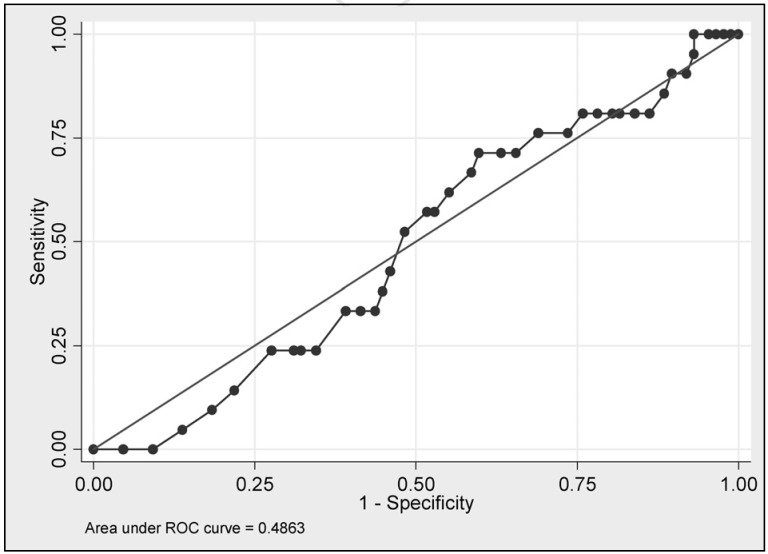
Severely anaemic Jehovah’s Witness (JW) patients who refuse blood transfusion on religious grounds have high mortality and morbidity1,2. Carson et al. have shown that the odds of death increase 2.5 times for every 10 g/L postoperative haemoglobin (Hb) decrease below 70 g/L3. The latest study4 demonstrated that an unadjusted nadir Hb concentration is a poor predictor of mortality of anaemic JW patients (Figure 1). Early risk factors including age ≥45 years of age, weight ≥90 kg, hypertension, cardiac arrhythmia, angina, previous myocardial infarction, valvular heart disease, heart failure, being on haemodialysis, acute admission and Hb ≤80 g/L on admission to hospital are associated with mortality of anaemic JW patients5. It was shown that an Auckland Anaemia Mortality Risk Score (Auckland AMRS), which is a composite score of the number of early risk factors each anaemic JW patient had, was associated with mortality. JW patients with Auckland AMRS of 0 to 3 had 4% mortality, Auckland AMRS 4 to 5, 32%, Auckland AMRS 6 to 7, 50% and Auckland AMRS ≥8, 83%5. During their hospital stay JW patients can develop anaemia-related (late) mortality risk factors including shock, acute gastro-intestinal bleeding, pneumonia, nadir Hb concentration ≤70 g/L, septicaemia, worsened congestive heart failure and neurologic complications4. Among these late risk factors, shock was the strongest predictor of mortality followed by acute gastro-intestinal bleeding and pneumonia.
Figure 1.
ROC curve: Nadir haemoglobin concentration as a predictor of Jehovah’s Witness patients mortality.
When weights of individual statistically significant anaemia-related risk factors of mortality were combined and a composite mortality risk score, the Hamilton Anaemia Mortality Risk Score (Hamilton AMRS), was calculated, it was shown that JW patients with Hamilton AMRS of 0 to 2 had 4% mortality, Hamilton AMRS of 3 to 4, 29%, Hamilton AMRS of 5, 40%, and Hamilton AMRS of ≥6, 67%.
On admission to hospital the trauma patient described by Lorentzen et al.6 had an Auckland AMRS of 3 (age, acute admission and Hb ≤80 g/L on admission to hospital) and a Hamilton AMRS of 6 (shock, ischaemic bowel perforation and the nadir Hb concentration ≤70 g/L) that estimated the patient’s mortality risk exceeding 70%. The patient underwent an open bowel resection and was managed in an intensive care unit (ICU) with physiologic parameters monitoring, ventilatory support, fluid resuscitation and an administration of vasopressors. The patient’s infective complications were treated with broad spectrum intravenous antibiotics, collection drainage, and wounds debridement and washout. Also the patient was treated with intravenous iron, B12 supplementation and subcutaneous administration of erythropoetin (EPO) in the dose of 10,000 units every second day.
JW patients accept EPO, which is an erythropoiesis stimulating agent, as an alternative to blood transfusion. EPO is a 165 amino-acid glycoprotein, which is mainly produced by renal peritubular capillary endothelial cells in response to hypoxia7. As a haematopoietic cytokine, EPO promotes proliferation, differentiation and survival of erythroid progenitor cells8. In addition, EPO exerts a potent protective effect against hypoxia through its anti-apoptotic action9. After binding to its receptor on the cell surface, EPO initiates a JAK2 signalling cascade leading to NF-kB- and STAT5-dependent transcription of anti-apoptotic genes, including Bcl-xL, Bcl-210. Furthermore, EPO exerts a potent vascular protection and induces neoangiogenesis11–13.
Hematopoietic effects of EPO
EPO has been used for many years to treat patients with anaemia of end-stage renal disease14,15 and it has been found to improve exercise tolerance and physical function16. More recently, EPO started to be used to alleviate chemotherapy-induced anaemia in cancer patients17, to treat anaemia in critically ill18–21, and surgical patients22,23.
In critically ill patients, compared with placebo, a subcutaneous administration of epoetin alfa in the dose of 40,000 units per week for three consecutive weeks had neutral effects on exposure to allogeneic red blood cell (ARBC) (48.3% vs 46.0%, respectively, p =0.34) and the mean number of red blood cell units transfused (4.3±4.8 units vs 4.5±4.6 units, p =0.42)24. Importantly, there were no differences in 29-day mortality (adjusted odds ratio (OR), 0.79, 95% confidence interval (CI), 0.56 to 1.10), the length of stay in intensive care unit (7 days in the placebo group and 8 days in epoetin alfa group, p =0.43), and in the duration of hospital admission (15 days in both groups, p =0.43)20. A recent meta-analysis demonstrated that in critically ill anaemic patients any dose of epoetin alfa or darbepoetin, compared with placebo, significantly reduced the odds of ARBC exposure (OR =0.73, 95% CI 0.64–0.84, I2 =54.7%), and the volume of ARBC transfusion per patient (weighted mean difference −0.41 units per patient, 95% CI −0.74 to −0.10, I2 =79.2%)25.
Cytoprotective effects of EPO
The survival benefit of subcutaneous administration of epoetin alfa in the dose of 40,000 units per week vs placebo in anaemic trauma patients was investigated in two randomised, double-blind, placebo-controlled trials. In the EPO-2 trial epoetin alfa reduced 29-day mortality between 54% (unadjusted OR=0.46, 95% CI, 0.24–0.89, p =0.017) and 50% (best-fit adjusted OR =0.50; 95% CI, 0.26–0.97)25. In the EPO-3 trial epoetin alfa reduced 29-day mortality between 49% (unadjusted OR =0.51, 95% CI, 0.27–0.98, p =0.039) and 64% (fully adjusted OR of 0.36; 95% CI, 0.19–0.74).
In EPO-2 study epoetin alfa-treated patients had improved 42-day survival by 54% (unadjusted OR =0.46, 95% CI, 0.25–0.85, p =0.011). Trauma patients of EPO-3 trial treated with epoetin alfa had a reduction of 42-day mortality between 49% (unadjusted OR =0.51; 95% CI, 0.27–0.95, p =0.030) and 65% (adjusted OR 0.35; 95% CI, 0.18–0.68). Day 140 survival of epoetin alfa treated patients in EPO-3 study, compared with placebo treated controls, was increased by 59% (adjusted OR 0.41; 95% CI, 0.24–0.70)26.
At present there is a paucity of data on clinical benefits, optimal doses and regimen of administration of EPO in severe anaemia, but an experts opinion holds that EPO therapy should be instituted early in the course of patient’s illness and in high doses27–30.
The case report of Lorentzen et al.6 highlights an importance of mortality risk stratification and management of high-risk anaemic JW patients in ICU with monitoring of their physiological parameters, ventilatory and circulatory support, prompt treatment of infective complications, and an administration of a high dose EPO therapy in combination with iron, B12 and folate supplementations.
Footnotes
The Author declares no conflicts of interest.
References
- 1.Tobian AAR, Ness PM, Noveck H, Carson JL. Time course and etiology of death in patients with severe anemia. Transfusion. 2009;49:1395–9. doi: 10.1111/j.1537-2995.2009.02134.x. [DOI] [PubMed] [Google Scholar]
- 2.Beliaev AM, Marshall RJ, Gordon M, et al. Clinical benefits and cost-effectiveness of allogeneic red-blood-cell transfusion in severe symptomatic anaemia. Vox Sang. 2012;103:18–24. doi: 10.1111/j.1423-0410.2011.01573.x. [DOI] [PubMed] [Google Scholar]
- 3.Carson JL, Noveck H, Berlin JA, Gould SA. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–8. doi: 10.1046/j.1537-2995.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 4.Beliaev AM, Marshall RJ, Smith W, Windsor JA. Treatment monitoring and mortality risk adjustment in anaemic Jehovah’s Witnesses. ANZ J Surg. 2013;83:161–4. doi: 10.1111/j.1445-2197.2012.06228.x. [DOI] [PubMed] [Google Scholar]
- 5.Beliaev AM, Marshall RJ, Smith W, Windsor JA. Mortality Risk Stratification in Severely Anaemic Jehovah’s Witness Patients. Intern Med J. 2012;42:e1–3. doi: 10.1111/j.1445-5994.2011.02699.x. [DOI] [PubMed] [Google Scholar]
- 6.Lorentzen K, Bjarne Kjær B, Jørgen Jørgensen J. Supportive treatment of severe anaemia in a Jehovah’s Witness with severe trauma. Blood Transfus. 2013;11:452–3. doi: 10.2450/2013.0263-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marzo F, Lavorgna A, Coluzzi G, et al. Erythropoietin in heart and vessels: focus on transcription and signalling pathways. J Thromb Thrombolysis. 2008;26:183–7. doi: 10.1007/s11239-008-0212-3. [DOI] [PubMed] [Google Scholar]
- 8.Bao H, Jacobs-Helber, Lawson A, et al. Protein Kinase B (c-Akt), Phosphatidylinositol 3-Kinase, and STAT5 Are Activated by Erythropoietin (EPO) in HCD57 Erythroid Cells But Are Constitutively Active in an EPO-Independent, Apoptosis-Resistant Subclone (HCD57-SREI Cells) Blood. 1999;93:3757–73. [PubMed] [Google Scholar]
- 9.Imamura R, Moriyama T, Isaka Y, et al. Erythropoietin protects the kidneys against ischemia reperfusion injury by activating hypoxia inducible factor-1alpha. Transplantation. 2007;83:1371–9. doi: 10.1097/01.tp.0000264200.38926.70. [DOI] [PubMed] [Google Scholar]
- 10.Kumral A, Tüzün F, Oner MG, et al. Erythropoietin in neonatal brain protection: The past, the present and the future. Brain Dev. 2011;33:632–43. doi: 10.1016/j.braindev.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Fliser D, Bahlmann FH. Erythropoietin and the endothelium - a promising link? European Journal of Clinical Investigation. 2008;38:457–61. doi: 10.1111/j.1365-2362.2008.01968.x. [DOI] [PubMed] [Google Scholar]
- 12.Hirata Y, Nagata D, Suzuki E, et al. Diagnosis and treatment of endothelial dysfunction in cardiovascular disease. Int Heart J. 2010;51:1–6. doi: 10.1536/ihj.51.1. [DOI] [PubMed] [Google Scholar]
- 13.Nogueras S, Merino A, Ojeda R, et al. Coupling of endothelial injury and repair: an analysis using an in vivo experimental model. American Journal of Physiology - Heart & Circulatory Physiology. 2008;294:H708–13. doi: 10.1152/ajpheart.00466.2007. [DOI] [PubMed] [Google Scholar]
- 14.Muirhead N, Laupacis A, Wong C. Erythropoietin for anaemia in haemodialysis patients: results of a maintenance study (the Canadian Erythropoietin Study Group) Nephrol Dial Transplant. 1992;7:811–6. [PubMed] [Google Scholar]
- 15.Muirhead N, Keown P, Churchill D, et al. Dialysis patients treated with Epoetin 3 show improved exercise tolerance and physical function: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int. 2010 doi: 10.1111/j.1542-4758.2010.00508.x. [DOI] [PubMed] [Google Scholar]
- 16.Johansen K, Finkelstein F, Revicki D, et al. Systematic review and meta-analysis of exercise tolerance and physical functioning in dialysis patients treated with erythropoiesis-stimulating agents. Am J Kidney Dis. 2010;55:535–48. doi: 10.1053/j.ajkd.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Auerbach M, Silberstein P, Webb R, et al. Darbepoetin alfa 300 or 500 μg once every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. Am J Hematol. 2010;85:655–63. doi: 10.1002/ajh.21779. [DOI] [PubMed] [Google Scholar]
- 18.Lundy J, Hetz K, Chung K, et al. Outcomes with the use of recombinant human erythropoietin in critically ill burn patients. Am Surg. 2010;76:951–6. [PubMed] [Google Scholar]
- 19.Napolitano L, Fabian T, Kelly K, et al. Improved survival of critically ill trauma patients treated with recombinant human erythropoietin. J Trauma. 2008;65:285–97. doi: 10.1097/TA.0b013e31817f2c6e. [DOI] [PubMed] [Google Scholar]
- 20.Corwin HL, Gettinger A, Fabian TC, et al. Efficacy and safety of epoetin alfa in critically ill patients. New England Journal of Medicine. 2007;357:965–76. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- 21.Corwin HL, Gettinger A, Pearl RG, et al. Efficacy of recombinant human erythropoietin in critically ill patients: a randomized controlled trial. JAMA. 2002;288:2827–35. doi: 10.1001/jama.288.22.2827. [DOI] [PubMed] [Google Scholar]
- 22.Bacuzzi A, Dionigi G, Piffaretti G, et al. Preoperative methods to improve erythropoiesis. Transplant Proc. 2011;43:324–6. doi: 10.1016/j.transproceed.2010.09.097. [DOI] [PubMed] [Google Scholar]
- 23.Parker WH, Wagner WH. Gynecologic surgery and the management of hemorrhage. Obstet Gynecol Clin North Am. 2010;37:427–36. doi: 10.1016/j.ogc.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Corwin HL, Gettinger A, Fabian T, et al. Efficacy and Safety of Epoetin Alfa in Critically Ill Patients. The New England Journal of Medicine. 2007;357:965–76. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- 25.Zarychanski R, Turgeon A, McIntyre L, Fergusson D. Erythropoietin-receptor agonists in critically ill patients: a meta-analysis of randomized controlled trials. CMAJ. 2007;177:725–34. doi: 10.1503/cmaj.071055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napolitano LM, Fabian TC, Kelly KM, et al. Improved survival of critically ill trauma patients treated with recombinant human erythropoietin. Journal of Trauma-Injury Infection & Critical Care. 2008;65:285–97. doi: 10.1097/TA.0b013e31817f2c6e. discussion 97–9. [DOI] [PubMed] [Google Scholar]
- 27.Ball A, Winstead P. Recombinant human erythropoietin therapy in critically ill Jehovah’s Witnesses. Pharmacotherapy. 2008;28:1383–90. doi: 10.1592/phco.28.11.1383. [DOI] [PubMed] [Google Scholar]
- 28.Casati V, D’Angelo A, Barbato L, et al. Perioperative management of four anaemic female Jehovah’s Witnesses undergoing urgent complex cardiac surgery. Br J Anaesth. 2007;99:349–52. doi: 10.1093/bja/aem170. [DOI] [PubMed] [Google Scholar]
- 29.Berend K, Levi M. Management of adult Jehovah’s Witness patients with acute bleeding. Am J Med. 2009;122:1071–6. doi: 10.1016/j.amjmed.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Schälte G, Janz H, Busse J, et al. Life-threatening postoperative blood loss in a Jehovah’s Witness, treated with high-dose erythropoietin. Br J Anaesth. 2005;94:442–4. doi: 10.1093/bja/aei068. [DOI] [PubMed] [Google Scholar]