Abstract
Spider dragline silk is a proteinaceous fiber with impressive physical characteristics making it attractive for use in advanced materials. The fiber is composed of two proteins (spidroins MaSp1 and MaSp2), each of which contains a large central repeat array flanked by non-repetitive N- and C-terminal domains. The repeat arrays appear to be largely responsible for the tensile properties of the fiber, suggesting that the N- and C-terminal domains may be involved in self-assembly. We recently isolated the MaSp1 and MaSp2 N-terminal domains from Nephila clavipes and have incorporated these into mini-silk genes for expression in transgenic systems. Current efforts involve the development of expression vectors that will allow purification using a removable affinity tag for scalable protein purification.
Key Terms: Major Ampullate Spidroin, Protein, Recombinant, Spider Silk
Introduction
Natural and synthetic fibrous molecules provide some of the most durable materials known. Much of the synthetic fiber industry has focused on the development and production of hydrocarbon-based materials including nylon, polyethylene, and polypropylene. While many of these high-performance fibers exhibit desirable physical properties, their production requires the use of hazardous organic solvents as well as elevated temperatures and pressures. In contrast, there are a number of naturally-occurring fibrous materials that are spun at ambient temperature and pressure from an aqueous solution. Among these are the silk protein fibers produced by numerous species of insects and spiders.
Silks are highly-insoluble proteinaceous fibers. The best-studied silks are those of the common silkworm (Bombyx mori)1–3 and those produced by the orb-weaving spiders Nephila clavipes4,5 and Araneus diadematus.4,5 While the silkworm produces a single type of silk, orb-weaving spiders (Araneidae) like Nephila clavipes can produce up to seven different types of silk that originate from different abdominal glands.6 These fibers vary widely in mechanical properties and have evolved to accommodate specific needs of the spider. The frame and radii of the web are made from dragline silk that exhibits appreciable elasticity and strength resulting in an incredibly tough fiber.4 Flagelliform silk is the most elastic of the silks and is used for the capture spiral of the web.7 Aciniform silk is used for wrapping prey—while intermediate with respect to strength when compared to the weaker flagelliform and stronger dragline silks, it is superior to both in toughness.8
Table I shows the impressive mechanical qualities of some naturally occurring silks compared to common man-made materials. It is notable that Nylon 6,6 displays properties very close to that of silkworm silk. However, with respect to the combination of tensile strength, breaking strain, and resilience, spider dragline silks are superior to synthetic materials and silkworm silk. For this reason, dragline fibers have often been described as one of nature’s “super-fibers.” Along with superior mechanical properties, spider dragline silks are proteinaceous fibers and are, therefore, biodegradable, making them very attractive for the development of new, advanced materials.
Table I.
Mechanical Properties of Silk and High-performance Synthetic Fibersa
| Fiber Type | Density (g/cm3) | Modulus of Elasticity (GPa) | Tensile Strength (GPa) | Breaking Strain (%) | Resilience (MJ/m3) |
|---|---|---|---|---|---|
| Spider dragline silk | |||||
| Argiope trifasciata | 1.3 | 1 – 10 | 1.2 | 30 | 100 |
| N. clavipes | 1.3 | 1 – 10 | 1.8 | 30 | 130 |
| Silkworm silk | 1.3 | 5 | 0.6 | 12 | 50 |
| Nylon 6,6 | 1.1 | 5 | 0.9 | 18 | 80 |
| Kevlar 49 | 1.4 | 130 | 3.6 | 3 | 50 |
| PBOb | 1.6 | 270 | 5.8 | 3 | 70 |
| Steel | 7.8 | 200 | 3 | 2 | 6 |
Modified from reference 36.
Polybenzoxazole
The majority of silk biomedical studies used regenerated silkworm silks as a research model. Relatively few evaluated spider silks despite their superior mechanical qualities. Silkworm silks are readily available due to their extensive use in the textile industry and the ability to farm the insects. In addition, it has been found that silkworm silks are more readily solubilized by high concentration LiBr solution.1 Unlike silkworms, the farming of spiders is unrealistic due to low yields (~0.8 mg per spider every other day)9 and territorial/cannibalistic behavior. Thus, several research groups have attempted to exploit recombinant expression systems for the production of spidroins. Spidroins have been expressed in bacteria,10 yeast,11 insect,12 and mammalian cell cultures.13 Transgenic mammal milk14 and transgenic plants15 have also been used as expression platforms.
Dragline Silks
Among the different types of spider silks, dragline silk is the best studied. Dragline silk is used not only to construct the outer frame and radii of the orb-shaped web but also as a hanging lifeline that allows the spider to evade and/or escape from predators. The core constituents of dragline silk are two fibrous proteins produced in the major ampullate gland called major ampullate spidroins 1 and 2 (MaSp1 and MaSp2).5,16,17 These proteins are stored at high concentration in the lumen of the gland as a spinning dope.18 When there is demand for dragline fiber, this spinning dope is drawn down a long, tapered duct in which there are documented changes in both pH and ionic environment.19
The repeat arrays of both MaSp1 and MaSp2 (and other spidroin proteins) are flanked by non-repetitive N-terminal and C-terminal domains of ~150 and ~100 amino acids, respectively. The deduced C-terminal domain sequences of MaSp proteins have been known for almost two decades.16,17 Functional studies using partial dragline silk spidroins have implicated the C-terminal domain in the organized transition from a soluble spidroin solution to an insoluble fiber during spinning.12,20–22 There is also a conserved cysteine residue in the C-terminal domain of most MaSp proteins that has been suggested to be important in assembly of the final fiber as a result of intermolecular crosslinking through disulfide bond formation.5 A recent study implicates this non-repetitive domain as playing a role in both spidroin storage and the self-assembly process.23
In contrast, N-terminal domain MaSp sequences were not known until more recently due, at least in part, to difficulties in cloning and/or sequencing the large and highly-repetitive spidroin genes and cDNAs.24,25 Analysis of these sequences demonstrates that they are the most highly conserved domains of spidroins. While a number of these reported sequences have been deduced from genomic DNA, it has allowed evaluation of the N-terminal domains for specific motifs that would support their authenticity. One of these motifs, a signal peptide that would be consistent with secretion of spidroins into the duct, now appears to be a clearly recognized component of essentially all known N-terminal domains.24,26 Recombinant expression of this domain as a recombinant protein has allowed biochemical characterization by circular dichroism (CD) spectroscopy that confirmed the predicted, largely α-helical secondary structure.27,28 The same studies also indicated that the N-terminal domain is capable of homodimerization as observed in size exclusion chromatography elution profiles.27,28 More recent work supports a role for this domain in spidroin solubility and the self assembly process that leads to fiber formation.28,29
Interestingly, the N-terminal and C-terminal domains found on mature spidroins are not only conserved between MaSp1 and MaSp2, but also among many silk types and spider species.24,26,30,31 Individual N- and C-terminal domains themselves bear little resemblance to each other except that they are both rich in serine (~13% for N-terminal and 23% for C-terminal) and both are predicted to exist as largely amphipathic, α-helical secondary structures.5,24,26 In both cases, this could be consistent with terminal globular domains resulting from the interaction of the amphipathic helices.
Results and Discussion
Expression Constructs Generation
Most reports of fibers spun from silk-like proteins used recombinant proteins that lack native N-terminal domains.22,32,33 One report that specifically includes an N-terminal domain describes it as “artificial” and likely not of spider origin.13 Conservation of the N-terminal sequence among different silk types suggests that, despite their relatively small size, they play an important role in the function of the silk. While little is known about their actual contribution to the final fiber, it is believed that they are intimately involved in the self-assembly process.
This research group recently reported isolation of native N-terminal MaSp1 and MaSp2 dragline sequences from N. clavipes and are incorporating these domains into their expression constructs.24 Using consensus sequences for the repeat domains of N. clavipes MaSp1 and MaSp2, this group assembled mini-silk genes that contained native N- and C-terminal domains flanking various numbers of repeat domains for both MaSp1 and MaSp2 (Fig. 1). Ideally, expression constructs would contain larger numbers of repeat domains to better approximate native silks. However, there are technical difficulties associated with manipulation of large, repetitive nucleic acid sequences. In addition, recent work using Escherichia coli shows that expression of large repeat domains also required specially engineered strains and even then only low levels of expression were achieved.34
Fig. 1.

General structure of mini-silk protein modules.
Mini-silk genes encoded native N- and C-terminal sequences for MaSp1 or MaSp2, between which were placed various numbers of the corresponding repeat domain. Constructs that contain 8-, 16-, and 32-mer repeat domains were generated for both MaSp1 and MaSp2. In some cases, there was a C-terminal polyhistidine tag (His-tag).
Expression Analyses
Initial expression analyses were performed in a yeast expression system (Pichia pastoris) using a pPICZα plasmid (Invitrogen) such that the expressed protein contained a C-terminal His-tag and was secreted into the culture medium.
Cells were cultured in a bioreactor and recombinant MaSp1 (rMaSp1) expression subsequently induced with methanol. Samples were harvested at the indicated times (U = Uninduced), clarified by centrifugation, and electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE, 20 μL of clarified culture supernatant per lane). The proteins were transferred to a polyvinylidene fluoride (PVDF) membrane and silk-like proteins detected with an anti-silk antibody (primary antibody = polyclonal rabbit anti-silk; secondary antibody = alkaline phosphatase-linked goat anti-rabbit).
Fig. 2 shows immunodetection results using an anti-silk antibody to detect expression, before and after induction with methanol, of an MaSp1 mini-silk protein containing eight copies of the MaSp1 repeat consensus (predicted molecular weight (MW) = 44.4 kDa). MW markers are shown to the left and the appropriate MW for rMaSp1 is indicated by an asterisk.
Fig. 2.

Immunodetection of recombinant MaSp1 (8-mer) protein expressed in P. pastoris.
There was a low level of cross-reactive material in the uninduced culture, although not of the correct MW for the rMaSp1 protein. After addition of methanol, however, there was rapid induction of a cross-reactive protein of the appropriate MW (see Fig. 2 asterisk). There were also additional protein species that reacted with the anti-silk antibody, both at higher and lower MWs than full length rMaSp1. Interestingly, only the protein that migrates at ~44 kDa (full-length monomeric rMaSp1) was detected by an anti-His antibody (data not shown). This suggests that the lower MW species detected by the anti-silk antibody is a degradation product of rMaSp1 that has been truncated at the C-terminal end. The higher MW species appear to migrate at multiples of ~44 kDa and may represent multimers of rMaSp1. This was observed by others who have expressed silk-like proteins in yeast.35 It is not clear why these proteins were also not recognized by the anti-His antibody. The epitope may be sequestered in the higher-order structure such that it is not available for binding.
Recombinant Protein Purification
Inclusion of an affinity tag is a useful means of recombinant protein purification from a variety of expression systems. One of the more common tags is a His-tag that allows recombinant protein purification by immobilization using metal affinity chromatography (IMAC). Unfortunately, rMaSp1 protein did not bind to commercially-available Ni+2 resin. This may be due to sequestration of the C-terminal polyhistidine in a configuration that is not available to the matrix. Exchange of Co+2 (stronger affinity) for Ni+2 did not improve recovery. Therefore, the culture supernatant was subjected to ion exchange chromatography (IEC).
Culture supernatant (50 mL) was diluted (1:4) in wash buffer (25 mM Tris pH 8.0, 500 mM NaCl) and applied to a wide-diameter Sephacryl SP cation exchange column (15 mL column volume) at a flow rate of 10 mL/min. Small scale sample evaluations showed that supernatant dilution was necessary to minimize binding of peptides from the complex media. The column was washed with 10 column volumes of wash buffer and rMaSp1 eluted in ~two column volumes of elution buffer (~30 mL of 25 mM Tris pH 8.0, 1 M NaCl).
Fig. 3 shows the elution profile of rMaSp1 culture supernatant passed over a wide diameter column packed with 15 mL Sephacryl SP cation exchange resin. The large off-scale peak at the beginning of the run represents absorbance of peptides present in the complex medium (solid line). The small peak at ~380–420 mL of elution volume represents rMaSp1 that eluted after increasing the salt concentration to 1M (dotted line).
Fig. 3.
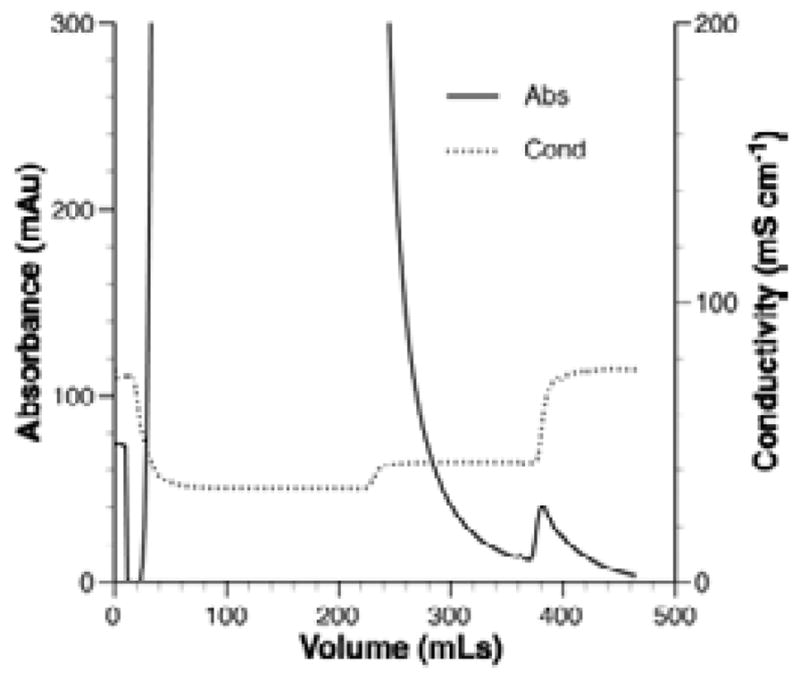
IEC of rMaSp1.
Purification of rMaSp1 was evaluated by SDS-PAGE electrophoresis. Fig. 4 shows the results from a representative IEC run. The filtered supernatant, before and after clarification, clearly contained multiple proteins but was of relatively low complexity compared to cell lysates. There was also no obvious insoluble protein in the filtrate. Most of the protein present in the filtrate supernatant came off the column in the flow-through fraction and some remaining protein was present in the wash fraction. Elution of protein from the column with 1 M salt revealed the presence of two or three prominent bands. One of these is the appropriate MW for rMaSp1 protein (asterisk in Fig. 4). The other two bands migrate at approximately 2× and 4× the MW of rMaSp1 (~88 and ~160 kDa, respectively).
Fig. 4.

Purification of rMaSp1 from yeast culture medium.
Filtered culture supernatant (> 10 kDa) was subjected to IEC as described in the Fig. 3 legend. Samples taken prior to, during, and after IEC were separated on a 10% SDS-PAGE gel electrophoresis. MW markers are shown to the left and the appropriate MW for rMaSp1 is indicated by an asterisk.
Conclusions
Spider dragline silk fibers have many desirable properties that make them attractive for materials applications. The results of this study demonstrate that recombinant silk-like proteins containing native N-terminal sequences, in addition to repeat domains and C-terminal sequences, can be expressed in a soluble form in yeast. In contrast, some silk-like proteins expressed without a native N-terminal domain display insolubility20,22.
Expression and purification of large quantities of recombinant silk-like proteins are crucial for the successful exploitation of new materials derived from them. To realize that goal, technologies must be developed that allow soluble expression and scalable purification. Current efforts are focused on building new expression constructs that include cleavable affinity tags to facilitate purification that should be amenable to use in numerous systems, including yeast and transgenic plants.
Footnotes
This paper was presented at the AATCC International Conference, March 10–12, 2009 in Myrtle Beach, S.C., USA.
References
- 1.Altman GH, et al. Biomaterials. 2003 Feb;24(3):401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 2.Craig CL, Riekel C. Comparative Biochemistry and Physiology B Biochemistry and Molecular Biology. 2002 Dec;133(4):493–507. doi: 10.1016/s1096-4959(02)00095-7. [DOI] [PubMed] [Google Scholar]
- 3.Fournier A. Biochimie. 1979 Apr;61(2):283–320. doi: 10.1016/s0300-9084(79)80073-5. [DOI] [PubMed] [Google Scholar]
- 4.Gosline JM, et al. Journal of Experimental Biology. 1999 Dec;202(23):3295–3303. doi: 10.1242/jeb.202.23.3295. [DOI] [PubMed] [Google Scholar]
- 5.Sponner A, et al. Biochemistry. 2005 Mar;44(12):4727–4736. doi: 10.1021/bi047671k. [DOI] [PubMed] [Google Scholar]
- 6.Lewis RV. Chemical Reviews. 2006 Sep;106(9):3762–3774. doi: 10.1021/cr010194g. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi CY, Lewis RV. Journal of Molecular Biology. 1998 Feb;275(5):773–784. doi: 10.1006/jmbi.1997.1478. [DOI] [PubMed] [Google Scholar]
- 8.Blackledge TA, Hayashi CY. Journal of Experimental Biology. 2006 Aug;209(16):3131–3140. doi: 10.1242/jeb.02327. [DOI] [PubMed] [Google Scholar]
- 9.Seidel A, Liivak O, Jelinski LW. Macromolecules. 1998 Aug;31(19):6733–6736. [Google Scholar]
- 10.Fahnestock SR, Irwin SL. Applied Microbiology and Biotechnology. 1997 Jan;47(1):23–32. doi: 10.1007/s002530050883. [DOI] [PubMed] [Google Scholar]
- 11.Fahnestock SR, Bedzyk LA. Applied Microbiology and Biotechnology. 1997 Jan;47(1):33–39. doi: 10.1007/s002530050884. [DOI] [PubMed] [Google Scholar]
- 12.Huemmerich D, et al. Current Biology. 2004 Nov;14(22):2070–2074. doi: 10.1016/j.cub.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Lazaris A, et al. Science. 2002 Jan;295(5554):472–476. doi: 10.1126/science.1065780. [DOI] [PubMed] [Google Scholar]
- 14.Karatzas CN, et al. Transgenic Research. 1999 Dec;8(6):463–494. [PubMed] [Google Scholar]
- 15.Scheller J. Nature Biotechnology. 2001 Jun;19(6):573–577. doi: 10.1038/89335. [DOI] [PubMed] [Google Scholar]
- 16.Hinman MB, Lewis RV. Journal of Biological Chemistry. 1992 Sep;267(27):19320–19324. [PubMed] [Google Scholar]
- 17.Xu M, Lewis RV. Proceedings of the National Academy of Sciences USA. 1990 Sep;87(18):7120–7124. doi: 10.1073/pnas.87.18.7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hijirida DH, et al. Biophysics Journal. 1996 Dec;71(6):3442–3447. doi: 10.1016/S0006-3495(96)79539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight DP, Vollrath F. Naturwissenschaften. 2001 Apr;88(4):179–182. doi: 10.1007/s001140100220. [DOI] [PubMed] [Google Scholar]
- 20.Ittah S, et al. Biomacromolecules. 2006 Jun;7(6):1790–1795. doi: 10.1021/bm060120k. [DOI] [PubMed] [Google Scholar]
- 21.Sponner A, et al. Biochemical and Biophysical Research Communications. 2005 Dec;338(2):897–902. doi: 10.1016/j.bbrc.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 22.Stark M. Biomacromolecules. 2007 May;8(5):1695–1701. doi: 10.1021/bm070049y. [DOI] [PubMed] [Google Scholar]
- 23.Hagn F, et al. Nature. 2010 May;465(7295):239–242. doi: 10.1038/nature08936. [DOI] [PubMed] [Google Scholar]
- 24.Gaines WA, Marcotte WR., Jr Insect Molecular Biology. 2008 Sep;17(5):465–474. doi: 10.1111/j.1365-2583.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motriuk–Smith D, et al. Biomacromolecules. 2005 Nov;6(6):3152–3159. doi: 10.1021/bm050472b. [DOI] [PubMed] [Google Scholar]
- 26.Rising A, et al. Biomacromolecules. 2006 Nov;7(11):3120–3124. doi: 10.1021/bm060693x. [DOI] [PubMed] [Google Scholar]
- 27.Hedhammar M, et al. Biochemistry. 2008 Mar;47(11):3407–3417. doi: 10.1021/bi702432y. [DOI] [PubMed] [Google Scholar]
- 28.Gaines WA, Sehorn MG, Marcotte WR., Jr Journal of Biological Chemistry. 2010 Dec;285(52):40745–40753. doi: 10.1074/jbc.M110.163121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Askarieh G, et al. Nature. 2010 May;465(7295):236–238. doi: 10.1038/nature08962. [DOI] [PubMed] [Google Scholar]
- 30.Beckwitt R, Arcidiacono S. Journal of Biological Chemistry. 1994 Mar;269(9):6661–6663. [PubMed] [Google Scholar]
- 31.Hayashi CY, Blackledge TA, Lewis RV. Molecular Biology and Evolution. 2004 Oct;21(10):1950–1959. doi: 10.1093/molbev/msh204. [DOI] [PubMed] [Google Scholar]
- 32.Lewis RV, et al. Protein Expression and Purification. 1996 Jun;7(4):400–406. doi: 10.1006/prep.1996.0060. [DOI] [PubMed] [Google Scholar]
- 33.Prince JT, et al. Biochemistry. 1995 Aug;34(34):10879–10885. doi: 10.1021/bi00034a022. [DOI] [PubMed] [Google Scholar]
- 34.Xia X-X, Qian Z-G, et al. Proceedings of the National Academy of Sciences. 2010 Aug;107(32):14059–14063. doi: 10.1073/pnas.1003366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teulé F. PhD Thesis. Clemson University; Aug, 2003. pp. 132–201. [Google Scholar]
- 36.Elices M, et al. [accessed September 2010];JOM. 2005 Feb;57(2):60. www.tms.org/pubs/journals/JOM/0502/Elices-0502.html. [Google Scholar]


