Abstract
Background
Blood loss during total joint arthroplasty strongly influences the time to recover after surgery and the quality of the recovery. Blood conservation strategies such as pre-operative autologous blood donation and post-operative cell salvage are intended to avoid allogeneic blood transfusions and their associated risks. Although widely investigated, the real effectiveness of these alternative transfusion practices remains controversial.
Materials and methods
The surgery reports of 600 patients undergoing total joint arthroplasty (312 hip and 288 knee replacements) were retrospectively reviewed to assess transfusion needs and related blood management at our institute. Evaluation parameters included post-operative blood loss, haemoglobin concentration measured at different time points, ASA score, and blood transfusion strategies.
Results
Autologous blood donation increased the odds of receiving a red blood cell transfusion. Reinfusion by a cell salvage system of post-operative shed blood was found to limit adverse effects in cases of severe post-operative blood loss. The peri-operative net decrease in haemoglobin concentration was higher in patients who had predeposited autologous blood than in those who had not.
Discussion
The strengths of this study are the high number of cases and the standardised procedures, all operations having been performed by a single orthopaedic surgeon and a single anaesthesiologist. Our data suggest that a pre-operative autologous donation programme may often be useless, if not harmful. Conversely, the use of a cell salvage system may be effective in reducing the impact of blood transfusion on a patient’s physiological status. Basal haemoglobin concentration emerged as a useful indicator of transfusion probability in total joint replacement procedures.
Keywords: total hip arthroplasty, total knee arthroplasty, pre-operative autologous blood donation, post-operative blood cell salvage system, blood management
Introduction
Elective total hip and knee arthroplasty (THA and TKA) are becoming increasingly frequent procedures due to population ageing and obesity, especially in the United States of America and Europe, where the number of such procedures is expected to double in the next 10 years1. An important cost element in these procedures is blood management, which accounts for 5.27% of overall costs in THA2 today and increased by 80% for TKA from 1991 to 20093.
Blood transfusion requirements depend directly on surgical technique, procedure duration, haemostatic accuracy and the patient’s characteristics4. Since non-functional recovery from total joint arthroplasty (TJA) is influenced by blood loss, the transfusion strategy adopted is a crucial factor in restoring the patient’s physiological status5,6. In the last decades a variety of approaches have been developed to minimise the effect of blood loss on surgical outcome. For example, minimally invasive surgery has a lesser impact on muscles and soft tissues, thus providing for faster recovery of limb function, and, compared with classical techniques, it is associated with significantly less blood loss5,6.
Other approaches intended to reduce the use of allogeneic blood and to address the related issues, in terms of blood availability and risks of infection, include pre-operative autologous blood donation (PABD) and post-operative cell salvage (PCS). Despite the enormous impact that these techniques can have on outcome after TJA, their real effectiveness is still unclear. The specific literature is limited and the results are often discrepant. Some studies report that PABD can significantly reduce allogeneic blood usage7,8, while others have criticised it because of the large number of wasted autologous blood units and the risk of orthopaedic-induced anemia9–11. With regard to PCS, some studies have shown that it is ineffective in restoring basal haemoglobin concentration12,13, while others have found the opposite14,15.
Against this background, the aim of the present study was to review the blood management and transfusion practices currently applied to TJA at our Institute in order to better understand what is useful and what is not in patients’ care and cost reduction. Specifically, we wanted to assess the real effectiveness of the PABD programme in place at our Institute and to test the predictive potential of haemoglobin concentration, patient’s age and gender in guiding blood requirements associated with TJA. To do this, we collected and retrospectively analysed the data from 600 surgery reports of patients who underwent TKA or THA at our Institute.
Materials and methods
Data collection
We reviewed the surgery reports of 600 patients who underwent TJA at the Prosthetic Surgery Centre of the IRCCS Galeazzi Orthopedic Institute between January 2010 and June 2011. A standardised procedure was ensured to the extent that all operations were performed by a single orthopaedic surgeon and a single anaesthesiologist. Parameters evaluated included: patient’s age and gender; type of surgery (TKA or THA); post-operative blood loss (shed blood volume); PCS use; haemoglobin concentration measured at three time points (baseline, post-operative, discharge); American Society of Anesthesiologists (ASA) score; co-morbidities; and transfusion strategy (PABD, allogeneic or neither).
The basal haemoglobin concentration was measured about 30 days before surgery, the post-operative haemoglobin concentration was measured within 6 hours after the operation, after PCS reinfusion if administered, and the last haemoglobin value was taken on the day of discharge (between 1 and 33 days after the operation). In addition, the haemoglobin concentration was measured immediately before blood administration in patients who received a transfusion.
Minimally invasive surgical technique
TKA was performed via a minimally invasive approach which involves a shorter skin incision and a mini-mid-vastus access to the joint and the use of extramedullary guides. No tourniquets were applied. The average operating time for TKA was 45 minutes. THA was performed using either a modified posterolateral approach passing through the gemellus inferior and the quadratus femoris muscles or an anterolateral Rottinger approach to minimise damage to muscle tissue, followed by implantation of neck-preserving short stems. The average operating time for THA was 40 minutes.
Anaesthesiology and transfusion
Hypotensive spinal anaesthesia was performed by the same anaesthesiologist on all patients. Immediately after surgery, the patients were transferred to the recovery room and monitored under the care of a blood management team. A drain was placed in each patient. A transfusion team leader was appointed to take decisions about blood reinfusion and transfusion. PCS blood was re-infused when the drainage volume was more than 300 mL within 6 hours after surgery, and allogeneic or autologous blood was transfused when the haemoglobin concentration fell to 8 g/dL or if signs and symptoms of anaemia developed. PCS blood was reinfused after blood washing (Autolog™, Medtronic, Minneapolis, MN, USA) and supernatant separation (Dry Wash™, Euroset, Medolla, MO, Italy) in order to obtain a high concentration of red blood cells in the re-infused PCS blood.
Patients were advised to participate in a PABD programme between 15 and 21 days before surgery. Each patient donated 1 blood unit and only ASA class I or II patients, with a basal haemoglobin concentration >12.5 g/dL were allowed to donate blood. The PABD programme did not include erythropoietin administration. No post-operative thromboembolic events occurred.
Statistical analysis
Data are presented as means ± standard deviation. Student’s t-test was used to compare the groups for quantitative variables and the χ2 test for qualitative variables. A P value <0.05 was considered statistically significant. Receiver operating characteristic (ROC) analysis was applied to assess the predictive potential of basal haemoglobin concentration on transfusion needs.
Results
Selection and demographic characteristics of the patients
A total of 600 surgery reports (288 TKA and 312 THA) were retrospectively reviewed and the data were manually entered into a computer database. The series was composed of 214 (37.5%) males (81 TKA and 133 THA) and 386 (62.5%) females (207 TKA and 179 THA); the mean age of the patients in the TKA group was 70.5±9.1 years, while that of the THA group was 65.3±12.4 years. Co-morbidities were present in 418 patients; hypertension was the most common (78.5%). The mean basal haemoglobin concentration was 13.9±1.3 g/dL and was higher in males. A total of 182 patients participated in the PABD programme. Co-morbidities, most often hypertension, were present in 93 of these patients. Allogeneic units of blood were reserved for 374 patients: one unit was reserved for 353 patients and more than one unit (2 to 4) was reserved for 21 patients. The mean basal haemoglobin concentration in this latter group was 12.1±1.6 g/dL, which was significantly lower than that in the overall sample (P <0.001). These patients were all transfused after surgery and were, therefore, excluded from further analysis, since their need for transfusion appeared obvious. No units of blood (either allogeneic or autologous) were reserved for 44 patients. These patients’ mean basal haemoglobin concentration (14.1±1.7 g/dL) was similar to that of the overall series. This category was mixed: some had no units of blood reserved because of the low risk of requiring transfusion, others because of blood supply shortage or PABD was not applicable or because religious beliefs (Jehovah’s Witness) precluded blood transfusion. Given this marked variability, this category was excluded from further analysis. Patients with a basal haemoglobin concentration <12.5 g/dL (n =68) and ASA III patients (n =13) were deemed not suitable for PABD and so were also excluded from further analysis.
PCS was applied to all the patients in the final analysis (n =461). These patients were sorted into two groups according to transfusion strategy: PCS patients (with 1 allogeneic unit of blood reserved; n =279) and PABD+PCS patients (n =182). Table I summarizes the demographic and baseline characteristics of the patients in each group. Overall, the PABD+PCS group was younger, healthier (lower ASA class and fewer co-morbidities) and had a higher basal haemoglobin concentration than the PCS group, but the time spent in hospital and the male:female ratio were similar for the two groups. THA was slightly more frequent than TKA in both groups.
Table I.
Characteristics of the patients at baseline.
| Overall | PABD+PCS | PCS | |
|---|---|---|---|
| N. of patients (%) | 461 (100) | 182 (39.5) | 279 (60.5) |
| Age (years) | 66.9±10.7 | 62.5±9.4*** | 69.7±10.5*** |
| Male sex (%) | 38.6 | 37.7 | 39.4 |
| Basal Hb (g/dL) | 14.2±1.1 | 14.5±1.0*** | 14.0±1.1*** |
| N. of days in hospital | 3.9±2.4 | 4.1±2.8 | 3.7±2.1 |
| Blood loss in TKA (mL) | 511±240£££ | 491±224 | 523±249£££ |
| Blood loss in THA (mL) | 436±184£££ | 442±205 | 431±167£££ |
| Decrease in Hb concentration after TKA (g/dL) | −2.2±0.9§§§ | −2.9±0.9*** | −1.8±0.8***;§§§ |
| Decrease in Hb concentration after THA (g/dL) | −2.6±1.0§§§ | −3.1±0.9*** | −2.2±0.9***;§§§ |
|
| |||
| Co-morbidities | |||
| Hypertension - n. (%) | 255 (55.3) | 89 (48.9)* | 166 (59.5)* |
| Diabetes - n. (%) | 36 (7.8) | 8 (4.4)* | 28(10.0)* |
| Coronary artery disease - n. (%) | 45 (9.8) | 1 (0.0)*** | 44 (15.8)*** |
| Kidney disease - n. (%) | 35 (7.6) | 11 (6.0) | 24 (8.6) |
|
| |||
| ASA Class | |||
| I - n. (%) | 84 (18.2) | 61 (33.5)*** | 23 (8.2)*** |
| II - n. (%) | 377 (81.8) | 121 (66.5)*** | 256 (91.8)*** |
|
| |||
| Procedure | |||
| Unilateral TKA - n. | 218 | 82 | 136 |
| Unilateral THA - n. | 243 | 100 | 143 |
Legend
P <0.001, between PABD+PCS and PCS only patients (same row);
p <0.001 between TKA and THA (same column).
Plus-minus values are means ± SD. PABD: pre-operative blood donation; PCS: post-operative cell salvage; TKA: total knee arthroplasty; THA: total hip arthroplasty; ASA: American Society of Anesthesiologists; Hb: haemoglobin.
Blood loss
The amount of shed blood refers to drainage volume. An additional index of blood loss is the difference in haemoglobin concentrations measured at baseline, after surgery and at discharge or at transfusion. The blood loss was higher in the TKA patients (P <0.001), whereas the post-operative decrease in haemoglobin concentration (post-operative haemoglobin minus basal haemoglobin concentration) was significantly greater in the THA patients (P <0.001), even if, after blood transfusion, the decrease in haemoglobin concentration at discharge was similar for both groups. The same results were seen for both groups (PABD+PCS and PCS), even if the differences in blood loss and in post-operative decrease in haemoglobin concentration between the TKA and THA patients in the PABD+PCS group were not statistically significant. The post-operative decrease in haemoglobin concentration was significantly greater in the PABD+PCS group than in the PCS group, irrespectively of whether the patients had undergone THA or TKA (P <0.001). Most probably, this difference stemmed directly from the autologous blood donation, as shown by the two haemoglobin values in the PABD+PCS group (Table I).
Transfusion rates
Overall, the transfusion rate was 9.11% (42 of 461 patients). Table II reports the transfusion rates by procedure type and transfusion strategy. None of the patients with an autologous blood unit received allogeneic blood transfusion. The transfusion rate was higher in the PABD+PCS group than in the PCS group, irrespectively of whether the patients had undergone THA or TKA. A statistically significant difference was observed only in the THA patients and in the overall series, probably because of the small number of TKA patients who were transfused. An analysis of transfusion rates based on the ASA score revealed that the rate was higher in the PABD+PCS group than in the PCS group across all ASA classes. It was statistically higher among ASA class II patients in the THA group who had predeposited autologous blood as compared to those who did not. Despite the higher transfusion rates in PABD patients, a total of 157 (86.3%) autologous blood units were wasted.
Table II.
Transfusion rates as percentages and total numbers according to ASA class, transfusion strategy and orthopaedic procedure.
| Procedure | Overall | PABD+PCS group | PCS group |
|---|---|---|---|
| Overall | 9.1 (42) | 13.7 (25)** | 6.1 (17)** |
| TKA | 6.8 (15) | 9.7 (8) | 5.0 (7) |
| THA | 11.1 (27) | 17 (17)* | 7.0 (10)* |
|
| |||
| ASA Class I | |||
| Overall | 11.9 (10) | 13.1 (8) | 8.7 (2) |
| TKA | 11.5 (3) | 11.1 (2) | 12.5 (1) |
| THA | 12.1 (7) | 14.0 (6) | 6.7 (1) |
|
| |||
| ASA Class II | |||
| Overall | 8.5 (32) | 14.0 (17) | 5.8 (15) |
| TKA | 6.2 (12) | 9.4 (6) | 4.7 (6) |
| THA | 10.8 (20) | 19.3 (11)* | 7.0 (9)* |
Legend
p <0.05;
p <0.01.
PABD: pre-operative blood donation; PCS: post-operative cell salvage; TKA: total knee arthroplasty; THA: total hip arthroplasty; ASA: American Society of Anesthesiologists.
Haemoglobin basal concentration as an index of transfusion requirements
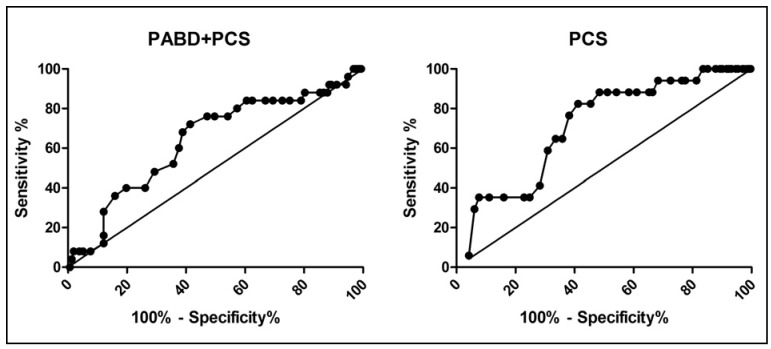
Basal haemoglobin values were compared in patients with and without PABD to identify differences, if any, between transfused and non-transfused patients. A ROC analysis was applied to assess the predictive potential of the basal haemoglobin level on transfusion requirements (Figure 1). For both the PCS and the PABD+PCS groups, the basal haemoglobin concentration emerged as a good predictor of the need for blood transfusion: the area under the curve (AUC) was significantly larger than a non-discriminating test (identity line) (P <0.01 in the PCS group and P <0.05 in the PABD+PCS group).
Figure 1.
ROC curve of basal haemoglobin concentration.
ROC curves with the basal haemoglobin concentrations of transfused and non-transfused patients was built for the PABD+PCS and PCS groups. For both transfusion strategies, basal haemoglobin concentration was predictive of transfusion, as it differed significantly from the non-discriminating test (straight line: identity). PABD+PCS: P <0.05; PCS: P <0.01.
The sensitivity threshold was 92% at a basal haemoglobin concentration of 15.85 g/dL in the PABD+PCS group while a slightly higher threshold of 94.12% was observed at a basal haemoglobin concentration of 14.65 g/dL in the PCS group. At these haemoglobin levels the specificity was lower in the PABD+PCS group than in the PCS group (11.46% vs 31.68%). This indicates that setting a threshold trigger of 15.85 g/dL, above which PABD would not be useful, would save only 11% of wasted PABD units, with 8% of patients at risk for non-reserved blood needs. In contrast, setting the threshold trigger at 14.65 g/dL, above which ordering allogeneic blood units would be unnecessary, would save about 30% of non-used reserved blood, with only 6% of patients needing non-reserved blood units.
Net decrease in haemoglobin concentration
Since the basal haemoglobin concentration was measured before PABD, the difference between the baseline and the haemoglobin concentrations at discharge could give a reasonable estimate of peri-operative blood loss and recovery after transfusion. Table III reports the difference (haemoglobin concentration at discharge minus basal haemoglobin concentration) for each category of patients. The patients in the PABD group had a greater net decrease in haemoglobin concentration than that of patients not in the PABD group, the non-transfused patients and, slightly, also those who received blood transfusions. The differences between the non-transfused patients were all statistically significant, while, probably because of the small number of transfused patients, a statistical significance in this subgroup was found only in the THA patients.
Table III.
Net decrease in haemoglobin concentration according to transfusion strategy and orthopaedic procedure in transfused vs non-transfused patients between discharge and baseline.
| Transfused patients | ||
|---|---|---|
| Overall (g/dL) | −4.9±1.2 | −4.3±1.2 |
| TKA (g/dL) | −4.3±1.1 | −4.4±1.2 |
| THA (g/dL) | −5.2±1.1* | −4.2±1.7 * |
|
| ||
| Non-transfused patients | ||
| Overall (g/dL) | −4.2±1.1*** | −3.6±1.2*** |
| TKA (g/dL) | −4.3±1.2*** | −3.7±1.2*** |
| THA (g/dL) | −4.2±1.1*** | −3.6±1.2*** |
Legend
p <0.001;
p <0.05.
PABD: pre-operative blood donation; PCS: post-operative cell salvage; TKA: total knee arthroplasty; THA: total hip arthroplasty.
Post-operative cell salvage
PCS blood was re-infused in 307/461 patients (66.6%); their shed blood volume was >300 mL and the difference between the blood loss in the re-infused and the non-re-infused patients was statistically significant for each group (P <0.001). Despite this obvious difference, the indicators of recovery of physiological status, such as transfusion rates and post-operative haemoglobin concentrations (measured after PCS reinfusion, if administered) and those observed at discharge were all similar between the patients who received reinfusion and those who did not. The only difference was the length of stay in hospital, which was slightly longer, even if not clinically relevant, in the re-infused patients (Table IV). These results remained unchanged when the patients were stratified according to type of procedure.
Table IV.
Physiological parameters after surgery in re-infused and not re-infused PCS patients, for the overall series and according to transfusion strategy.
| Overall | Re-infused | Not re-infused |
|---|---|---|
| N. of patients %) | 307 (66.6) | 154 (33.4) |
| Shed blood in drainage (mL) | 560±200*** | 296±112*** |
| Difference between post-operative and basal Hb concentrations (g/dL) | −2.4±1.1 | −2.5±1.0 |
| Difference between at discharge and basal Hb concentrations (g/dL) | −3.9±1.3 | −3.9±1.1 |
| N. of days in hospital | 4.0±2.6* | 3.5±2.1* |
| Transfusion rate (%) | 9.1 | 9.0 |
|
| ||
| PABD+PCS | ||
| N. of patients (%) | 117 (64.3) | 65 (35.7) |
| Shed blood in drainage (mL) | 561±197*** | 289±111*** |
| Difference between post-operative and basal Hb concentrations (g/dL) | −3.0±0.9 | −3.1±0.9 |
| Difference between at discharge and basal Hb concentrations (g/dL) | −4.3±1.1 | −4.3±1.2 |
| N. of days in hospital | 4.4±3.4 | 3.6±1.3 |
| Transfusion rate (%) | 14.5% | 12.3% |
|
| ||
| PCS | ||
| N. of patients (%) | 190 (68.1) | 89 (31.9) |
| Shed blood in drainage (mL) | 559±202*** | 300±113*** |
| Difference between post-operative and basal Hb concentrations (g/dL) | −2.0±0.9 | −2.0±0.9 |
| Difference between at discharge and basal Hb concentrations (g/dL) | −3.7±1.3 | −3.6±1.0 |
| No. of days in hospital | 3.8±1.9 | 3.5±2.5 |
| Transfusion rate (%) | 5.8% | 6.7% |
Legend
p <0.05;
p <0.001
PABD: pre-operative blood donation; PCS: post-operative cell salvage; Hb: haemoglobin.
Discussion
Due to its retrospective nature, this study has several limitations, including the non-randomised design, which did not allow for complete control of peri-operative variables such as demographics and co-morbidities. Moreover, the procedures carried out at our Institute, like the PABD programme which involves a single blood donation within 1 month before surgery, minimally invasive surgery and hypotensive anaesthesia, may limit the generalisability of our findings to other settings. Despite these limitations, our analysis focused on a large number of cases, and the variables were minimised to the extent that the patients were all treated by a single orthopaedic surgeon and a single anaesthesiologist, thus ensuring standardised procedures. Moreover, we were able to pinpoint transfusion needs in TJA, whereas the current literature on blood management in orthopaedics often reports contradictory statements without definitive conclusions.
None of the patients in the PABD group required allogeneic blood transfusion. Due to marked variability in techniques, patients’ characteristics and transfusion strategies, reported transfusion rates differ widely. The overall transfusion rate for TJA at our institute between January 2010 and June 2011 was 14.7% (all patients, n =600), which is comparable to other recent data16–19. This rate is, however, considerably lower than that found in older studies10,20,21, suggesting that modern surgical techniques, such as minimally invasive surgery5,6, are effective in reducing blood requirements in TJA.
None of the algorithms to predict blood loss and transfusion needs in TJA proposed so far7,22–26 appear to be widely effective16,27. According to our data, the simple measurement of basal haemoglobin concentration could be sufficient to effectively reduce non-used autologous and allogeneic blood units, as already suggested elsewhere28,29. Specifically, if autologous blood were requested only for patients with a baseline haemoglobin concentration below a threshold of 14.65 g/dL, a total of 83 requested and non-used allogeneic blood units (31.7%) would have been saved, while only one patient (0.4%, THA) would have needed an emergency request for blood. The threshold calculated for the PABD patients was higher (15.85 g/dL). Use of this threshold trigger in blood transfusion decision-making would have saved only 9.5% of discarded units (15 out of 157), while two patients (1.1%) would have needed an emergency request for blood units. Furthermore, there appears no reason to adopt different threshold triggers for PABD+PCS and PCS only patients, as their starting conditions are identical.
Accordingly, the higher threshold value calculated for the PABD+PCS patients is consistent with the observation that PABD is a risk factor that increases the transfusion probability in younger and healthier (according to ASA score, basal haemoglobin concentration and presence of co-morbidities) patients. Moreover, patients who had predeposited blood had a greater net decrease in haemoglobin concentration at discharge and after surgery, even though donated autologous blood had been transfused. These observations, taken together, demonstrate that PABD has a negative effect on patients, and are consistent with the decline in participation in PABD protrammes in recent years9,30–34.
The basal haemoglobin concentration was, however, characterised by a low specificity (30%) albeit still sufficient for informing a policy for down-sizing blood transfusion procedures before surgery: the percentage of patients at risk of needing blood during and after surgery is acceptable in a setting of major surgery and TJA.
Moreover, no clear differences were observed between the patients re-infused and not re-infused with PCS, in terms of decrease in haemoglobin concentration and transfusion rate, while the re-infused patients had a much greater blood loss than the non-re-infused ones; therefore, PCS blood administration seems to be effective in preventing further complications. Previous studies demonstrated a decrease in transfusion rates after PCS blood reinfusion35, while we did not observed any difference: this disagreement could be due to the lack of a control group in our study, in which the patients who lost more than 300 mL were not re-infused. Unlike other studies, however, the shed blood volume was accurately evaluated and measured in this series. For example, we observed that the drainage volume was higher in the TKA group, while the decrease in haemoglobin concentration was greater in the THA group. This difference may be explained by the greater efficacy of drainage, and subsequently PCS reinfusion, in TKA as compared to THA, in which blood collection in drainage is more difficult because of tissue characteristics.
In conclusion, the current PABD programme at our institute may be regarded as useless if not harmful. Pharmacological support or prolonging the time between donation and surgery could enhance its effectiveness, but this might drive up costs without providing benefits for patients. In contrast, PCS reinfusion seems to be useful in reducing the impact of surgery on a patient’s physiological status. Finally, baseline haemoglobin concentration could be a useful indicator of transfusion requirements; nonetheless, the transfusion thresholds will need to be defined for the facility and the blood transfusion team, and regularly audited.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Iorio R, Davis CM, III, Healy WL, et al. Impact of the economic downturn on adult reconstruction surgery. J Arthroplasty. 2010;25:1005–14. doi: 10.1016/j.arth.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Healy WL, Rana AJ, Iorio R. Hospital economics of primary total knee arthroplasty at a teaching hospital. Clin Orthop. 2011;469:87–94. doi: 10.1007/s11999-010-1486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rana AJ, Iorio R, Healy WL. Hospital Economics of Primary THA Decreasing Reimbursement and Increasing Cost, 1990 to 2008. Clin Orthop. 2011;469:355–61. doi: 10.1007/s11999-010-1526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noticewala MS, Nyce JD, Wang W, et al. Predicting need for allogeneic transfusion after total knee arthroplasty. J Arthroplasty. 2012;27:961–7. doi: 10.1016/j.arth.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Thienpont E. Faster recovery after minimally invasive surgery in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012 doi: 10.1007/s00167-012-1978-6. [DOI] [PubMed] [Google Scholar]
- 6.Wojciechowski P, Kusz D, Kopec K, Borowski M. Minimally invasive approaches in total hip arthroplasty. Ortop Traumatol Rehabil. 2007;9:1–7. [PubMed] [Google Scholar]
- 7.Wong CJ, Vandervoort MK, Vandervoort SL, et al. A cluster-randomized controlled trial of a blood conservation algorithm in patients undergoing total hip joint arthroplasty. Transfusion. 2007;47:832–41. doi: 10.1111/j.1537-2995.2007.01197.x. [DOI] [PubMed] [Google Scholar]
- 8.Atay EF, Güven M, Altintaş F, et al. Allogeneic blood transfusion decreases with post-operative autotransfusion in hip and knee arthroplasty. Acta Orthop Turc. 2010;44:306–12. doi: 10.3944/AOTT.2010.2417. [DOI] [PubMed] [Google Scholar]
- 9.Rock G, Berger R, Bormanis J, et al. A review of nearly two decades in an autologous blood programme: the rise and fall of activity. Transfus Med. 2006;16:307–11. doi: 10.1111/j.1365-3148.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 10.Goldman M, Savard R, Long A, et al. Declining value of pre-operative autologous donation. Transfus. 2002;42:819–23. doi: 10.1046/j.1537-2995.2002.00132.x. [DOI] [PubMed] [Google Scholar]
- 11.Cushner FD, Hawes T, Kessler D, et al. Orthopaedic-induced anemia: the fallacy of autologous donation programs. Clin Orthop. 2005;431:145–9. [PubMed] [Google Scholar]
- 12.Horstmann WG, Kuipers BM, Slappendel R, et al. Postoperative autologous blood transfusion drain or no drain in primary total hip arthroplasty? A randomised controlled trial. Int Orthop. 2012;36:2033–9. doi: 10.1007/s00264-012-1613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avall A, Hyllner M, Bengtson JP, et al. Greater increase in cytokine concentration after salvage with filtered whole blood than with washed red cells, but no difference in post-operative hemoglobin recovery. Transfusion. 1999;39:271–6. doi: 10.1046/j.1537-2995.1999.39399219283.x. [DOI] [PubMed] [Google Scholar]
- 14.Rao VK, Dyga R, Bartels C, Waters JH. A cost study of postoperative cell salvage in the setting of elective primary hip and knee arthroplasty. Transfusion. 2012;52:1750–60. doi: 10.1111/j.1537-2995.2011.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muñoz M, Ariza D, Campos A, et al. The cost of post-operative shed blood salvage after total knee arthroplasty: an analysis of 1,093 consecutive procedures. Blood Transfus. 2012 doi: 10.2450/2012.0139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karger R, Bornmann A, Kretschmer V. Limited utility of algorithms predicting blood transfusions. Blood Transfus. 2012 doi: 10.2450/2012.0048-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pietsch M, Djahani O, Zweiger C, et al. Custom-fit minimally invasive total knee arthroplasty: effect on blood loss and early clinical outcomes. Knee Surg Sports Traumatol Arthrosc. 2012 doi: 10.1007/s00167-012-2284-z. [DOI] [PubMed] [Google Scholar]
- 18.Thomassen BJ, Pilot P, Scholtes VA, et al. Limit allogeneic blood use with routine re-use of patient’s own blood: a prospective, randomized, controlled trial in total hip surgery. PLoS One. 2012;7:e44503. doi: 10.1371/journal.pone.0044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SH, Cho KY, Khurana S, Kim KI. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2012 doi: 10.1007/s00167-012-2213-1. [DOI] [PubMed] [Google Scholar]
- 20.Rosencher N, Kerkkamp HEM, Macheras G, et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43:459–69. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 21.Bierbaum BE, Callaghan JJ, Galante JO, et al. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81:2–10. doi: 10.2106/00004623-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of pre-operative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mercuriali F, Inghilleri G. Proposal of an algorithm to help the choice of the best transfusion strategy. Curr Med Res Opin. 1996;13:465–78. doi: 10.1185/03007999609115227. [DOI] [PubMed] [Google Scholar]
- 24.Nuttall GA, Santrach PJ, Oliver WC, et al. Possible guidelines for autologous red blood cell donation before total hip arthroplasty based on the surgical blood order equation. Mayo Clin Proc. 2000;75:10–7. doi: 10.4065/75.1.10. [DOI] [PubMed] [Google Scholar]
- 25.Palmer T, Wahr JA, O’Reilly M, Greenfield ML. Reducing unnecessary cross-matching: a patient-specific blood ordering system is more accurate in predicting who will receive a blood transfusion than the maximum blood ordering system. Anesth Analg. 2003;96:369–75. doi: 10.1097/00000539-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Martinez V, Monsaingeon-Lion A, Cherif K, et al. Transfusion strategy for primary knee and hip arthroplasty: impact of an algorithm to lower transfusion rates and hospital costs. BJA. 2007;99:794–800. doi: 10.1093/bja/aem266. [DOI] [PubMed] [Google Scholar]
- 27.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130:515–24. doi: 10.7326/0003-4819-130-6-199903160-00016. [DOI] [PubMed] [Google Scholar]
- 28.Guerin S, Collins C, Kapoor H, et al. Blood transfusion requirement prediction in patients undergoing primary total hip and knee arthroplasty. Transfus Med. 2007;17:37–43. doi: 10.1111/j.1365-3148.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- 29.Kotzé A, Carter LA, Scally AJ. Effect of a patient blood management programme on pre-operative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108:943–52. doi: 10.1093/bja/aes135. [DOI] [PubMed] [Google Scholar]
- 30.Kim S, Altneu E, Monsef JB, et al. Nonanemic patients do not benefit from autologous blood donation before total knee replacement. HSS J. 2011;7:141–4. doi: 10.1007/s11420-011-9200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy C, Leonard M, Devitt A, et al. Efficacy of pre-operative autologous blood donation for elective posterior lumbar spinal surgery. Spine (Phila Pa 1976) 2011;36:E1736–43. doi: 10.1097/BRS.0b013e3182194a42. [DOI] [PubMed] [Google Scholar]
- 32.Kleinert K, Theusinger OM, Nuernberg J, et al. Alternative procedures for reducing allogeneic blood transfusion in elective orthopedic surgery. HSS J. 2010;6:190–8. doi: 10.1007/s11420-009-9151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boettner F, Altneu EI, Williams BA, et al. Nonanemic patients do not benefit from autologous blood donation before total hip replacement. HSS J. 2010;6:66–70. doi: 10.1007/s11420-009-9145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabibbo S, Garozzo G, Antolino A, et al. Continuous improvement of our autologous blood donation program carried out during 10 years in 1198 orthopaedic patients. Transfus Apher Sci. 2009;40:13–7. doi: 10.1016/j.transci.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Carless PA, Henry DA, Moxey AJ, et al. Cell salvage for minimising peri-operative allogeneic blood transfusion. Cochrane Database Syst Rev. 2010;4:CD001888. doi: 10.1002/14651858.CD001888.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]