Abstract
Background
On 12 May 2012, over 200 college students with acute diarrhoea were reported to the Guizhou Center for Disease Control and Prevention. We conducted an investigation to identify the agent and mode of transmission and to recommend control measures.
Methods
A suspected case was a person at the college with onset of ≥ two of the following symptoms: diarrhoea (more than three loose stools in 24 hours), abdominal pain, vomiting or fever (> 37.5C) between 6 and 15 May 2012. A confirmed case also had a positive Aeromonas hydrophila culture from a stool sample. A retrospective-cohort study of 902 students compared attack rates (AR) by dining place, meals and food history. We reviewed the implicated premise, its processes and preparation of implicated food.
Results
We identified 349 suspected cases (AR = 14%) and isolated Aeromonas hydrophila from three stools of 15 cases. Students who ate in cafeteria A were more likely to be ill compared to those eating in other places (relative risk [RR]: 3.1, 95% confidence interval [CI]: 2.0–4.8). The cohort study implicated cold cucumber (RR: 2.6, 95% CI: 2.0–3.3) and houttuynia dishes (RR: 1.8, 95% CI: 1.4–2.3). Environmental investigation showed that vegetables were washed in polluted water from a tank close to the sewage ditch, then left at 30 °C for two hours before serving. The Escherichia coli count of the tank was well above the standard for drinking-water.
Conclusion
This outbreak of Aeromonas hydrophila was most probably caused by salad ingredients washed in contaminated tank water. We recommended enhancing training of foodhandlers, ensuring tanks and sewerage systems comply with appropriate standards and adequate monitoring of drinking-water sources.
Introduction
On 12 May 2012, a college in Xingyi City, China notified the Guizhou Center for Disease Control and Prevention (CDC) of over 200 students sick with acute diarrhoea. The illness was reported as gastroenteritis-like with diarrhoea, abdominal pain, headache, vomiting and fever being common symptoms. An outbreak investigation was conducted to identify the agent, the mode of transmission and to recommend control measures. This paper describes the public health investigation.
Methods
Cases
A suspected case was defined as any person in the college with two or more of the following symptoms: diarrhoea (more than three loose stools in 24 hours), abdominal pain, vomiting or fever (> 37.5 °C) between 6 and 15 May 2012. A confirmed case was a suspected case with a positive Aeromonas hydrophila culture from a stool specimen.
Case-finding was conducted through outpatient and inpatient records from the school infirmary and county hospital. Student dormitories were visited and students interviewed to find further unreported cases. A structured questionnaire comprised questions about detailed food and water consumption for the three days preceding illness onset. Students, teachers and cafeteria staff were interviewed using the standardized questionnaire to identify illness and exposure details.
We were able to obtain computerized data that recorded meals served at college cafeterias by student name. Data were collected for all meals from 6 to 11 May.
Cohort study
A retrospective cohort study was initiated to compare attack rates (AR) for dining place, meals and food history. We selected 40 dormitories by AR-stratified sampling. The cohort study population comprised the 989 students that had lunch at the college on 8 May. We calculated relative risks (RR) with 95% confidence intervals (CI), comparing all foods consumed at that meal. We also calculated a χ2 test for trend for the dose–response data for the cucumber salad.
Laboratory investigation
Faecal specimens were cultured at laboratories at Guizhou CDC. Food and environmental samples were tested at Xingyi CDC. The World Health Organization (WHO) definition of safe drinking-water, Escherichia coli not detectable in 100 ml of water,1 was used to assess the water.
Environmental investigation
Site visits were made to the suspected cafeteria and bottled-water factory to identify the possible sources and causes of contamination. The entire production process in the cafeteria, from the purchase of raw ingredients to preparation of implicated food, was thoroughly reviewed with management.
Results
Cases
There was a total of 349 suspected cases (AR = 14%). After diarrhoea, the main symptoms were abdominal pain (80%), headache (55%), vomiting (29%) and fever (18%); 14% of cases reported acute diarrhoea with blood and mucus. Median duration of illness was seven days (range: two to 18 days) with 40% having self-limiting symptoms. The first case’s onset date was 8 May and cases continued until 15 May (Fig. 1).
Fig. 1.
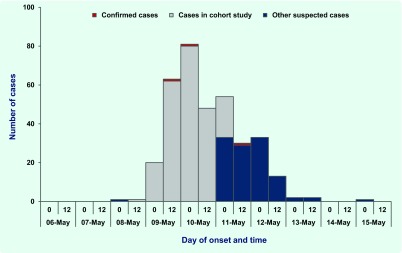
Epidemic curve by onset date and time, Aeromonas hydrophila outbreak, Guizhou Province, China, 2012 (n = 349)
The ARs for students (14%) and cafeteria staff (21%) were much higher than that of teachers (1%). Boarding students had a significantly higher risk than those who were on graduation field work and did not live at the school (relative risk [RR] = 6.9, 95% CI = 4.4–11). There were four dormitories (A, B, C and D) and ARs among dormitory A, B and C were higher than dormitory D. The AR among students who ate in cafeteria A was 64% compared to the 21% who ate in other places (RR: 3.1, 95% CI: 2.0–4.8). From analysis of the electronic cafeteria records, four meals were associated with illness: lunch on 8 May (RR: 1.8, 95% CI: 1.3–2.4); supper on 8 May (RR: 1.5, 95% CI: 1.1–2.1); supper on 9 May (RR: 1.5, 95% CI: 1.1–2.1); and supper on 10 May (RR: 1.5, 95% CI: 1.1–2.1).
Cohort study
Questionnaire responses were returned for 902 (91%) students in the cohort study of all students who had lunch on 8 May at the college. We found that the cold cucumber dish made from Chinese cucumbers (RR: 2.6, 95% CI: 2.0–3.3) and the cold houttuynia dish (a wild heartleaf vegetable that grows in the south-western of China [RR: 1.8, 95% CI: 1.4–2.3]) were associated with illness. Illness was not statistically significantly different among those exposed and not exposed to the other 28 dishes (data not shown). The RR of the cucumber dish increased by 1.4 for each tablespoon consumed (P = 0.016, Table 1).
Table 1. Dose–response analysis of Chinese cucumber salad in an outbreak of Aeromonas hydrophila, Guizhou Province, China, May 2012.
| Tablespoons | Sick | Not sick | AR (%) | RR | 95% CI |
|---|---|---|---|---|---|
| 2 | 73 | 105 | 41.0 | 1.8 | 1.1–2.0 |
| 1 | 59 | 94 | 38.6 | 1.6 | 1.1–1.9 |
| ½ | 39 | 102 | 27.7 | Ref | – |
AR – attack rate, RR – rate ratio; CI – confidence interval.
Laboratory findings
We collected 15 stool specimens from 15 cases, of which three were culture positive for Aeromonas hydrophila and negative for all other common gastroenteritis agents, such as Salmonella, Shigella, Vibrio cholera, Vibrio parahemolyticus, Typhoid bacillus, Bacillus paratyphosus, Campylobacteria and toxigenic Escherichia coli.
The testing of the tank water showed that the Escherichia coli count was > 1600MPN/100ml, higher than the WHO standard.
Environmental findings
Bottled water is the most common drink at the college. There were 300 bottles of water restocked between 4 and 8 May, and the same batches were also restocked in 56 nearby villages. This bottled water was not considered a source of the outbreak as there were no increases in notifications of acute diarrhoea in these villages and because interviews with the teachers found that although they drank bottled water at the college, they had a much lower morbidity (1%).
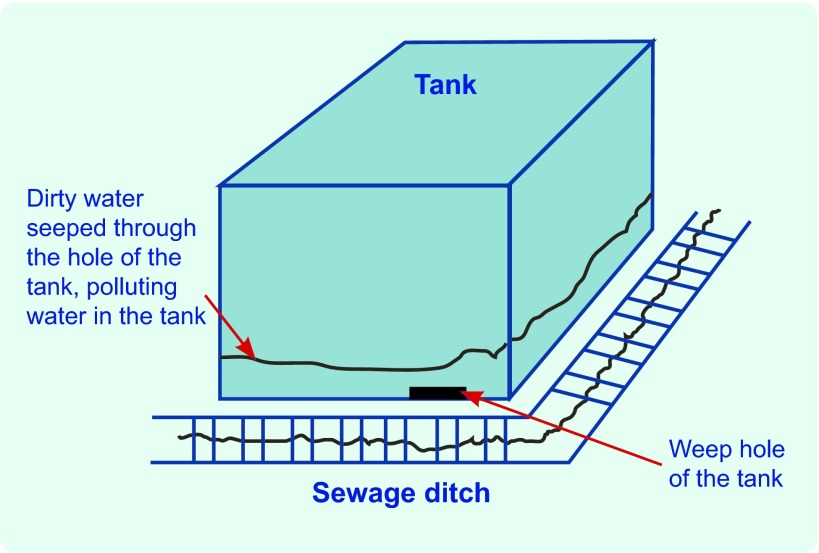
Environmental investigation of cafeteria A showed that when the supply of tap water in the kitchen stopped, a water tank was used for washing vegetables. The tank was located close to the sewage ditch of the cafeteria (< 10 cm) and there was a weep hole at the bottom of tank (Fig. 2). Interviews with school staff identified that between 8 and 10 May the cafeteria stopped supplying tap water and that the sewage ditch was blocked on 8 May, resulting in the overflow of dirty water into the weep hole of the tank. Cafeteria staff then washed the vegetables for the cold dishes using water from the tank. The cold dishes were then left at 30 °C for two hours before serving.
Fig. 2.
The tank used to wash the vegetables in an outbreak of Aeromonas hydrophila, Guizhou Province, China, May 2012
Discussion
This serious outbreak of Aeromonas hydrophila was most likely caused by contaminated cold dishes. The possible source was contaminated water from the tank used for washing vegetables. The inappropriate food storage and display allowed for the reproduction of Aeromonas hydrophila.
Aeromonas hydrophila is a gram-negative facultatively anaerobic bacteria with an optimal growth temperature of 35%–37 °C.2–4 The incubation period of Aeromonas hydrophila is typically 24–48 hours,2 and the main symptoms are diarrhoea, gastroenteritis, abdominal pain, vomiting and fever. Duration of illness can range from three days to six months but can be self-limiting.5–8 Aeromonas is ubiquitous to water, with the ability to form biofilms in and subsequently colonize water systems.3 The heterotrophic plate count calculated that Aeromonas can make up 1–27% of total bacteria in samples of drinking-water, implicating drinking-water as a possible source of infection.9,10
The symptoms, duration and incubation of the illness in this outbreak conformed to that of Aeromonas hydrophila. Three stools were positive for Aeromonas hydrophila and all 15 stools tested were negative for other gastroenteritis pathogens. The epidemiological evidence implicating cold salads as the vehicle of transmission was supported by environmental findings. Cucumbers and houttuynia used in the implicated salads were washed in water from a tank that had an extremely high Escherichia coli count. It was likely that the water of the tank was contaminated by the sewage ditch located nearby (< 10 cm). There were also anecdotal reports from school staff that students were sick with diarrhoea the last time the sewage ditch was blocked.
The local temperature was rather high before and during the outbreak. This and the inappropriate operations in the cafeteria may have caused reproduction of Aeromonas hydrophila. The cold dishes sold well in the students’ cafeteria, which may be why so many students were involved in this outbreak.
This was not the first large outbreak of Aeromonas hydrophila. An outbreak involving 381 children from two day-care centres were reported in 1992.11 Another outbreak of Aeromonas hydrophila occurred in 1993 in China with 82 cases, and the source of infection was found to be drinking-water contaminated by sewage.12 In recent years, the number of outbreaks caused by Aeromonas hydrophila has been significantly increasing, mostly due to contaminated drinking-water and food.
Our investigation had some limitations. Less than 10% of the reported cases had their stools examined for Aeromonas hydrophila because most cases either self-medicated or were treated as outpatients. Collecting specimens after antibiotic use may explain the low positive proportion of Aeromonas hydrophila. The investigation was unable to isolate Aeromonas hydrophila from the salads since there were no leftovers. There was also the potential for recall bias in the three-day food histories with some students not supplying detailed information because they could not remember.
Gastroenteritis occurs frequently in summer, and nonstandard cold salad preparation and storage could make pathogens easy to spread and breed. This outbreak highlighted the importance of enhancing the training of kitchen staff on correct operations such as using clean water to wash vegetables and keeping food in cold storage before serving. Moreover, tanks and sewage ditches should comply with the Code for Design of Dietetic Buildings.13 The supervision department also should strengthen monitoring and sterilizing drinking-water to guarantee food safety.
Conflicts of interest
None declared.
Funding
None.
References
- 1.Guidelines for drinking-water quality, 4th edition. Geneva: World Health Organization; 2011. http://www.who.int/water_sanitation_health/publications/2011/dwq_chapters/en/index.html accessed 20 May 2012. [Google Scholar]
- 2.Addendum to Guidelines for drinking-water quality, 2nd edition. Geneva: World Health Organization; 1998. Aeromonas.http://www.who.int/water_sanitation_health/dwq/en/admicrob2.pdf accessed 20 May 2012. [Google Scholar]
- 3.Parker JL, Shaw JG. Aeromonas spp. clinical microbiology and disease. The Journal of Infection. 2011;62:109–18. doi: 10.1016/j.jinf.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Igbinosa IH, et al. Emerging Aeromonas species infections and their significance in public health. The Scientific World Journal. 2012;2012:625023. doi: 10.1100/2012/625023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyer NP. Clinical significance of Aeromonas species isolated from patients with diarrhea. Journal of Clinical Microbiology. 1987;25:2044–8. doi: 10.1128/jcm.25.11.2044-2048.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang SM, Wand MS. Aeromonas hydrophila and its pathogensis to humans. Chinese Journal of Disease Control and Prevention. 2006;10:511–4. [Google Scholar]
- 7.Morinaga Y, et al. Clinical characteristics of seven patients with Aeromonas septicemia in a Japanese hospital. The Tohoku Journal of Experimental Medicine. 2011;225:81–4. doi: 10.1620/tjem.225.81. [DOI] [PubMed] [Google Scholar]
- 8.Llopis F, et al. Epidemiological and clinical characteristics of bacteraemia caused by Aeromonas spp. as compared with Escherichia coli and Pseudomonas aeruginosa. Scandinavian Journal of Infectious Diseases. 2004;36:335–41. doi: 10.1080/00365540410020631. [DOI] [PubMed] [Google Scholar]
- 9.Rusin PA, et al. Risk assessment of opportunistic bacterial pathogens in drinking water. Reviews of Environmental Contamination and Toxicology. 1997;152:57–83. doi: 10.1007/978-1-4612-1964-4_2. [DOI] [PubMed] [Google Scholar]
- 10.Egorov AI, et al. Occurrence of Aeromonas spp. in a random sample of drinking water distribution systems in the USA. Journal of Water and Health. 2011;9:785–98. doi: 10.2166/wh.2011.169. [DOI] [PubMed] [Google Scholar]
- 11.de la Morena ML, et al. Diarrhea associated with Aeromonas species in children in day care centers. The Journal of Infectious Diseases. 1993;168:215–8. doi: 10.1093/infdis/168.1.215. [DOI] [PubMed] [Google Scholar]
- 12.Qing-wen J, et al. An acute diarrhea outbreak associated with E. Aeromonas caused by contaminated drinking water. Journal of Environmental Health. 1993;10:99–101. [Google Scholar]
- 13.Code for Design of Dietetic Buildings. (JGJ 64–89). Professional Standard of People’s Republic of China. Beijing: Ministry of Commerce and Ministry of Health; 1989. [Google Scholar]