Introduction
T regulatory cells (Treg) are a specialised subset of T cells that engage in the maintenance of immunological self-tolerance by actively suppressing the activation, expansion of self-reactive lymphocytes, suppression of immune response1,2 and constitute a central mechanism in immune regulation3. There is no doubt that vital organ transplantation has prolonged the survival of patients with terminal diseases and end-stage organ failure. This great advance in transplantation could not have been achieved without the rapid development of immunosuppressive agents; although the side effects of immunosuppression remain one of the major concerns in clinical transplantation. As more patients benefit from organ transplantation we also face the worldwide problem of the shortage of donor organs, and long-term graft loss. It is almost evident that conventional T cells are involved in transplant injury, and their toxic effects on the health of transplants could, therefore, be manipulated by intervening through immunotherapies4,5.
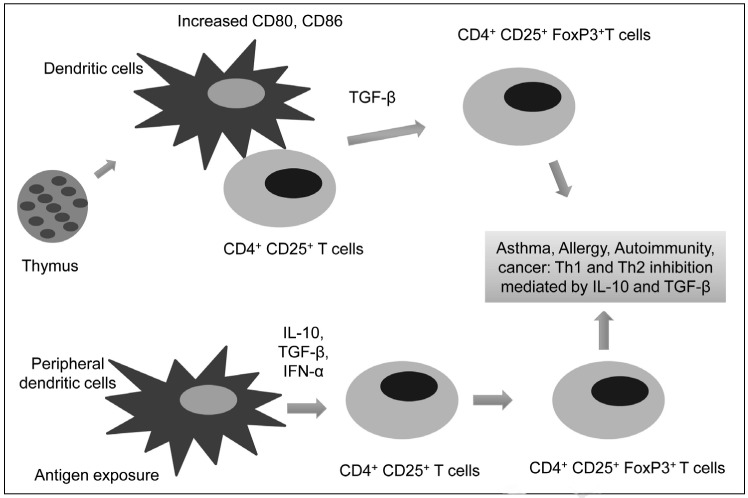
The presence of Treg allows the human immune system to maintain self-tolerance against self-destruction while sustaining the ability to mount robust immune responses against invading microorganisms and cancerous cells3,6. Cell-mediated suppression has been shown to play a central role in immune regulation although a suitable marker for identifying, and characterising the suppresser cells involved was not known7. In 1995, Sakaguchi and his colleagues identified a group of T cells from the thymus, which they showed to be responsible for protection against the development of autoimmune diseases8,9. Reconstitution of CD4+CD25+ cells in nude mice prevented the development of autoimmune diseases associated with the transfer of the CD4+CD25− cells8. These findings confirmed the contribution of CD4+CD25+ cells in maintaining self-tolerance by down-regulating the immune response of CD4+CD25− cells, and this became a significant stepping-stone for Treg research8–10. The development of natural Treg in the thymus was thought to occur in the Hassall’s corpuscles10. The thymus is the central organ for immunological self- and non-self-discrimination, and Treg are produced in the thymus as a mechanism for maintaining self-tolerance11. Thymocytes, which originate from bone marrow, undergo two selection processes during their development in the thymus before being released into the circulation10,12,13. These rigorous selection processes ensure that lymphocytes obtain a broad range of reactivity to foreign antigens yet lack reactivity to self-antigens3,11. During the selection processes, most of the self-reactive lymphocytes eliminated, nevertheless, a fraction of reactive T lymphocytes do escape clonal deletion (the negative selection) in the thymus and are released into the circulation14. In vitro studies have shown that the suppression by Treg occurs in a cell-contact manner, and is likely to be cytokine-independent15. The occurrence of many autoimmune diseases is prevented by active suppression of naturally occurring Treg in the periphery9. These findings have also been supported by the observation that elimination of the CD4+CD25+ Treg population led to the loss of self-tolerance, and the induction of autoimmune diseases in animals. Several classes of induced Treg have been identified and shown to be capable of suppressing antigen-specific immune responses via cell-contact or of secreting soluble factors such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β)16,17. These Treg are thought to arise from CD4+CD25− T cells either during an immune response or after encountering a tolerogenic dendritic cell (Figure 1). Chen et al. demonstrated that, under the co-stimulation of T-cell receptors (TCR) and in the presence of TGF-β, peripheral CD4+CD25− naive T cells were converted to CD4+CD25+ T cells, acquiring the expression of the Treg transcription factor Foxp3 and inhibiting T-cell proliferation in vitro18. Other studies also indicated that these Treg might be antigen-specific and inhibit CD4+ T-cell proliferation via a mechanism involving the soluble factors IL-10 and TGF-β16,18. Following the study in 1995 by Sakaguchi et al., several additional studies investigating the function of CD4+CD25+ cells in murine models of auto-immune diseases further demonstrated a close correlation between the development and function of Treg and their expression of Foxp32,8. In 2001, Brunkow et al. demonstrated that the genetic cause of a fatal lymphoproliferative disorder was a defect in a gene called Foxp3 in the X chromosome19. The frame-shift mutation in this gene results in disruption of Foxp3 protein production, causing a disproportionate increase in CD4+CD8− T lymphocyte activity19,20. Recent studies have revealed that different genetic defects in the Foxp3 gene (single amino acid deletions in the leucine zipper domain and missense and nonsense mutations and deletions in the fork head homology domain) result in uncontrolled activation and expansion of CD4+ T cells, causing the human IPEX/XLAAD diseases21,22. Studies have also shown that, in a number of allergic and autoimmune disorders, loss-of-function mutations in the Foxp3 gene cause congenital deficiency of Treg; triggering the onset of lymphoproliferation, myeloproliferation, autoimmunity, and allergic dysregulation3. Studies of Foxp3 expression in CD4+CD25+ Treg also demonstrated that Foxp3 is associated with the development and function of Treg1,6. Using real-time quantitative polymerase chain reaction, Hori et al. showed that the expression of Foxp3 was primarily on the CD4+CD25+ T cells in mouse thymus1. Other studies have also indicated that Foxp3 expression is predominantly restricted to the CD4+CD25+ Treg population in both mice thymus and in the periphery6,23. Moreover, the induction of Foxp3 expression in CD4+CD25− cells result in these cells acquiring a Treg-like phenotype and suppressive activity1. Given the evidence that the expression of Foxp3 converts CD4+CD25− T cells into cells that are phenotypically and functionally like Treg1,24 and that Foxp3 expression is confined to CD4+CD25+ Treg, Foxp3 is thought to be necessary and sufficient for mouse CD4+CD25+ Treg development and function6. Treg can also be induced in mice via the stimulation of CD4+ T cells with anti-CD3/28 in the presence of TGF-β or all-trans-retinoic acid. These cells acquire Foxp3 expression and potent immunosuppressive effects25. Though, the generation and expansion of Treg for clinical applications still require further investigation, these cells may be of benefit in future cellular therapy for human diseases and transplantation25.
Figure 1.
A model illustrating generation of T regulatory cells from the thymus.
The CD4+ CD25+ T-cell interaction with dendritic cells facilitates expression of CD4+ CD25+ FoxP3+ cells. The development of Treg takes place in the thymus and in the periphery (converted from CD4+CD25− T cells) under stimulation from activated dendritic cells.
Phenotype of T regulatory cells
Apart from CD4+CD25+Foxp3+ Treg, several other types of Treg have been identified in the immune system with distinct phenotypes and mechanisms of action26. The immunological function and importance of each type remains speculative and under investigation. T cells with “regulatory function” can be derived from the CD4+CD8+ T cell populations or even from other minor T-cell populations such as non-polymorphic CD1d-responsive natural killer T cells26. CD4+Tr1, CD8+, CD8+CD28− and TCR+CD4− CD8− Treg have been shown in other studies to exert regulatory effects in different transplantation models17,27.
CD25 expression
After the discovery of CD4+CD25+ Treg, CD25 was thought to be an indispensable molecule for the generation and maintenance of natural CD4+ Treg28. Sakaguchi et al. showed that the reconstitution of BALB/c athymic nude mice with CD4+CD25+ T cells could normalise the autoimmune disease caused by the transfer of naïve BALB/c splenic cells depleted of CD4+CD25+ T cells8. The co-transfer of a small number of CD4+CD25+ T cells with CD4+CD25− T cells could prevent lethal systemic autoimmunity8,29. Furthermore, lethal autoimmunity in CD25− deficient mice could be prevented by the inoculation of CD4+CD25+ Treg from normal syngeneic mice29. Similar phenotypes and properties have also been observed in human CD4+CD25+ T cells as these cells have been shown to suppress the activation and proliferation of CD4+ and CD8+ T cells30. Thus, the expression of CD25 seemed to be linked to the development, maintenance and function of Treg31,32. CD25 is not expressed on resting CD4+ T cells; however, upon exposure to antigen and T-cell activation, CD25 can be detected on activated CD4+ T cells, which have no regulatory functions. Although CD25 has been very useful in identifying and enriching Treg. The reliability of CD25 as a marker for Treg has been questioned27. Other studies have also suggested that the actual level of CD25 expression on CD4+ T cells is critical for distinguishing between regulatory and non-regulatory CD4+ T cells27,33. Baecher-Allan et al. found that only CD4+CD25high cells that constitute approximately 2–3% of total CD4+ T cells, consistently had in vitro regulatory activity closely resembling murine Treg30,34. The CD4+CD25low cells, on the other hand, contained a more heterogeneous mixture of cells with different cell surface phenotypes and did not show suppressive activity30.
CD28 expression
CD28 is one of the main T-cell co-stimulatory molecules, which binds to CD80/CD86 molecules on antigen-presenting cells to deliver a positive signal for T-cell survival and proliferation35,36. This signal promotes the secretion of cytokines, such as IL-2, and cell expansion, and prevents the induction of anergy and cell death35. Recent studies have shown that CD28 interactions are essential for the development of Treg in the thymus and for their survival in the periphery3. Both CD28 and CD80/CD86 knockout mice on a non-obese diabetic background rapidly develop severe spontaneous autoimmune diabetes and have a substantial reduction in the CD4+CD25+ Treg population in both the thymus and periphery37. Adoptive transfer of CD4+CD25+ Treg from a naïve animal delayed the onset of diabetes in the mice37. In the transplant setting, emerging data show that CD28 signalling is required for the induction of unresponsiveness to donor allo-antigens in vivo27. Soligo et al. demonstrated that unique CD28-induced NF-κB-dependent signals were sufficient to activate Foxp3 transcription in human CD4+CD25+ T cells38. It has also been shown that CD28 is critical for the generation of CD4+CD25+Foxp3+ Treg from CD4+CD25+ T cells both in vitro and in vivo. Thus CD28 plays a critical role in Treg and the regulation of peripheral tolerance38. However, the importance of CD28 signalling in the development and homeostasis of Treg has been demonstrated, CD28 is also found on other T lymphocytes39 and, therefore, can not be used as a unique Treg marker. Furthermore, CD4+CD25+ Treg from CD28 knockout mice were found to have a similar phenotype and a potent suppressive effect comparable to that of the CD4+CD25+ Treg extracted from CD28+ animals in in vitro assays40. In brief, the role and influence of CD28 signalling in Treg homeostasis need further defining.
CD62L is another surface molecule that is expressed constitutively at a high level by both human and mouse peripheral CD4+CD25+ Treg30,41. Indeed, in human, more than 95% of the CD4+CD25high Treg were found to be CD62L-positive30,34. In an in vivo murine model of allogeneic bone marrow transplantation, Ermann et al. showed that CD4+CD25+CD62L+ T cells inhibited the expansion of alloreactive CD4+CD25− T cells more efficiently than CD4+CD25+CD62L− T cells42. Only the adoptive transfer of donor CD4+CD25+CD62L+ Treg protected the recipient mice from lethal acute GVHD induced by donor CD4+CD25− T cells42. Due to the different expression of various memory/or activation markers of these two subsets of CD4+CD25+ cells, it was proposed that the CD4+CD25+CD62L+ cell population contained more Treg and less recently activated CD4+ T cells than the CD4+CD25+CD62L− population42. The variation seen in different CD62L subsets of Treg indicated that they might differ in their ability to enter the priming sites and that CD62L expression might be important for Treg function. Furthermore, in vitro-expanded CD4+CD25high T cells with suppressive function and allo-antigen-specific CD4+CD25+ Treg have also been found to express CD62L on their surface27. Although CD62L has been shown to be expressed on human and mice CD4+CD25+ Treg, the expression of CD62L is not unique to Treg and can also be found on other cell types: i.e. “B cells”, naïve, memory T cells, granulocytes and thymocytes30.
CTLA-4 expression
Peripheral CD4+CD25+ Treg from both humans and mice have been shown to express CTLA-4 constitutively30. T cells from CTLA-4−/− mice are resistant to gamma-irradiation-induced apoptosis, as observed in NOD mice, suggesting that CTLA-4 might contribute to the pathogenesis of autoimmune diabetes43. CTLA-4−/− mice and Foxp3−/− mice also share similar systemic autoimmune-like syndromes, and both have a short life span due to massive lymphoproliferation. The induced expression of Foxp3 delays disease and prevents mortality in CTLA-4−/− mice indicating the potential correlation of Foxp3 and CTLA-4 pathways23. The link between CLTA-4 and Treg, however, remains controversial. The analysis of Treg in CTLA-4 deficient mice indicated that CLTA-4 might not be required for the development or function of peripheral Treg while other studies showed that CTLA-4 plays a vital role in controlling the homeostasis and suppressive capacity of Treg in both mice and human44–46. Boden et al. showed that non-activating anti-CTLA-4 antibodies could block the suppressive activity of Treg in vitro47. Like other Treg markers described above, CTLA-4 expression is not restricted to Treg and has also been reported on activated T and B cells27. Foxp3 has been widely demonstrated to be expressed in mice and human CD4+CD25+ Treg, and to be critically important for the development and function of Treg10.
Foxp3 expression
Foxp3 is currently the most widely used Treg marker27,33,46,48,49. It has, however, been demonstrated that Foxp3 is not necessarily an indicator of suppressive capacity in human, as has been shown for mice23. Studies showed that stimulation via TCR in the presence of IL-2 could induce transient Foxp3 expression in human CD4+CD25− T cells, although the level of expression was significantly lower than that in suppressive naïve Tr eg50. The transient expression of Foxp3 in these CD4+CD25− T cells did not induce a Treg-like phenotype or confer suppressive activity on freshly isolated CD4+CD25− T cells50. Furthermore, several studies have discovered different types of Treg, which do not express Foxp3, but are still able to exert inhibitory effects on the in vitro proliferation of CD4+CD25− T cells with a similar efficiency as the CD4+CD25+ Treg27. Thus, the correlation of Foxp3 with the suppressive ability in Treg in humans may not be the same as that observed in mice, in which expression of Foxp3 correlates strictly with regulatory activity50. In contrast to the prevention of transplant rejection by Treg in animal models, clinical studies examining Foxp3 mRNA expression as a marker of Treg revealed a correlation of Treg with acute rejection of renal transplants51. A small study examining CD4+CD25high cells rather than Foxp3 mRNA expression as a marker for Treg showed that the cells from recipients of rejected organs exerted greater inhibition on CD4+CD25− cells than the cells from recipients of non-rejected organs in a small number of heart transplants52. The emerging information on Treg in human indicates that these cells are more divergent, less well defined, and more complex than was originally thought. Thus caution should be taken with regards to their characterisation and isolation.
Discussion
High-dose immunosuppression is used to prevent acute graft rejection and is designed according to the drug levels, graft function, and biopsy outcomes of the patient53. Though, the immunosuppressive regimen is tailored for each individual patient the health risks related to the use of immunosuppression remain a major global problem. The side effects depend on the nature and amount of the immunosuppressive drugs used and also the duration of the treatment. Current immunosuppressive drugs are non-specific and damage transplanted organs31. In most cases, transplant recipients require lifelong treatment with immunosuppressive drugs31, even though, this favours the development of opportunistic infections and tumours31,54–57. Indeed, the adverse effects of immunosuppressive treatment contribute substantially to morbidity and mortality among transplant recipients57,31,58, becoming a burden on medical resources and lowering the frequency of transplantation58–60. In an effort to improve outcome on the basis of individualised treatment, there is an urgent need for non-invasive assays, as well as biomarkers, to more accurately monitor allogeneic responses and predict the risk of acute and chronic allograft rejection in transplant recipients53. However, the potential impact of current immunosuppression regimens on the development and function of Treg in transplant recipients is still unclear and requires further elucidation. CD4+CD25+Foxp3+ Treg can act as critical mediators of peripheral self-tolerance. However, Treg can avert immune reactions against tumours and pathogens. Treg were originally identified as a CD4+CD25+ T-cell population with the ability to suppress immune responses. The identification of Foxp3 as the “master-regulator” of Treg was a critical step in defining Treg as a distinct T-cell lineage.
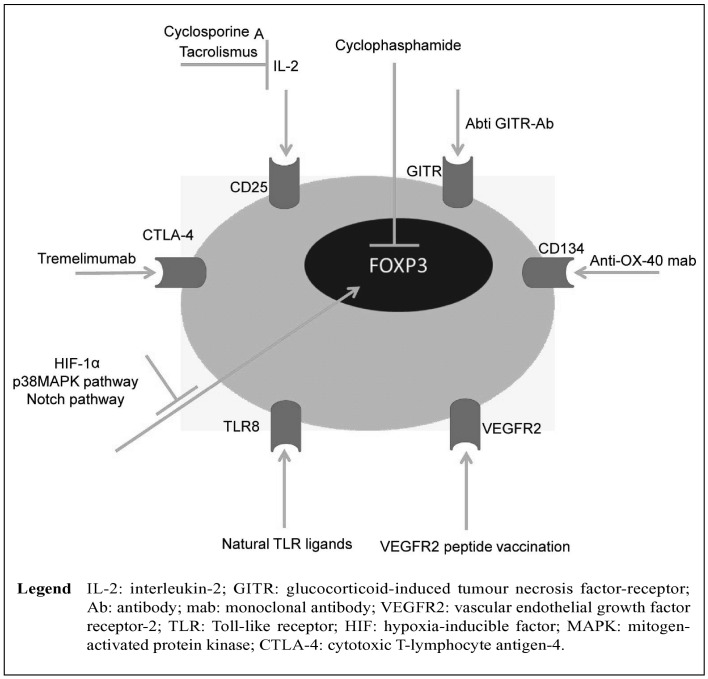
Proliferation and cytokine production of CD4+CD25− conventional T cells can be inhibited directly by Treg. In addition, Treg can indirectly suppress T-cell activation via inhibition of the stimulatory capacity of antigen-presenting cells (Figure 2). The immunosuppressive mechanism of Treg remains obscure, although there is increasing evidence that these cells exert their effects through a variety of mechanisms that include the secretion of immunosuppressive soluble factors such as IL-9, IL-10 and TGF-β, while cell-mediated regulation, via the high affinity TCR and other co-stimulatory molecules such as CTLA-4, GITR (glucocorticoid-induced tumour necrosis factor receptor-related protein), cytolytic activity and, therefore, a number of different immunotherapies, has been adopted to target Treg61. As illustrated in Figure 2, most Treg-directed immunotherapies use anti-CD2562, anti CTLA-463, anti-VEGFR264, anti-CD13465 and anti-GITR monoclonal antibodies66. Additional therapies, however, have been implemented using drugs such ascyclophosphamide67, tacrolis mus68, cyclosporine A69, and other research is targeting different cell signalling pathways involved in Treg development such as the p38MAPK pathway70 and the Notch pathway71. The impact of anti-CD25 monoclonal antibody therapy on circulating Treg in transplant recipients has been examined. In a conversion study by Demirkiran et al. a single dose of IL-2-receptor (CD25) blocking antibody (daclizumab) reduced the levels of CD4+CD25+ cells but not the levels of CD4+CD25+Foxp3+ cells in the circulation of liver transplant recipients72. These findings explain why immunosuppression has complex effects on Treg in clinical transplantation. The IL-2 signalling pathway is crucial for the development and function of Treg; thus treatments targeting or affecting this pathway might potentially influence the level or function of Treg in transplant recipients. However, the potential impact of current immunosuppression regimens on the development and function of Treg in transplant recipients is still unclear and requires further elucidation. The important role of Treg in the modulation of the immune response has ensured that these cells continue to generate significant research interest. As has been shown in numerous studies, the role of Treg in transplant recipients is diverse27. Understanding the mechanisms by which Treg cells exert their effects is an area of global research with broad implications for the development of therapeutic strategies for many disease processes including pulmonary hypertension, solid organ transplants, cancer, diabetes, and other immune-mediated diseases.
Figure 2.
A model of the various cell markers and specific immunotherapeutic strategies to eliminate and inhibit T regulatory cells.
In summary, this review highlights important information about immunosuppressive treatment in transplant recipients, and provides some basis for the potential use of Treg as diagnostic and therapeutic tools in clinical transplantation.
Acknowledgements
The Authors thank to Dr. Naveen Sharma, Indian Agriculture Research Institute, for critically reviewing this manuscript.
Footnotes
Authors’ contributions
Mohammad A. Khan planned the manuscript and wrote the first draft; Sana Moeez and Suhail Akhtar contributed in designing the figures. All the Authors read and approved the final version of manuscript.
The Authors declare no conflict of interests.
References
- 1.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 2.Chatila TA. Role of regulatory T cells in human diseases. J Allergy Clin Immunol. 2005;116:949–59. doi: 10.1016/j.jaci.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 3.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 4.Khan MA, Jiang X, Dhillon G, et al. CD4+ T cells and complement independently mediate graft ischemia in the rejection of mouse orthotopic tracheal transplants. Circ Res. 2011;109:1290–301. doi: 10.1161/CIRCRESAHA.111.250167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan MA, Nicolls MR. Complement-mediated microvascular injury leads to chronic rejection. Adv Exp Med Biol. 2013;734:233–46. doi: 10.1007/978-1-4614-4118-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 7.Penhale WJ, Irvine WJ, Ingli JR, Farmer A. Thyroiditis in T cell-depleted rats: suppression of the autoallergic response by reconstitution with normal lymphoid cells. Clin Exp Immunol. 1976;25:6–16. [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 9.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinen J, Finkelman F, Shearer WT. Advances in basic and clinical immunology. J Allergy Clin Immunol. 2006;118:489–95. doi: 10.1016/j.jaci.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Griesemer AD, Sorenson EC, Hardy MA. The role of the thymus in tolerance. Transplantation. 2010;90:465–74. doi: 10.1097/TP.0b013e3181e7e54f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–67. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 13.Kawahata K, Misaki Y, Yamauchi M, et al. Generation of CD4(+)CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002;168:4399–405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 14.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–40. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 15.Shevach EM, McHugh RS, Piccirillo CA, Thornton AM. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev. 2001;182:58–67. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 16.Oberg HH, Juricke M, Kabelitz D, Wesch D. Regulation of T cell activation by TLR ligands. Eur J Cell Biol. 2011;90:582–92. doi: 10.1016/j.ejcb.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Hoglund P. Induced peripheral regulatory T cells: the family grows larger. Eur J Immunol. 2006;36:264–6. doi: 10.1002/eji.200535797. [DOI] [PubMed] [Google Scholar]
- 18.Chen YA, Almeida JS, Richards AJ, et al. A nonparametric approach to detect nonlinear correlation in gene expression. J Comput Graph Stat. 2010;19:552–68. doi: 10.1198/jcgs.2010.08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 20.Lahl K, Loddenkemper C, Drouin C, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacchetta R, Passerini L, Gambineri E, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713–22. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 23.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi S. Regulatory T cells: history and perspective. Methods Mol Biol. 2011;707:3–17. doi: 10.1007/978-1-61737-979-6_1. [DOI] [PubMed] [Google Scholar]
- 25.Hippen KL, Merkel SC, Schirm DK, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11:1148–57. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassis L, Aiello S, Noris M. Natural versus adaptive regulatory T cells. Contrib Nephrol. 2005;146:121–31. doi: 10.1159/000082072. [DOI] [PubMed] [Google Scholar]
- 27.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 28.Lin HC, Wang CH, Liu CY, et al. Erythromycin inhibits beta2-integrins (CD11b/CD18) expression, interleukin-8 release and intracellular oxidative metabolism in neutrophils. Respir Med. 2000;94:654–60. doi: 10.1053/rmed.1999.0781. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 30.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Issa F, Wood KJ. CD4+ regulatory T cells in solid organ transplantation. Curr Opin Organ Transplant. 2010 doi: 10.1097/MOT.0b013e32834017ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 33.Schmetterer KG, Neunkirchner A, Pickl WF. Naturally occurring regulatory T cells: markers, mechanisms, and manipulation. FASEB J. 2012;26:2253–76. doi: 10.1096/fj.11-193672. [DOI] [PubMed] [Google Scholar]
- 34.Baecher-Allan C, Wolf E, Hafler DA. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+ CD25+ T cells. Clin Immunol. 2005;115:10–8. doi: 10.1016/j.clim.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 35.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–52. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 36.van der Merwe PA, Bodian DL, Daenke S, et al. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 38.Soligo M, Camperio C, Caristi S, et al. CD28 costimulation regulates FOXP3 in a RelA/NF-kappaB-dependent mechanism. Eur J Immunol. 2011;41:503–13. doi: 10.1002/eji.201040712. [DOI] [PubMed] [Google Scholar]
- 39.Bour-Jordan H, Blueston JA. CD28 function: a balance of costimulatory and regulatory signals. J Clin Immunol. 2002;22:1–7. doi: 10.1023/a:1014256417651. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Geboes K, Hellings P, et al. B7 interactions with CD28 and CTLA-4 control tolerance or induction of mucosal inflammation in chronic experimental colitis. J Immunol. 2001;167:1830–8. doi: 10.4049/jimmunol.167.3.1830. [DOI] [PubMed] [Google Scholar]
- 41.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 42.Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–6. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 43.Bergman ML, Cilio CM, Penha-Goncalves C, et al. CTLA-4−/− mice display T cell-apoptosis resistance resembling that ascribed to autoimmune-prone non-obese diabetic (NOD) mice. J Autoimmun. 2001;16:105–13. doi: 10.1006/jaut.2000.0474. [DOI] [PubMed] [Google Scholar]
- 44.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Josefowicz SZ, Niec RE, Kim HY, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–9. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samstein RM, Arvey A, Josefowicz SZ, et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151:153–66. doi: 10.1016/j.cell.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boden E, Tang Q, Bour-Jordan H, Bluestone JA. The role of CD28 and CTLA4 in the function and homeostasis of CD4+CD25+ regulatory T cells. Novartis Found Symp. 2003;252:55–63. 106–14. doi: 10.1002/0470871628.ch5. discussion 63–6. [DOI] [PubMed] [Google Scholar]
- 48.Greaves P, Clear A, Coutinho R, et al. Expression of FOXP3, CD68, and CD20 at Diagnosis in the Microenvironment of Classical Hodgkin Lymphoma Is Predictive of Outcome. J Clin Oncol. 2013;31:256–62. doi: 10.1200/JCO.2011.39.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–58. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 50.Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 51.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363:1451–62. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt-Lucke C, Aicher A, Romagnani P, et al. Specific recruitment of CD4+CD25++ regulatory T cells into the allograft in heart transplant recipients. Am J Physiol Heart Circ Physiol. 2007;292:H2425–31. doi: 10.1152/ajpheart.01197.2006. [DOI] [PubMed] [Google Scholar]
- 53.Bohmig GA, Wahrmann M, Saemann MD. Detecting adaptive immunity: applications in transplantation monitoring. Mol Diagn Ther. 2010;14:1–11. doi: 10.1007/BF03256348. [DOI] [PubMed] [Google Scholar]
- 54.Sandrin MS. Transplantation immunobiology: two important themes. Immunol Cell Biol. 2009;87:194. doi: 10.1038/icb.2009.6. [DOI] [PubMed] [Google Scholar]
- 55.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56:23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009;69:2227–43. doi: 10.2165/11319260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 57.Fandrich F. Tolerance in clinical transplantation: progress, challenge or just a dream? Langenbecks Arch Surg. 2011;396:475–87. doi: 10.1007/s00423-011-0757-z. [DOI] [PubMed] [Google Scholar]
- 58.Hernandez-Fuentes MP, Lechler RI. A ‘biomarker signature’ for tolerance in transplantation. Nat Rev Nephrol. 2010;6:606–13. doi: 10.1038/nrneph.2010.112. [DOI] [PubMed] [Google Scholar]
- 59.Moore R, Ravindran V, Baboolal K. The burden of new-onset diabetes mellitus after transplantation. Clin Transplant. 2006;20:755–61. doi: 10.1111/j.1399-0012.2006.00565.x. [DOI] [PubMed] [Google Scholar]
- 60.Wadei HM, Textor SC. Hypertension in the kidney transplant recipient. Transplant Rev (Orlando) 2010;24:105–20. doi: 10.1016/j.trre.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Onishi H, Morisaki T, Katano M. Immunotherapy approaches targeting regulatory T-cells. Anticancer Res. 2012;32:997–1003. [PubMed] [Google Scholar]
- 62.Bluestone JA, Liu W, Yabu JM, et al. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant. 2008;8:2086–96. doi: 10.1111/j.1600-6143.2008.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Rezende LC, Silva IV, Rangel LB, Guimaraes MC. Regulatory T cell as a target for cancer therapy. Arch Immunol Ther Exp (Warsz) 2010;58:179–90. doi: 10.1007/s00005-010-0075-0. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki H, Onishi H, Wada J, et al. VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur J Immunol. 2010;40:197–203. doi: 10.1002/eji.200939887. [DOI] [PubMed] [Google Scholar]
- 65.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4:420–31. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 66.McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4(+) CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 67.Lutsiak ME, Semnani RT, De Pascalis R, et al. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 68.Shibutani S, Inoue F, Aramaki O, et al. Effects of immunosuppressants on induction of regulatory cells after intratracheal delivery of alloantigen. Transplantation. 2005;79:904–13. doi: 10.1097/01.tp.0000158023.21233.de. [DOI] [PubMed] [Google Scholar]
- 69.Kawai M, Kitade H, Mathieu C, et al. Inhibitory and stimulatory effects of cyclosporine A on the development of regulatory T cells in vivo. Transplantation. 2005;79:1073–7. doi: 10.1097/01.tp.0000153505.73700.32. [DOI] [PubMed] [Google Scholar]
- 70.Ohkusu-Tsukada K, Toda M, Udono H, et al. Targeted inhibition of IL-10-secreting CD25− Treg via p38 MAPK suppression in cancer immunotherapy. Eur J Immunol. 2010;40:1011–21. doi: 10.1002/eji.200939513. [DOI] [PubMed] [Google Scholar]
- 71.Ou-Yang HF, Zhang HW, Wu CG, et al. Notch signaling regulates the FOXP3 promoter through RBP-J- and Hes1-dependent mechanisms. Mol Cell Biochem. 2009;320:109–14. doi: 10.1007/s11010-008-9912-4. [DOI] [PubMed] [Google Scholar]
- 72.Demirkiran A, Sewgobind VD, van der Weijde J, et al. Conversion from calcineurin inhibitor to mycophenolate mofetil-based immunosuppression changes the frequency and phenotype of CD4+FOXP3+ regulatory T cells. Transplantation. 2009;87:1062–8. doi: 10.1097/TP.0b013e31819d2032. [DOI] [PubMed] [Google Scholar]