Abstract
Sensorimotor inhibition, or the ability to filter out excessive or irrelevant information, theoretically supports a variety of higher-level cognitive functions. Impaired inhibition may be associated with increased impulsive and risky behavior in everyday life. Individuals infected with HIV frequently show impairment on tests of neurocognitive function, but sensorimotor inhibition in this population has not been studied and may be a contributor to the profile of HIV-associated Neurocognitive Disorders (HAND). 37 HIV-infected individuals (15 with HAND) and 48 non-infected comparison subjects were assessed for prepulse inhibition (PPI), an eyeblink startle paradigm measuring sensorimotor gating. Although HIV status alone was not associated with PPI deficits, HIV-positive participants meeting criteria for HAND showed impaired PPI compared to cognitively intact HIV-positive subjects. In HIV-positive subjects, PPI was correlated with working memory but was not associated with antiretroviral therapy or illness factors. In conclusion, sensorimotor disinhibition in HIV accompanies deficits in higher-order cognitive functions, though the causal direction of this relationship requires investigation. Subsequent research on the role of sensorimotor gating on decision-making and risk behaviors in HIV may be indicated.
Keywords: sensorimotor gating, AIDS dementia complex, cognition, startle, working memory, impulsivity
Introduction
Human Immunodeficiency Virus (HIV) infection is associated with a pattern of mild-to moderate neurocognitive deficits linked to fronto-striatal dysfunction (Heaton et al., 1995; H. P. Martin, 1995; Rippeth et al., 2004). HIV-associated neurocognitive disorders (HAND) are observed in approximately 40% of HIV-positive individuals and more frequently in persons with histories of immunosuppression (Heaton et al., 2010). One cognitive domain that is of central importance in the regulation of behavior and everyday functioning is inhibition (Hofmann, Schmeichel, & Baddeley, 2012), or the ability to withhold or attenuate an action or a thought. Prior studies of inhibition in HIV have focused largely on self-report measures and traditional neuropsychological tasks (e.g., Stroop paradigms). Even in the era of antiretroviral treatment (ART), persons with HIV report elevated rates of cognitive and behavioral disinhibition which are associated with impairment in basic cognitive processes such as attention (Hardy, Castellon, Hinkin, Levine, & Lam, 2008), difficulty with activities of daily living (Kamat, Woods, Marcotte, Ellis, & Grant, in press), and engagement in high risk behaviors such as unprotected sex (Semple, Zians, Grant, & Patterson, 2006). HIV-infected individuals also exhibit inhibitory deficits on standard neuropsychological tests (Hinkin, Castellon, Hardy, Granholm, & Siegle, 1999). For example, one study (E. M. Martin et al., 2004) reported a disproportionate reaction time slowing during the interference trial of a computerized Stroop paradigm. Cognitive disinhibition in HIV has been linked to elevated glial activation in frontal white matter which may occur as a result of systemic changes in immune system function (Chang et al., 2002)
One aspect of inhibition that has not been studied in HIV is sensorimotor gating, or the automatic filtering of excessive or irrelevant stimuli. Sensorimotor gating is commonly measured with prepulse inhibition (PPI) paradigms, in which a relatively weak sensory stimulus such as a sound (a prepulse) is presented shortly before a startle-inducing stimulus and results in a reduction of the startle reflex (Braff, Geyer, & Swerdlow, 2001). PPI is thought to be regulated by a complex network of neural pathways that includes a cortico-striato-pallido-thalamic loop (N.R. Swerdlow, 1996) and frontal-striatal inhibitory circuitry impacted by HIV infection (Chung et al., 2007). Sensorimotor gating is putatively an early, involuntary inhibitory function (Braff et al., 2001; Geyer, Krebs-Thomson, Braff, & Swerdlow, 2001), thus distinct from self-report measures and neuropsychological tests which are impacted by the individual’s effort, motivation, and fatigue, all of which may be factors that affect performance in persons with HIV. Therefore PPI may be a more specific indicator of early inhibitory deficits compared to traditional neurocognitive measures (Feifel, Minassian, & Perry, 2009). Higher sensorimotor inhibition as measured by PPI has been associated with better cognitive performance in healthy subjects (Bitsios, Giakoumaki, Theou, & Frangou, 2006; Csomor et al., 2008; Giakoumaki, Bitsios, & Frangou, 2006), but the association between sensorimotor inhibition and cognition in HIV is unknown. An additional advantage of sensorimotor gating is that it can be measured in animals using a comparable paradigm to further the understanding of neuropathological processes that underlie HIV, contribute to the refinement of much-needed animal models of this condition, and advance the development of novel treatments. Although PPI has not been assessed previously in HIV-infected subjects, individuals with the disease exhibit abnormalities on related measures of sensory processing, such as event related potentials, that correlate with general cognitive impairment (Fein, Biggins, & MacKay, 1995).
The current investigation examined HIV and inhibitory deficits as measured by PPI in an effort to understand the impact of the virus on the adaptive inhibitory function of sensorimotor gating. We hypothesized that individuals with HIV infection (HIV+) would exhibit PPI deficits compared to non HIV-infected (comparison) individuals. Furthermore, we predicted that HIV+ subjects who met criteria for HAND would demonstrate more severe PPI deficits relative to HIV+ subjects without HAND. HAND status was determined using a Global Deficit Score (GDS), a summary measure of neurocognitive impairment commonly used in HIV research (Blackstone et al., 2012) by our group (Heaton et al., 2010; Heaton et al., 2011; Letendre et al., 2006) as well as others (Joska et al., 2012; Overton et al., 2012; Sun, Abadjian, Rempel, Monto, & Pulliam, 2013). The GDS is thought to provide an advantage over traditional clinical ratings of neurocognitive impairment because it reduces a conglomerate of measures into one summary score, thus decreasing the chance of interpreting spurious findings of impairment on isolated neuropsychological tests (Carey et al., 2004).
Method
Subjects
This investigation was a component of the Translational Methamphetamine AIDS Research Center (TMARC), which is a multi-project center grant focused on translational approaches to understanding the combined effects of HIV and methamphetamine dependence on brain structure and function. The UCSD Human Research Protections Program approved the study. For the purposes of the current study, three groups of TMARC human subjects were examined: Individuals with HIV infection who did not meet criteria for HAND (HIV+/HAND− subjects, n = 22), individuals with HIV infection who met criteria for HAND (HAND+ subjects, n = 15), and individuals who tested negative for HIV (comparison subjects, n = 48). HIV infection was determined by Enzyme-linked immunosorbent assay (ELISA) and a confirmatory Western blot, which are standard diagnostic tests for HIV. Potential subjects were excluded if they reported histories of psychosis (e.g., schizophrenia) or significant medical (e.g., hepatitis C infection) or neurological (e.g., head injury with loss of consciousness > 30 min, seizure disorders, stroke, multiple sclerosis) conditions known to affect cognitive functions. Participants were also excluded if they met diagnostic criteria for current abuse or dependence on alcohol or any illicit drugs. Subjects were excluded if they had a positive urine toxicology screen for any drug except marijuana. The parent study from which this investigation was derived, TMARC, does not exclude subjects with positive marijuana urine toxicology tests for the following reasons: 1) some ART medications produce false positive results on marijuana toxicology screens; and 2) since marijuana can be detected in the urine for up to one month after use, positive toxicology screens do not necessarily indicate very recent use. On the day of evaluation, six subjects had a urine toxicology test positive for marijuana (3 comparison subjects, 2 HIV+/HAND−subjects, and 1 HIV+/HAND+ subject). As Table 2 illustrates, the groups were equivalent for prevalence of positive marijuana urine toxicology tests. Again, these subjects did not meet diagnostic criteria for cannabis abuse or dependence. When the primary analyses of the study were repeated with these six subjects removed, the essential findings were unchanged. Otherwise, all individuals provided negative urine toxicology screenings for illicit drug use on the day of evaluation. HAND diagnoses were provided by the TMARC Neuropsychiatric Core, who performed comprehensive testing of neuropsychological, psychiatric, and everyday living functions consistent with the recommendations of the Frascati criteria for diagnosing HAND (Antinori et al., 2007) which were formulated by Antinori and colleagues in Frascati, Italy and emphasize that the essential feature of HAND is cognitive disturbance (versus neuromotor problems or symptoms of psychiatric disease). The neurocognitive battery included the tests listed in Table 1 and was designed to assess cognitive domains known to be most affected by HIV, namely speed of information processing, attention/working memory, executive functions, learning, memory, verbal fluency, and motor functions (Carey et al., 2004). Raw scores were converted to T-scores using published, demographically adjusted normative standards. T-scores were then converted to deficit scores, which range from 0 (T > 39) to 5 (T < 20), with higher scores reflected greater neurocognitive disturbance. Deficit scores from primary test measures were then averaged to derive the GDS, for which values greater than or equal to 0.5 are considered an indication of HAND (Carey et al., 2004).
Table 2.
Demographic, psychiatric, and HIV characteristics of the study sample
| HIV− (n = 48) |
HIV+/HAND − (n = 22) |
HIV+/HAND+ (n = 15) |
Group Difference Statistic |
p- value |
|
|---|---|---|---|---|---|
| Age (yrs) | 37.0 (13.0) | 40.1 (9.5) | 40.5 (11.7) | F = .46 | ns |
| Education (yrs) | 13.3 (1.9) | 13.6 (2.6) | 13.7 (2.6) | F = .79 | ns |
| WRAT Reading Standard Score | 102.9 (11.9) | 104.0 (10.2) | 102.0 (11.7) | F = .14 | ns |
| Sex (% male) | 79.2% | 95.5% | 73.3% | Fisher’s Exact | ns |
| Ethnicity (% Caucasian) | 60.4% | 68.2% | 66.7% | Fisher’s Exact | ns |
| % with Lifetime Substance Dependence | 33.3% | 54.5% | 40.0% | Chi-Square = 2.8 | ns |
| % with current Major Depression | 14.6% | 22.7% | 26.6% | Fisher’s Exact | ns |
| % with Lifetime Major Depression | 29.2% | 54.5% | 66.7% | Fisher’s Exact | .01b |
| % with positive marijuana urine toxicology result | 6.3% | 9.1% | 7.1% | Fisher’s Exact | ns |
| POMS score | 45.3 (34.5) | 57.0 (44.7) | 59.3 (40.4) | F = 1.2 | ns |
| Current CD4a | -- | 560.0 (395.0, 746.3) | 426.0 (308.8, 602.3) | F = 1.1 | ns |
| Nadir CD4a | -- | 250.0 (78.8, 454.0) | 174.0 (67.0, 450.0) | F = .77 | ns |
| % detectable plasma HIV RNA | 35.0% | 48.5% | Fisher’s Exact | ns | |
| % with AIDS | -- | 36.4% | 66.7% | Fisher’s Exact | <.001 |
| % on ART | -- | 54.5% | 73.3% | Chi-Square = 43.4 | < .001 |
| GDSa | .26 (.07, .41) | .16 (.05, .32) | .74 (.63, .95) | F = 27.1 | < .001c |
Note: Data represent means and standard deviations unless otherwise footnoted. WRAT = Wide Range Achievement Test; POMS = Profile of Mood States- Total Mood Disturbance; ART = antiretroviral therapies; GDS = Global Deficit Score
Data represent medians with interquartile ranges
HIV− vs. HIV+/HAND+, p = .04, other group comparisons not statistically significant
HIV− vs. HIV+/HAND−, p = .05; HIV− vs. HIV+/HAND+ and HIV+/HAND− vs. HIV+/HAND=, p < .001
Table 1.
Cognitive domains and associated neuropsychological tests that comprise the Global Deficit Score (GDS)
| Domain | Tests |
|---|---|
| Speed of information processing | WAIS-III Digit Symbol WAIS-III Symbol Search Trail Making Test- Part A |
| Attention/Working memory | PASAT-200 WAIS-III Letter-Number Sequencing |
| Executive functions | Wisconsin Card Sorting Test-64 card version Trail Making Test-Part B Stroop interference Category Test |
| Learning | HVLT-R Trials 1–3 BVMT-R Trials 1–3 |
| Memory | HVLT-R Delay |
| Verbal fluency | COWAT-FAS Animal Fluency |
| Motor | Grooved Pegboard Dominant Nondominant |
Note: WAIS-III= Wechsler Adult Intelligence Scale, Third Edition; PASAT=Paced Auditory Serial Addition Test, HVLT=Hopkins Verbal Learning Test, BVMT=Brief Visuospatial Memory Test, COWAT=Controlled Oral Word Association Test.
Demographic data, illness-related variables, mood and substance abuse history, and GDS for subjects are presented in Table 2. The groups did not differ significantly in age, education, ethnicity, or gender. As expected based on group definitions, GDS were higher in the HIV+/HAND+ group than the HIV+/HAND− group and the comparison group. A higher percentage of HIV+/HAND+ subjects met criteria for AIDS and were prescribed antiretroviral therapy (ART) compared to HIV+/HAND− subjects.
Procedure
Written informed consent for all study procedures was obtained by the TMARC Administrative Core. As above, subjects underwent neurocognitive testing to derive the GDS and subsequently determine HAND status. Demographic information and urine toxicology were obtained on the day of the study visit, and subjects then underwent PPI testing. Standard procedures for PPI were implemented, e.g., participants refrained from nicotine and caffeine use 30 minutes prior to startle testing (Minassian, Feifel, & Perry, 2007). All participants underwent a brief hearing screening using an audiometer to ensure that they could hear tones bilaterally at 500, 1000, and 6000 Hz. Participants were seated comfortably in a reclining chair. Acoustic startle and prepulse stimuli were presented binaurally through headphones. The eyeblink component of the auditory startle reflex was measured using electromyography (EMG) of the orbicularis oculi muscle and EMG activity was recorded and filtered per our established methods (Braff, Grillon, & Geyer, 1992; Perry, Feifel, Minassian, Bhattacharjie, & Braff, 2002; Perry, Minassian, Feifel, & Braff, 2001).
The startle session was similar to previous methodology (Ahmari, Risbrough, Geyer, & Simpson, 2012; Minassian et al., 2007), beginning with a 5-minute acclimation period of 70 dB(A) white noise followed by four blocks of trials. The first and last blocks consisted of five pulse-alone trials of 40 msec 115-dB(A) startle stimuli. Blocks two and three consisted of 12 pulse-alone and prepulse-pulse trials presented in pseudorandom order. The 20 msec prepulse stimuli preceded the startle stimulus by 60 msec (onset-to-onset) and were either 74, 78, or 86 dB(A) (i.e., 4, 8, and 16 dB(A)) above the 70 dB(A) background noise). The inter-trial interval averaged 15 sec with a range of 11 to 21 sec. All blocks contained hidden “no stimulus” trials where no sound was delivered but EMG data were collected. The session duration was approximately 15 minutes.
Data Processing and Statistical Analyses
The startle measures were: 1) amplitude of the startle response to pulse alone trials as measured in digital units. 2) habituation of the startle response was measured by assessing the percentage decrement in the amplitude of the startle response to pulse alone trials (Block 1 and Block 4). 3) prepulse inhibition (PPI), calculated as the percent decrement in startle amplitude in the presence of the prepulse compared to the amplitude without the prepulse [100− (prepulse amplitude/pulse amplitude × 100)]. Average PPI was calculated over Blocks 2 and 3 (the two blocks containing prepulse trials). Subjects with an average amplitude to the pulse alone trials that was less than three times the average amplitude for no-stimulus trials in any of the four blocks were classified as startle non-responders (Ahmari et al., 2012) and were excluded from further analyses.
The primary hypotheses were: 1) HIV+ subjects would have lower percent PPI than HIV− subjects; and 2) HIV+/HAND+ subjects would have lower percent PPI than HIV+/HAND− subjects. These two pairwise comparisons were tested using Generalized Estimating Equations (GEE) (Zeger & Liang, 1986), with group as an independent variable and PPI condition (74 dB, 78 dB, 86 dB) as a categorical variable. An unstructured GEE working correlation was used. This model is appropriate for the PPI repeated measure, allows for the testing of main effects and interactions, and is more efficient and robust than the traditional repeated measures analysis. The GEE appropriately accounts for the within-subject correlations. The GEE model tested for interactive effects (group-by-PPI condition); in the absence of a significant interaction, the group and PPI condition effects were evaluated from a GEE model with additive main effects (group, PPI condition). The effects of AIDS, ART, and lifetime substance dependence on the outcomes were analyzed post-hoc by evaluating the effects of these covariates in the GEE model.
Data were inspected for normality and homogeneity. PPI data were normally distributed. Startle amplitude data were moderately positively skewed, thus a natural log transformation was applied. The analysis on the logarithmic scale allows the interpretation of the group and PPI effects in terms of multiplicative factors of the mean startle amplitude response. Neurocognitive deficit scores and illness factors such as nadir CD4 and viral load were not normally distributed, thus the correlational analyses with these measures were non-parametric Spearman’s rho coefficients as in previous studies (Carey et al., 2004; Moore et al., 2011).
All statistical analyses were performed with SPSS 21. Significance level was set at p < 0.05. Effect sizes were calculated using Cohen’s d.
Results
A total of 109 subjects received startle EMG testing, but 24 subjects were excluded from the analyses because they were startle non-responders, leaving a total sample size of 85 subjects for the PPI analyses. In the HIV+/HAND− group, 8 subjects (27%) were startle non-responders, in the HAND group, 4 subjects (21%) were non-responders, and in the HIV− group, 12 subjects (20%) were non-responders (Chi Square = .53, ns). The non-responder and responder groups were not significantly different on GDS scores, AIDS status, or whether they were taking ARTs.
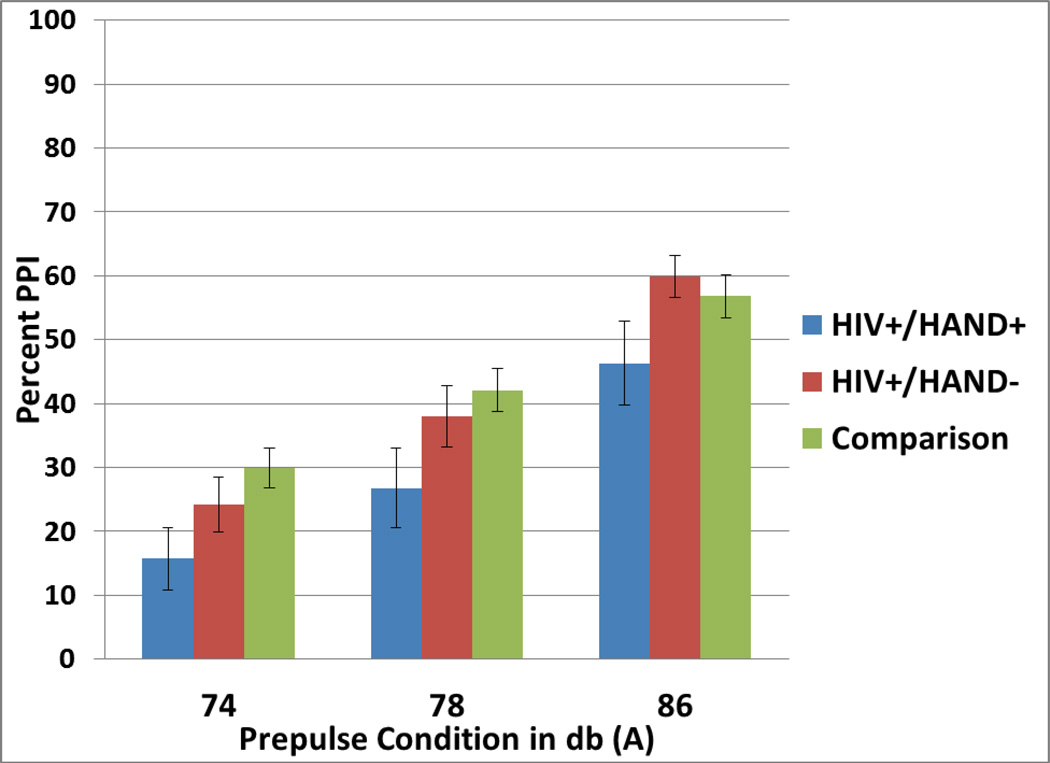
The GEE comparing all HIV+ subjects to HIV− subjects revealed a significant main effect of PPI condition (Wald Chi-square = 174.0, df=2, p<0.001) such that percent PPI increased as prepulse intensity increased: PPI 78 dB versus 74dB = 12.4% (95% CI 8.5, 16.4), PPI 86 dB versus 74 dB = 29.8%, 95% CI = (25.3, 34.4). There was no significant main effect of HIV status [HIV+ versus HIV− = −6.8%, 95% CI (−14.8, 1.3), Cohen’s d = −0.30, 95% CI (−0.65, 0.06), Wald Chi-square = 2.7, ns]. Thus, the first hypothesis was not supported. There was no significant PPI-by-HIV status interaction (Wald Chi-square = 3.3, df=2, ns). Cohen’s d effect sizes for the differences between HIV+ and HIV− subjects at each PPI condition were small-to-medium [74 dB: −9.2%, 95% CI (−17.9, −0.5), Cohen’s d = −0.42, 95% CI (−0.81, −0.02); 78 dB: −8.6%, 95% CI(−18.52, 1.35), Cohen’s d = −0.39, 95% CI (−0.83, 0.06); 86 dB: −2.4%, 95% CI (−11.7, 6.9); Cohen’s d = −0.11 (−0.53, 0.31)]. (Figure 1)
Figure 1.
Mean percent prepulse inhibition (PPI) at the three prepulse conditions for HIV+/HAND+ subjects (n = 15), HIV+/HAND− subjects (n = 22), and comparison subjects (n = 48).
The GEE comparing HIV+/HAND+ subjects to HIV+/HAND− subjects revealed a significant main effect of PPI condition (Wald Chi-Square = 97.6, df=2, p < .001) such that PPI increased as prepulse intensity increased: PPI 78 dB versus 74 dB = 12.8% (95% CI 5.9, 19.6), PPI 86 dB versus 74 dB = 33.7%, 95% CI = (26.5, 40.8). There was a significant main effect of HAND status such that HIV+ subjects with HAND had decreased PPI compared to HIV+ subjects without HAND [HAND+ vs. HAND− = −11.1%, 95% CI (−21.7, −.42), Cohen’s d = .53, 95% CI (−.14, −0.02), Wald Chi-Square = 4.2, df = 1, p=.04]. Thus, the second hypothesis was supported. There was no PPI-by-HAND status interaction (Wald Chi-Square = 0.4, df=2, ns). Cohen’s d effect sizes for the differences between HIV+ subjects with and without HAND at each PPI condition were medium [74 dB: −8.5%, 95% CI (−21.9, 4.9), Cohen’s d = −0.41, 95% CI (−1.1, −0.2); 78 dB: −11.3%, 95% CI(−27.0, 4.4), Cohen’s d =−.54, 95% CI (−1.3, 0.2); 86 dB: −13.7%, 95% CI (−27.3, 0.00); Cohen’s d = −0.66 (−1.3, 0.0)].
Because AIDS and current ART status were significantly different in the two HIV+ groups, two post-hoc GEEs were conducted on PPI where AIDS status and HAND status (HIV+/HAND−, HIV+/HAND+) were the predictors in the first GEE, and ART status and HAND status were the predictors in the second GEE. There were no significant main effects or interactions involving AIDS (main effect of AIDS Wald Chi-Square = 0.5, df=1, ns; HAND × AIDS interaction Wald Chi-Square = 0.1, df=1, ns) or ART status (main effect of ART Wald Chi-Square = 0.4, df=1, ns; HAND × ART interaction Wald Chi-Square = 0.1, df=1, ns). As Table 2 displays, the groups were matched on a history of lifetime substance dependence. Nevertheless, to further determine the potential impact of substance dependence, two additional post-hoc GEEs were conducted on PPI. Presence or absence of lifetime substance dependence and HIV status were entered as predictors in the first GEE. There was neither a main effect of lifetime substance dependence (Wald Chi-Square = 0.1, df=1, ns) nor a HIV status-by-substance dependence interaction (Wald Chi-Square = 0.9, df=1, ns). Presence or absence of lifetime substance dependence and HAND status were entered as predictors in the second GEE. Again there was neither a main effect of lifetime substance dependence (Wald Chi-Square = 0.6, df=1, ns) nor a HAND status-by-substance dependence interaction (Wald Chi-Square = 2.4, df=1, ns). As Table 2 also displays, there was no difference among the groups in terms of presence of a positive marijuana urine toxicology screen.
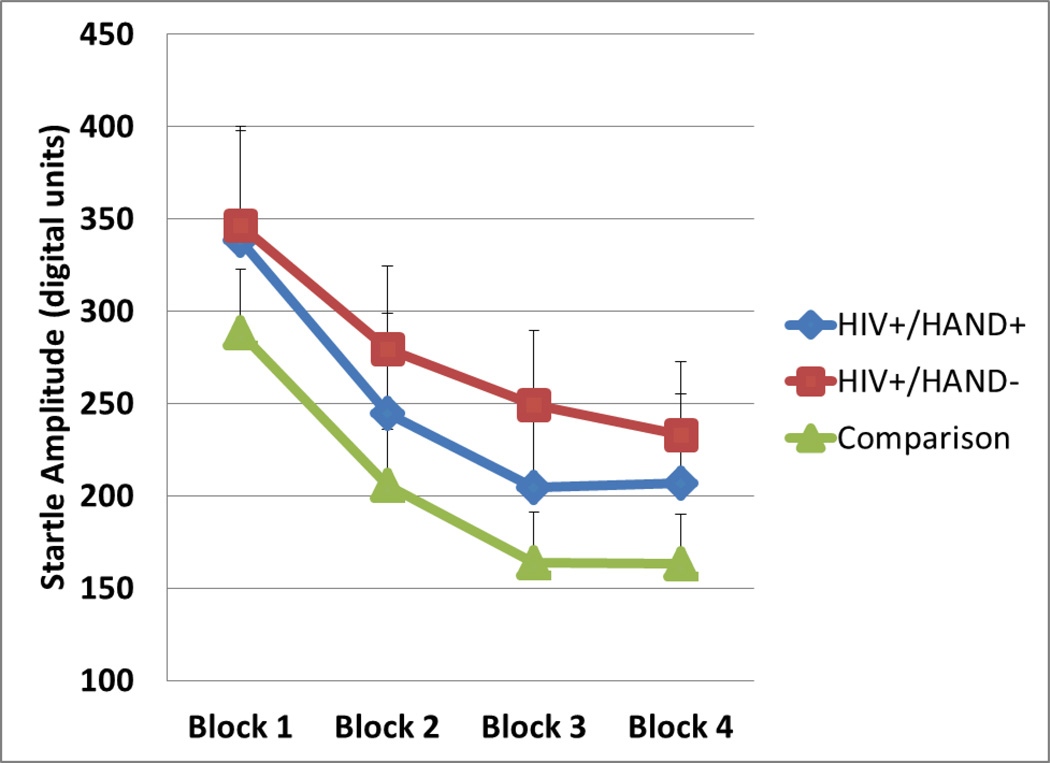
Post-hoc GEEs on startle amplitude in each of the four blocks revealed no differences between HIV+ and HIV− subjects (main effect of HIV Wald Chi-Square = 0.5, df=1,ns; HIV × block interaction Wald Chi-Square = 2.1, df=3, ns) nor between HIV+/HAND+ subjects and HIV+/HAND− subjects (main effect of HAND Wald Chi-Square = 0.4, df=1, ns; HAND × block interaction Wald Chi-Square = 1.6, df=3,ns). Figure 2 displays mean startle amplitudes in the groups.
Figure 2.
Mean startle amplitude in digital units for HIV+/HAND+ subjects (n = 15), HIV+/HAND− subjects (n = 22), and comparison subjects (n = 48).
Spearman rho correlation coefficients in the overall group of HIV+ subjects (n = 37) yielded significant negative correlations between two of the three PPI conditions and deficit scores in the domain of Working Memory (Table 3). There were no significant correlations in the HIV− subjects. There were no significant correlations or trends for PPI conditions compared to current or nadir CD4 counts, viral load, or mood symptoms as measured by the Profile of Mood States (POMS). Among the overall group of HIV+ subjects, the correlation between current CD4 count and GDS approached statistical significance (rho =−.33, p = .05), suggesting that lower CD4 counts were associated with higher GDS scores. There were no significant relationships between GDS and nadir CD4 count or viral load.
Table 3.
Spearman rho correlation coefficients between cognitive domains and percent prepulse inhibition (PPI) in all HIV+ subjects (n = 37).
| PPI at 74 dB | PPI at 80 dB | PPI at 86 dB | |
|---|---|---|---|
| Speed of information processing | −.32 | −.23 | −.19 |
| Attention/Working memory | −.12 | −.39* | −.50** |
| Executive functions | −.24 | −.13 | −.10 |
| Learning | −.25 | −.05 | .09 |
| Memory | −.25 | −.07 | −.04 |
| Verbal fluency | −.10 | −.05 | −.09 |
| Motor | −.14 | −.13 | −.22 |
p < .05,
p < .01
Post-hoc t-tests were conducted in the overall group of HIV+ subjects (n=37) on whether GDS differed based on AIDS or ART status. Subjects with AIDS had higher GDS than subjects without AIDS [t (35) = 2.4, p = .02, Cohen’s d = 0.8]. The difference in GDS between subjects taking ARTs versus not taking ARTs did not reach statistical significance [t (35) = 1.4, ns, Cohen’s d = 0.4].
Discussion
Neurocognitive impairment has been observed in some individuals with HIV and has implications for day-to-day functioning and the ability to appropriately regulate behavior. Sensorimotor gating is thought to serve a critical inhibitory function that helps maintain normal cognition and has been found to be compromised across a range of neuropsychiatric conditions, including schizophrenia (Braff & Geyer, 1990; Minassian et al., 2007), the mania of bipolar disorder (Perry et al., 2001), Huntington’s Disease (Neal R. Swerdlow, Paulsen, Braff, & Butters, 1995), and other neurocognitive disorders (Neal R. Swerdlow, Benbow, Zisook, & Geyer, 1993). Given that the fronto-striatal circuitry regulating PPI is also thought to be impacted by HIV infection, we anticipated PPI deficits in this population. Although the current report is, to our knowledge, the first on PPI in HIV, previous studies on event-related potentials do suggest decreased arousal and cognitive decline in this population (Chao, Lindgren, Flenniken, & Weiner, 2004; Polich, Ilan, Poceta, Mitler, & Darko, 2000). In the current investigation, contrary to our hypothesis, HIV+ individuals as a group did not show prominent PPI impairment when compared to non-infected individuals. HIV+ subjects who also met criteria for HAND, however, did show less sensorimotor gating compared to HIV+ subjects with non-impaired cognition. These findings suggest that sensorimotor gating impairment does not appear to be a global deficit in HIV, rather it is present in those individuals with HIV who also show evidence of neurocognitive impairment, perhaps more prominently in cognitive functions such as working memory. As a group, HIV-infected people with relatively intact cognition demonstrate normal levels of PPI.
It is not surprising that PPI deficits would be associated with impaired neurocognitive function (Geyer, 2006), and in healthy subjects higher sensorimotor inhibition has been related to better performance on neurocognitive tests of executive function (Bitsios et al., 2006; Giakoumaki et al., 2006) and working memory (Csomor et al., 2008). In individuals with schizophrenia however, where PPI has been proposed as an endophenotype of the disease, some studies show relationships between sensorimotor inhibition deficits and impaired cognition (Butler, Jenkins, Sprock, & Braff, 1992; Rabin, Sacco, & George, 2009), while others do not (Hasenkamp et al., 2011; Kishi et al., 2012; Molina et al., in press; N. R. Swerdlow et al., 2006). For example, in a well-powered study using multiple statistical methods, Swerdlow and colleagues found that schizophrenia patients with low PPI were not the same subjects that exhibited poor neuropsychological performance. These authors have posited that, at least in schizophrenia, the neural circuitry underlying PPI does not directly and consistently correspond to that of general neurocognitive impairment. In the case of HIV, one may speculate that acquisition of the HIV virus does not in and of itself directly impair sensorimotor gating function; rather the deficit is seen in the context of broader neurocognitive impairment that is observed in some, but not all, individuals with the disease. Thus PPI may simply be another indicator of frontal systems impairment in those HIV subjects who have difficulties with executive functions, impulsivity, and risk-taking. Alternatively, given that sensorimotor gating is considered a measure of early and largely involuntary inhibition (Braff et al., 2001; Geyer et al., 2001), deficits in early information processing may actually impede higher-order executive functions. Both speculations have been suggested by others (Bitsios et al., 2006), but the current results do not provide substantial support for either, since the relationships between PPI and neurocognitive measures were modest and were not consistently seen across all the domains of frontally mediated cognitive tasks. Not unexpectedly, subjects with AIDS did have higher global neurocognitive impairment scores than those without AIDS, and a lower current CD4 count was modestly associated with higher GDS. The mere presence of AIDS was not, however, associated with PPI deficits. The finding that a summary score of neurocognitive impairment differentiated AIDS status but PPI did not again suggests that decreased sensorimotor inhibition in and of itself does not inevitably accompany the progression of this disease.
A third factor may cause both PPI and neurocognitive deficits. For example, although has been suggested that antiretroviral therapy may slow or delay neurocognitive decline in HIV (Deutsch et al., 2001; Dore et al., 1999; Maschke et al., 2000; Sacktor et al., 2001), the prevalence of HIV-associated cognitive impairment has not decreased since the advent of antiretroviral therapies (Sacktor et al., 2002). Additionally, there is evidence from rodent studies that ARTs can actually induce cognitive impairment, possibly via metabolic changes (Pistell et al., 2010). The effect of ARTs on sensorimotor inhibition is not known. Consistent with prior work (Heaton et al., 2010), a higher percentage of HIV+ subjects with HAND were taking ARTs in this study compared to HIV+ subjects without HAND, although when all HIV+ subjects were examined collectively, GDS were not robustly different in those taking ARTs versus those not taking them. Furthermore, current ART status did not explain the decreased PPI in the individuals with HAND. Clearly an evaluation of ART penetrance of the central nervous system (Letendre et al., 2004) is needed to elucidate the potential impact of ARTs on important cognitive functions such as inhibition.
Limitations of this study include relatively smaller sample sizes for some of the comparisons with HIV+/HAND+ subjects; thus the lack of significant findings in those analyses could be attributed to low power. The finding of lower PPI in the HAND+ subjects should be interpreted with caution given the large confidence intervals around the effect sizes when the PPI conditions are examined separately. As noted above, since the relationships between PPI and the cognitive domains were modest and not observed consistently across all domains, they should be interpreted with caution. Although no subject met current criteria for abuse or dependence on any illicit substance, six subjects had positive urine toxicology results for marijuana. While the proportions of these subjects did not differ among the groups of interest, average PPI was relatively lower in this small group. Small sample sizes preclude us from drawing conclusions about the effect of marijuana use on PPI in HIV from these results. Nevertheless, this question is important given reports of marijuana use impacting PPI (Kedzior & Martin-Iverson, 2006, 2007; Mathias et al., 2012) and the prevalence of marijuana use due to its decriminalization and medicinal uses, which is not limited to the HIV population. Finally, a not inconsequential limitation is the restriction of gender to almost entirely men, curtailing our ability to draw conclusions about sensorimotor gating features in women with HIV. Underscoring the importance of studying women are the previously identified relationships between PPI and menstrual cycle (Neal R. Swerdlow, Hartman, & Auerbach, 1997) and findings in rodents that PPI varies according to estrus cycle (Koch, 1998),
In conclusion, sensorimotor gating deficits do not appear to be a global feature of HIV. Instead, they manifest in the context of neurocognitive impairment and thus may be influenced by frontal system impairments which also cause “downstream” cognitive problems. Using a translational measure of a critical cognitive function such as sensorimotor gating in parallel human and animal studies may help shed further light on the unique neural circuitry features of this acquired illness and its interaction with other potentially neurotoxic factors such as substance use.
Acknowledgments
Funding for this study was provided by NIH grants P50-DA026306 and R01-MH91716; the NIH had no further role in study design, in the collection, analysis, and interpretation of data, in the writing of this report, or in the decision to submit the paper for publication.
We hereby declare we have no conflicts of interest involving this research and did not receive funds from a commercial sponsor. M.A. Geyer holds an equity interest in San Diego Instruments.
The Translational Methamphetamine AIDS Research Center (TMARC) is affiliated with the University of California San Diego (UCSD) and the Sanford-Burnham Medical Research Institute (SBMRI). The TMARC is comprised of: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Cristian L. Achim, M.D., Ph.D., and Scott L. Letendre, M.D.; Center Manager – Steven Paul Woods, Psy.D.; Assistant Center Manager – Aaron M. Carr, B.A.; Clinical Assessment and Laboratory (CAL) Core: Scott L. Letendre, M.D. (Core Director), Ronald J. Ellis, M.D., Ph.D., Rachel Schrier, Ph.D.; Neuropsychiatric (NP) Core: Robert K. Heaton, Ph.D. (Core Director), J. Hampton Atkinson, M.D., Mariana Cherner, Ph.D., Thomas D. Marcotte, Ph.D.; Neuroimaging (NI) Core: Gregory Brown, Ph.D. (Core Director), Terry Jernigan, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A.; Neurosciences and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Eliezer Masliah, M.D., Stuart Lipton, M.D., Ph.D.; Administrative Coordinating Core (ACC) – Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Leader), Clint Cushman (Unit Manager); ACC – Statistics Unit: Ian Abramson, Ph.D. (Unit Leader), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC – Participant Unit: J. Hampton Atkinson, M.D. (Unit Leader), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark Geyer, Ph.D., Brook Henry, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Martin Paulus, M.D., Ronald J. Ellis, M.D., Ph.D.; Project 3: Sheldon Morris, M.D., M.P.H. (Project Director), David M. Smith, M.D., M.A.S., Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director), Athina Markou, Ph.D., James Kesby, Ph.D.; Project 5: Marcus Kaul, Ph.D. (Project Director). The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
References
- Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology. 2012;37(5):1216–1223. doi: 10.1038/npp.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsios P, Giakoumaki SG, Theou K, Frangou S. Increased prepulse inhibition of the acoustic startle response is associated with better strategy formation and execution times in healthy males. Neuropsychologia. 2006;44(12):2494–2499. doi: 10.1016/j.neuropsychologia.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, Heaton RK. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clinical Neuropsychology. 2012;26(6):894–908. doi: 10.1080/13854046.2012.694479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Archives of General Psychiatry. 1990;47(2):181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156(2–3):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Archives of General Psychiatry. 1992;49(3):206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Butler RW, Jenkins MA, Sprock J, Braff DL. Wisconsin Card Sorting Test deficits in chronic paranoid schizophrenia: Evidence for a relatively discrete subgroup? Schizophrenia Research. 1992;7(2):169–176. doi: 10.1016/0920-9964(92)90047-9. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004;26(3):307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage. 2002;17(3):1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- Chao LL, Lindgren JA, Flenniken DL, Weiner MW. ERP evidence of impaired central nervous system function in virally suppressed HIV patients on antiretroviral therapy. Clinical Neurophysiology. 2004;115(7):1583–1591. doi: 10.1016/j.clinph.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, Renshaw PF. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. International Journal of Neuropsychopharmacology. 2007;10(6):765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- Csomor PA, Stadler RR, Feldon J, Yee BK, Geyer MA, Vollenweider FX. Haloperidol differentially modulates prepulse inhibition and p50 suppression in healthy humans stratified for low and high gating levels. Neuropsychopharmacology. 2008;33(3):497–512. doi: 10.1038/sj.npp.1301421. [DOI] [PubMed] [Google Scholar]
- Deutsch R, Ellis RJ, McCutchan JA, Marcotte TD, Letendre S, Grant I. AIDS-associated mild neurocognitive impairment is delayed in the era of highly active antiretroviral therapy. Aids. 2001;15(14):1898–1899. doi: 10.1097/00002030-200109280-00027. [DOI] [PubMed] [Google Scholar]
- Dore GJ, Cooper DA, Barrett C, Goh LE, Thakrar B, Atkins M. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. The Journal of Infectious Diseases. 1999;180(3):607–613. doi: 10.1086/314942. [DOI] [PubMed] [Google Scholar]
- Feifel D, Minassian A, Perry W. Prepulse inhibition of startle in adults with ADHD. Journal of Psychiatric Research. 2009;43(4):484–489. doi: 10.1016/j.jpsychires.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Biggins CA, MacKay S. Delayed latency of the event-related brain potential P3A component in HIV disease. Progressive effects with increasing cognitive impairment. Archives of Neurology. 1995;52(11):1109–1118. doi: 10.1001/archneur.1995.00540350103022. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Are cross-species measures of sensorimotor gating useful for the discovery of procognitive cotreatments for schizophrenia? Dialogues in Clinical Neuroscience. 2006;8(1):9–16. doi: 10.31887/DCNS.2006.8.1/mgeyer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156(2–3):117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Giakoumaki SG, Bitsios P, Frangou S. The level of prepulse inhibition in healthy individuals may index cortical modulation of early information processing. Brain Research. 2006;1078(1):168–170. doi: 10.1016/j.brainres.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Castellon SA, Hinkin CH, Levine AJ, Lam MN. Sensation seeking and visual selective attention in adults with HIV/AIDS. AIDS and Behavior. 2008;12(6):930–934. doi: 10.1007/s10461-007-9288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Kelley M, Egan G, Green A, Wilcox L, Boshoven, Duncan E. Lack of relationship between acoustic startle and cognitive variables in schizophrenia and control subjects. Psychiatry Research. 2011;187(3):324–328. doi: 10.1016/j.psychres.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, Abramson I. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. Journal of the International Neuropsychological Society. 1995;1(3):231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Hardy DJ, Granholm E, Siegle G. Computerized and traditional stroop task dysfunction in HIV-1 infection. Neuropsychology. 1999;13(2):306–316. doi: 10.1037//0894-4105.13.2.306. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends in Cognitive Sciences. 2012;16(3):174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Joska JA, Westgarth-Taylor J, Hoare J, Thomas KG, Paul R, Myer L, Stein DJ. Neuropsychological outcomes in adults commencing highly active anti-retroviral treatment in South Africa: a prospective study. BMC Infectious Diseases. 2012;12:39. doi: 10.1186/1471-2334-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat R, Woods SP, Marcotte TD, Ellis RJ, Grant I. Implications of Apathy for Everyday Functioning Outcomes in Persons Living with HIV Infection. Archives of Clinical Neuropsychology. 27(5):520–531. doi: 10.1093/arclin/acs055. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzior KK, Martin-Iverson MT. Chronic cannabis use is associated with attention-modulated reduction in prepulse inhibition of the startle reflex in healthy humans. Journal of Psychopharmacology. 2006;20(4):471–484. doi: 10.1177/0269881105057516. [DOI] [PubMed] [Google Scholar]
- Kedzior KK, Martin-Iverson MT. Attention-dependent reduction in prepulse inhibition of the startle reflex in cannabis users and schizophrenia patients--a pilot study. European Journal of Pharmacology. 2007;560(2–3):176–182. doi: 10.1016/j.ejphar.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Kishi T, Fukuo Y, Okochi T, Kawashima K, Moriwaki M, Furukawa O, Iwata N. The relationship between acoustic startle response measures and cognitive functions in Japanese patients with schizophrenia. Neuromolecular Medicine. 2012;14(2):131–138. doi: 10.1007/s12017-012-8177-y. [DOI] [PubMed] [Google Scholar]
- Koch M. Sensorimotor gating changes across the estrous cycle in female rats. Physiology and Behavior. 1998;64(5):625–628. doi: 10.1016/s0031-9384(98)00098-5. [DOI] [PubMed] [Google Scholar]
- Letendre SL, McCutchan JA, Childers ME, Woods SP, Lazzaretto D, Heaton RK, Ellis RJ. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Annals of Neurology. 2004;56(3):416–423. doi: 10.1002/ana.20198. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Woods SP, Ellis RJ, Atkinson JH, Masliah E, van den Brande G, Everall I. Lithium improves HIV-associated neurocognitive impairment. Aids. 2006;20(14):1885–1888. doi: 10.1097/01.aids.0000244208.49123.1b. [DOI] [PubMed] [Google Scholar]
- Martin EM, Novak RM, Fendrich M, Vassileva J, Gonzalez R, Grbesic S, Sworowski L. Stroop performance in drug users classified by HIV and hepatitis C virus serostatus. Journal of the International Neuropsychological Society. 2004;10(2):298–300. doi: 10.1017/S135561770410218X. [DOI] [PubMed] [Google Scholar]
- Martin HP. Mild cognitive impairment in HIV disease. Nurse Practitioner. 1995;20(8):94–97. [PubMed] [Google Scholar]
- Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART) Journal of Neurology, Neurosurgery and Psychiatry. 2000;69(3):376–380. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias CW, Blumenthal TD, Dawes MA, Liguori A, Richard DM, Bray B, Dougherty DM. Failure to sustain prepulse inhibition in adolescent marijuana users. Drug and Alcohol Dependence. 2012;116(1–3):110–116. doi: 10.1016/j.drugalcdep.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Feifel D, Perry W. The relationship between sensorimotor gating and clinical improvement in acutely ill schizophrenia patients. Schizophrenia Research. 2007;89(1–3):225–231. doi: 10.1016/j.schres.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina V, Cortes B, Perez J, Martin C, Villa R, Lopez DE, Sancho C. No association between prepulse inhibition of the startle reflex and neuropsychological deficit in chronic schizophrenia. European Archives of Psychiatry and Clinical Neurosciences. 260(8):609–615. doi: 10.1007/s00406-010-0102-5. (in press) [DOI] [PubMed] [Google Scholar]
- Moore DJ, Arce M, Moseley S, McCutchan JA, Marquie-Beck J, Franklin DR, Grant I. Family history of dementia predicts worse neuropsychological functioning among HIV-infected persons. The Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23(3):316–323. doi: 10.1176/appi.neuropsych.23.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton ET, Kauwe JS, Paul R, Tashima K, Tate DF, Patel P, Clifford DB. Performances on the CogState and standard neuropsychological batteries among HIV patients without dementia. AIDS and Behavior. 2012;15(8):1902–1909. doi: 10.1007/s10461-011-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Feifel D, Minassian A, Bhattacharjie I, Braff DL. Information processing deficits in acutely psychotic schizophrenia patients medicated and unmedicated at the time of admission. The American Journal of Psychiatry. 2002;159(8):1375–1381. doi: 10.1176/appi.ajp.159.8.1375. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biological Psychiatry. 2001;50(6):418–424. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Gupta S, Knight AG, Domingue M, Uranga RM, Ingram DK, Bruce-Keller AJ. Metabolic and neurologic consequences of chronic lopinavir/ritonavir administration to C57BL/6 mice. Antiviral Research. 2010;88(3):334–342. doi: 10.1016/j.antiviral.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Ilan A, Poceta JS, Mitler MM, Darko DF. Neuroelectric assessment of HIV: ERP, and viral load. International Journal of Psychophysiology. 2000;38(1):97–108. doi: 10.1016/s0167-8760(00)00133-1. [DOI] [PubMed] [Google Scholar]
- Rabin RA, Sacco KA, George TP. Correlation of prepulse inhibition and Wisconsin Card Sorting Test in schizophrenia and controls: effects of smoking status. Schizophrenia Research. 2009;114(1–3):91–97. doi: 10.1016/j.schres.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, McArthur JC. HIV-associated neurologic disease incidence changes:: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56(2):257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. Journal of Neurovirology. 2002;8(2):136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Zians J, Grant I, Patterson TL. Methamphetamine use, impulsivity, and sexual risk behavior among HIV-positive men who have sex with men. Journal of Addictive Diseases. 2006;25(4):105–114. doi: 10.1300/J069v25n04_10. [DOI] [PubMed] [Google Scholar]
- Sun B, Abadjian L, Rempel H, Monto A, Pulliam L. Differential Cognitive Impairment in HCV Coinfected Men With Controlled HIV Compared to HCV Monoinfection. Journal of Acquired Immune Deficiency Syndromes. 2013;62(2):190–196. doi: 10.1097/QAI.0b013e31827b61f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR. Cortico-striatal substrates of cognitive, motor and sensory gating: Speculations and implications for psychological function and dysfunction. In: Panksepp J, editor. Advances in Biological Psychiatry. Vol. 2. Greenwich, CT: JAI Press Inc.; 1996. pp. 179–208. [Google Scholar]
- Swerdlow NR, Benbow CH, Zisook S, Geyer MA. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biological Psychiatry. 1993;33(4):298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: Implications for neuropsychiatric disorders. Biological Psychiatry. 1997;41(4):452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Archives of General Psychiatry. 2006;63(12):1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Paulsen J, Braff DL, Butters N. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington's disease. Journal of Neurology, Neurosurgery and Psychiatry. 1995;58(2):192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]