Abstract
B cells can mediate protective responses against nematode parasites by supporting Th2 cell development and/or by producing Abs. To examine this, B cell-deficient mice were inoculated with Nippostrongylus brasiliensis or Heligmosomoides polygyrus. B cell-deficient and wild type mice showed similar elevations in Th2 cytokines and worm expulsion after N. brasiliensis inoculation. Worm expulsion was inhibited in H. polygyrus-inoculated B cell-deficient mice, although Th2 cytokine elevations in mucosal tissues were unaffected. Impaired larval migration and development was compromised as early as day 4 after H. polygyrus challenge, and administration of immune serum restored protective immunity in B cell-deficient mice, indicating a primary role for Ab. Immune serum even mediated protective effects when administered to naive mice prior to inoculation. This study suggests variability in the importance of B cells in mediating protection against intestinal nematode parasites, and it indicates an important role for Ab in resistance to tissue-dwelling parasites.
Helminth infection is a major source of morbidity worldwide, contributing to anemia and malnutrition. Drug treatment does not protect against reinfection, and attempts are currently underway to develop effective vaccines and immunotherapies (1, 2). Increasing evidence suggests that the Th2-type response characterized by elevated IL-4 and IL-13 may be important in mediating protection against certain helminths (3, 4). The relevant targets for IL-4 and IL-13 can vary with the particular infecting parasite and can include both immune and nonimmune cells (4, 5). Each species of helminthic parasite has a distinct lifecycle, including migration to different niches in the host. The immune mechanisms that actually mediate worm expulsion from the intestine or destruction in tissues are not well understood. Understanding how the Th2-type response mediates helminth parasite resistance can provide important insights into the development of next generation vaccines and immunotherapies.
Two widely used experimental models of intestinal nematode infection include inoculation of mice with either Nippostrongylus brasiliensis or Heligmosomoides polygyrus (6). The infective third-stage larvae (L3) of N. brasiliensis are inoculated s.c. and migrate briefly through the skin and lungs before reaching the lumen of the small intestine, where adult worms are expulsed by day 10 after primary inoculation. In contrast, infective H. polygyrus larvae enter the intestine after oral inoculation, quickly penetrate intestinal tissue and, during a tissue-dwelling phase, develop into adults that return to the lumen by day 8 after inoculation. The primary immune response results in chronic infection while the memory response following a drug-induced expulsion and secondary challenge infection triggers worm expulsion by 2 wk after inoculation. Both intestinal nematode parasites stimulate CD4 T cell-dependent Th2-type polarized immune responses with potentially overlapping effector mechanisms.
Several studies suggest that B cells can promote the Th2-type response and that they might be particularly important in controlling opposing Th1-type responses (7–9). B cells can produce cytokines (10–12) and express costimulatory molecules (13–15), which may sustain T helper effector cells and other components of the Th2-type response (13, 16), and also support the development of memory T cells (17). Few studies have examined the role of B cells in mediating protection and in promoting the development of Th2-type responses evoked by infectious agents.
Recent studies established a role for B cells in mediating protective responses against H. polygyrus, but differed with regards to B cell-dependent mechanisms. One report suggested that B cells contributed to the protective response against H. polygyrus primarily through their production of Ab (18), and another concluded that Th2 effector cell function may also be defective in B cell-deficient hosts (19). To examine whether B cells and Abs are generally important in protective responses against intestinal nematode parasites, we studied the development of the protective response to N. brasiliensis and H. polygyrus using B cell-deficient JHD mice. We showed that multiple parameters of the mucosal in vivo Th2-type primary and memory responses are unaffected by the absence of B cells after inoculation with either N. brasiliensis or H. polygyrus, and successful parasite expulsion is not impaired in B cell-deficient mice inoculated with N. brasiliensis, but it is compromised after secondary H. polygyrus inoculation. We then demonstrated that B cells, through their production of Ab, are essential in the host protective memory response to H. polygyrus during the tissue-dwelling phase as early as 4 d after secondary inoculation.
Materials and Methods
Mice
Six- to 8-wk-old wild type (WT) BALB/c female mice were obtained from the National Cancer Institute (Frederick, MD). B cell-deficient BALB/c Jhdtm1 (JHD) mice from Taconic Laboratories (Hudson, NY) were maintained and bred in a specific pathogen-free facility at the New Jersey Medical School, University of Medicine and Dentistry of New Jersey (Newark, NJ) research animal facility. The studies have been reviewed and approved by the Institutional Animal Care and Use Committee at New Jersey Medical School. The experiments in this study were conduced according to the principles set forth in the Guide for the Care and Use of Laboratory Animal, Institute of Animal Resources, National Research Council, Department of Health, Education and Welfare (National Institutes of Health, Bethesda, MD) 78–23.
Parasite inoculation, serum transfer, and anti-CD4 treatment
For N. brasiliensis infection, both 6–8-wk-old WT and JHD female mice were inoculated s.c. with 500 infective L3, and worm expulsion was detected on day 7 or 10 postinoculation as described previously (20). For secondary N. brasiliensis inoculation, WT and JHD naive mice were inoculated with 500 L3, and at either 3 wk or 3 mo later were secondarily inoculated with N. brasiliensis. Worm fecundity was determined 2 and 7 d later. For H. polygyrus infection, mice were inoculated periorally with 200 L3, and 2 wk later worms were expelled by administration of pyrantel pamoate (1–2 mg) (21). Four weeks later, the mice were challenged with H. polygyrus (Hp2′) while naive WT and JHD mice were inoculated with H. polygyrus as controls (Hp1′). Serum transfer used immune serum (IS) collected from WT mice on day 14 of Hp2′ and naive serum (NS) from WT mice unexposed to H. polygyrus. Serum was administered i.p. every third day beginning one day prior to H. polygyrus inoculation, with a dose of 0.5 ml for the first week following inoculation and 0.3 ml subsequently. For serum transfer experiments with N. brasiliensis, IS was collected from WT mice inoculated twice with N. brasiliensis, 10 d apart, and exsanguinated on day 7 after the second inoculation. Serum was administered i.p. one day prior to N. brasiliensis inoculation at a dose of 0.4 ml. To deplete the CD4 T cell function in vivo, 1 mg of anti-CD4 mAb (GK1.5) was given by i.v. administration once a week starting the day before parasite inoculation. This dose has previously been shown to effectively deplete CD4+ T cells in vivo (21).
Parasite health and burden
Quantitative counts of adult worms and egg production were determined (21) at 14 d postinoculation. At day 4 postinoculation, tissue-dwelling H. polygyrus larvae were counted in situ in 2-cm intervals along the entire small intestine. The mean larval position was calculated as a weighted average: sum of (number of larvae per segment × distance of segment from stomach) divided by (total larvae × intestine length). Also at day 4, larvae were isolated from the proximal intestine, and their lengths measured using fine forceps under a dissecting microscope at ×40 magnification. Sex was determined by presence of bursa rays at the caudal end of female larvae.
ELISPOT
ELISPOT assays were performed from single-cell lymph node suspensions (20, 22).
Cytokine gene expression by RT-PCR
For RT-PCR, total RNA was extracted from tissue and then reverse transcribed as previously described (20). Real-time PCR kits (Applied Bio-systems, Foster City, CA), specific for individual cytokines or rRNA, were used to determine quantitative differences in gene expression, and all data were normalized to constitutive rRNA values. For gene expression of granulomas surrounding the tissue phase of H. polygyrus larvae, individual granulomas were collected using a capillary tube to selectively excise individual granulomas and the tissues processed as described above.
Flow cytometry
Mesenteric lymph node (MLN) cell suspensions were collected and prepared from untreated and inoculated mice, washed, blocked with Fc Block (BD Pharmingen, San Diego, CA), and stained with anti–CD4-FITC (BD Pharmingen) and anti–CD69-PE (BD Pharmingen) and analyzed by flow cytometric analysis (20). To analyze T regulatory (Treg) cells, the cells were surface stained with anti–CD4-Percp and anti–CD25-FITC (Caltag, Burlingame, CA) and intracellular stained with Foxp3-APC (eBioscience, San Diego, CA). For intracellular cytokine detection, MLN cell suspensions were incubated for 4 h with PMA (50 ng/ml) and ionomycin (0.5 μg/ml), stained for IL-4 or IFN-γ, and analyzed by flow cytometry (20). For CD4 T cell sorting, MLN cell suspensions were prepared from N. brasiliensis or H. polygyrus inoculated mice and incubated with anti-CD4 microbeads (Miltenyi Biotec, Auburn, CA). CD4+ T cells were isolated by positive selection with >98% purity. RNAs were extracted from sorted cells using an RNA isolation kit (Stratagene Cloning Systems, La Jolla, CA).
Quantitative levels of serum immunoglobulin
Serum IgE levels were detected by ELISA (20).
Cell transfer
B cells were purified from spleens and lymph nodes from WT untreated or H. polygyrus-inoculated mice by using negative selection kits (Miltenyi Biotec); 15 ×106 B cells from WT or H. polygyrus-inoculated mice were transferred i.v. into H. polygyrus-primed and drug-treated JHD mice. One day later, these mice were challenged with H. polygyrus again. On day 14 after challenge, worm fecundity was determined. To isolate and transfer memory T cells, WT mice were inoculated with H. polygyrus orally, treated with pyrantel pamoate 2 wk later; 2 mo later, the mice were sacrificed and CD4+ T cells were positively purified by using anti-CD4 beads (Miltenyi Biotec) from spleens and lymph nodes as memory T cells. Memory T cells (5 million cells per mouse) and/or memory B cells (15 million cells per mouse) were then transferred into different groups of naive JHD mice (five mice per group). One day later, these mice were inoculated with H. polygyrus orally. On day 14 after H. polygyrus inoculation, the mice were examined for worm fecundity.
Statistical analysis
Statistical differences between groups were assessed using ANOVA and Fisher’s protected least significant difference test for pairwise comparisons. In experiments in which only two groups were evaluated, Student t test was used to detect differences. If data failed the assumptions of normality or equal variance, then the Kruskal-Wallis nonparametric ANOVA was used with a Mann-Whitney U test for pairwise comparisons. The software program SigmaStat (Jandel, San Rafael, CA) was used for all statistical analyses.
Results
N. brasiliensis-induced Th2 response and worm expulsion are B cell independent
Our previous studies have shown that B cells are required for the development of Th2 cells in the draining nonmucosal cervical lymph node after intracutaneous inoculation in the ear with N. brasiliensis (16). Because the host protective response to N. brasiliensis is dependent on Th2 cells (6), we investigated whether B cell-deficient mice could generate intact protective immunity to N. brasiliensis, which in WT mice culminates in worm expulsion from the intestine by day 10 after inoculation. JHD and WT mice (five mice per treatment group) were inoculated s.c. with 500 N. brasiliensis L3 and sacrificed at day 10 after inoculation. No adult worms were detected in either N. brasiliensis-inoculated WT or N. brasiliensis-inoculated JHD mice (data not shown), indicating that B cells are not required for host protection leading to N. brasiliensis adult worm expulsion.
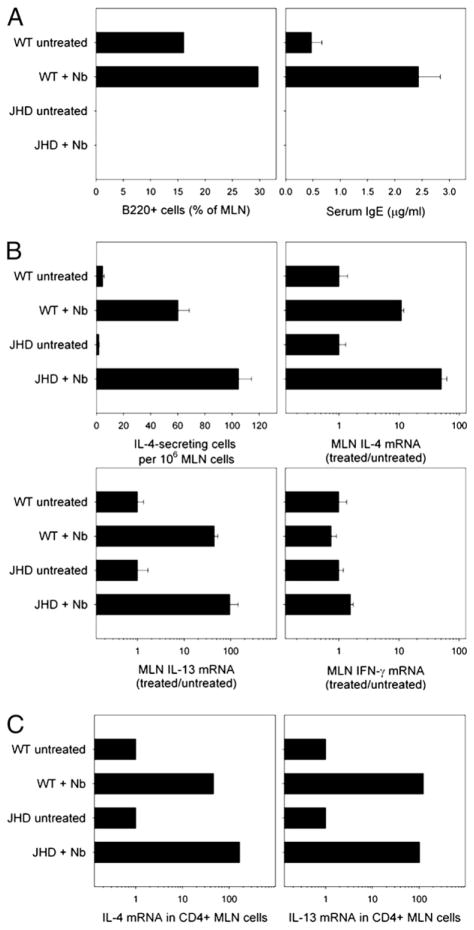
To address whether production of Th2 cytokines in the mucosal tissues was affected in B cell-deficient mice, MLNs were collected at day 10 postinoculation and examined for cytokine expression. Comparable elevations in IL-4 mRNA were observed in both N. brasiliensis-inoculated WT and JHD mice (data not shown). To confirm that the JHD mice were B cell-deficient and could not make Abs, MLN cells were stained with anti-B220 (6B2) mAb for FACS analysis. B220+ cells were detected in WT but not JHD mice (Fig. 1A). Similarly, WT mice had significantly increased serum IgE levels after N. brasiliensis inoculation, as measured by ELISA, whereas JHD mice had no detectable serum IgE (Fig. 1A). Absence of B220+ cells and IgE production confirmed that these mice are B cell deficient.
FIGURE 1.
The development of Th2 cells in draining MLNs after N. brasiliensis inoculation does not require B cells. JHD and WT mice were inoculated s.c. with 500 N. brasiliensis L3 and sacrificed 10 d (A) or 7 d later (B–C). A, Frequency of B220+ cells in MLNs was analyzed by flow cytometry. Serum IgE levels were determined by ELISA. Results are representative of two independent experiments. B, The number of IL-4–producing cells in 1 million MLN cells was determined by ELISPOT assay. IL-4, IL-13, and IFN-γ mRNA expression in MLN cells were detected by real-time quantitative PCR. C, CD4+ T cells were isolated from MLN cells of N. brasiliensis-inoculated and untreated JHD and WT mice, and quantitative PCR was used to measure IL-4 and IL-13 mRNA. Results are representative of two independent experiments.
Previous studies have demonstrated that N. brasiliensis is a potent inducer of Th2-type responses (20) and that host resistance to N. brasiliensis is dependent on Th2 cytokines, especially IL-13 (23, 24). Studies in other systems have showed that the absence of B cells can shift the Th2-type response toward a Th1-type response in vivo (13). To study whether B cell-deficient JHD mice can generate a normal Th2-type mucosal immune response after N. brasiliensis s.c. inoculation, WT and JHD mice were inoculated with N. brasiliensis and sacrificed 7 d later, a time when the Th2 cytokine response peaks in WT mice. MLNs were collected and analyzed for cytokine protein and gene expression. The number of IL-4–secreting MLN cells, as determine by ELISPOT assay, was not reduced in N. brasiliensis-inoculated JHD mice compared with N. brasiliensis-inoculated WT mice (Fig. 1B). Real-time quantitative RT-PCR was used to assess changes in cytokine mRNA gene expression. Pronounced increases in IL-4 and IL-13 mRNA were detected in the MLNs of N. brasiliensis-inoculated JHD mice that were at least as elevated as in WT mice. Overexpression of IL-4 and IL-13 in MLNs of JHD mice may be caused by increased frequency of CD4 T cells in MLNs because of the absence of B cells. IFN-γ mRNA remained low in all groups (data not shown), suggesting that the absence of B cells in N. brasiliensis-inoculated JHD mice does not shift the Th2-type response to N. brasiliensis toward a Th1-type phenotype.
During intestinal nematode parasite infection, other cells besides T cells can produce Th2 cytokines, including eosinophils, basophils, B cells, NK cells, and mast cells (5). To examine the changes in CD4 T cell cytokine expression in JHD mice after N. brasiliensis inoculation, MLN CD4+ T cells were isolated from WT and JHD mice and analyzed for IL-4 and IL-13 mRNA gene expression. As shown in Fig. 1C, CD4 T cell IL-4 and IL-13 mRNAs were comparably elevated in WT and JHD mice postinoculation, suggesting that B cells are not required for the development of Th2 cells in MLNs after N. brasiliensis inoculation.
Host resistance to N. brasiliensis in JHD mice remains CD4-dependent
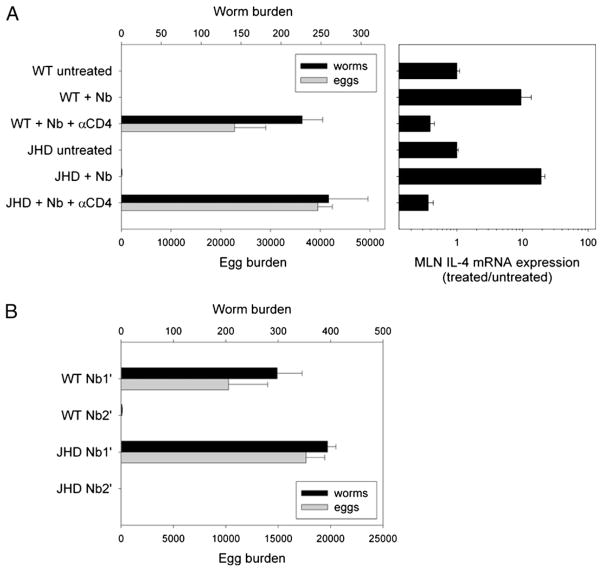
Previous studies have demonstrated that the host protective response against N. brasiliensis is dependent on CD4 T cells in WT mice. To address whether host protection in B cell-deficient mice remained dependent on CD4 T cells, anti-mouse CD4 mAb was used to deplete CD4 T cells in vivo in WT and JHD mice starting on the day of N. brasiliensis inoculation. On day 10 postinoculation, adult worms and eggs were recovered from the intestine (Fig. 2A). Again, both WT and JHD mice expelled N. brasiliensis successfully. However, depletion of CD4 T cells abrogated host protection in both WT and JHD mice, indicating that resistance to N. brasiliensis in JHD mice is still dependent on CD4 T cells. To examine whether IL-4 mRNA elevations were similarly CD4 T cell-dependent in WT and JHD mice, MLNs were collected and analyzed. N. brasiliensis inoculation markedly increased MLN IL-4 mRNA expression in WT and JHD mice, and anti-CD4 Ab treatment significantly suppressed IL-4 mRNA expression below untreated levels in both groups (Fig. 2A). These results suggest that host protection and the IL-4–dominant, Th2-type immune response in JHD mice are CD4 dependent. Our findings demonstrate that, following N. brasiliensis inoculation, host resistance and the Th2-type immune response associated with mucosal immunity in lymph nodes draining the gut do not require the presence of B cells.
FIGURE 2.
The host protective response to N. brasiliensis inoculation depends on CD4 cells but not B cells. A, JHD and WT mice were inoculated s.c. with N. brasiliensis L3, and some mice were administered an additional 1 mg anti-CD4 mAb once per week starting the day before inoculation. Mice were sacrificed 12 d postinoculation, and worm burden in small intestine and egg burden in large intestine were measured. IL-4 mRNA expression of MLN was detected by real-time quantitative PCR. B, JHD and WT mice were primed with N. brasiliensis L3 s.c. Two months later, these mice were given a secondary challenge (Nb2′) and compared with naive mice given a primary inoculation (Nb1′). At day 7 after challenge, mice were sacrificed and assayed for worm burden in the small intestine and egg burden in the large intestine. Results are representative of two independent experiments.
Host protection following N. brasiliensis secondary inoculation does not require B cells
Because previous studies have shown that B cells may be required for the development of memory CD4+ T cells in vivo (13), we next examined whether the more rapid expulsion of N. brasiliensis after a secondary inoculation requires B cells. WT and JHD mice were inoculated with N. brasiliensis. Two months later, the primed mice were administered a secondary N. brasiliensis inoculation (Nb2′), and naive mice were simultaneously given a primary inoculation (Nb1′). Mice were sacrificed at day 7 after inoculation, a point at which mice given a secondary inoculation have expelled all parasites whereas mice given a primary inoculation have not. Comparable worm and egg burdens were detected after the primary N. brasiliensis inoculation, and both mouse strains successfully expelled worms after secondary inoculation (Fig. 2B). In a separate experiment, WT and JHD mice examined for worm burden showed no significant difference in parasite number in the lungs at day 2 after secondary inoculation (data not shown). Thus, the more rapid memory response to secondary N. brasiliensis inoculation is also not dependent on B cells.
B cell-deficient JHD mice have impaired immunity following H. polygyrus challenge
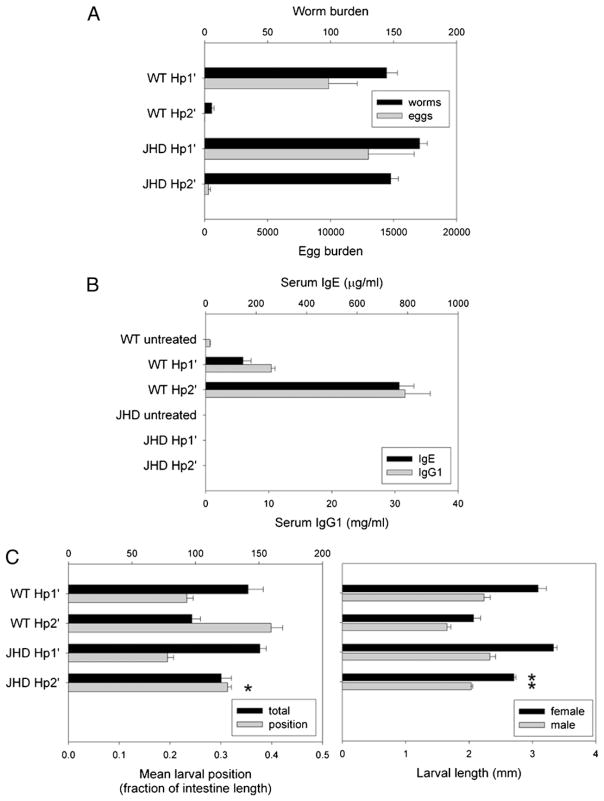
Our findings with N. brasiliensis suggest that B cells play no role in the development of the Th2-type enteric immune response and associated protective immunity. We next investigated whether B cells are required for the development of host protection to H. polygyrus, which is a strictly enteric helminth parasite that includes a tissue-dwelling stage in the small intestine required for the development of adult worms. Primary inoculation with H. polygyrus results in chronic infection, but secondary inoculation after drug-induced parasite clearance results in a host-protective, Th2-type memory response culminating in worm expulsion by 2 wk after inoculation (5). WT and JHD mice were primed with H. polygyrus L3 and drug-cured (see Materials and Methods). Primed mice were given a secondary H. polygyrus inoculation (Hp2′ group), and naive controls were given a primary inoculation (Hp1′ group) on day 0 of each experiment. Mice from all treatment groups were sacrificed at day 14. As expected (Fig. 3A), the number of adult worms was markedly reduced in WT Hp2′ compared with WT Hp1′. In contrast, adult worm expulsion was impaired in the H. polygyrus-challenged JHD mice, indicating that JHD mice have a defect in the development of a host protective memory immune response to H. polygyrus. Egg production was negligible in JHD Hp2′, despite the presence of adult worms, suggesting that fecundity can be controlled by B cell-independent effectors during the memory response. These findings suggest that the H. polygyrus memory response generated in the absence of B cells is unable to support effective host protection leading to parasite clearance.
FIGURE 3.
The host protection to acute or chronic H. polygyrus infection is B cell-dependent. JHD and WT mice were orally inoculated with 200 H. polygyrus L3, 2 wk later treated with antihelminthic, and then four weeks after drug cure rechallenged with H. polygyrus (Hp2′). Other mice were given primary challenge alone (Hp1′). A and B, The mice were sacrificed at day 14 after final inoculation. Worms and eggs were counted in the small intestinal lumen and fecal contents, respectively (A), and serum IgE and IgG1 levels were determined by ELISA (B). Results are representative of three independent experiments. C, Primary and secondary challenged mice were sacrificed at day 4 postinoculation. Tissue-dwelling larvae were counted in situ to determine total number and distribution along small intestine. Individual larvae were removed, sexed, and measured. Means represent at least 10 larvae per mouse, 4 mice per group. Asterisks mark significant difference (p <0.01) from WT Hp2′ group.
Previous studies have demonstrated that B cells can produce serum IgG1 and IgE, which are dependent on the presence of CD4 T cells during both the primary and secondary immune responses to H. polygyrus (25, 26). To determine whether the humoral immune response was blocked in JHD mice, total serum Ig levels were measured by ELISA at day 14 following H. polygyrus challenge inoculation of WT and JHD mice. As shown in Fig. 3B, elevations in total serum IgE and IgG1 levels were significantly increased in WT Hp2′ compared with WT Hp1′ or untreated mice (p <0.001). Serum IgE and IgG1 were not detected in JHD mice, consistent with an absence of Ab-producing B cells in JHD mice during both the primary and secondary immune responses to H. polygyrus.
Our observation that JHD mice do not expel H. polygyrus parasites following secondary H. polygyrus inoculation suggested an important role for B cells and perhaps Abs in the protective response. To examine whether B cells contribute to host protective effects at early stages of the response when developing larvae are at the tissue-dwelling stage, larval development was compared after H. polygyrus inoculation of JHD and WT mice. Primed mice and naive mice were inoculated with H. polygyrus and then sacrificed at day 4 after challenge, a point when larvae have already migrated through the intestinal mucosa and taken residence in the submucosal tissue near the muscularis. Larvae were counted in situ, and their distribution across the length of the small intestine was determined (mean larval position, see Materials and Methods). Individual larvae were extracted and their length, as an indicator of development, measured by sex (Fig. 3C). Our results show that larvae in primary infections cluster in the duodenum and proximal intestine, whereas the WT secondary response causes the distribution to be more uniform (mean larval position closer to 0.5) as a result of parasites invading more distal regions of the small intestine. Although the WT Hp1′ and JHD Hp1′ groups were similar in distribution, JHD Hp2′ had a significantly lower mean larval position than WT Hp2′ (p <0.01). Furthermore, larvae of each sex were significantly longer in mice of the JHD Hp2′ group than WT Hp2′ (p <0.001), albeit not as long as in the Hp1′ groups. These results indicate that protective effects of the early memory response that result in disruption of H. polygyrus larval migration and impairment of larval growth in the tissue-dwelling phase are partly dependent on B cells.
T cell activation, Treg cell frequency, and the Th2-type immune response in MLN following H. polygyrus challenge are comparable in WT and JHD mice
Previous studies have demonstrated that the host protective response to H. polygyrus is primarily dependent on IL-4 and IL-13 produced by CD4+ Th2 cells (5, 27, 28). The defective immunity in JHD mice against H. polygyrus infection might be caused by impaired T cell activation or Th2 cell development in JHD mice. To address these possibilities, WT and JHD mice were given a primary inoculation of H. polygyrus, drug treated, and re-challenged with H. polygyrus larvae (Hp2′). These mice were compared with untreated controls and mice given a primary inoculation (Hp1′). To assess T cell activation and cytokine expression, MLNs were collected at day 7 after challenge, a point at which optimal levels of cytokines are expressed (29). Cells were stained with anti–CD4-FITC and anti–CD69-PE. After primary or secondary H. polygyrus inoculation, CD69 expression was increased on CD4 T cells in both WT and JHD mice (Fig. 4A), suggesting that the CD4 T cell activation in MLNs following H. polygyrus inoculation is not dependent on B cells.
FIGURE 4.
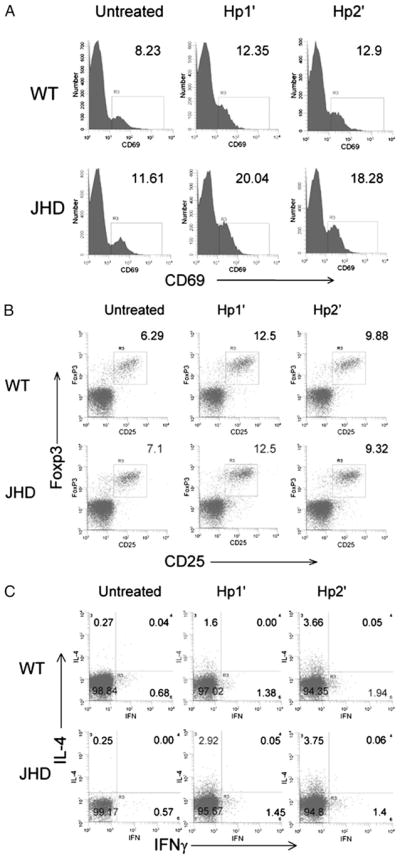
T cell activation, Treg cell frequency, and Th2 differentiation in draining MLNs were comparable in H. polygyrus-inoculated JHD and WT mice. Primary (Hp1′) and secondary (Hp2′) H. polygyrus challenges were administered to WT and JHD mice as described in Fig. 3. On day 7 after challenge, the mice were sacrificed, MLNs were collected, and cell suspensions were prepared. A, The cells were stained for anti-CD4 and anti-CD69. The expression of CD69 on CD4+ populations is shown. B, Cell suspensions were stained for surface CD4 and CD25 expression and intracellular Foxp3. Cells were gated on CD4+ population, and the frequency of CD25+ Foxp3+ Treg cells is shown. C, Cells were stimulated with PMA and inomycin for 6 h and stained for surface CD4 and intracellular IL-4 and IFN-γ. The IL-4 and IFN-γ production by CD4+ T cells is shown.
It has been shown previously that B cell deficiency causes an increased frequency of CD4+ CD25+ Foxp3+ Treg cells in humans and mice (30, 31), suggesting that the impaired immunity in JHD mice is caused by increased Treg cells that downregulate the Th2 response and other immune cell functions. To address whether Treg cells increase in H. polygyrus-inoculated JHD mice, MLNs were collected from untreated, Hp1′, and Hp2′ WT and JHD mice at day 7 after inoculation. Single-cell suspensions from pooled MLNs were prepared and stained for surface CD4 and CD25 and intracellular Foxp3. Cells were gated on the CD4+ population. Primary H. polygyrus inoculation increased the frequency of CD4+ CD25+ Foxp3+ Treg cells to the same level in WT and JHD mice (Fig. 4B). Similarly, secondary H. polygyrus inoculation comparably boosted the percentage of CD4+ CD25+ Foxp3+ Treg cells in WT and JHD mice. These findings suggest that the impaired immunity in H. polygyrus-inoculated JHD mice is not associated with changes in the frequency of CD4+ CD25+ Foxp3+ Treg cells.
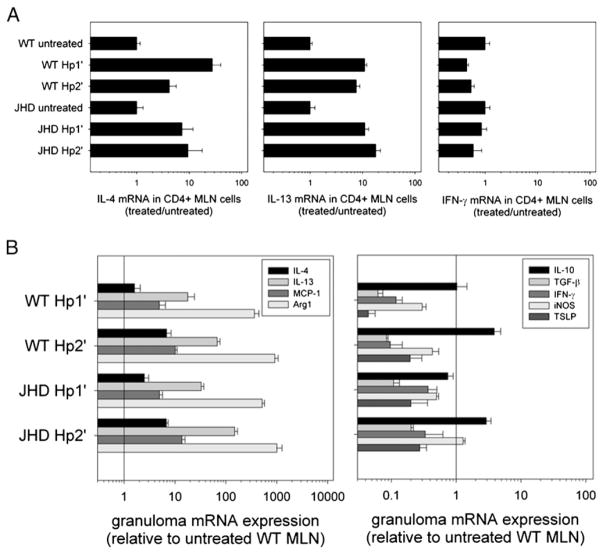
To investigate Th2 cell development, intracellular cytokine staining was used to determine cytokine production by CD4+ T cells. As shown in Fig. 4C, CD4 T cells from untreated WT or JHD mice did not produce IL-4. After primary H. polygyrus inoculation, pronounced increases in IL-4 were detected in both WT and JHD mice, with even greater IL-4 cytoplasmic staining following secondary H. polygyrus inoculation. Absence of B cells did not increase the percentage of IFN-γ–producing cells in MLNs after primary or secondary H. polygyrus inoculation (data not shown). As confirmation, we purified CD4+ T cells from MLN cells by CD4 positive selection and isolated RNA for the measurement of cytokine gene expression (Fig. 5A). Comparable expression of IL-4 and IL-13 was found in CD4+ T cells from WT and JHD MLNs following either primary or secondary H. polygyrus inoculation. IFN-γ gene expression remained low in all groups. Thus, our findings suggest that the defective immunity to H. polygyrus infection in JHD mice is not due to impairment in T cell activation, Th2 or Treg cell development, or deviation toward Th1 cell differentiation.
FIGURE 5.
The cytokine profile at the host–parasite interface is intact in JHD mice. WT and JHD mice were challenged with primary and secondary H. polygyrus inoculation as previously described. A, CD4 T cells were purified from MLNs by positive selection and RNA was isolated. IL-4, IL-13, and IFN-γ mRNA expression by these CD4 T cells were detected by quantitative PCR. Results are representative of two independent experiments. B, On day 7 after challenge, granulomas were collected and RNA was isolated. IL-4, IL-13, MCP-1, Arg1, IL-10, TGF-β, IFN-γ, iNOS, and thymic stromal lymphopoietin mRNA expression were detected by real-time quantitative PCR. Results are representative of two independent experiments.
Cytokine response in granulomas from JHD mice is comparable to that from WT mice following H. polygyrus challenge
Our results show that JHD mice generate comparable levels of T cell activation, CD4+ CD25+ Foxp3+ Treg cell expansion, and Th2 cell development with WT; however, it remains possible that the peripheral Th2-type response at the host–parasite interface is impaired in B cell-deficient mice. In the H. polygyrus life cycle, infective L3 penetrate the small intestinal wall and migrate to the submucosal region to develop into parasitic L4 on day 4; by day 8 these larvae develop into adult worms that return to the intestinal lumen and produce eggs. Our previous studies have shown that CD4+ T cells and alternatively activated macrophages (AAMacs) accumulate in the granulomas that surround tissue-dwelling L4 and that more rapid accumulation of these cells after secondary H. polygyrus inoculation contributes to the protective memory response (21). To examine whether cytokines, particularly those produced by infiltrating CD4+ T cells or AAMacs, are reduced in H. polygyrus granulomas from B cell-deficient H. polygyrus-inoculated mice, we collected cells from primary and secondary granulomas of both WT and JHD mice at day 7 postinoculation. RNA was isolated from these granuloma cells for cytokine analysis by quantitative real-time PCR (Fig. 5B). WT and JHD mice expressed similar levels of IL-4, IL-13, MCP-1, and Arg1 in the granulomas containing tissue-dwelling H. polygyrus L4, suggesting that the Th2-type response and accumulation of AAMacs at the host–parasite interface is not dependent on B cells or Abs. Levels of IFN-γ and inducible NO synthase (iNOS) mRNA remained low in all groups, consistent with a Th2-polarized response. Thymic stromal lymphopoietin mRNA also remained low. Expression of the inhibitory cytokines IL-10 and TGF-β was similar in the H. polygyrus granulomas from WT and JHD mice. Using immunofluorescence staining, AAMacs and other immune cell types accumulated in the granuloma similar to that of H. polygyrus inoculated WT mice, although some increase in neutrophils in H. polygyrus-inoculated JHD mice compared with inoculated WT mice was observed (data not shown). Because accumulation of AAMacs is dependent on IL-4 signaling (21), these studies show that Th2 cytokine effector function was intact. These data suggest that the development of the pronounced Th2-type response and the associated formation of the Th2-type granuloma at the site of parasite invasion in the intestinal submucosa are B cell independent.
Transfer of H. polygyrus memory B cells or IS from WT mice to H. polygyrus-primed JHD mice restores host protection following H. polygyrus challenge
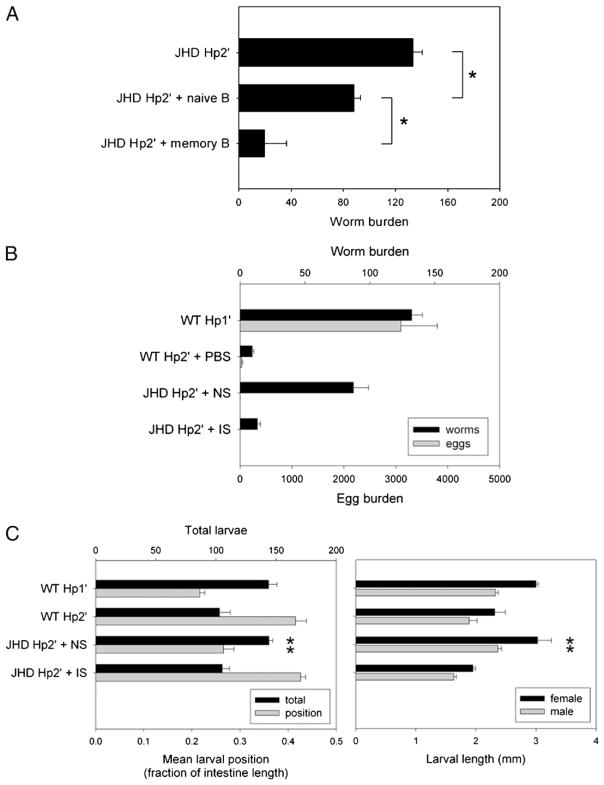
Our results suggest that B cells contribute to host defense against H. polygyrus during the tissue-dwelling stage, but that their absence does not affect the development of cytokine-expressing Th2 cells either in the MLNs or at peripheral sites of inflammation in the submucosa of the intestine. To further address the function of B cells in the protective response to H. polygyrus, transfer experiments of cells or serum from immune WT mice into JHD mice were performed. B cells from either untreated WT or H. polygyrus-primed WT donors were purified using magnetic bead cell sorting. JHD recipients were inoculated with H. polygyrus followed by drug cure. Three months later, naive or memory B cells were transferred i.v. to JHD recipients, which were then challenged again with H. polygyrus 2 d later and sacrificed at day 14 after secondary inoculation. As shown in Fig. 6A, transfer of naive B cells to H. polygyrus-primed JHD recipients only moderately enhanced host resistance to H. polygyrus secondary inoculation. However, transfer of memory B cells to H. polygyrus-primed JHD recipients significantly increased host immunity to secondary H. polygyrus infection (p <0.001). These findings confirm that memory B cells play a significant role in mediating host resistance to H. polygyrus infection.
FIGURE 6.
B cells and serum can restore the protective memory response against acute or chronic H. polygyrus infection in JHD mice. IS, NS, and memory B cells were collected from H. polygyrus-challenged WT mice. Naive B cells were isolated from untreated WT mice. JHD mice were primed with H. polygyrus and drug-cured in preparation for secondary challenge. B cells were transferred i.v. 2 d before secondary challenge, or serum was transferred i.p. every 3 d beginning 1 d before secondary challenge. A, Parasite burden at day 14 postinoculation was compared between groups of JHD Hp2′ mice given memory B, naive B, or no cells. B, Day 14 worm and egg burdens were compared for JHD Hp2′ with IS or NS versus WT and primary control groups. C, Day 4 larval burden, distribution, and development was measured as previously described for JHD Hp2′ given IS or NS. Results are representative of two independent experiments. Asterisks mark significant difference (p <0.01) from WT Hp2′ group, or as indicated by brackets.
Recent reports of the memory response to H. polygyrus infection have implicated both Ab-dependent and Ab-independent functions of B cells (18, 19). To clarify the role of memory B cells in our transfer system, we repeated the previous experiment with serum instead of B cells. IS was collected from WT mice at day 14 after secondary H. polygyrus inoculation, and NS was collected from untreated WT mice. Serum was administered i.p. every 3 d starting at day – 1 before secondary H. polygyrus inoculation of JHD recipients. At day 14 after challenge, the JHD group receiving NS with secondary inoculation had a poorly controlled chronic infection, whereas the JHD secondary group given IS exhibited effective worm expulsion, comparable to WT mice given a secondary H. polygyrus inoculation (Fig. 6B). This result indicates that Ab from memory B cells is sufficient to restore the protective memory response to H. polygyrus in B cell-deficient mice that culminates in worm expulsion.
Because the effects of the impairment in the JHD response to H. polygyrus challenge were observed during the tissue-dwelling larval phase (Fig. 3C), we investigated whether transferred IS could restore the pattern of larval distribution and impaired development seen in the WT secondary (Hp2′) versus primary (Hp1′) response. Primed JHD mice were given serum transfers with secondary H. polygyrus inoculation and sacrificed at day 4 (Fig. 6C). The usual pattern of fewer larvae, more distal distribution, and reduced larval length was observed in WT Hp2′ mice compared with WT Hp1′ mice or JHD Hp2′ mice. Transfer of NS to JHD Hp2′ mice failed to restore the characteristic pattern of an effective memory response. However, IS transfer to JHD Hp2′ mice resulted in a response comparable to that observed with WT Hp2′ mice. These results suggest that Ab produced during the memory response can affect worm invasion and development as early as 4 d after inoculation.
Transfer of memory T cells, B cells, or serum from inoculated WT mice to naive WT mice promotes protective immunity
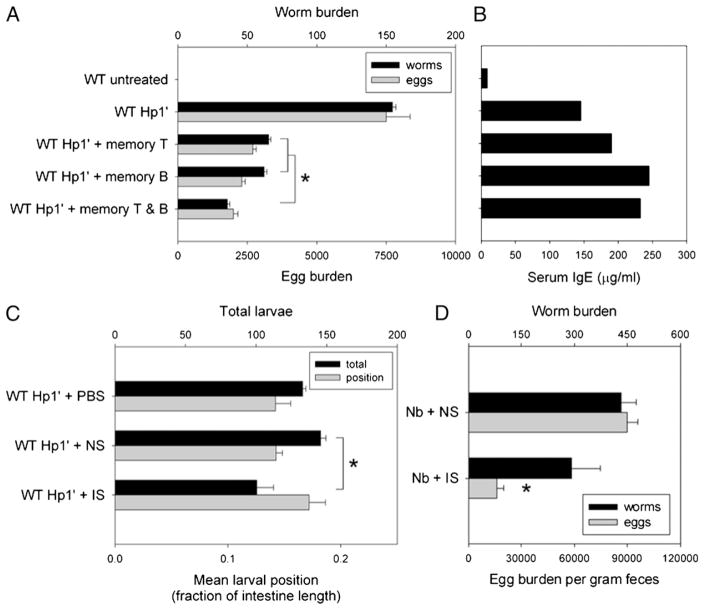
We next investigated whether the protective effect of transferred memory B cells on secondary H. polygyrus inoculation in JHD mice requires memory T cell help. CD4+ T cells and B cells were isolated from spleens and MLNs from H. polygyrus-primed WT mice and used as memory T cells or B cells for transfer into naive WT recipient mice. One day after i.v. transfer of memory T and/or memory B cells, the recipient mice were inoculated with H. polygyrus, and at day 14 postinoculation the mice were assessed for worm expulsion and egg production (Fig. 7A). Compared with primary inoculation alone, the parasite burden was significantly lower in H. polygyrus-inoculated mice receiving either memory T cell or memory B cell transfer (p <0.001). Moreover, when recipient naive WT mice received both memory T cells and memory B cells prior to H. polygyrus inoculation, the protective response was further enhanced (p <0.001). This finding suggests that H. polygyrus memory T cells and H. polygyrus memory B cells can act independently or in an additive fashion to mediate host resistance to H. polygyrus inoculation.
FIGURE 7.
T cells, B cells, and serum from immunized mice can transfer protection against acute or chronic primary H. polygyrus infection. WT mice were primed and drug-cured with H. polygyrus as described. Two months later, some mice were sacrificed for harvest of T and B cells (memory T, memory B). Other mice were rechallenged with H. polygyrus inoculation and exsanguinated at day 14 after secondary inoculation with IS. NS from untreated mice and PBS were used as control interventions. A and B, Memory B and/or T cells were transferred i.v. into naive WT mice 2 d before primary H. polygyrus inoculation. Worm and egg burden (A) and serum IgE (B) were assayed 14 d later as measurements of the response to chronic infection. C, WT mice were inoculated with H. polygyrus on day 0, administered PBS, NS, or IS on days – 1 and 2, and the distribution of tissue-dwelling larvae (L4) was observed as an indicator of the early immune response. D, WT mice were inoculated with N. brasiliensis on day 0, administered NS or IS on day – 1, and examined for worm burden on day 7 after inoculation. Asterisks mark significant difference (p <0.01) from the NS group, or as indicated by brackets.
H. polygyrus-inoculated WT mice produce high levels of IgE and IgG1, with significantly more H. polygyrus-specific Ab seen after secondary inoculation than after primary inoculation (32). We investigated IgE production in WT mice receiving memory T and/or memory B cell transfer (Fig. 7B). Transfer of WT memory T cells boosted IgE production in H. polygyrus-inoculated recipient WT mice. Transfer of WT memory B cells with or without WT memory T cells markedly promoted IgE production in WT recipient mice following H. polygyrus inoculation. These data suggest that the B cell contribution to host protection to H. polygyrus inoculation can be mediated through the production of Abs.
To determine whether H. polygyrus-specific Ab can induce protective responses in the absence of memory B or T cells, we tested the effect of IS transfer during primary H. polygyrus inoculation. Naive WT recipients of IS or NS were inoculated with H. polygyrus and sacrificed at day 4, early during the tissue-dwelling larval phase of infection (Fig. 7C). The group receiving NS transfer had a larval total and distribution similar to the control group with PBS injections, whereas the group receiving IS transfer had significantly decreased larval total (p <0.01) and a distribution that trended more distal. This result suggests that H. polygyrus-specific Ab, in the absence of memory B or T cells, is sufficient to induce modest protective effects early during infection.
To examine whether N. brasiliensis-specific Ab could accelerate protective immunity during the primary immune response to N. brasiliensis, IS was collected from N. brasiliensis-inoculated mice (see Materials and Methods). WT mice were administered NS or IS 1 d prior to s.c. inoculation with 500 N. brasiliensis L3 and sacrificed at day 7. No difference was found in total worm burden between the IS- and NS-treated groups, suggesting that Ab alone is not sufficient to mediate protective immunity against primary N. brasiliensis inoculation. However, a significant reduction in worm fecundity, as measured by fecal egg count, was observed in IS-treated mice (p <0.01; Fig. 7D), indicating that exogenous Ab administration can affect worm fecundity during N. brasiliensis infection.
Discussion
Our findings demonstrate that the Th2-type mucosal immune response is intact in the absence of B cells during the immune response to the intestinal nematodes H. polygyrus and N. brasiliensis. Although N. brasiliensis worm expulsion was similar in WT and B cell-deficient mice, the protective memory immune response to H. polygyrus was compromised and the B cell effect was primarily mediated by Abs affecting early larval migration and development during the tissue-dwelling phase.
Previous studies of the immune response to N. brasiliensis in B cell-deficient mice showed a reduced Th2-type immune response. However, these studies examined the nonmucosal immune response in the draining ear lymph node after intracutaneous inoculation in the ear (16). Our studies now show that the mucosal in vivo immune response, including the development of IL-4–producing Th2 cells, was intact in the intestine draining MLNs and effectively mediated worm expulsion. A number of previous studies showed increased permissiveness of Th2 cell differentiation in mucosal tissues (33, 34), and our findings are consistent with a mucosal microenvironment supporting Th2 cell differentiation, even in the absence of B cells during the immune response to N. brasiliensis.
The Th2-type mucosal response was also intact in both the primary and secondary mucosal immune response to H. polygyrus. These latter results are consistent with recent studies (18) that suggested an intact Th2-type cytokine response in the spleens of H. polygyrus-inoculated B cell-deficient mice. Our studies expand on these previous findings, demonstrating that in the draining MLNs and at the peripheral site of parasite invasion of the intestinal mucosa, Th2 cytokine expression was comparable between H. polygyrus-inoculated WT and H. polygyrus-inoculated B cell-deficient mice. In contrast, another recent report (19) concluded that the Th2-type immune response to H. polygyrus was compromised in B cell-deficient mice. However, these studies relied primarily on lethally irradiated and reconstituted chimeric mice, which can provide important insights into B cell function, but may not reflect the natural in vivo immune response to H. polygyrus that also requires that structural and physiologic components work in concert. The limited studies that examined Th2 cytokines during the natural immune response to H. polygyrus relied on cytokine measurements of cell populations restimulated overnight in vitro (19), which might bias the cytokine response toward increased B cell dependence. Our studies confirm and extend those of McCoy et al. (18), indicating that the protective memory response leading to H. polygyrus expulsion from the intestinal lumen is compromised by the absence of B cells despite unimpaired development of the mucosal Th2-type response to intestinal nematode parasites.
Our studies further identified an important role for B cells and Abs at the early tissue-dwelling stages of H. polygyrus development. Direct measurements of larval distribution, number, sex, and length showed quantitative impairment of early parasite development during the memory response compared with the primary response. These observations indicated that the protective effects of the memory response were significantly reduced in B cell-deficient mice and were restored after transfer of IS, as early as day 4 after inoculation. These findings suggested that Abs mediate protective effects at the tissue-dwelling stage of development or earlier. In an experimental model of Strongyloides infection, in which larvae are housed in diffusion chambers, Abs have also been shown to play an essential role in mediating protective effects during the memory response (35). In addition, other studies have suggested an important role for Abs in protective responses against intestinal nematode parasites (36, 37). In the natural H. polygyrus model, parasite-specific Ab can bind the migrating larvae shortly after inoculation, impairing their penetration or identification of the intestinal niche and their subsequent migration to preferred sites, and thereby affecting their distribution in the small intestine. This may also be one mechanism through which protective immunity to neonates was conferred after transfer of serum from adult mice immunized multiple times with H. polygyrus (38). IS transfer was also shown to impair larval migration of hookworms in naive mice (39). Our additional observation that parasites in the submucosa showed B cell-dependent reduced length further suggests that Ab also affects development after migration when the parasite is maturing within the granuloma. In contrast, our finding—that the cytokine expression and immune cell architecture (data not shown) in the granuloma surrounding the developing parasite were largely unchanged in B cell-deficient mice compared with inoculated WT controls—suggests that B cells have only modest effects in terms of regulating the peripheral Th2-type immune response. Previous studies have shown a significant role for memory Th2 cells and AAMacs in immune protection (21). It will be of interest in future studies to examine whether macrophage- and Ab-mediated protective effects are interactive or operate independently.
The observation that effective worm expulsion required B cells after H. polygyrus inoculation but not N. brasiliensis inoculation suggests that B cells are not always an essential component of the host protective response against intestinal nematode parasites. Because an absence of B cells did not affect the accelerated protective memory response to N. brasiliensis, it is unlikely that specific Abs play a significant role in protective host responses against this parasite. These findings are consistent with other studies indicating that specific components of the Th2-type response, such as mast cells or goblet cells, might be more effective against some helminth parasites than others (4, 40). Our studies now implicate B cells as also showing variability in their capacity to mediate protective responses against different nematode parasites. It should be noted though that increasing specific Ab levels by exogenous administration of IS during primary infection of WT mice with either N. brasiliensis or H. polygyrus can contribute to protective immunity.
Acknowledgments
This work was supported by National Institutes of Health Grant AI031678.
Abbreviations used in this paper
- AAMac
alternatively activated macrophage
- Hp1′
primary Heligmosomoides polygyrus inoculation
- Hp2′
secondary H. polygyrus inoculation
- iNOS
inducible NO synthase
- IS
immune serum
- L3
third-stage larvae
- MLN
mesenteric lymph node
- NS
naive serum
- Treg
T regulatory
- WT
wild type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diemert DJ, Bethony JM, Hotez PJ. Hookworm vaccines. Clin Infect Dis. 2008;46:282–288. doi: 10.1086/524070. [DOI] [PubMed] [Google Scholar]
- 3.Scales HE, Ierna MX, Lawrence CE. The role of IL-4, IL-13 and IL-4Ralpha in the development of protective and pathological responses to Trichinella spiralis. Parasite Immunol. 2007;29:81–91. doi: 10.1111/j.1365-3024.2006.00920.x. [DOI] [PubMed] [Google Scholar]
- 4.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 5.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gause WC, Urban JF, Jr, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–277. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez HJ, Wang Y, Stadecker MJ. In infection with Schistosoma mansoni, B cells are required for T helper type 2 cell responses but not for granuloma formation. J Immunol. 1997;158:4832–4837. [PubMed] [Google Scholar]
- 8.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192:475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackwell NM, Else KJ. B cells and antibodies are required for resistance to the parasitic gastrointestinal nematode Trichuris muris. Infect Immun. 2001;69:3860–3868. doi: 10.1128/IAI.69.6.3860-3868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson-Lindbom B, Borrebaeck CA. Germinal center B cells constitute a predominant physiological source of IL-4: implication for Th2 development in vivo. J Immunol. 2002;168:3165–3172. doi: 10.4049/jimmunol.168.7.3165. [DOI] [PubMed] [Google Scholar]
- 11.Harris DP, Goodrich S, Mohrs K, Mohrs M, Lund FE. Cutting edge: the development of IL-4-producing B cells (B effector 2 cells) is controlled by IL-4, IL-4 receptor alpha, and Th2 cells. J Immunol. 2005;175:7103–7107. doi: 10.4049/jimmunol.175.11.7103. [DOI] [PubMed] [Google Scholar]
- 12.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linton PJ, Bautista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, Padrick RC, Bradley LM. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu P, Urban JF, Zhou XD, Chen SJ, Madden K, Moorman M, Nguyen H, Morris SC, Finkelman FD, Gause WC. CD40-mediated stimulation contributes to lymphocyte proliferation, antibody production, eosinophilia, and mastocytosis during an in vivo type 2 response, but is not required for T cell IL-4 production. J Immunol. 1996;156:3327–3333. [PubMed] [Google Scholar]
- 15.Lu P, Zhou X, Chen SJ, Moorman M, Morris SC, Finkelman FD, Linsley P, Urban JF, Gause WC. CTLA-4 ligands are required to induce an in vivo interleukin 4 response to a gastrointestinal nematode parasite. J Exp Med. 1994;180:693–698. doi: 10.1084/jem.180.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, Liu Z, Rozo CT, Hamed HA, Alem F, Urban JF, Jr, Gause WC. The role of B cells in the development of CD4 effector T cells during a polarized Th2 immune response. J Immunol. 2007;179:3821–3830. doi: 10.4049/jimmunol.179.6.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 18.McCoy KD, Stoel M, Stettler R, Merky P, Fink K, Senn BM, Schaer C, Massacand J, Odermatt B, Oettgen HC, et al. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe. 2008;4:362–373. doi: 10.1016/j.chom.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, Kusser K, Honjo T, Mohrs K, Mohrs M, Randall T, Lund FE. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Liu Q, Pesce J, Whitmire J, Ekkens MJ, Foster A, VanNoy J, Sharpe AH, Urban JF, Jr, Gause WC. Nippostrongylus brasiliensis can induce B7-independent antigen-specific development of IL-4-producing T cells from naive CD4 T cells in vivo. J Immunol. 2002;169:6959–6968. doi: 10.4049/jimmunol.169.12.6959. [DOI] [PubMed] [Google Scholar]
- 21.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Liu Z, Whitmire J, Alem F, Hamed H, Pesce J, Urban JF, Jr, Gause WC. IL-18 stimulates IL-13-mediated IFN-gamma-sensitive host resistance in vivo. Eur J Immunol. 2006;36:1187–1198. doi: 10.1002/eji.200535668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasiteNippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 24.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, Murray R, Grencis R, McKenzie AN. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9:423–432. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 25.Finkelman FD, I, Katona M, Urban JF, Jr, Holmes J, Ohara J, Tung AS, Sample JV, Paul WE. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335–2341. [PubMed] [Google Scholar]
- 26.Urban JF, Jr, Katona IM, Finkelman FD. Heligmosomoides polygyrus: CD4+ but not CD8+ T cells regulate the IgE response and protective immunity in mice. Exp Parasitol. 1991;73:500–511. doi: 10.1016/0014-4894(91)90074-7. [DOI] [PubMed] [Google Scholar]
- 27.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 28.Urban JF, Jr, Katona IM, Paul WE, Finkelman FD. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci USA. 1991;88:5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svetić A, Madden KB, Zhou XD, Lu P, Katona IM, Finkelman FD, Urban JF, Jr, Gause WC. A primary intestinal helminthic infection rapidly induces a gut-associated elevation of Th2-associated cytokines and IL-3. J Immunol. 1993;150:3434–3441. [PubMed] [Google Scholar]
- 30.Sfikakis PP, V, Souliotis L, Fragiadaki KG, Moutsopoulos HM, Boletis JN, Theofilopoulos AN. Increased expression of the FoxP3 functional marker of regulatory T cells following B cell depletion with rituximab in patients with lupus nephritis. Clin Immunol. 2007;123:66–73. doi: 10.1016/j.clim.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomchik MJ, Wen L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekkens MJ, Liu Z, Liu Q, Whitmire J, Xiao S, Foster A, Pesce J, VanNoy J, Sharpe AH, Urban JF, Gause WC. The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus. J Immunol. 2003;170:384–393. doi: 10.4049/jimmunol.170.1.384. [DOI] [PubMed] [Google Scholar]
- 33.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herbert DR, Nolan TJ, Schad GA, Abraham D. The role of B cells in immunity against larval Strongyloides stercoralis in mice. Parasite Immunol. 2002;24:95–101. doi: 10.1046/j.0141-9838.2001.00441.x. [DOI] [PubMed] [Google Scholar]
- 36.Gu Y, Li J, Zhu X, Yang J, Li Q, Liu Z, Yu S, Li Y. Trichinella spiralis: characterization of phage-displayed specific epitopes and their protective immunity in BALB/c mice. Exp Parasitol. 2008;118:66–74. doi: 10.1016/j.exppara.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Bethony J, Loukas A, Smout M, Brooker S, Mendez S, Plieskatt J, Goud G, Bottazzi ME, Zhan B, Wang Y, et al. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. FASEB J. 2005;19:1743–1745. doi: 10.1096/fj.05-3936fje. [DOI] [PubMed] [Google Scholar]
- 38.Harris NL, Spoerri I, Schopfer JF, Nembrini C, Merky P, Massacand J, Urban JF, Jr, Lamarre A, Burki K, Odermatt B, et al. Mechanisms of neonatal mucosal antibody protection. J Immunol. 2006;177:6256–6262. doi: 10.4049/jimmunol.177.9.6256. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh K, Hotez PJ. Antibody-dependent reductions in mouse hookworm burden after vaccination with Ancylostoma caninum secreted protein 1. J Infect Dis. 1999;180:1674–1681. doi: 10.1086/315059. [DOI] [PubMed] [Google Scholar]
- 40.Patel N, Kreider T, Urban JF, Jr, Gause WC. Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites. Int J Parasitol. 2009;39:13–21. doi: 10.1016/j.ijpara.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]