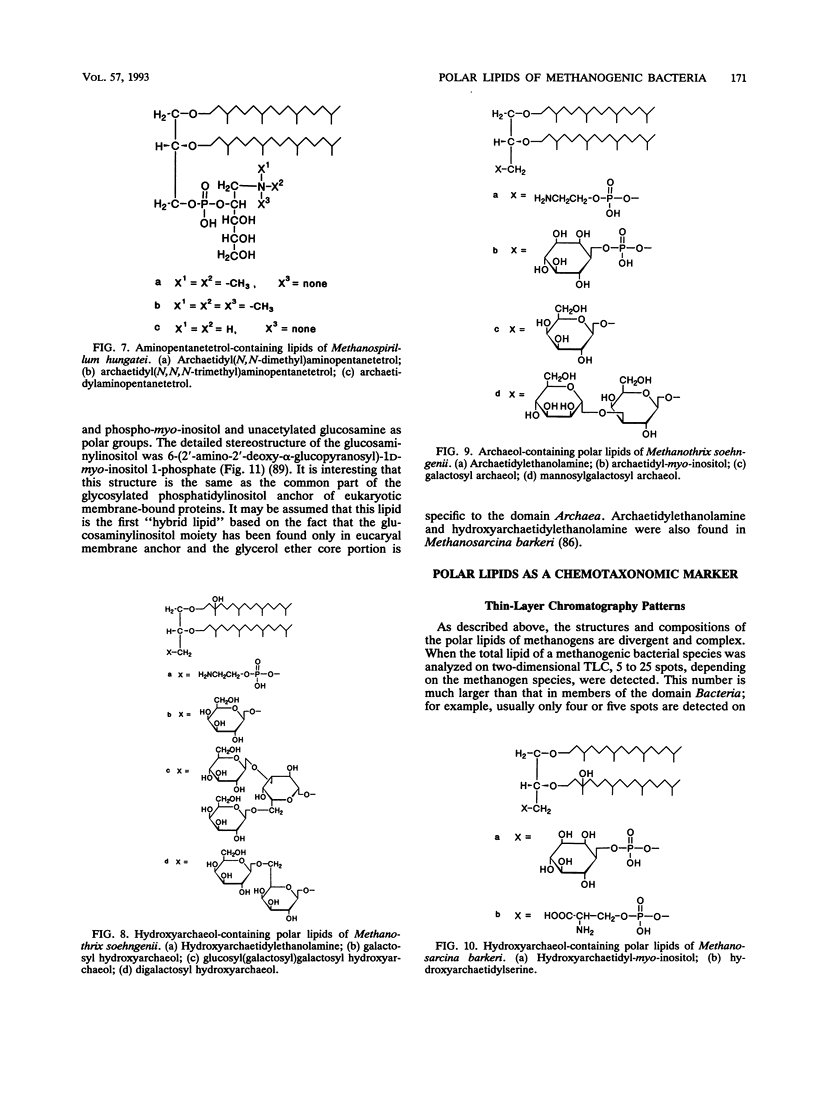
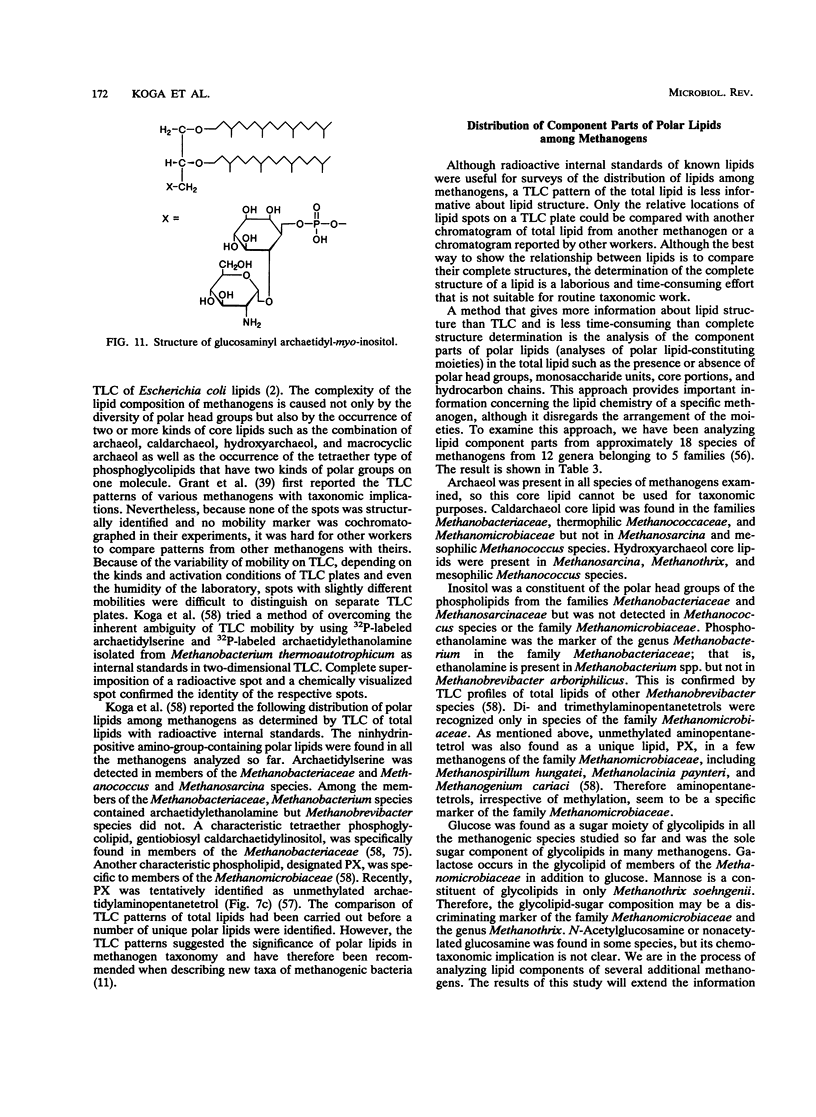
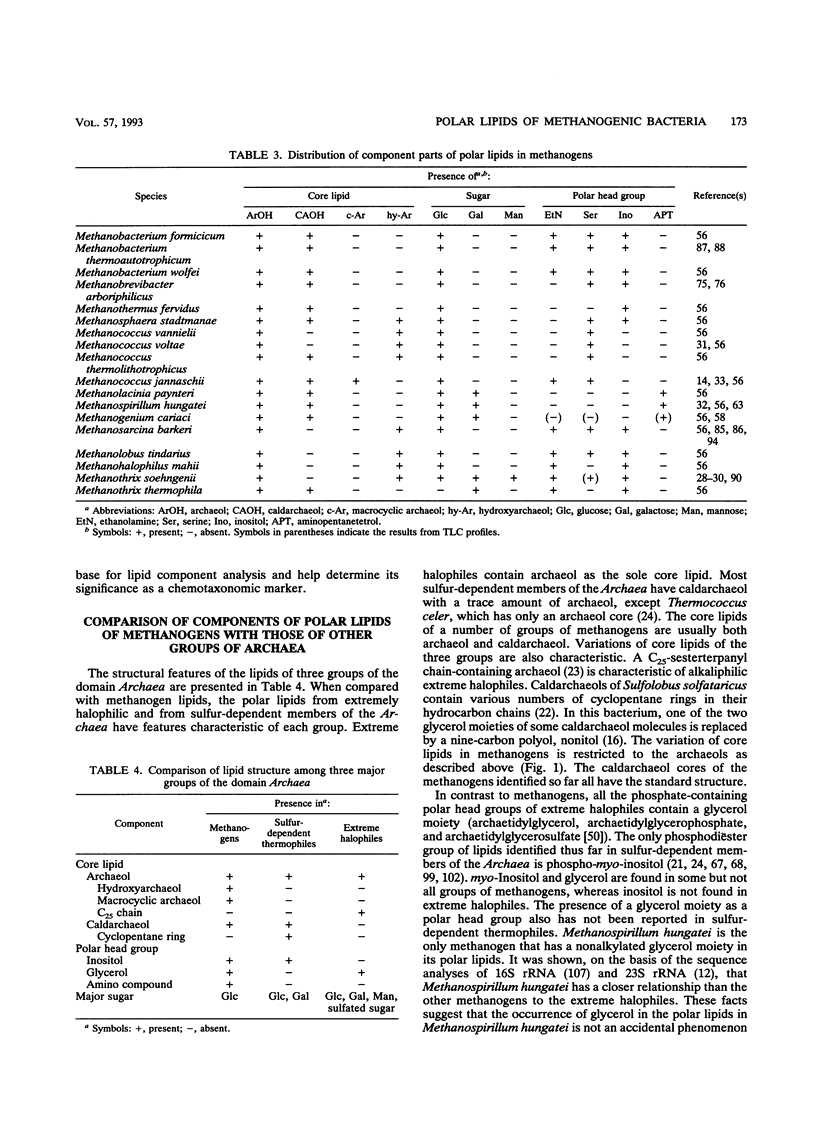
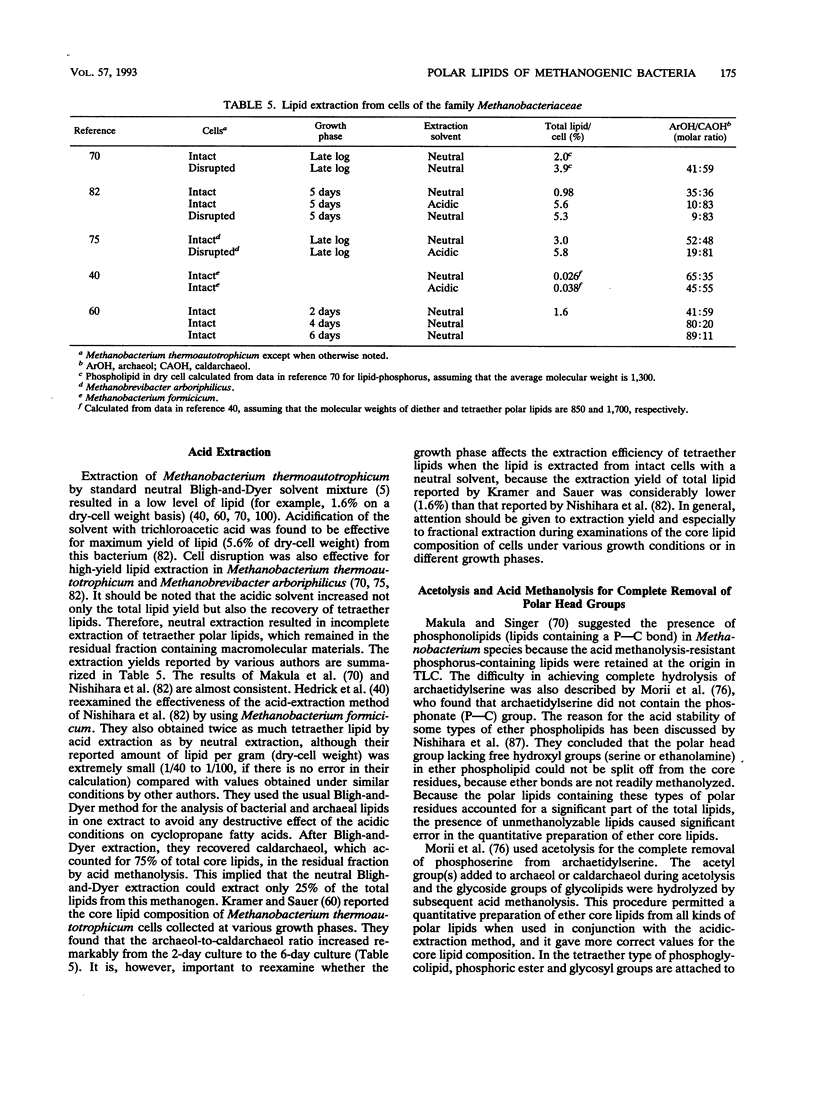
Complete structures of nearly 40 ether polar lipids from seven species of methanogens have been elucidated during the past 10 years. Three kinds of variations of core lipids, macrocyclic archaeol and two hydroxyarchaeols, were identified, in addition to the usual archaeol and caldarchaeol (for the nomenclature of archaeal [archaebacterial] ether lipids, see the text). Polar head groups of methanogen phospholipids include ethanolamine, serine, inositol, N-acetylglucosamine, dimethyl- and trimethylaminopentanetetrol, and glucosaminylinositol. Glucose is the sole hexose moiety of glycolipids in most methanogens, and galactose and mannose have been found in a few species. Methanogen lipids are characterized by their diversity in phosphate-containing polar head groups and core lipids, which in turn can be used for chemotaxonomy of methanogens. This was shown by preliminary simplified analyses of lipid component residues. Core lipid analysis by high-pressure liquid chromatography provides a method of determining the methanogenic biomass in natural samples. There has been significant progress in the biosynthetic studies of methanogen lipids in recent years. In vivo incorporation experiments have led to delineation of the outline of the synthetic route of the diphytanylglycerol ether core. The mechanisms of biosynthesis of tetraether lipids and various polar lipids, and cell-free systems of either lipid synthesis, however, remain to be elucidated. The significance and the origin of archaeal ether lipids is discussed in terms of the lipid composition of bacteria living in a wide variety of environments, the oxygen requirement for biosynthesis of hydrocarbon chains, and the physicochemical properties and functions of lipids as membrane constituents.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achenbach-Richter L., Gupta R., Stetter K. O., Woese C. R. Were the original eubacteria thermophiles? Syst Appl Microbiol. 1987;9:34–39. doi: 10.1016/s0723-2020(87)80053-x. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggraf S., Ching A., Stetter K. O., Woese C. R. The sequence of Methanospirillum hungatei 23S rRNA confirms the specific relationship between the extreme halophiles and the Methanomicrobiales. Syst Appl Microbiol. 1991;14:358–363. doi: 10.1016/s0723-2020(11)80310-3. [DOI] [PubMed] [Google Scholar]
- Clarke N. G., Hazlewood G. P., Dawson R. M. Structure of diabolic acid-containing phospholipids isolated from Butyrivibrio sp. Biochem J. 1980 Nov 1;191(2):561–569. doi: 10.1042/bj1910561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comita P. B., Gagosian R. B., Pang H., Costello C. E. Structural elucidation of a unique macrocyclic membrane lipid from a new, extremely thermophilic, deep-sea hydrothermal vent archaebacterium, Methanococcus jannaschii. J Biol Chem. 1984 Dec 25;259(24):15234–15241. [PubMed] [Google Scholar]
- De Rosa M., Gambacorta A., Gliozzi A. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol Rev. 1986 Mar;50(1):70–80. doi: 10.1128/mr.50.1.70-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco A. A., Bobik T. A., Wolfe R. S. Unusual coenzymes of methanogenesis. Annu Rev Biochem. 1990;59:355–394. doi: 10.1146/annurev.bi.59.070190.002035. [DOI] [PubMed] [Google Scholar]
- Ekiel I., Smith I. C., Sprott G. D. Biosynthetic pathways in Methanospirillum hungatei as determined by 13C nuclear magnetic resonance. J Bacteriol. 1983 Oct;156(1):316–326. doi: 10.1128/jb.156.1.316-326.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiel I., Sprott G. D., Patel G. B. Acetate and CO2 assimilation by Methanothrix concilii. J Bacteriol. 1985 Jun;162(3):905–908. doi: 10.1128/jb.162.3.905-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante G., Brisson J. R., Patel G. B., Ekiel I., Sprott G. D. Structures of minor ether lipids isolated from the aceticlastic methanogen, Methanothrix concilii GP6. J Lipid Res. 1989 Oct;30(10):1601–1609. [PubMed] [Google Scholar]
- Ferrante G., Ekiel I., Sprott G. D. Structural characterization of the lipids of Methanococcus voltae, including a novel N-acetylglucosamine 1-phosphate diether. J Biol Chem. 1986 Dec 25;261(36):17062–17066. [PubMed] [Google Scholar]
- Ferrante G., Richards J. C., Sprott G. D. Structures of polar lipids from the thermophilic, deep-sea archaeobacterium Methanococcus jannaschii. Biochem Cell Biol. 1990 Jan;68(1):274–283. doi: 10.1139/o90-038. [DOI] [PubMed] [Google Scholar]
- Fulco A. J. Metabolic alterations of fatty acids. Annu Rev Biochem. 1974;43(0):215–241. doi: 10.1146/annurev.bi.43.070174.001243. [DOI] [PubMed] [Google Scholar]
- Giovannoni S. J., Britschgi T. B., Moyer C. L., Field K. G. Genetic diversity in Sargasso Sea bacterioplankton. Nature. 1990 May 3;345(6270):60–63. doi: 10.1038/345060a0. [DOI] [PubMed] [Google Scholar]
- Goldfine H. The evolution of oxygen as a biosynthetic reagent. J Gen Physiol. 1965 Sep;49(1 Suppl):253–274. doi: 10.1085/jgp.49.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick D. B., Guckert J. B., White D. C. Archaebacterial ether lipid diversity analyzed by supercritical fluid chromatography: integration with a bacterial lipid protocol. J Lipid Res. 1991 Apr;32(4):659–666. [PubMed] [Google Scholar]
- Hensel R., Zwickl P., Fabry S., Lang J., Palm P. Sequence comparison of glyceraldehyde-3-phosphate dehydrogenases from the three urkingdoms: evolutionary implication. Can J Microbiol. 1989 Jan;35(1):81–85. doi: 10.1139/m89-012. [DOI] [PubMed] [Google Scholar]
- Iwabe N., Kuma K., Hasegawa M., Osawa S., Miyata T. Evolutionary relationship of archaebacteria, eubacteria, and eukaryotes inferred from phylogenetic trees of duplicated genes. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9355–9359. doi: 10.1073/pnas.86.23.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- KATES M., YENGOYAN L. S., SASTRY P. S. A DIETHER ANALOG OF PHOSPHATIDYL GLYCEROPHOSPHATE IN HALOBACTERIUM CUTIRUBRUM. Biochim Biophys Acta. 1965 Apr 5;98:252–268. doi: 10.1016/0005-2760(65)90119-0. [DOI] [PubMed] [Google Scholar]
- Kates M. Structure, physical properties, and function of archaebacterial lipids. Prog Clin Biol Res. 1988;282:357–384. [PubMed] [Google Scholar]
- Kates M. The phytanyl ether-linked polar lipids and isoprenoid neutral lipids of extremely halophilic bacteria. Prog Chem Fats Other Lipids. 1978;15(4):301–342. doi: 10.1016/0079-6832(77)90011-8. [DOI] [PubMed] [Google Scholar]
- Kates M., Wassef M. K., Pugh E. L. Origin of the glycerol moieties in the glycerol diether lipids of Halobacterium cutirubrum. Biochim Biophys Acta. 1970 Feb 10;202(1):206–208. doi: 10.1016/0005-2760(70)90238-9. [DOI] [PubMed] [Google Scholar]
- Klein R. A., Hazlewood G. P., Kemp P., Dawson R. M. A new series of long-chain dicarboxylic acids with vicinal dimethyl branching found as major components of the lipids of Butyrivibrio spp. Biochem J. 1979 Dec 1;183(3):691–700. doi: 10.1042/bj1830691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Sprott G. D., Smith I. C. Novel polar lipids from the methanogen Methanospirillum hungatei GP1. Biochim Biophys Acta. 1981 Apr 23;664(1):156–173. doi: 10.1016/0005-2760(81)90038-2. [DOI] [PubMed] [Google Scholar]
- Langworthy T. A. Long-chain diglycerol tetraethers from Thermoplasma acidophilum. Biochim Biophys Acta. 1977 Apr 26;487(1):37–50. doi: 10.1016/0005-2760(77)90042-x. [DOI] [PubMed] [Google Scholar]
- Makula R. A., Singer M. E. Ether-containing lipids of methanogenic bacteria. Biochem Biophys Res Commun. 1978 May 30;82(2):716–722. doi: 10.1016/0006-291x(78)90933-6. [DOI] [PubMed] [Google Scholar]
- Mancuso C. A., Nichols P. D., White D. C. A method for the separation and characterization of archaebacterial signature ether lipids. J Lipid Res. 1986 Jan;27(1):49–56. [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Methanosphaera stadtmaniae gen. nov., sp. nov.: a species that forms methane by reducing methanol with hydrogen. Arch Microbiol. 1985 Mar;141(2):116–122. doi: 10.1007/BF00423270. [DOI] [PubMed] [Google Scholar]
- Morii H., Nishihara M., Ohga M., Koga Y. A diphytanyl ether analog of phosphatidylserine from a methanogenic bacterium, Methanobrevibacter arboriphilus. J Lipid Res. 1986 Jul;27(7):724–730. [PubMed] [Google Scholar]
- Nishihara M., Koga Y. Extraction and composition of polar lipids from the archaebacterium, Methanobacterium thermoautotrophicum: effective extraction of tetraether lipids by an acidified solvent. J Biochem. 1987 Apr;101(4):997–1005. doi: 10.1093/oxfordjournals.jbchem.a121969. [DOI] [PubMed] [Google Scholar]
- Nishihara M., Koga Y. Hydroxyarchaetidylserine and hydroxyarchaetidyl-myo-inositol in Methanosarcina barkeri: polar lipids with a new ether core portion. Biochim Biophys Acta. 1991 Mar 12;1082(2):211–217. doi: 10.1016/0005-2760(91)90196-o. [DOI] [PubMed] [Google Scholar]
- Nishihara M., Koga Y. Quantitative conversion of diether or tetraether phospholipids to glycerophosphoesters by dealkylation with boron trichloride: a tool for structural analysis of archaebacterial lipids. J Lipid Res. 1988 Mar;29(3):384–388. [PubMed] [Google Scholar]
- Nishihara M., Morii H., Koga Y. Structure determination of a quartet of novel tetraether lipids from Methanobacterium thermoautotrophicum. J Biochem. 1987 Apr;101(4):1007–1015. doi: 10.1093/oxfordjournals.jbchem.a121942. [DOI] [PubMed] [Google Scholar]
- Nishihara M., Utagawa M., Akutsu H., Koga Y. Archaea contain a novel diether phosphoglycolipid with a polar head group identical to the conserved core of eucaryal glycosyl phosphatidylinositol. J Biol Chem. 1992 Jun 25;267(18):12432–12435. [PubMed] [Google Scholar]
- Patel G. B. A contrary view of the proposal to assign a neotype strain for Methanothrix soehngenii. Int J Syst Bacteriol. 1992 Apr;42(2):324–326. doi: 10.1099/00207713-42-2-324. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Ekiel I., Dicaire C. Novel, acid-labile, hydroxydiether lipid cores in methanogenic bacteria. J Biol Chem. 1990 Aug 15;265(23):13735–13740. [PubMed] [Google Scholar]
- Sprott G. D., Meloche M., Richards J. C. Proportions of diether, macrocyclic diether, and tetraether lipids in Methanococcus jannaschii grown at different temperatures. J Bacteriol. 1991 Jun;173(12):3907–3910. doi: 10.1128/jb.173.12.3907-3910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornabene T. G., Wolfe R. S., Balch W. E., Holzer G., Fox G. E., Oro J. Phytanyl-glycerol ethers and squalenes in the archaebacterium Methanobacterium thermoautotrophicum. J Mol Evol. 1978 Aug 2;11(3):259–266. doi: 10.1007/BF01734487. [DOI] [PubMed] [Google Scholar]
- Touzel J. P., Conway de Macario E., Nölling J., De Vos W. M., Zhilina T., Lysenko A. M. DNA relatedness among some thermophilic members of the genus Methanobacterium: emendation of the species Methanobacterium thermoautotrophicum and rejection of Methanobacterium thermoformicicum as a synonym of Methanobacterium thermoautotrophicum. Int J Syst Bacteriol. 1992 Jul;42(3):408–411. doi: 10.1099/00207713-42-3-408. [DOI] [PubMed] [Google Scholar]
- Wassef M. K., Sarner J., Kates M. Stereospecificity of the glycerol kinase and the glycerophosphate dehydrogenase in Halobacterium cutirubrum. Can J Biochem. 1970 Jan;48(1):69–73. doi: 10.1139/o70-012. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Fox G. E. Archaebacteria. J Mol Evol. 1978 Aug 2;11(3):245–251. doi: 10.1007/BF01734485. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Olsen G. J. Archaebacterial phylogeny: perspectives on the urkingdoms. Syst Appl Microbiol. 1986;7:161–177. doi: 10.1016/s0723-2020(86)80001-7. [DOI] [PubMed] [Google Scholar]
- Zellner G., Stackebrandt E., Messner P., Tindall B. J., Conway de Macario E., Kneifel H., Sleytr U. B., Winter J. Methanocorpusculaceae fam. nov., represented by Methanocorpusculum parvum, Methanocorpusculum sinense spec. nov. and Methanocorpusculum bavaricum spec. nov. Arch Microbiol. 1989;151(5):381–390. doi: 10.1007/BF00416595. [DOI] [PubMed] [Google Scholar]
- Zillig W. Comparative biochemistry of Archaea and Bacteria. Curr Opin Genet Dev. 1991 Dec;1(4):544–551. doi: 10.1016/s0959-437x(05)80206-0. [DOI] [PubMed] [Google Scholar]
- Zillig W., Klenk H. P., Palm P., Pühler G., Gropp F., Garrett R. A., Leffers H. The phylogenetic relations of DNA-dependent RNA polymerases of archaebacteria, eukaryotes, and eubacteria. Can J Microbiol. 1989 Jan;35(1):73–80. doi: 10.1139/m89-011. [DOI] [PubMed] [Google Scholar]


