Abstract
Background
Haemodilution during resuscitation after massive haemorrhage may worsen the coagulopathy and perpetuate bleeding.
Materials and methods
Blood samples from healthy donors were diluted (30 and-60%) using crystalloids (saline, Ringer’s lactate, PlasmalyteTM) or colloids (6% hydroxyethylstarch [HES130/0.4], 5% human albumin, and gelatin). The effects of haemodilution on platelet adhesion (Impact R), thrombin generation (TG), and thromboelastometry (TEM) parameters were analysed as were the effects of fibrinogen, prothrombin complex concentrates (PCC), activated recombinant factor VII (FVIIa), and cryoprecipates on haemodilution.
Results
Platelet interactions was already significantly reduced at 30% haemodilution. Platelet reactivity was not improved by addition of any of the concentrates tested. A decrease in TG and marked alterations of TEM parameters were noted at 60% haemodilution. HES130/0.4 was the expander with the most deleterious action. TG was significantly enhanced by PCC whereas rFVIIa only caused a mild acceleration of TG initiation. Fibrinogen restored the alterations of TEM parameters caused by haemodilution including those caused by HES 130/0.4. Cryoprecipitates significantly improved the alterations caused by haemodilution on TG and TEM parameters; the effects on TG disappeared after ultracentrifugation of the cryoprecipitates.
Discussion
The haemostatic alterations caused by haemodilution are multifactorial and affect both blood cells and coagulation. In our in vitro approach, HES 130/0.4 had the most deleterious effect on haemostasis parameters. Coagulation factor concentrates did not improve platelet interactions in the Impact R, but did have favourable effects on coagulation parameters measured by TG and TEM. Fibrinogen notably improved TEM parameters without increasing thrombin generation, suggesting that this concentrate may help to preserve blood clotting abilities during haemodilution without enhancing the prothrombotic risk.
Keywords: haemodilution, coagulation factor concentrates, platelet function, thrombin generation, thromboelastometry
Introduction
Haemorrhage continues to be one of the main causes of death following severe trauma. Massive bleeding is a leading cause of death worldwide standing already as the most important cause of death in individuals aged 5 to 45 years after severe trauma1. Trauma patients are particularly susceptible to the early development of coagulopathy and the most severely injured patients are coagulopathic on admission to hospital. Uncontrolled bleeding initially causes loss of coagulation factors, red blood cells and platelets. Hypothermia, acidosis, and dilution from standard resuscitation practices can worsen this coagulopathy and perpetuate bleeding2. Moreover, trauma-induced exposure of tissue factor to flowing blood induces the activation of coagulation, which may trigger consumptive coagulopathy3. Administration of fluids for resuscitation after a massive blood loss causes clotting factor levels to decrease significantly, exacerbating the initial coagulopathy1,2,4. The type of fluid and, more specifically, some colloids are being blamed for an additional deleterious effect on haemostasis, with altered fibrin polymerisation and decreased platelet adhesive and aggregating properties5. The vicious cycle of coagulopathy, fluid resuscitation and massive transfusion results in thrombocytopenia, delayed clot generation due to various clotting factor deficiencies, alterations of clot firmness, fibrin polymerization and impaired clot stability due to hyperfibrinolysis6.
Guidelines for the management of massive blood loss recommend that patients receive packed red blood cells, platelet concentrates and fresh frozen plasma (FFP). Although the proportions are not precisely defined6,7,8, it has been suggested that current practice with FFP could be insufficient to reverse severe coagulopathies9. Chowdary et al.10 demonstrated that the standard doses of FFP recommended by current guidelines should be doubled or tripled to correct severe coagulation factor deficiencies in critically ill patients. Since the volume of transfused products contributes to the coagulopathy, the rationale for a change in current practices towards an earlier and more resolute use of concentrates of coagulation factors (fibrinogen, activated recombinant factor VII [rFVIIa], and prothrombin complex concentrates [PCC]) could reverse severe coagulopathy more efficiently and consequently reduce transfusion needs.
Evaluating the alteration in haemostasis and decision making during haemodilutional coagulopathy is a controversial subject. Standard coagulation tests such as prothrombin time (PT) and activated partial thromboplastin time (aPTT) do not seem to correlate with bleeding symptoms11. In recent years, several reports have encouraged the use of algorithms based on thromboelastometry (TEM) methodology to support the use of coagulation factor concentrates12–15. Unfortunately, there is a lack of comparative studies of TEM technologies with routine coagulation tests which could validate such algorithms. We must accept that the emergency of the clinical situation in severe trauma patients - with isolated exceptions16 - imposes limitations to the design of prospective, randomised, comparative clinical trials that could generate evidence-based decisions for transfusion strategies to treat coagulopathy developing after massive bleeding.
Alterations in haemostasis caused by dilutional coagulopathy imply the interaction of complex cellular and plasma mechanisms. With this rationale in mind, we analysed modifications on platelet adhesive and cohesive functions, viscoelastic properties of clots and thrombin generation parameters induced by experimental haemodilution. Different crystalloid and colloid solutions currently used for resuscitation were administered to produce levels of haemodilution levels of 30 and 60%, theoretically corresponding to situations of severe bleeding equivalent to blood losses of 1.5 L or 3 L, respectively, for an average-sized adult. Once we had characterised the alterations caused by haemodilution on the different parameters studied, we evaluated the potential action of different factor concentrates: fibrinogen, PCC, rFVIIa, or cryoprecipitate in the correction or reversion of the experimentally induced coagulopathy.
Materials and methods
Study material
Following approval from the local Ethics Committee and written informed consent from each participant, the study group consisted of eight healthy volunteers. Individuals who had received acetylsalicylic acid, non-steroidal anti-inflammatory or antiplatelet drugs within 7 days before blood sampling were excluded. Blood samples were collected into tubes (BD Vacutainer, Franklin Lakes, United States of America) containing citrate (final concentration of 13 mM).
Experimental design
Samples of whole blood were diluted in vitro with different crystalloids, including isotonic saline (NaCl 0.9%, Baxter S.L., Valencia, Spain), Ringer’s lactate (LacRingerTM, Braun Medical S.A., Barcelona, Spain), and PlasmalyteTM (Baxter S.L.); or colloids such as 6% hydroxyethylstarch HES 130/0.4 (VoluvenTM, Fresenius Kabi S.A, Barcelona, Spain), 5% human albumin (Grifols S.A., Barcelona, Spain), and gelatin (GelafundinaTM, B. Braun Medical S.A). Diluted samples were used to evaluate: (i) platelet interactions using the cone platelet analyser method (Impact RTM, Matis Medical, Beersel, Belgium); (ii) clot firmness by thromboelastometry (ROTEM, Tem International GmbH, Munich, Germany); and iii) thrombin generation using the fluorogenic assay Technothrombin TGA (Technoclone GmBH, Vienna, Austria). One crystalloid and one colloid were selected to continue with the second stage of the study.
In the second stage, the following haemostatic components were spiked to the haemodiluted sample to evaluate their potential corrective effect on above mentioned technologies: fibrinogen concentrate (HaemocomplettanTM, CSL Behring GmbH, Marburg, Germany), PCC (BeriplexTM, CSL Behring GmbH), rFVIIa-NovoSevenTM (NovoNordisk, Bagsvaerd, Denmark), and cryoprecipitates (Bang de Sang i Teixits, Barceloma, Spain). All haemostatic components were investigated at concentrations comparable to those commonly used in patients presenting with massive bleeding: fibrinogen at 2 g/L, PCC at 35 IU/kg, rFVIIa at 6 μg/mL, and cryoprecipitates at 2 units/10 kg of weight).
Routine laboratory determinations
Platelet count, haematocrit, and other haematological parameters were determined in an Advia 2120 Haematology System (Siemens, Deerfield, Illinois, United States of America). Fibrinogen levels, PT, and aPTT were determined in the BCSTM XP system (Siemens, Deerfield, Illinois, United States of America). The von Willebrand factor antigen (VWF:Ag) was evaluated by and enzyme-linked immunosorbent assay (ELISA; DG-EIA vWF, Grifols).
Platelet interaction: the cone platelet analyser procedure
The cone platelet analyser method was used as previously described17. Briefly, 130 μL of whole blood or haemodiluted blood samples were placed in a well polystyrene plate and subjected to a shear rate of 1,800 s 1 for 2 minutes using a rotating TeflonTM (Beersel, Belgium) cone. The wells were subsequently washed thoroughly with tap water, stained with May-Grünwald stain and analysed with an inverted light microscope connected to an image analysis system. Two parameters of platelet adhesion were evaluated: percentage of surface coverage (% SC), which is the percentage of total area covered by platelets (single platelets and clusters/aggregates), and the average size (AS, measured as μm2) of the polystyrene-bound platelet clusters/aggregates.
Thromboelastometry studies
In order to evaluate the influence of haemodilution, and the effect of the different coagulation factor concentrates on the clot formation, we used the ROTEM analyser18. The technique was performed according to the manufacturer’s instructions. For simplicity, we focused on the analysis of the exTEM test in TEM studies. Briefly, exTEM uses tissue factor as an activator and is sensitive for measuring changes to the extrinsic pathway of coagulation, fibrinogen and fibrin polymerisation as well as platelet function. This technique provides information through different parameters. We assessed four variables. The clotting time (CT), defined as the time elapsed from the start of the measurement until the amplitude of the forming clot reaches 2 mm. The clot formation time (CFT) is the time from the start of clot formation until the tracing reaches 20 mm of amplitude. The clot amplitude after 10 min (A10) and the maximum clot firmness (MCF) were evaluated as measures of clot firmness. In the experiments performed in the presence of the different plasma concentrates, only the A10 was measured to assess clot firmness. Recently, it has been reported that A10 is a good linear predictor of MCF19. CT and CFT are indicators of the dynamics of clot formation. The clot amplitude gives information about clot strength and stability, which is largely dependent on fibrinogen and platelets. All analyses were performed for a minimum of 45 min.
Thrombin generation assay
We evaluated thrombin generation in citrated platelet-rich plasma (PRP) samples, haemodiluted PRP, and haemodiluted PRP with the coagulation factor concentrates at the appropriate dosage. For specific experimental purposes, cryoprecipitates were subjected to ultarcentrifugation at 6 × 105 × g for 10 min at 10 ºC in an ultracentrifuge (Sorval MTX 150 Micro-UltraCentrifuge, Thermo Fisher Scientific, Kanagawa Prefecture, Japan).
Thrombin generation on citrated PRP was assessed with the fluorogenic assay Technothrombin TGATM following the manufacturer’s instructions and indications in previous publications20,21. This assay is based on the fluorescence generated by the cleavage of a fluorogenic substrate by thrombin over time. The activation of the coagulation cascade was triggered by a reagent (RCL) consisting of low concentration micelles of negatively charged phospholipids containing 71.6 pM of recombinant human tissue factor and CaCl2. The fluorescence generated was measured at 1 min intervals throughout 90 min. The assay provides thrombin concentration as well as other parameters, such as the lag time (min) to trigger thrombin generation (lag phase), peak of maximal thrombin concentration (nM) and the velocity index of thrombin generation.
Statistics
Data are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed with raw data using ANOVA. The SPSS statistical package 17.0.0 (SPSS Inc, Chicago, IL, United States of America) was used for all analyses. P values less than 0.05 were considered statistically significant.
Results
Description of the effect of haemodilution
Table I summarises the effects of 30% and 60% haemodilution with Ringer’s lactate and HES 130/0.4 on platelet counts, haematocrit, fibrinogen, PT, aPTT, and VWF levels. Similar results were observed with other diluents used in our studies (data not shown). Alterations in these parameters followed the severity of the haemodilution, reaching maximal differences at 60% haemodilution. No significant differences were observed depending on whether the haemodilution was produced with crystalloids or colloids.
Table I.
Effects of haemodilution with Ringer’s lactate and HES 130/04 on various haematological parameters.
| Platelet count (103/μL) | Hematocrit (%) | Fibrinogen (g/L) | PT (ratio) | aPTT (s) | VWF (U/dL) | |
|---|---|---|---|---|---|---|
| Baseline | 170.8±16.8 | 42.4±1.8 | 2.8±0.3 | 1.05±0.03 | 28.4±1.5 | 154.7±6.1 |
| Ringer’s lactate | ||||||
| 30% Haemodilution | 117.0±2.7* | 30.0±1.3*** | 1.7±0.2* | 1.48±0.08** | 35.9±2.5* | 95.0±8.0* |
| 60% Haemodilution | 69.0±6.4* | 17.0±0.5*** | 1.0±0.1** | 2.55±0.13** | 104.0±45.4 | 26.1±3.4** |
| HES 130/0.4 | ||||||
| 30% Haemodilution | 139.0±14.2** | 30.2±1.3*** | 2.1±0.2* | 1.40±0.08* | 86.1±51.3 | 102.7±21.9 |
| 60% Haemodilution | 84.3±6.9** | 17.0±0.2*** | 1.4±0.2* | 1.92±0.2** | 109.0±44.0 | 34.2±5.3** |
Legend Data are expressed as mean ± S.E.M (n=8).
P <0.05;
P <0.01;
P<0.001 vs baseline.
Influence on platelet interactions
Haemodilution had a dramatic influence on platelet interactions, as assessed by Impact-R technology. Platelet surface coverage in citrated whole blood samples reached values of 9.1±1.2% (mean ± S.E.M, n =8). A 30% haemodilution with any of the crystalloids or colloids caused reductions in platelet interactions with coverage values declining to 6%. As an example, 30% haemodilution with Ringer’s lactate reduced the surface coverage to 5.5±0.8% (n =8; P <0.05). Undetectable levels of platelet coverage were observed at more intense levels of haemodilution (60%) with any of the diluents used in our experiments (data not shown).
None of the concentrates tested improved reductions in platelet surface coverage observed after haemodilution. Figure 1 exemplifies representative microscopic fields obtained in experiments before, after 30% hemodilution with Ringer’s lactate and following in vitro addition of fibrinogen to the haemodiluted sample. Figure 2 summarises independent values for all the concentrates tested in blood samples haemodiluted with Ringer’s lactate (fibrinogen, PCC, rFVIIa, and cryoprecipitates). Similar tendencies were observed for haemodilution with other crystalloids or colloids or after in vitro addition of the different concentrates.
Figure 1.
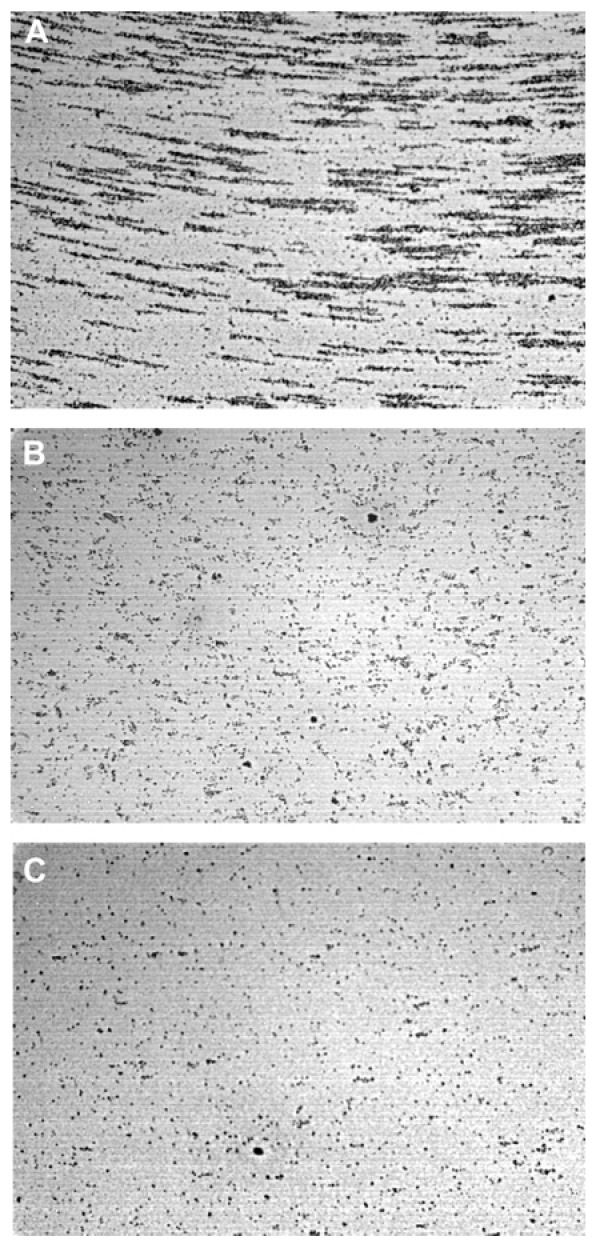
Micrographs showing platelet interactions as observed with the cone and plate technology (Impact R).
Representative microscopic fields in: (A) Experiments performed with whole blood; (B) a 30% haemodiluted sample using Ringer’s lactate; (C) a 30% haemodiluted sample using Ringer’s Lactate after in vitro addition of fibrinogen (2 mg/mL).
Figure 2.
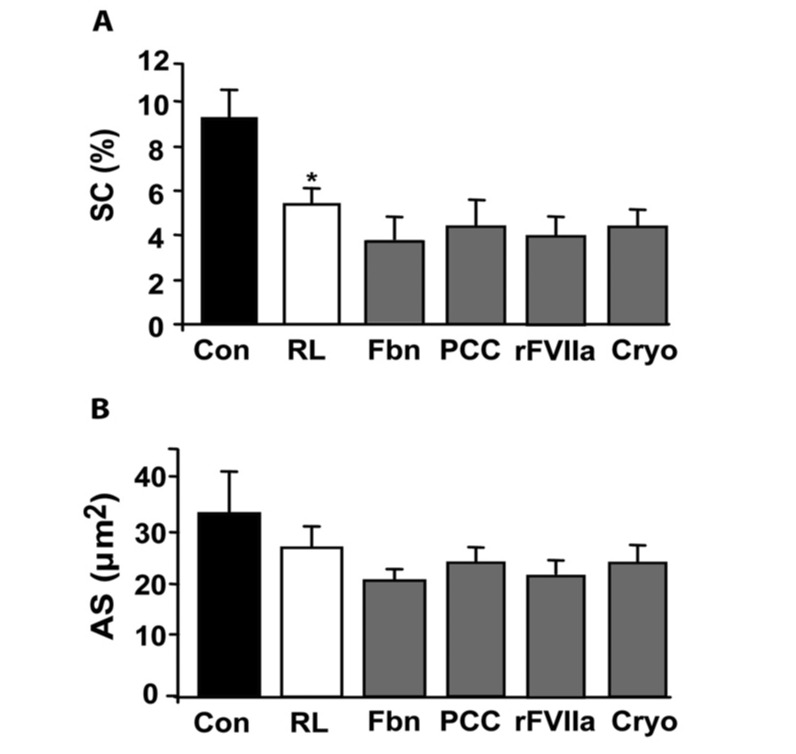
Influence of different concentrates on platelet interactions using the cone and platelet technology.
Bar diagrams indicate the percentage of surface covered by platelets with whole blood (Con), after 30% haemodilution using Ringer’s lactate (RL), and after in vitro addition of fibrinogen (Fbn) at 2 mg/mL, prothrombin complex concentrates (PCC) at 35 IU/kg, activated recombinant factor VII (rFVIIa) at 6 μg/mL, and cryoprecipitates (cryo) at a dose of 2 cryo/10 kg of weight. Panel A shows the total percentage of surface covered (SC) by platelets and panel B shows the average size (AS) of the aggregates formed, expressed in μm2. Results are expressed as mean ± SEM, n=8 (*P <0.05 vs whole blood).
Modifications in viscoelastic properties of clots
The viscoelastic properties of clots were progressively altered with the intensity of the haemodilution, always being more evident at 60%. Figure 3 summarises the most significant alterations in TEM parameters observed at a 60% haemodilution for each of the solutions tested. HES 130/0.4 proved to be the expander with the most deleterious effect (P <0.001). Crystalloids produced a delay in the initiation of clot formation, showing significant increases of CT and CFT. Decreased levels of A10 and MCF indicate a reduction of the clot firmness (see Table II). Haemodilution with colloids resulted in more significant impairment as reflected by the greater delay in CT and more intense reduction of the clot firmness than those observed with crystalloids (see Table II).
Figure 3.
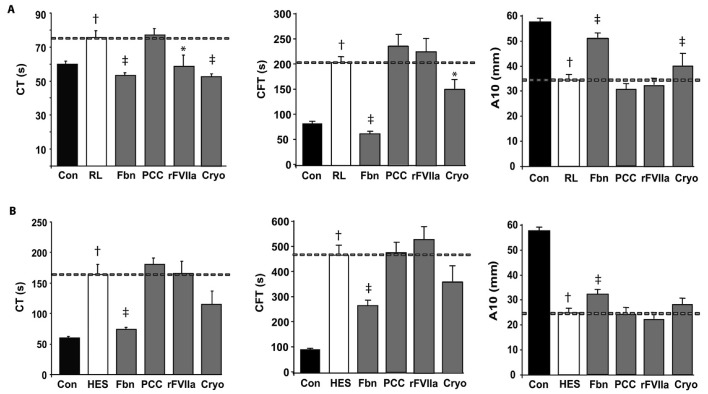
Effects of different plasma concentrates on the viscoelastic properties of clots evaluated by ROTEM.
Each panel corresponds to a different fluid used for haemodilution of the sample: Ringer’s lactate (A), representative of crystalloids, and HES 130/0.4 (B), representative of colloids. Each panels shows the clotting time (CT), clot formation time (CFT) both expressed in seconds; and clot amplitude after 10 min (A10) expressed in mm. The dashed line provides the reference for undiluted control samples (Con), samples 60% haemodiluted with Ringer’s lactate (RL) or HES 130/0.4 (HES), before and after addition of: 2 mg/mL of fibrinogen (Fbn), 35 IU/kg of prothrombin complex concentrates (PCC), 6 μg/mL of activated recombinant factor VII (rFVIIa), or cryoprecipitates (Cryo) at an equivalent dose of 2 cryo/10 kg of weight. Results are expressed as mean ± S.E.M (n=8); (†) P <0.01 vs control samples, (*) P <0.05 vs haemodilution (‡), and P <0.01 vs haemodilution.
Table II.
Effect of 60% haemodilution with different crystalloids and colloids on viscoelastic properties of forming clots.
| Baseline | Saline | Ringer lactate | Plasmalyte | HES 130/0.4 | 5% Albumin | Gelatin | |
|---|---|---|---|---|---|---|---|
| CT (s) | 60.9±2.0 | 88.6±3.0** | 75.5±4.3* | 70.3±3.2* | 163.5±17.1** | 111.3±4.6** | 104.3±22.7* |
| CFT (s) | 79.6±3.6 | 205.4±16.5** | 202.9±15.4** | 210.7±17.1** | 457.9±42.5** | 243.7±16.4** | 344.0±63.2** |
| A10 (mm) | 57.0±1.1 | 33.7±1.3** | 34.3±1.1 ** | 33.8±1.3** | 23.6±1.3** | 31.3±1.0** | 28.7±3.5** |
| MCF (mm) | 65.0±1.6 | 42.7±2.3** | 44.7±2.3 ** | 41.0±2.1** | 36.5±3.5** | 40.7±1.8** | 40.8±2.3** |
Legend Data are expressed as mean ± S.E.M. (n= 8).
P <0.05;
P <0.001 vs baseline.
As shown in Figure 3, the in vitro addition of fibrinogen significantly improved the alterations caused by haemodilution with any of the diluents tested, even those with HES 130/0.4. Cryoprecipitates significantly improved alterations of TEM parameters induced by haemodilution with crystalloids but seemed less efficient than fibrinogen at correcting the alterations caused by haemodilution with HES 130/0.4. The effect of rFVIIa only was evident in the first stage of clot formation, shortening the CT when crystalloids were used. The in vitro addition of PCC did not have any effect on TEM parameters (Figure 3).
Influence on thrombin generation
Table III summarises the effects of the greatest haemodilution on thrombin generation. The lag phase preceding the initiation of thrombin generation was significantly delayed (P <0.05) at 60% haemodilution with saline, plasmalyteTM, HES 130/0.4, and 5% albumin. Lag phases were also slightly delayed after haemodilution with Ringer’s lactate and gelatin although the differences did not reach the levels of statistical significance. Significant reductions in thrombin peaks and slower velocity indices were observed after 60% haemodilution with the different crystalloids and colloids tested (P <0.05 vs baseline conditions). Haemodilution with albumin seemed to have a more profound influence on thrombin generation as confirmed by the lowest maximal concentration of thrombin reached at peak (P <0.01 vs baseline).
Table III.
Modifications in thrombin generation parameters after 60% haemodilution with different crystalloids and colloids.
| Baseline | Saline | Ringer lactate | Plasmalyte | HES 130/0.4 | 5% Albumin | Gelatin | |
|---|---|---|---|---|---|---|---|
| Lag phase (min) | 10.9±0.2 | 13.6±0.4* | 12.0±0.6 | 13.1±0.5* | 14.1±0.9* | 12.1±0.3* | 11.6±0.2 |
| Thrombin peak (nM) | 471.0±48.7 | 346.8±36.0* | 375.9±38.5* | 325.8±37.5* | 355.6±40.5* | 230.1±24.9** | 368.5±37.9* |
| Velocity index | 85.5±10.7 | 47.9±8.2** | 51.8±7.8* | 40.4±6.3* | 47.3±7.4* | 37.6±6.4* | 62.9±9.1* |
Legend Data are expressed as mean ± S.E.M. (n=8).
P <0.05;
P <0.001 vs baseline.
With regards to the different concentrates studied, the thrombin generation peak was significantly elevated by PCC and cryoprecipitates (Figure 4). In vitro addition of rFVIIa to haemodiluted samples caused a moderate acceleration of thrombin generaiton. Addition of fibrinogen to haemodiluted blood samples caused a slight delay of the initiation of thrombin generation without any significant effect on total thrombin generation. Interestingly, cryoprecipitates significantly improved the alterations caused by haemodilution on thrombin generation (Figure 4). However, the previously mentioned effect disappeared when cryoprecipitate was ultracentrifuged, suggesting that contaminating particulates could account for this effect (Figure 5).
Figure 4.
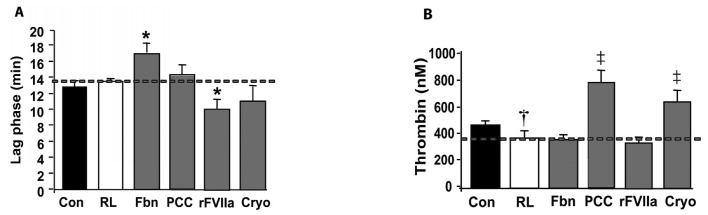
Effect of the different factor concentrates on thrombin generation.
Bar diagrams summarise modifications in (A) lag time (min) to trigger thrombin generation (lag phase) and (B): the peak of maximal thrombin concentration (nM) in samples of platelet-rich plasma (PRP) from blood haemodiluted to 60% with Ringer’s lactate. The different bars represent values in control undiluted samples (Con), haemodiluted samples before (RL), and after addition of 2 mg/mL of fibrinogen (Fbn), 35 IU/kg of prothrombin complex concentrates (PCC), 6 μg/mL of activated recombinant factor VII (rFVIIa), or cryoprecipitates (Cryo) at a dose equivalent to 2 cryo/10 kg of weight. Results are expressed as mean ± S.E.M (n=8); (*) P <0.05 vs haemodiluted samples, (†) P <0.01 vs control samples, and (‡) P <0.01 vs haemodilution.
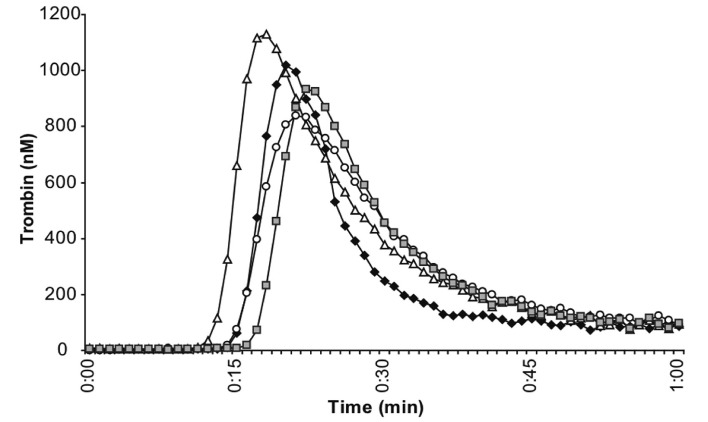
Figure 5.
Effect of ultracentrifugation of cryoprecipitate on thrombin generation using samples of platelet-rich plasma (PRP) from blood haemodiluted to 60% with Ringer’s lactate.
Graphs display representative thrombin generation kinetics (n=4) in control undiluted samples (◆), haemodiluted PRP (○), haemodiluted PRP plus cryoprecipitate at a dose of 2 cryo/10 kg of weight (△), and haemodiluted PRP plus supernatant of ultracentrifuged cryoprecipitates at equivalent doses (■). Enhanced thrombin generation induced by cryoprecipitates disappeared after ultracentrifugation.
Discussion
Our results demonstrate that haemodilution has a dramatic impact on platelet-mediated haemostasis at high shear with an additional influence on thrombin generation and subsequent alterations of the viscoelastic properties of clots generated under low shear conditions. All the concentrates showed favourable effects, correcting different phases of the coagulation process, but failed to compensate the platelet defect. Fibrinogen and cryoprecipitates clearly improved the viscoelastic properties of forming clots, cryoprecipitates and PCC increased thrombin generation, whereas rFVIIa accelerated the initiation of thrombin generation initially delayed by the haemodilution.
There is a general agreement that alterations in haemostasis induced by haemodilution are multifaceted and affect both cellular and coagulation mechanisms. It is also accepted that routine standard coagulation tests (PT, aPTT) have limited value for predicting bleeding22 or for monitoring replacement therapy4,7 in patients subjected to surgery. Although haemostatic alterations caused by haemodilution should be evaluated through specific laboratory tests, the emergency of the situation in critical bleeding patients and the need for rapid management of the coagulopathy have promoted the introduction of point of care technologies with an ability to evaluate haemostasis globally14,23,24. TEM technology has rapidly become established as a useful method for quick monitoring of coagulopathies and applied to transfusional decision-making in the care of perioperative bleeding.
In the present study we used an experimental model of dilutional coagulopathy that was characterszed through three different laboratory approaches to investigate platelet interactions, thrombin generation and clot formation after haemodilution. The experimental dilutional coagulopathy we produced is compatible with the clinical situation in severely bleeding patients who may require intensive transfusion. Special attention was paid to results produced at 60% haemodilution considering that this is the situation in which clotting factor concentrates would have the potential for fast reversal.
A dramatic reduction in platelet interactions with adhesive surfaces was observed in our studies at the elevated shear rates of the cone and plate analyser. None of the concentrates tested improved adhesive or aggregating properties of platelets in a system that is also sensitive to the negative haemorrheological influence of the haematocrit reduction, though we cannot exclude possible limitations of the cone and plate technology in our experimental setting. It is accepted that the haemorrheological effect of red blood cells is critical for the maintenance of correct haemostasis under flow conditions25,26. The transfusion of packed red blood cells with the objective of raising the haematocrit above 30% has a favourable impact on the haemostatic performance of circulating platelets27,28.
An additional finding of our present studies, which could explain the dramatic reduction in platelet interactions was the significant reduction in VWF levels after severe haemodilution. Levels of VWF reached values compatible with a diagnose of type 1 von Willebrand’s disease. It is interesting in this respect that transfusion algorithms based on TEM technology promote the use of fibrinogen or PCC to correct alterations in TEM parameters, but disregard the use of concentrates containing VWF to compensate the obvious deficiency in this adhesive protein.
Haemodilution with colloids or crystalloids caused significant alterations in the viscoelastic properties of forming clots as evaluated by TEM technology. Viscoelastic parameters were more severely affected when HES 130/0.4 was used as the haemodilution fluid. Fibrinogen and cryoprecipitate were found to be very effective at improving the alterations of the viscoelastic properties of thrombi induced by haemodilution as evaluated by TEM. These findings are basically in agreement with those of previous experimental and clinical studies13,29. Interestingly, PCC or rFVIIa did not show any relevant correction of the alterations of viscoelastic clot parameters produced in our experimental model of haemodilution. The favourable results in the TEM parameters in our studies were not significantly potentiated by further addition of PPC or rFVIIa (data not shown).
With the exception of fibrinogen, all the products tested in our experimental setting (PCC, rFVIIa and cryoprecipitate) had a favourable action, enhancing and accelerating alterations in thrombin generation caused by haemodilution. PCC appeared to be the most efficacious at reversing the alterations caused by haemodilution. The modifications induced by rFVIIa on thrombin generation were apparently milder than those observed with PCC, but correlated with the shorter CT produced by rFVIIa in the TEM. Previous studies demonstrated that rFVIIa had a marked impact on thrombin generation in plasma from patients with haemophilia30. It is very likely that the by-passing effects of rFVIIa on thrombin generation are less evident when there is an overall haemodilution of all coagulation factors as would be the case during severe haemodilutional coagulopathy.
Cryoprecipitates were the only concentrate that improved the viscoelastic properties of the clots and, surprisingly, thrombin generation. Previous studies by George et al.31 revealed that platelet membrane microparticles, highly concentrated in cryoprecipitates, could contribute to their therapeutic effect. The fact that the enhanced thrombin generation we observed in our studies disappeared after ultracentrifugation suggests that contaminant materials present in the cryoprecipitates could have been responsible for the effects on thrombin generation. Cryoprecipitate is a heterogeneous plasma component32 that may exert a complex haemostatic action other than restoring fibrinogen concentration. On the other hand, cryoprecipitates are the only concentrate, used in our studies, providing an additional supply of VWF, an adhesive protein that we found significantly to be reduced after severe haemodilution.
Our results indicate that TEM technology, currently applied for a global evaluation of coagulation in emergency, surgical or intensive care units, is very sensitive at detecting the effects of fibrinogen and cryoprecipitates, but less sensitive for detecting those of PCC or rFVIIa. According to our findings, point-of-care TEM devices are not designed to evaluate the mechanism of primary haemostasis with regards to the adhesive functions of platelets. Moreover, in our experimental setting the exTEM seemed to have limited sensitivity for identifying the effects of some concentrates (PCC and FVIIa) which, on the other hand, did have a definite action on thrombin generation. The information provided by the point-of-care TEM devices is useful for providing an early diagnose of the coagulopathy and for facilitating transfusion decisions in emergency situations33, but haemostatic alterations in the critically ill patient are far more complex than revealed by this technology.
Our findings have implications that can be extrapolated to clinical situations. First, the amounts of fluids used for resuscitation in severe trauma situations should be kept to a minimum to avoid dilutional coagulopathy. Second, hydroxyethyl starches may have an additional deleterious effect on coagulation parameters, as measured by TEM. Third, fibrinogen concentrates may be useful for improving or restoring blood clotting without increasing thrombin generation if the severity of bleeding has resulted in haemodilution. Finally, PCC, rFVIIa or cryoprecipitates could be used to boost thrombin generation in patients with uncontrollable bleeding resistant to the previous transfusional approach. Recent evidence indicates that the combined use of concentrates capable of enhancing thrombin generation may result in thrombotic complications34,35.
Our experimental studies indirectly suggest that fibrinogen concentrates might be useful to preserve blood clotting capacities during transportation of severely injured patients who require resuscitation with fluids. Fibrinogen concentrates could help to prevent the alterations of viscoelastic properties of clots altered by haemodilution without increasing thrombin generation.
Limitations of our studies
Our experimental model of haemodilution cannot reproduce the additional consumptive coagulopathy and associated hyperfibrinolysis related to the exposure of damaged tissue components occurring in severe trauma patients.
Acknowledgements
The Authors thank Dr. Jose Aznar for his scientific advice and assistance during the preparation of this manuscript. This work was mainly supported by a grant (PET 2008_0231) from the Spanish government and partially by grants SAF2009-10365, FIS (CP04-00112, PS09/00664), and Red HERACLES RD06/0009/1003 from the Spanish government. Dr. Ana Maria Galan is part of the Researchers’ Stabilisation Programme of the “Instituto de Salud Carlos III” of the Spanish government and the “Direcció d’Estratègia i Coordinació del Departament de Salut” of the Generalitat de Catalunya.
Footnotes
Conflicts of interest disclosure
Gines Escolar has received honoraria/consultant fees from CSL Behring and from NovoNordisk. None of the other Authors have any potential conflicts of interest.
References
- 1.Hess JR. Blood and coagulation support in trauma care. Hematology Am Soc Hematol Educ Program. 2007:187–91. doi: 10.1182/asheducation-2007.1.187. [DOI] [PubMed] [Google Scholar]
- 2.Tieu BH, Holcomb JB, Schreiber MA. Coagulopathy: its pathophysiology and treatment in the injured patient. World J Surg. 2007;31:1055–64. doi: 10.1007/s00268-006-0653-9. [DOI] [PubMed] [Google Scholar]
- 3.Levi M, Ten CH. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–92. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- 4.Bolliger D, Gorlinger K, Tanaka KA. Pathophysiology and treatment of coagulopathy in massive hemorrhage and hemodilution. Anesthesiology. 2010;113:1205–19. doi: 10.1097/ALN.0b013e3181f22b5a. [DOI] [PubMed] [Google Scholar]
- 5.Fenger-Eriksen C, Tonnesen E, Ingerslev J, Sorensen B. Mechanisms of hydroxyethyl starch-induced dilutional coagulopathy. J Thromb Haemost. 2009;7:1099–105. doi: 10.1111/j.1538-7836.2009.03460.x. [DOI] [PubMed] [Google Scholar]
- 6.Kozek-Langenecker S. Management of massive operative blood loss. Minerva Anestesiol. 2007;73:401–15. [PubMed] [Google Scholar]
- 7.Rossaint R, Bouillon B, Cerny V, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14:R52. doi: 10.1186/cc8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stainsby D, MacLennan S, Hamilton PJ. Management of massive blood loss: a template guideline. Br J Anaesth. 2000;85:487–91. doi: 10.1093/bja/85.3.487. [DOI] [PubMed] [Google Scholar]
- 9.Ho AM, Dion PW, Cheng CA, et al. A mathematical model for fresh frozen plasma transfusion strategies during major trauma resuscitation with ongoing hemorrhage. Can J Surg. 2005;48:470–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol. 2004;125:69–73. doi: 10.1111/j.1365-2141.2004.04868.x. [DOI] [PubMed] [Google Scholar]
- 11.Kitchens CS. To bleed or not to bleed? Is that the question for the PTT? J Thromb Haemost. 2005;3:2607–11. doi: 10.1111/j.1538-7836.2005.01552.x. [DOI] [PubMed] [Google Scholar]
- 12.Fenger-Eriksen C, Tonnesen E, Ingerslev J, Sorensen B. Recombinant factor VIIa and fibrinogen display additive effect during in vitro haemodilution with crystalloids. Acta Anaesthesiol Scand. 2009;53:332–8. doi: 10.1111/j.1399-6576.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 13.Fries D, Haas T, Klingler A, et al. Efficacy of fibrinogen and prothrombin complex concentrate used to reverse dilutional coagulopathy - a porcine model. Br J Anaesth. 2006;97:460–7. doi: 10.1093/bja/ael191. [DOI] [PubMed] [Google Scholar]
- 14.Schochl H, Nienaber U, Hofer G, et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14:R55. doi: 10.1186/cc8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theusinger OM, Baulig W, Asmis LM, et al. In vitro factor XIII supplementation increases clot firmness in Rotation Thromboelastometry (ROTEM) Thromb Haemost. 2010;104:385–91. doi: 10.1160/TH09-12-0858. [DOI] [PubMed] [Google Scholar]
- 16.Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 17.Shenkman B, Savion N, Dardik R, et al. Testing of platelet deposition on polystyrene surface under flow conditions by the cone and plate(let) analyzer: role of platelet activation, fibrinogen and von Willebrand factor. Thromb Res. 2000;99:353–61. doi: 10.1016/s0049-3848(00)00255-3. [DOI] [PubMed] [Google Scholar]
- 18.Anderson L, Quasim I, Soutar R, et al. An audit of red cell and blood product use after the institution of thromboelastometry in a cardiac intensive care unit. Transfus Med. 2006;16:31–9. doi: 10.1111/j.1365-3148.2006.00645.x. [DOI] [PubMed] [Google Scholar]
- 19.Blasi A, Beltran J, Pereira A, et al. An assessment of thromboelastometry to monitor blood coagulation and guide transfusion support in liver transplantation. Transfusion. 2012 doi: 10.1111/j.1537-2995.2011.03526.x. [DOI] [PubMed] [Google Scholar]
- 20.Hron G, Kollars M, Binder BR, et al. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. JAMA. 2006;296:397–402. doi: 10.1001/jama.296.4.397. [DOI] [PubMed] [Google Scholar]
- 21.Varadi K, Turecek PL, Schwarz HP. Thrombin generation assay and other universal tests for monitoring haemophilia therapy. Haemophilia. 2004;10(Suppl 2):17–21. doi: 10.1111/j.1365-2516.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- 22.van Veen JJ, Spahn DR, Makris M. Routine preoperative coagulation tests: an outdated practice? Br J Anaesth. 2011;106:1–3. doi: 10.1093/bja/aeq357. [DOI] [PubMed] [Google Scholar]
- 23.Fries D, Innerhofer P, Schobersberger W. Time for changing coagulation management in trauma-related massive bleeding. Curr Opin Anaesthesiol. 2009;22:267–74. doi: 10.1097/ACO.0b013e32832678d9. [DOI] [PubMed] [Google Scholar]
- 24.Johansson PI, Stissing T, Bochsen L, Ostrowski SR. Thrombelastography and thromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med. 2009;17:45. doi: 10.1186/1757-7241-17-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aarts PA, van den Broek SA, Prins GW, et al. Blood platelets are concentrated near the wall and red blood cells, in the center in flowing blood. Arteriosclerosis. 1988;8:819–24. doi: 10.1161/01.atv.8.6.819. [DOI] [PubMed] [Google Scholar]
- 26.Jordan A, David T, Homer-Vanniasinkam S, et al. The effects of margination and red cell augmented platelet diffusivity on platelet adhesion in complex flow. Biorheology. 2004;41:641–53. [PubMed] [Google Scholar]
- 27.Escolar G, Garrido M, Mazzara R, et al. Experimental basis for the use of red cell transfusion in the management of anemic-thrombocytopenic patients. Transfusion. 1988;28:406–11. doi: 10.1046/j.1537-2995.1988.28588337325.x. [DOI] [PubMed] [Google Scholar]
- 28.Valeri CR, Cassidy G, Pivacek LE, et al. Anemia-induced increase in the bleeding time: implications for treatment of nonsurgical blood loss. Transfusion. 2001;41:977–83. doi: 10.1046/j.1537-2995.2001.41080977.x. [DOI] [PubMed] [Google Scholar]
- 29.Fenger-Eriksen C, Jensen TM, Kristensen BS, et al. Fibrinogen substitution improves whole blood clot firmness after dilution with hydroxyethyl starch in bleeding patients undergoing radical cystectomy: a randomized, placebo-controlled clinical trial. J Thromb Haemost. 2009;7:795–802. doi: 10.1111/j.1538-7836.2009.03331.x. [DOI] [PubMed] [Google Scholar]
- 30.Hedner U. Mechanism of action of factor VIIa in the treatment of coagulopathies. Semin Thromb Hemost. 2006;32(Suppl 1):77–85. doi: 10.1055/s-2006-939557. [DOI] [PubMed] [Google Scholar]
- 31.George JN, Pickett EB, Heinz R. Platelet membrane microparticles in blood bank fresh frozen plasma and cryoprecipitate. Blood. 1986;68:307–9. [PubMed] [Google Scholar]
- 32.Sorensen B, Bevan D. A critical evaluation of cryoprecipitate for replacement of fibrinogen. Br J Haematol. 2010;149:834–43. doi: 10.1111/j.1365-2141.2010.08208.x. [DOI] [PubMed] [Google Scholar]
- 33.Davenport R, Khan S. Management of major trauma haemorrhage: treatment priorities and controversies. Br J Haematol. 2011;155:537–48. doi: 10.1111/j.1365-2141.2011.08885.x. [DOI] [PubMed] [Google Scholar]
- 34.Mitterlechner T, Innerhofer P, Streif W, et al. Prothrombin complex concentrate and recombinant prothrombin alone or in combination with recombinant factor X and FVIIa in dilutional coagulopathy: a porcine model. J Thromb Haemost. 2011;9:729–37. doi: 10.1111/j.1538-7836.2011.04211.x. [DOI] [PubMed] [Google Scholar]
- 35.O’Connell NM, Perry DJ, Hodgson AJ, et al. Recombinant FVIIa in the management of uncontrolled hemorrhage. Transfusion. 2003;43:1711–6. doi: 10.1046/j.0041-1132.2003.00577.x. [DOI] [PubMed] [Google Scholar]