Abstract
Background
Transfusion therapy remains the main treatment for patients with severe haemoglobinopathies, but can cause adverse reactions which may be classified as immediate or delayed. The use of targeted prevention with drugs and treatments of blood components in selected patients can contribute to reducing the development of some reactions.
The aim of our study was to develop an algorithm capable of guiding behaviours to adopt in order to reduce the incidence of immediate transfusion reactions.
Materials and methods
Immediate transfusion reactions occurring over a 7-year period in 81 patients with transfusion-dependent haemoglobinopathies were recorded. The patients received transfusions with red cell concentrates that had been filtered prestorage. Various measures were undertaken to prevent transfusion reactions: leucoreduction, washing the red blood cells, prophylactic administration of an antihistamine (loratidine 10 mg tablet) or an antipyretic (paracetamol 500 mg tablet).
Results
Over the study period 20,668 red cell concentrates were transfused and 64 adverse transfusion reactions were recorded in 36 patients. The mean incidence of reactions in the 7 years of observation was 3.1‰. Over the years the incidence gradually decreased from 6.8‰ in 2004 to 0.9‰ in 2010.
Discussion
Preventive measures are not required for patients who have an occasional reaction, because the probability that such a type of reaction recurs is very low. In contrast, the targeted use of drugs such as loratidine or paracetamol, sometimes combined with washing and/or double filtration of red blood cells, can reduce the rate of recurrent (allergic) reactions to about 0.9‰. The system for detecting adverse reactions and training staff involved in transfusion therapy are critical points for reliable collection of data and standardisation of the detection system is recommended for those wanting to monitor the incidence of all adverse reactions, including minor ones.
Keywords: transfusion, adverse reactions, washed red blood cells, haemovigilance
Introduction
Transfusion therapy is currently still the main treatment for patients with severe haemoglobinopathies1–6, although it can cause adverse reactions which may be classified as immediate, when occurring during the transfusion or within minutes or hours of it being completed, or delayed, when developing days, months or years after the administration of the blood.
The acute adverse reactions that are most common, but also usually the less severe, are febrile non-haemolytic transfusion reactions (FNHTR) and allergic reactions. FNHTR are manifested by fever (defined as an increase in temperature of ≥1 °C with respect to the baseline value) and/or shivers which develop within 3 hours of the transfusion. Allergic reactions are more often associated with the development of urticaria or other skin rashes, pruritus, laboured breathing, or angioedema which manifests within a few hours of the transfusion7–15. These reactions are usually not prolonged, often resolve spontaneously and normally carry a low risk of causing permanent damage.
Mild FNHTR are limited to a modest increase in temperature without other symptoms, while severe reactions manifested by the development of shivers and high fever can be associated with other general symptoms. Allergic reactions may present with a localised urticarial rash in the mildest cases, while in more severe cases the urticaria may be extensive and painful and be accompanied by systemic or respiratory symptoms.
It is not always possible to predict the evolution of urticaria or a FNHTR from the type of onset and, furthermore, it is often not possible to distinguish symptoms of mild reactions from the early symptoms of more serious problems, such as sepsis, haemolytic reactions or anaphylaxis.
The development of repeated reactions, even if mild, in patients chronically receiving blood transfusions may be reduced by subjecting the blood components to some treatments such as leucodepletion or washing red blood cells. These treatment do, however, require considerable organisational commitment, involve costs and lower the final quality of the product with repercussions on the transfusion yield. Thus, given that allergic and non-febrile haemolytic reactions affect the quality of the transfusion and, therefore, the patient’s wellbeing, with consequences for the organisation and management costs, their prevention is a priority.
The most common approach to preventing FNHTR and allergic reactions is to give the patient premedication with an antipyretic such as paracetamol and an anti-histamine such as diphenydramine. There is very widespread use of these drugs prior to a transfusion. Ezidiegwu et al.7 reported that the rate of premedication in a large hospital in the USA was 80%, while Patterson et al.8 reported a rate of 73% in a Canadian institution with a decrease of 50% in this rate after the implementation of institutional guidelines. In an American paediatric hospital, Geiger and Howard9 recorded that the percentage use of premedication in childhood cancer patients undergoing transfusions was 68%. Extrapolating these results it can be estimated that millions of transfused subjects are pre-treated each year to prevent transfusion reactions. Nevertheless, the efficacy of this premedication has never been rigorously tested.
In our institute, in which most transfusions are administered to patients with haemoglobinopathies, the incidence of FNHTR and allergic reactions before 2004 was 0.68%. Pharmacological premedication (paracetamol, antihistamines and corticosteroids) were given by various members of staff, but not in a standardised manner.
With a view to improving haemovigilance procedures, aimed at detecting and monitoring severe or unexpected adverse reactions and incidents inherent to the transfusion process16–17, we decided that we should not limit ourselves to recording and notifying adverse events, but that we should pro-actively identify all possible strategies to prevent transfusion reactions, including minor ones, particularly in patients having recurrent reactions.
The implementation of procedures aimed at unequivocal identification of the patient and the assigned blood components (active recognition of the patient, identification bracelets, biometric parameters) is fundamental to prevent ABO transfusion errors, which are the most feared adverse reactions because of their possible fatal complications18–21.
The use of filtered red blood cells to prevent febrile or hyperthermic type reactions11,22–24, prestorage filtration to reduce the detrimental effects of cytokines released during storage of blood components25–27 and washing red cells to avoid allergic-type reactions caused by the presence of residual plasma proteins28–30 are obvious choices for lowering the incidence of reactions.
The prophylactic, targeted use of drugs, together with the above treatments, can contribute, in selected subjects, to further reducing the development of adverse reactions7,9–10,31–35.
The aim of our study was to develop a decisional algorithm able to guide the choices to make with the purpose of reducing the incidence of transfusion reactions, through technical measures (selecting the most appropriate blood component) and/or pharmacological measures (administration of premedication).
Materials and methods
Our study is based on a 7-year follow-up (from 2004 to 2010) of 81 patients with haemoglobinopathies receiving periodic transfusions of red blood cells. The mean age of the patients at the start of the study was 32.6 years (minimum 12, maximum 74). This group comprised 43 males (53%) and 38 females (47%). Of the 81 patients, 70 had beta-thalassaemia major, while the other 11 had sickle cell disease (receiving standard transfusion treatment or red cell exchange).
All the patients were transfused with prestorage leucodepleted red blood cells collected into Composelect bags with an in-line filter for whole blood (Fresenius Kabi Italia Srl, Isola della Scala, Italy), according to the protocol in use in our institute which requires not only matching for the ABO Rh (D) system, but also for the phenotype of the Rh and Kell systems.
Since July 2007, in addition to proactive recognition procedures aimed at the unequivocal identification of the patient, the SecurBlood (BBS Srl, San Donato Milanese, Italy) biometric system has also been used, both to prevent ABO transfusion errors and to record any immediate adverse reactions18. The data on the transfusions performed and any immediate adverse reactions are transferred to the EmoNet information system (Insiel Mercato SpA, Trieste, Italy) which is interfaced with the biometric system in use in all the units in the hospital.
From a methodological point of view, we considered it appropriate and useful to classify the transfusion reactions into occasional and recurrent, choosing, in a completely arbitrary manner, 24 months as the interval discriminating between the two categories: (i) occasional reaction: a single adverse event to a transfusion in 24 months; (ii) recurrent reaction: two or more adverse events to transfusions in 24 months.
The reactions were recorded in accordance with current legislation17 and were categorised as grade 1 (mild symptoms, no therapeutic intervention required), grade 2 (symptoms requiring a therapeutic intervention) and grade 3 (symptoms necessitating resuscitation).
Over the 7 years various measures, targeting both the recipient and the blood components used, were taken to prevent adverse transfusion reactions:
no action;
premedication with an antihistamine (loratadine 10 mg), taken by the patient at home for 3 days prior to the transfusion;
premedication with paracetamol 500 mg tablet taken 30 minutes before the transfusion;
double filtration: red blood cells filtered prestorage and then filtered a second time at the bedside (BioR 01 Max BS Fresenius Kabi);
until 2005 use of washed red blood cells prepared manually36;
from 2006 use of washed red blood cells prepared with an automated instrument, ACP 215® Haemonetics (Haemonetics, Braintree, MA, USA)37.
Over the course of the 7 years, in order to prevent the most frequent adverse reactions, such as allergic reactions and FNHTR, the following decisional steps were progressively adopted:
in the case of a single grade 1 or 2 transfusion-related adverse event (occasional reaction), allergy or FNHTR within a 24-month period, only follow up of the patient;
in the case of grade 1 or 2 recurrent allergic reactions, premedication with loratidine 10 mg tablets;
in the case of a grade 3 allergic reaction or persistent grade 1 or 2 allergic reactions despite preventive pharmacological treatment, premedication with loratidine 10 mg tablets and washed red blood cells;
in the case of a single episode of grade 3 FNHTR or at least two grade 1 or 2 episodes within 24 months, premedication with paracetamol;
in the case of persistent FNHTR, despite preventive pharmacological therapy, double filtration of the red blood cells (prestorage and post-storage);
in the presence of allergic reactions and FNHTR, a combination of the above-listed preventive measures;
in the case that adverse reactions no longer occur in the subsequent 24 months, gradual suspension of the various preventive measures adopted.
Results
During the 7 years of the study, the 81 patients were transfused a total of 20,668 units of red cell concentrates; 18,714 were for patients with beta-thalassaemia and the other 1,954 were transfused into patients with sickle cell disease. Sixty-four adverse reactions to a transfusion were recorded; these reactions occurred in 36 patients (44% of the whole cohort), of whom 19 were males (53%) and 17 females (47%). Of the 64 reactions recorded, 58 developed in 30 thalassaemic patients and the other 6 in patients with sickle cell disease. The types of reactions are presented in Table I.
Table I.
Number and type of adverse reactions.
| Type of reaction | N. of events |
|---|---|
| Allergic reaction | 34 |
| Non-haemolytic febrile reaction | 28 |
| Headache | 1 |
| Haemolytic reaction | 1 |
| Total n. of events | 64 |
The mean incidence of adverse reactions in the 7 years of observation was 3.1‰. The break down of the number of adverse reactions/units of red blood cells transfused in each year is as follows: 2004 18/2,625; 2005 10/2,878; 2006 9/2,970; 2007 7/3,050; 2008 8/3,120; 2009 8/3,007; and 2010 3/3,018.
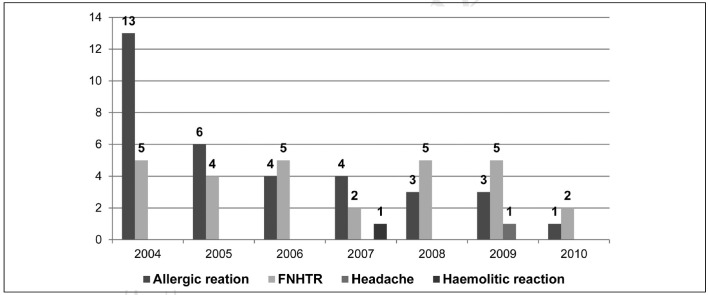
Over the years there was a gradual reduction in the incidence, which decreased from 6.8‰ in 2004 to 0.9‰ in 2010, as shown in Figure 1, which also presents the relative incidences.
Figure 1.
Adverse transfusion reactions in the period 2004–2010: 64 reactions to 20,668 units transfused (0.31%).
Most of the reactions (n =60) were grade 1 or 2 (mild or moderate symptoms); only 4 were more severe (grade 3), necessitating resuscitation interventions.
Of the 36 patients who had reactions, 18 had a single grade 1 or 2 adverse event (occasional reaction), 4 had one grade 3 allergic reaction and 14 had two or more reactions (recurrent reactions) (Table II).
Table II.
Adverse reactions: pharmacological prevention and treatment of blood components.
| N. of patients | Type of reaction | Preventive pharmacological treatment | Treatment of blood components | |
|---|---|---|---|---|
| Patients with an occasional reaction never treated (n =18) | 1 | Headache | None | None |
| 1 | Haemolytic reaction | None | None | |
| 11 | Allergy | None | None | |
| 5 | FNHTR | None | None | |
|
| ||||
| Patients with a single grade 3 allergic reaction receiving preventive treatment (n =4) | 4 | One grade 3 allergic reaction | Loratidine | Washed red blood cells |
|
| ||||
| Patients with recurrent reactions receiving preventive treatment (n =11) | 4 | Two grade 1 or 2 allergic reactions | Loratidine | |
| 2 | Three or more allergic reactions of various grades | Loratidine | Washed red blood cells | |
| 2 | Three or more FNHTR | Paracetamol | Double filtration | |
| 1 | Two FNHTR + three or more grade 1 or 2 allergic reactions | Loratidine Paracetamol |
Washed red blood cells | |
| 2 | Three or more FNHTR + three or more grade 1 or 2 allergic reactions | Paracetamol Loratidine |
Double filtration Washed red blood cells |
|
|
| ||||
| Patients in whom preventive measures have been suspended (n =3) | 2 | Allergy | None | None |
| 1 | Allergy and FNHTR | None | None | |
|
| ||||
| Total n. of patients | 36 | 15 | 11 | |
The preventive measures adopted in the 36 patients with adverse reactions were as follows:
- the 18 patients who had a single, grade 1 or 2 episode (occasional reaction) were kept under observation but no preventive measures were taken;
- the 4 patients with grade 3 allergic episodes were given preventive pharmacological treatment with loratidine 10 mg tablets for 3 days prior to the transfusion and were transfused with washed red blood cells;
- the 14 patients who had two or more reactions in 2 years (recurrent reactions) were managed with various preventive measures, as presented in Table II. During the observation period of the study, all preventive measures were stopped for three of these patients since they had no further reactions in 2 consecutive years.
Only 15 patients are currently being treated and receive the preventive measures listed in Table II.
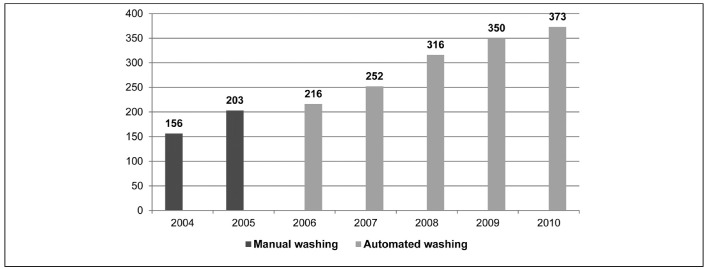
Nine patients (11%) were the recipients of 1,866 washed red blood cells, with a protein content of less than 0.5 g/unit. Over the period of 7 years, the doubling in the use of washed red blood cells (Figure 2) was accompanied by a drastic reduction in allergic reactions, which fell from 13 in 2004 to 1 in 2010 (Figure 1).
Figure 2.
Washed red blood cells from 2004 to 2010 (1,866 of 20,668 units =9%).
Discussion
The recording of adverse reactions, including mild ones, was implemented following careful training of all the staff at the Microcythemia Centre, which is part of the Transfusion Medicine Service with which it shares the same directorate and the same quality management system.
We believe that a system for detecting adverse reactions, particularly the grade 1 or 2 ones which are often not recorded, is critical for the purposes of correct, reliable data surveys. The lack of standardisation of detection forms makes it impossible to compare results between similar studies.
Since 2007 adverse reactions have been managed through the use of the Securblood system which, besides preventing ABO transfusion errors thanks to biometric recognition of staff and patients and the pairing of the blood components with their recipients, allows the registration of haemovigilance data and their electronic transmission to the Immunohaematology and Transfusion Medicine Service in real time, eliminating the need for movement of paper documentation.
The introduction of prestorage filtration of red blood cells in the Immunohaematology and Transfusion Medicine Service in 2004 gave us the opportunity to implement a 7-year follow-up using the same basic blood component for all patients. Double filtration and washing the red blood cells were the only treatments used to prevent recurrent reactions. The aim was to minimise the use of subsequent treatments of the red blood cell concentrates, aware of the fact that every manipulation of blood components exposes the cells to stress, introduces a risk of contamination and increases costs, sometimes notably.
The use of targeted preventive measures in individual patients has led to the almost complete resolution of allergic reactions with the most substantial results being obtained in the first year following the introduction of pharmacological prevention and washing red blood cell concentrates. During the subsequent 6 years, using antihistamine treatment in only 16% of patients (13/81), there was a progressive reduction in allergic reactions, such that only one event was recorded in 2010.
As far as concerns FNHTR, the incidence of these remained stable over the years, despite pharmacological prevention being used in only five patients (6%). Three patients received dual pharmacological prevention with paracetamol and loratidine.
Unlike the findings of some studies7–9, in which rates of premedication use prior to transfusion varied from 50 to 80%, in our experience drugs were used in just under 20% of patients, all of whom were multiply transfused, but this almost completely eliminated allergic reactions and the incidence of FNHTR was in line with those reported for other international populations, taking into account all patients and not only multiply transfused ones38,39.
In essence, targeted use of drugs such as loratidine and paracetamol, sometimes together with the administration of washed and/or double filtered red blood cells, has decreased the rate of adverse reactions to 0.09% (1 reaction every 1,000 units of red blood cells transfused).
Washed red blood cells
The Italian Standards of Transfusion Medicine40 and some international guidelines30,41–42 propose that the only indications for washed red blood cells are the presence of antibodies to plasma proteins, in particular anti IgA, and a history of severe post-transfusion allergic reactions. For this reason, the use of this treatment was evaluated with particular care and was reserved exclusively for patients with these indications.
In the 7 years of the study, the number of patients who received washed red blood cells was very limited, passing from 5/81 (6.1%) in 2004 to 9/81 (11%) in 2008. The number of patients transfused with washed red blood cells in the last 3 years of the study remained stable, although guaranteeing a progressive reduction in reactions. The combination of administering washed red blood cells and targeted, preventive use of antihistamines (loratidine 10 mg tablets) led to almost complete elimination of allergic reactions despite transfusing as many as 72/81 patients (89%) with red blood cells that had only been filtered prestorage without additional treatment, with substantial savings in time and resources.
Double filtration
Double filtration, administering the already prestorage leucodepleted blood component through a bedside filter, was used for only four patients: the decision to use double filtered products was made indispensable because FNHTR persisted in all four patients despite the fact that they had been transfused with units of blood that had been filtered prestorage and contained very low numbers of leucocytes (mean 0.05×106/unit), as determined by flow cytometric counting.
Preventive administration of drugs
Preventive, targeted use of drugs contributed, in selected subjects, to further reducing the development of adverse reactions.
Steroids were reserved exclusively for the treatment of adverse reactions and not for their prevention. This decision was taken for two reasons: (i) steroids can slow the onset of any haemolytic reactions delaying the implementation of more specific and more effective preventive measures; (ii) periodic preventive administration of steroids exposes patients to undesirable side effects, which is particularly important in the growth phase.
Premedication with loratidine 10 mg tablets, taken by the patient at home, for 3 days prior to the transfusion was easy to perform and did not cause any side effects worthy of note.
Premedication with paracetamol 500 mg tablets, taken 30 minutes before the transfusion, was managed directly by the staff at the Centre and was found to be very useful in preventing recurrent reactions such as shivers and hyperthermia which in the past would have required the administration of generous amounts of steroids.
Conclusions
The experience gained in this study on the prevention of adverse reactions to transfusions leads us to make the following considerations and proposals:
it is not useful to administer steroids to prevent adverse reactions;
premedication with antihistamines and paracetamol should not be given to all patients, but only to those with recurrent adverse reactions;
red blood cells do not need to be washed routinely; washed red cells should be reserved for a limited proportion of patients, which in our cohort was about 11%;
implementation of all the preventive measures we have described only reduced the number of cases of FNHTR slightly. With the exception of 2007 and 2010 in which only two cases were recorded, in the other years the number of FNHTR always remained constant, around five, suggesting that it is difficult to achieve a further lowering of the incidence of this type of reaction;
patients with grade 1 or 2 occasional reactions, who accounted for half of the 36 patients who had reactions, do not need require any preventive measure since the probability of a recurrence is very low. Observation is sufficient for these patients;
an important element for guaranteeing reliable data collection is to train all transfusion staff in the detection of adverse reactions using standardised procedures;
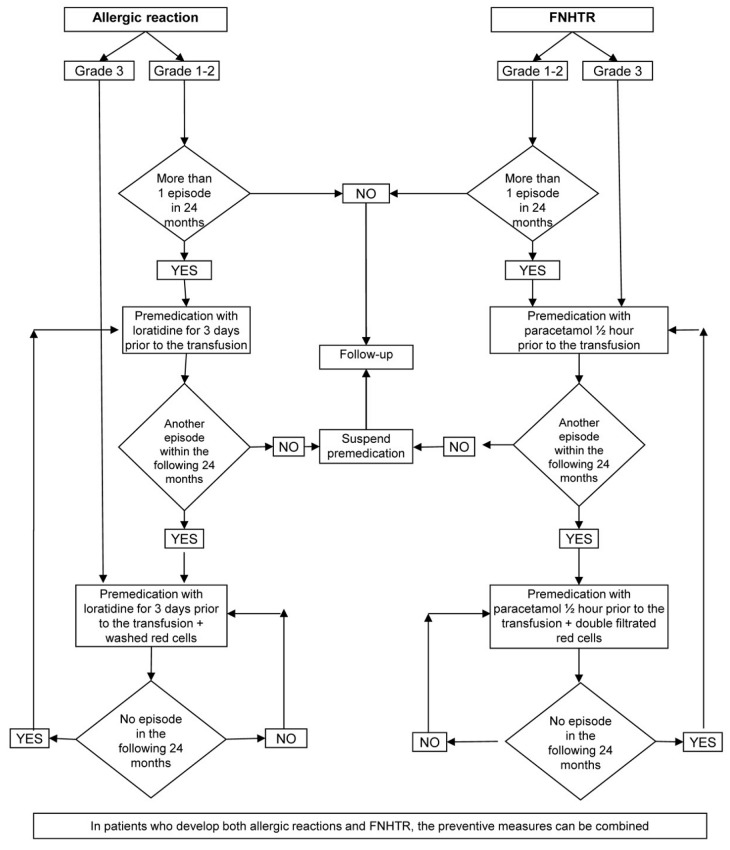
from the results obtained over the course of the 7 years, we suggest adopting the decisional algorithm shown in Figure 3, which summarises the main behaviours we introduced to progressively reduce the incidence of adverse reactions. The algorithm suggested may appear too rigid and not always easy to apply in all clinical situations, which deserve specific, targeted evaluation by the doctor. For example, in our experience four of the nine patients who received washed red blood cells have had a single, grade 3, severe allergic reaction now more than 2 years ago and, according to the algorithm that we proposed, should no longer be receiving washed red blood cells. However, particularly in the absence of definitive scientific evidence, we did not consider it appropriate to return to using unwashed red cells, which probably would have caused no harm, because of a possible emotional resistance by the patients, whose opinions should be held in due consideration. Despite this, we consider it useful to propose this model, which we reserve the right to apply fully after a future, definitive validation, ideally shared by other study groups.
Figure 3.
Flow chart of the management of adverse reactions to transfusions of red cell concentrates.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Cappellini MD, Cohen A, Eleftheriou A, et al. Guidelines for the clinical management of thalassemia. 2nd ed. Cyprus: Thalassemia International Federation; 2008. [PubMed] [Google Scholar]
- 2.Fidone C, Travali S, Bonomo P, et al. Clinical effects of different types of red cell concentrates in patients with thalassemia. Blood Transfus. 2006;4:311–26. doi: 10.1016/j.tracli.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Rund D, Rachmilewitz E. β-Thalassemia. New Engl J Med. 2005;353:1135–46. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 4.Di Gregorio F, Romeo MA, Pizzarelli G. La terapia trasfusionale nella β-talassemia major. In: Maggio A, Caronia F, Russo G, editors. Clinica e terapia della talassemia. Firenze: SEE; 2000. pp. 203–19. [Google Scholar]
- 5.Matteocci A, Scocchera R, Cianciulli P, et al. Reazioni trasfusionali non emolitiche e citochine in pazienti beta-talassemici. La Trasf del Sangue. 2002;47:470–6. [Google Scholar]
- 6.Aessopos A, Farmakis D, Deftereos S, et al. Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest. 2005;127:1523–30. doi: 10.1378/chest.127.5.1523. [DOI] [PubMed] [Google Scholar]
- 7.Ezidiegwu CN, Lauenstein KJ, Rosales LG. Febrile nonhemolytic transfusion reactions. Management by premedication and cost implications in adult patients. Arch Pathol Lab Med. 2004;128:991–5. doi: 10.5858/2004-128-991-FNTR. [DOI] [PubMed] [Google Scholar]
- 8.Patterson BJ, Freedman J, Blanchette V. Effect of premedication guidelines and leucoreduction on the rate of febrile nonhaemolytic platelet transfusion reactions. Transfus Med. 2000;10:199–206. doi: 10.1046/j.1365-3148.2000.00253.x. [DOI] [PubMed] [Google Scholar]
- 9.Geiger TL, Howard SC. Acetaminophen and diphenhydramine premedication for allergic and febrile non-hemolytic transfusion reactions: good prophylaxis or bad practice? Transfus Med Rev. 2007;21:1–12. doi: 10.1016/j.tmrv.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders RP, Maddirala SD, Geiger TL. Premedication with acetaminophen or diphenhydramine for transfusion with leucoreduced blood products in children. Br J Haematol. 2005;130:781–7. doi: 10.1111/j.1365-2141.2005.05670.x. [DOI] [PubMed] [Google Scholar]
- 11.Yazer MH, Podlosky L, Clarke G. The effect of prestorage WBC reduction on the rates of febrile nonhemolytic transfusion reactions to platelet concentrates and RBC. Transfusion. 2004;44:10–5. doi: 10.1046/j.0041-1132.2003.00518.x. [DOI] [PubMed] [Google Scholar]
- 12.Paglino JC, Pomper GJ, Fisch GS. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leucoreduction. Transfusion. 2004;44:16–24. doi: 10.1046/j.0041-1132.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 13.King KE, Shirey RS, Thoman SK. Universal leucoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44:25–9. doi: 10.1046/j.0041-1132.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 14.Ibojie J, Greiss M, Urbaniak SJ. Limited efficacy of universal leucodepletion in reducing the incidence of febrile non-haemolytic reactions in red cell transfusion. Transfus Med. 2002;12:387–9. doi: 10.1046/j.1365-3148.2002.00401_2.x. [DOI] [PubMed] [Google Scholar]
- 15.Pitocco C, Sexton TR. Alleviating blood shortages in a resource-constrained environment. Transfusion. 2005;45:1118–26. doi: 10.1111/j.1537-2995.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 16.Decreto del ministro della Salute 21 dicembre 2007. Gazzetta Ufficiale della Repubblica Italiana, Serie Generale n. 18 del 22/01/2008. Nuova disciplina delle attività trasfusionali e della produzione nazionale di emoderivati (Istituzione del sistema informativo dei servizi trasfusionali) [Google Scholar]
- 17.Decreto Legislativo 6 novembre 2007, n. 207. Attuazione della direttiva 2005/61/CE che applica la direttiva 2002/98/CE per quanto riguarda la prescrizione in tema di rintracciabilità del sangue e degli emocomponenti destinati a trasfusioni e la notifica di effetti indesiderati ed incidenti gravi. Gazzetta Ufficiale della Repubblica Italiana, Serie Generale n. 261 del 9 novembre 2007 - Supplemento ordinario n. 228.
- 18.Bennardello F, Fidone C, Bonomo P, et al. Use of an identification system based on biometric data for patients requiring transfusions guarantees transfusion safety and traceability. Blood Transfus. 2009;7:193–203. doi: 10.2450/2009.0067-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stainsby D. ABO incompatible transfusions - experience from the UK Serious Hazards of Transfusion (SHOT) scheme Transfusions ABO incompatible. Transfus Clin Biol. 2005;12:385–8. doi: 10.1016/j.tracli.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Stainsby D, Russell J, Cohen H, Lilleyman J. Reducing adverse events in blood transfusion. Br J Haematol. 2005;131:8–12. doi: 10.1111/j.1365-2141.2005.05702.x. [DOI] [PubMed] [Google Scholar]
- 21.Sazama K. Reports of 355 transfusion-associated deaths:1976 through 1985. Transfusion. 1990;30:583–90. doi: 10.1046/j.1537-2995.1990.30790385515.x. [DOI] [PubMed] [Google Scholar]
- 22.BCSH British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines on the clinical use of leucocyte depleted blood-components. Transfus Med. 1996;8:59–71. [PubMed] [Google Scholar]
- 23.AABB Bulletin: Leukocyte reduction. Bethesda, MD: American Association of Blood Bank; 1999. [Accessed on 05/04/2013]. Available at: http://www.cbbsweb.org/enf/attachments/aabb997lr.html. [Google Scholar]
- 24.Mukagatare I, Monfort M, de Marchin J, Gerard C. The effect of leucocyte-reduction on the transfusion reactions to red blood cells concentrates. Transfus Clin Biol. 2010;17:14–9. doi: 10.1016/j.tracli.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Pruss A, Kalus U, Radtke H, et al. Universal leucodepletion of blood components results in a significant reduction of febrile non-hemolytic but not allergic transfusion reactions. Transfus Apher Sci. 2004;30:41–6. doi: 10.1016/j.transci.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Wadhwa M, Seghatchian MJ, Dilger P, et al. Cytochine accumulation in stored cell concentrates: effect of buffy-coat removal and leucoreduction. Transfus Sci. 2000;23:7–16. doi: 10.1016/s0955-3886(00)00049-7. [DOI] [PubMed] [Google Scholar]
- 27.Chudziak D, Sireis W, Pfeiffer HU, et al. Accumulation of soluble inflammatory mediators between blood donation and prestorage leucocyte depletion. Vox Sang. 2009;96:163–6. doi: 10.1111/j.1423-0410.2008.01132.x. [DOI] [PubMed] [Google Scholar]
- 28.Westphal R. Washed RBC to prevent transfusion reactions. Transfusion. 1982;22:82. doi: 10.1046/j.1537-2995.1982.22182154229.x. [DOI] [PubMed] [Google Scholar]
- 29.Council of Europe. Guide to the Preparation, Use and Quality Assurance of Blood Components Recommendation No R (95) 15 on the Preparation, Use and Quality Assurance of Blood Components. 16th ed. Strasbourg: Council of Europe Press; 2010. [Google Scholar]
- 30.Mertes PM, Bazin A, Alla F, et al. Hypersensitivity Reactions to Blood Components: Document Issued by the Allergy Committee of the French Medicines and Healthcare Products Regulatory Agency. J Investig Allergol Clin Immunol. 2011;21:171–8. [PubMed] [Google Scholar]
- 31.Kennedy LD, Case LD, Hurd DD, et al. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48:2285–91. doi: 10.1111/j.1537-2995.2008.01858.x. [DOI] [PubMed] [Google Scholar]
- 32.Martí-Carvajal AJ, Solà I, González LE, et al. Pharmacological interventions for the prevention of allergic and febrile non-haemolytic transfusion reactions. Cochrane Database Syst Rev. 2010;16(6):CD007539. doi: 10.1002/14651858.CD007539.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bazin A. Recipients adverse reactions: guidance supports. Transfus Clin Biol. 2010;17:366–74. doi: 10.1016/j.tracli.2010.09.148. [DOI] [PubMed] [Google Scholar]
- 34.Tobian AA, King KE, Ness PM. Prevention of febrile nonhemolytic and allergic transfusion reactions with pretransfusion medication: is this evidence-based medicine? Transfusion. 2008;48:2274–6. doi: 10.1111/j.1537-2995.2008.01924.x. [DOI] [PubMed] [Google Scholar]
- 35.Gilstad CW. Anaphylactic transfusion reactions. Curr Opin Hematol. 2003;10:419–23. doi: 10.1097/00062752-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Brecher ME. Technical Manual. 15th ed. Bethesda, MD: American Association of Blood Banks; 2005. [Google Scholar]
- 37.Grabmer C, Holmberg J, Popovsky M, et al. Up to 21-day banked red blood cells collected by apheresis and stored for 14 days after automated wash at different times of storage. Vox Sang. 2006;90:40–4. doi: 10.1111/j.1423-0410.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 38.AFSSAPS - Agence Française de Securité Sanitarie des Produit de Santé. France Annual Haemovigilance report 2010. [Accessed on 05/04/2012]. Available at: http://www.afssaps.fr/Activites/Hemovigilance/Hemovigilance/(offset)/0)
- 39.New Zealand Blood Service Haemovigilance Steering Group. National Haemovigilance Programme Annual Report 2010. [Accessed on 05/04/2012]. Available at: http://www.nzblood.co.nz/Clinical-information/Haemovigilance-programme/Annual-haemovigilance-report.
- 40.Società Italiana di Medicina Trasfusionale ed Immunoematologia (SIMTI) Standard di Medicina Trasfusionale. 2nd ed. Milano: Edizioni SIMTI; 2010. [Google Scholar]
- 41.Società Italiana di Medicina Trasfusionale ed Immunoematologia (SIMTI) Raccomandazioni SIMTI sul corretto utilizzo degli emocomponenti e dei plasmaderivati. Milano: Edizioni SIMTI; 2009. [Google Scholar]
- 42.British Committee for Standards in Haematology Blood Transfusion Task Force. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31. [Google Scholar]