Abstract
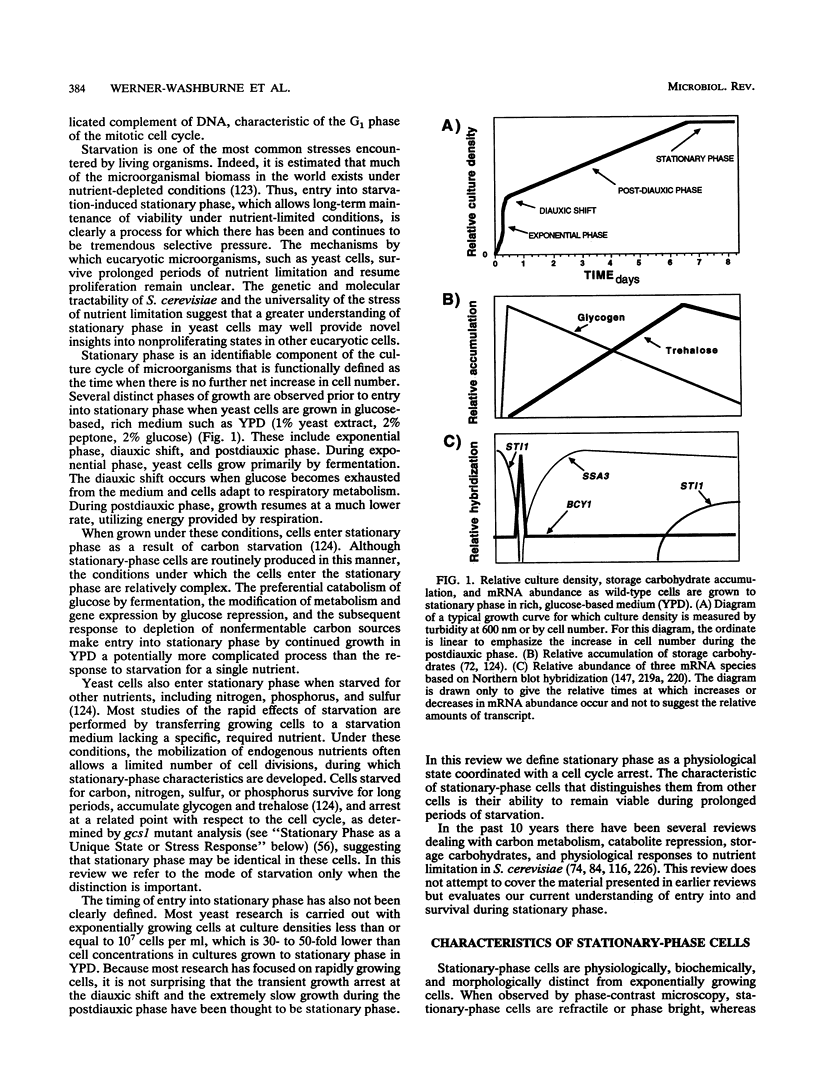
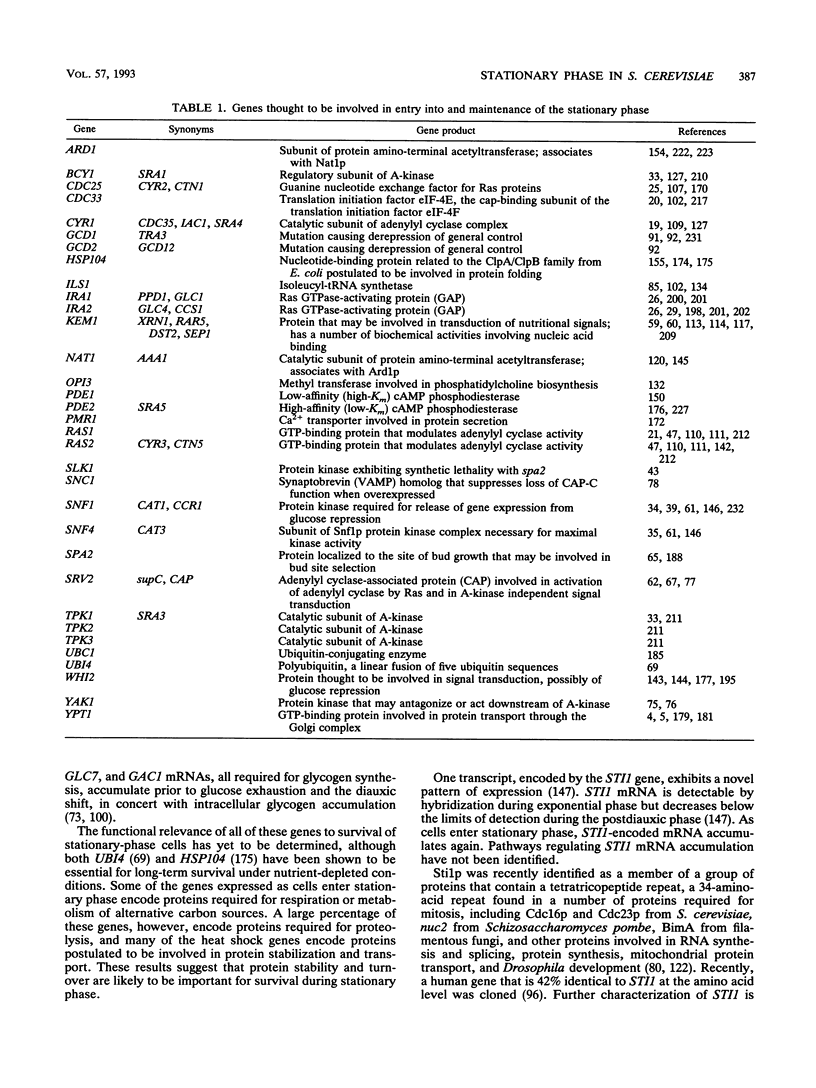
Growth and proliferation of microorganisms such as the yeast Saccharomyces cerevisiae are controlled in part by the availability of nutrients. When proliferating yeast cells exhaust available nutrients, they enter a stationary phase characterized by cell cycle arrest and specific physiological, biochemical, and morphological changes. These changes include thickening of the cell wall, accumulation of reserve carbohydrates, and acquisition of thermotolerance. Recent characterization of mutant cells that are conditionally defective only for the resumption of proliferation from stationary phase provides evidence that stationary phase is a unique developmental state. Strains with mutations affecting entry into and survival during stationary phase have also been isolated, and the mutations have been shown to affect at least seven different cellular processes: (i) signal transduction, (ii) protein synthesis, (iii) protein N-terminal acetylation, (iv) protein turnover, (v) protein secretion, (vi) membrane biosynthesis, and (vii) cell polarity. The exact nature of the relationship between these processes and survival during stationary phase remains to be elucidated. We propose that cell cycle arrest coordinated with the ability to remain viable in the absence of additional nutrients provides a good operational definition of starvation-induced stationary phase.
Full text
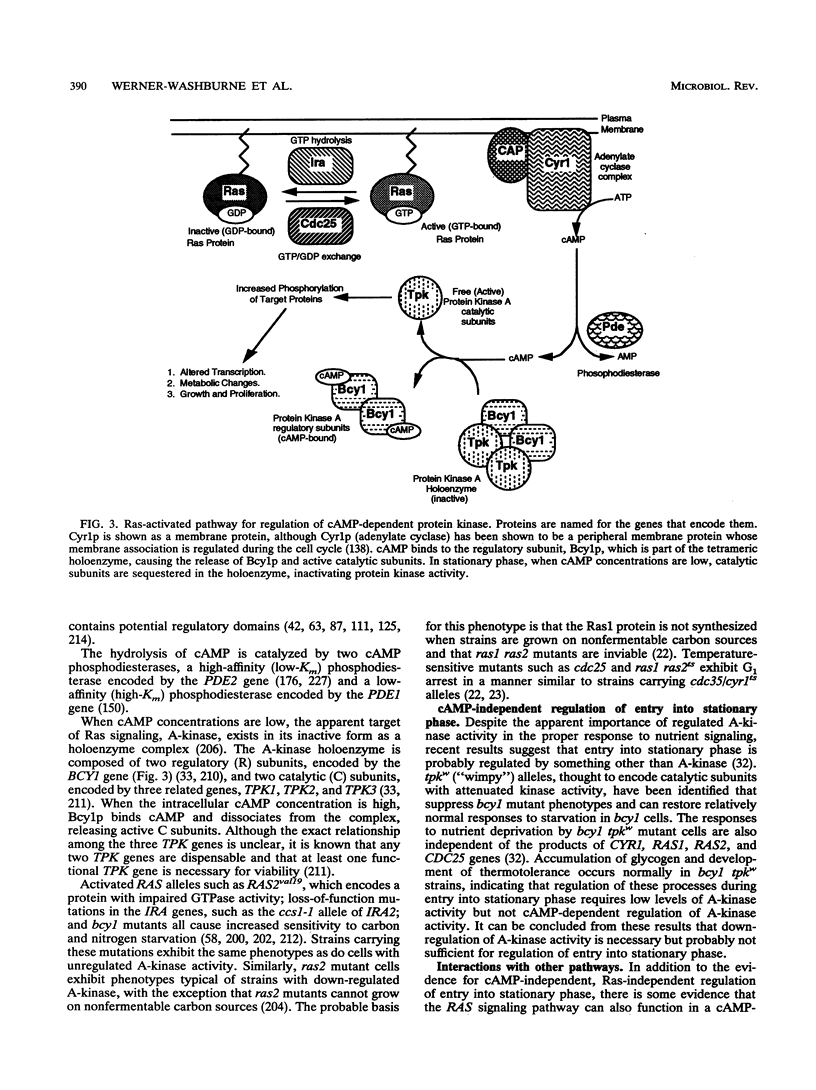
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstetter T., Ehmann C., Wolf D. H. Proteolysis in eucaryotic cells: aminopeptidases and dipeptidyl aminopeptidases of yeast revisited. Arch Biochem Biophys. 1983 Oct 1;226(1):292–305. doi: 10.1016/0003-9861(83)90296-5. [DOI] [PubMed] [Google Scholar]
- Altmann M., Sonenberg N., Trachsel H. Translation in Saccharomyces cerevisiae: initiation factor 4E-dependent cell-free system. Mol Cell Biol. 1989 Oct;9(10):4467–4472. doi: 10.1128/mcb.9.10.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attfield P. V., Raman A., Northcott C. J. Construction of Saccharomyces cerevisiae strains that accumulate relatively low concentrations of trehalose, and their application in testing the contribution of the disaccharide to stress tolerance. FEMS Microbiol Lett. 1992 Jul 15;73(3):271–276. doi: 10.1016/0378-1097(92)90642-2. [DOI] [PubMed] [Google Scholar]
- Bacon R. A., Salminen A., Ruohola H., Novick P., Ferro-Novick S. The GTP-binding protein Ypt1 is required for transport in vitro: the Golgi apparatus is defective in ypt1 mutants. J Cell Biol. 1989 Sep;109(3):1015–1022. doi: 10.1083/jcb.109.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Wuestehube L., Schekman R., Botstein D., Segev N. GTP-binding Ypt1 protein and Ca2+ function independently in a cell-free protein transport reaction. Proc Natl Acad Sci U S A. 1990 Jan;87(1):355–359. doi: 10.1073/pnas.87.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. V. GCR1 of Saccharomyces cerevisiae encodes a DNA binding protein whose binding is abolished by mutations in the CTTCC sequence motif. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9443–9447. doi: 10.1073/pnas.88.21.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. V. Glycolytic gene expression in Saccharomyces cerevisiae: nucleotide sequence of GCR1, null mutants, and evidence for expression. Mol Cell Biol. 1986 Nov;6(11):3774–3784. doi: 10.1128/mcb.6.11.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Belazzi T., Wagner A., Wieser R., Schanz M., Adam G., Hartig A., Ruis H. Negative regulation of transcription of the Saccharomyces cerevisiae catalase T (CTT1) gene by cAMP is mediated by a positive control element. EMBO J. 1991 Mar;10(3):585–592. doi: 10.1002/j.1460-2075.1991.tb07985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis L. T., Denis C. L. Identification of functional regions in the yeast transcriptional activator ADR1. Mol Cell Biol. 1988 May;8(5):2125–2131. doi: 10.1128/mcb.8.5.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissinger P. H., Wieser R., Hamilton B., Ruis H. Control of Saccharomyces cerevisiae catalase T gene (CTT1) expression by nutrient supply via the RAS-cyclic AMP pathway. Mol Cell Biol. 1989 Mar;9(3):1309–1315. doi: 10.1128/mcb.9.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorstein W. R., Craig E. A. Regulation of a yeast HSP70 gene by a cAMP responsive transcriptional control element. EMBO J. 1990 Aug;9(8):2543–2553. doi: 10.1002/j.1460-2075.1990.tb07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorstein W. R., Craig E. A. Transcriptional regulation of SSA3, an HSP70 gene from Saccharomyces cerevisiae. Mol Cell Biol. 1990 Jun;10(6):3262–3267. doi: 10.1128/mcb.10.6.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich K. A., Farrelly F. W., Finkelstein D. B., Taulien J., Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989 Sep;9(9):3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucherie H. Protein synthesis during transition and stationary phases under glucose limitation in Saccharomyces cerevisiae. J Bacteriol. 1985 Jan;161(1):385–392. doi: 10.1128/jb.161.1.385-392.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Boutelet F., Petitjean A., Hilger F. Yeast cdc35 mutants are defective in adenylate cyclase and are allelic with cyr1 mutants while CAS1, a new gene, is involved in the regulation of adenylate cyclase. EMBO J. 1985 Oct;4(10):2635–2641. doi: 10.1002/j.1460-2075.1985.tb03981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C., Nakayama N., Goebl M., Tanaka K., Toh-e A., Matsumoto K. CDC33 encodes mRNA cap-binding protein eIF-4E of Saccharomyces cerevisiae. Mol Cell Biol. 1988 Aug;8(8):3556–3559. doi: 10.1128/mcb.8.8.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breviario D., Hinnebusch A., Cannon J., Tatchell K., Dhar R. Carbon source regulation of RAS1 expression in Saccharomyces cerevisiae and the phenotypes of ras2- cells. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4152–4156. doi: 10.1073/pnas.83.12.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broach J. R., Deschenes R. J. The function of ras genes in Saccharomyces cerevisiae. Adv Cancer Res. 1990;54:79–139. doi: 10.1016/s0065-230x(08)60809-x. [DOI] [PubMed] [Google Scholar]
- Broach J. R. RAS genes in Saccharomyces cerevisiae: signal transduction in search of a pathway. Trends Genet. 1991 Jan;7(1):28–33. doi: 10.1016/0168-9525(91)90018-l. [DOI] [PubMed] [Google Scholar]
- Broek D., Toda T., Michaeli T., Levin L., Birchmeier C., Zoller M., Powers S., Wigler M. The S. cerevisiae CDC25 gene product regulates the RAS/adenylate cyclase pathway. Cell. 1987 Mar 13;48(5):789–799. doi: 10.1016/0092-8674(87)90076-6. [DOI] [PubMed] [Google Scholar]
- Buchberg A. M., Cleveland L. S., Jenkins N. A., Copeland N. G. Sequence homology shared by neurofibromatosis type-1 gene and IRA-1 and IRA-2 negative regulators of the RAS cyclic AMP pathway. Nature. 1990 Sep 20;347(6290):291–294. doi: 10.1038/347291a0. [DOI] [PubMed] [Google Scholar]
- Burke D. J., Church D. Protein synthesis requirements for nuclear division, cytokinesis, and cell separation in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Jul;11(7):3691–3698. doi: 10.1128/mcb.11.7.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussereau F., Dupont C. H., Boy-Marcotte E., Mallet L., Jacquet M. The CCS1 gene from Saccharomyces cerevisiae which is involved in mitochondrial functions is identified as IRA2 an attenuator of RAS1 and RAS2 gene products. Curr Genet. 1992 Apr;21(4-5):325–329. doi: 10.1007/BF00351690. [DOI] [PubMed] [Google Scholar]
- Cabib E., Bowers B., Sburlati A., Silverman S. J. Fungal cell wall synthesis: the construction of a biological structure. Microbiol Sci. 1988 Dec;5(12):370–375. [PubMed] [Google Scholar]
- Cabib E., Roberts R., Bowers B. Synthesis of the yeast cell wall and its regulation. Annu Rev Biochem. 1982;51:763–793. doi: 10.1146/annurev.bi.51.070182.003555. [DOI] [PubMed] [Google Scholar]
- Cameron S., Levin L., Zoller M., Wigler M. cAMP-independent control of sporulation, glycogen metabolism, and heat shock resistance in S. cerevisiae. Cell. 1988 May 20;53(4):555–566. doi: 10.1016/0092-8674(88)90572-7. [DOI] [PubMed] [Google Scholar]
- Cannon J. F., Tatchell K. Characterization of Saccharomyces cerevisiae genes encoding subunits of cyclic AMP-dependent protein kinase. Mol Cell Biol. 1987 Aug;7(8):2653–2663. doi: 10.1128/mcb.7.8.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986 Sep 12;233(4769):1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- Celenza J. L., Eng F. J., Carlson M. Molecular analysis of the SNF4 gene of Saccharomyces cerevisiae: evidence for physical association of the SNF4 protein with the SNF1 protein kinase. Mol Cell Biol. 1989 Nov;9(11):5045–5054. doi: 10.1128/mcb.9.11.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. R., Johnson T. R., Dollard C., Shuster J. R., Denis C. L. Cyclic AMP-dependent protein kinase phosphorylates and inactivates the yeast transcriptional activator ADR1. Cell. 1989 Feb 10;56(3):409–419. doi: 10.1016/0092-8674(89)90244-4. [DOI] [PubMed] [Google Scholar]
- Choder M. A general topoisomerase I-dependent transcriptional repression in the stationary phase in yeast. Genes Dev. 1991 Dec;5(12A):2315–2326. doi: 10.1101/gad.5.12a.2315. [DOI] [PubMed] [Google Scholar]
- Ciriacy M. Isolation and characterization of yeast mutants defective in intermediary carbon metabolism and in carbon catabolite derepression. Mol Gen Genet. 1977 Jul 20;154(2):213–220. doi: 10.1007/BF00330840. [DOI] [PubMed] [Google Scholar]
- Ciriacy M. Isolation and characterization of yeast mutants defective in intermediary carbon metabolism and in carbon catabolite derepression. Mol Gen Genet. 1977 Jul 20;154(2):213–220. doi: 10.1007/BF00330840. [DOI] [PubMed] [Google Scholar]
- Clifton D., Fraenkel D. G. The gcr (glycolysis regulation) mutation of Saccharomyces cerevisiae. J Biol Chem. 1981 Dec 25;256(24):13074–13078. [PubMed] [Google Scholar]
- Cohen G., Fessl F., Traczyk A., Rytka J., Ruis H. Isolation of the catalase A gene of Saccharomyces cerevisiae by complementation of the cta1 mutation. Mol Gen Genet. 1985;200(1):74–79. doi: 10.1007/BF00383315. [DOI] [PubMed] [Google Scholar]
- Colicelli J., Field J., Ballester R., Chester N., Young D., Wigler M. Mutational mapping of RAS-responsive domains of the Saccharomyces cerevisiae adenylyl cyclase. Mol Cell Biol. 1990 Jun;10(6):2539–2543. doi: 10.1128/mcb.10.6.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan C., Gehrung S., Snyder M. A synthetic lethal screen identifies SLK1, a novel protein kinase homolog implicated in yeast cell morphogenesis and cell growth. Mol Cell Biol. 1992 Mar;12(3):1162–1178. doi: 10.1128/mcb.12.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet M., Urdaci M., Dulau L., Aigle M. Yeast mutant affected for viability upon nutrient starvation: characterization and cloning of the RVS161 gene. Yeast. 1991 Oct;7(7):727–743. doi: 10.1002/yea.320070708. [DOI] [PubMed] [Google Scholar]
- Culbertson M. R., Henry S. A. Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics. 1975 May;80(1):23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C., Ossig R., Gallwitz D., Schmitt H. D. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol. 1991 Feb;11(2):872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nobel J. G., Klis F. M., Munnik T., Priem J., van den Ende H. An assay of relative cell wall porosity in Saccharomyces cerevisiae, Kluyveromyces lactis and Schizosaccharomyces pombe. Yeast. 1990 Nov-Dec;6(6):483–490. doi: 10.1002/yea.320060605. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D., Tatchell K., Robinson L. C., Sigal I. S., Vass W. C., Lowy D. R., Scolnick E. M. Mammalian and yeast ras gene products: biological function in their heterologous systems. Science. 1985 Apr 12;228(4696):179–184. doi: 10.1126/science.3883495. [DOI] [PubMed] [Google Scholar]
- Denis C. L., Audino D. C. The CCR1 (SNF1) and SCH9 protein kinases act independently of cAMP-dependent protein kinase and the transcriptional activator ADR1 in controlling yeast ADH2 expression. Mol Gen Genet. 1991 Oct;229(3):395–399. doi: 10.1007/BF00267461. [DOI] [PubMed] [Google Scholar]
- Denis C. L., Gallo C. Constitutive RNA synthesis for the yeast activator ADR1 and identification of the ADR1-5c mutation: implications in posttranslational control of ADR1. Mol Cell Biol. 1986 Nov;6(11):4026–4030. doi: 10.1128/mcb.6.11.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis C. L. Identification of new genes involved in the regulation of yeast alcohol dehydrogenase II. Genetics. 1984 Dec;108(4):833–844. doi: 10.1093/genetics/108.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis C. L., Malvar T. The CCR4 gene from Saccharomyces cerevisiae is required for both nonfermentative and spt-mediated gene expression. Genetics. 1990 Feb;124(2):283–291. doi: 10.1093/genetics/124.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Dever T. E., Feng L., Wek R. C., Cigan A. M., Donahue T. F., Hinnebusch A. G. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992 Feb 7;68(3):585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- Drebot M. A., Barnes C. A., Singer R. A., Johnston G. C. Genetic assessment of stationary phase for cells of the yeast Saccharomyces cerevisiae. J Bacteriol. 1990 Jul;172(7):3584–3589. doi: 10.1128/jb.172.7.3584-3589.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drebot M. A., Johnston G. C., Singer R. A. A yeast mutant conditionally defective only for reentry into the mitotic cell cycle from stationary phase. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7948–7952. doi: 10.1073/pnas.84.22.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont C. H., Rigoulet M., Aigle M., Guérin B. Isolation and genetic study of triethyltin-resistant mutants of Saccharomyces cerevisiae. Curr Genet. 1990 Jun;17(6):465–472. doi: 10.1007/BF00313073. [DOI] [PubMed] [Google Scholar]
- Dykstra C. C., Hamatake R. K., Sugino A. DNA strand transfer protein beta from yeast mitotic cells differs from strand transfer protein alpha from meiotic cells. J Biol Chem. 1990 Jul 5;265(19):10968–10973. [PubMed] [Google Scholar]
- Dykstra C. C., Kitada K., Clark A. B., Hamatake R. K., Sugino A. Cloning and characterization of DST2, the gene for DNA strand transfer protein beta from Saccharomyces cerevisiae. Mol Cell Biol. 1991 May;11(5):2583–2592. doi: 10.1128/mcb.11.5.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entian K. D., Zimmermann F. K. New genes involved in carbon catabolite repression and derepression in the yeast Saccharomyces cerevisiae. J Bacteriol. 1982 Sep;151(3):1123–1128. doi: 10.1128/jb.151.3.1123-1128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor-Chaiken M., Deschenes R. J., Broach J. R. SRV2, a gene required for RAS activation of adenylate cyclase in yeast. Cell. 1990 Apr 20;61(2):329–340. doi: 10.1016/0092-8674(90)90813-t. [DOI] [PubMed] [Google Scholar]
- Feger G., De Vendittis E., Vitelli A., Masturzo P., Zahn R., Verrotti A. C., Kavounis C., Pal G. P., Fasano O. Identification of regulatory residues of the yeast adenylyl cyclase. EMBO J. 1991 Feb;10(2):349–359. doi: 10.1002/j.1460-2075.1991.tb07956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z. H., Wilson S. E., Peng Z. Y., Schlender K. K., Reimann E. M., Trumbly R. J. The yeast GLC7 gene required for glycogen accumulation encodes a type 1 protein phosphatase. J Biol Chem. 1991 Dec 15;266(35):23796–23801. [PubMed] [Google Scholar]
- Field C., Schekman R. Localized secretion of acid phosphatase reflects the pattern of cell surface growth in Saccharomyces cerevisiae. J Cell Biol. 1980 Jul;86(1):123–128. doi: 10.1083/jcb.86.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Vojtek A., Ballester R., Bolger G., Colicelli J., Ferguson K., Gerst J., Kataoka T., Michaeli T., Powers S. Cloning and characterization of CAP, the S. cerevisiae gene encoding the 70 kd adenylyl cyclase-associated protein. Cell. 1990 Apr 20;61(2):319–327. doi: 10.1016/0092-8674(90)90812-s. [DOI] [PubMed] [Google Scholar]
- Finley D., Chau V. Ubiquitination. Annu Rev Cell Biol. 1991;7:25–69. doi: 10.1146/annurev.cb.07.110191.000325. [DOI] [PubMed] [Google Scholar]
- Finley D., Ozkaynak E., Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation, and other stresses. Cell. 1987 Mar 27;48(6):1035–1046. doi: 10.1016/0092-8674(87)90711-2. [DOI] [PubMed] [Google Scholar]
- Flick J. S., Johnston M. Two systems of glucose repression of the GAL1 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Sep;10(9):4757–4769. doi: 10.1128/mcb.10.9.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François J. M., Thompson-Jaeger S., Skroch J., Zellenka U., Spevak W., Tatchell K. GAC1 may encode a regulatory subunit for protein phosphatase type 1 in Saccharomyces cerevisiae. EMBO J. 1992 Jan;11(1):87–96. doi: 10.1002/j.1460-2075.1992.tb05031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François J., Eraso P., Gancedo C. Changes in the concentration of cAMP, fructose 2,6-bisphosphate and related metabolites and enzymes in Saccharomyces cerevisiae during growth on glucose. Eur J Biochem. 1987 Apr 15;164(2):369–373. doi: 10.1111/j.1432-1033.1987.tb11067.x. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M. Carbon catabolite repression in yeast. Eur J Biochem. 1992 Jun 1;206(2):297–313. doi: 10.1111/j.1432-1033.1992.tb16928.x. [DOI] [PubMed] [Google Scholar]
- Garrett S., Broach J. Loss of Ras activity in Saccharomyces cerevisiae is suppressed by disruptions of a new kinase gene, YAKI, whose product may act downstream of the cAMP-dependent protein kinase. Genes Dev. 1989 Sep;3(9):1336–1348. doi: 10.1101/gad.3.9.1336. [DOI] [PubMed] [Google Scholar]
- Garrett S., Menold M. M., Broach J. R. The Saccharomyces cerevisiae YAK1 gene encodes a protein kinase that is induced by arrest early in the cell cycle. Mol Cell Biol. 1991 Aug;11(8):4045–4052. doi: 10.1128/mcb.11.8.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst J. E., Ferguson K., Vojtek A., Wigler M., Field J. CAP is a bifunctional component of the Saccharomyces cerevisiae adenylyl cyclase complex. Mol Cell Biol. 1991 Mar;11(3):1248–1257. doi: 10.1128/mcb.11.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst J. E., Rodgers L., Riggs M., Wigler M. SNC1, a yeast homolog of the synaptic vesicle-associated membrane protein/synaptobrevin gene family: genetic interactions with the RAS and CAP genes. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4338–4342. doi: 10.1073/pnas.89.10.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Marshall M. S. The ras oncogene--an important regulatory element in lower eucaryotic organisms. Microbiol Rev. 1989 Jun;53(2):171–185. doi: 10.1128/mr.53.2.171-185.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebl M., Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci. 1991 May;16(5):173–177. doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
- Hadwiger J. A., Wittenberg C., Richardson H. E., de Barros Lopes M., Reed S. I. A family of cyclin homologs that control the G1 phase in yeast. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6255–6259. doi: 10.1073/pnas.86.16.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima S., Hannig E. M., Hinnebusch A. G. Interactions between positive and negative regulators of GCN4 controlling gene expression and entry into the yeast cell cycle. Genetics. 1987 Nov;117(3):409–419. doi: 10.1093/genetics/117.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima S., Hinnebusch A. G. Multiple GCD genes required for repression of GCN4, a transcriptional activator of amino acid biosynthetic genes in Saccharomyces cerevisiae. Mol Cell Biol. 1986 Nov;6(11):3990–3998. doi: 10.1128/mcb.6.11.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. Mutants of yeast with temperature-sensitive isoleucyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1968 Feb;59(2):422–428. doi: 10.1073/pnas.59.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heideman W., Casperson G. F., Bourne H. R. Adenylyl cyclase in yeast. Hydrodynamic properties and activation by trypsin. J Biol Chem. 1987 May 25;262(15):7087–7091. [PubMed] [Google Scholar]
- Heideman W., Casperson G. F., Bourne H. R. Adenylyl cyclase in yeast: antibodies and mutations identify a regulatory domain. J Cell Biochem. 1990 Apr;42(4):229–242. doi: 10.1002/jcb.240420406. [DOI] [PubMed] [Google Scholar]
- Henry S. A., Atkinson K. D., Kolat A. I., Culbertson M. R. Growth and metabolism of inositol-starved Saccharomyces cerevisiae. J Bacteriol. 1977 Apr;130(1):472–484. doi: 10.1128/jb.130.1.472-484.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Donahue T. F., Culbertson M. R. Selection of spontaneous mutants by inositol starvation in yeast. Mol Gen Genet. 1975 Dec 30;143(1):5–11. doi: 10.1007/BF00269415. [DOI] [PubMed] [Google Scholar]
- Hill D. E., Struhl K. Molecular characterization of GCD1, a yeast gene required for general control of amino acid biosynthesis and cell-cycle initiation. Nucleic Acids Res. 1988 Oct 11;16(19):9253–9265. doi: 10.1093/nar/16.19.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Mechanisms of gene regulation in the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Microbiol Rev. 1988 Jun;52(2):248–273. doi: 10.1128/mr.52.2.248-273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland M. J., Holland J. P., Thill G. P., Jackson K. A. The primary structures of two yeast enolase genes. Homology between the 5' noncoding flanking regions of yeast enolase and glyceraldehyde-3-phosphate dehydrogenase genes. J Biol Chem. 1981 Feb 10;256(3):1385–1395. [PubMed] [Google Scholar]
- Holland M. J., Yokoi T., Holland J. P., Myambo K., Innis M. A. The GCR1 gene encodes a positive transcriptional regulator of the enolase and glyceraldehyde-3-phosphate dehydrogenase gene families in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Feb;7(2):813–820. doi: 10.1128/mcb.7.2.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann M. J., Poole M. A., Gaynor P. M., Ho C. T., Carman G. M. Effect of growth phase on phospholipid biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1987 Feb;169(2):533–539. doi: 10.1128/jb.169.2.533-539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré B., Leffers H., Madsen P., Rasmussen H. H., Vandekerckhove J., Celis J. E. Molecular cloning and expression of a transformation-sensitive human protein containing the TPR motif and sharing identity to the stress-inducible yeast protein STI1. J Biol Chem. 1992 Apr 25;267(12):8485–8491. [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Regulatory role of phosphatidate phosphatase in triacylglycerol synthesis of Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Oct 24;796(1):110–117. [PubMed] [Google Scholar]
- Hubbard E. J., Yang X. L., Carlson M. Relationship of the cAMP-dependent protein kinase pathway to the SNF1 protein kinase and invertase expression in Saccharomyces cerevisiae. Genetics. 1992 Jan;130(1):71–80. doi: 10.1093/genetics/130.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang P. K., Tugendreich S., Fletterick R. J. Molecular analysis of GPH1, the gene encoding glycogen phosphorylase in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Apr;9(4):1659–1666. doi: 10.1128/mcb.9.4.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörtner H., Ammerer G., Hartter E., Hamilton B., Rytka J., Bilinski T., Ruis H. Regulation of synthesis of catalases and iso-1-cytochrome c in Saccharomyces cerevisiae by glucose, oxygen and heme. Eur J Biochem. 1982 Nov;128(1):179–184. doi: 10.1111/j.1432-1033.1982.tb06949.x. [DOI] [PubMed] [Google Scholar]
- Iida H., Yahara I. Durable synthesis of high molecular weight heat shock proteins in G0 cells of the yeast and other eucaryotes. J Cell Biol. 1984 Jul;99(1 Pt 1):199–207. doi: 10.1083/jcb.99.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jigami Y., Toshimitsu N., Fujisawa H., Uemura H., Tanaka H., Nakasato S. Analysis of expression of yeast enolase 1 gene containing a longer pyrimidine-rich region located between the TATA box and transcription start site. J Biochem. 1986 Apr;99(4):1111–1125. doi: 10.1093/oxfordjournals.jbchem.a135575. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Pringle J. R., Hartwell L. H. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977 Mar 1;105(1):79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- Jones E. W. The synthesis and function of proteases in Saccharomyces: genetic approaches. Annu Rev Genet. 1984;18:233–270. doi: 10.1146/annurev.ge.18.120184.001313. [DOI] [PubMed] [Google Scholar]
- Jones S., Vignais M. L., Broach J. R. The CDC25 protein of Saccharomyces cerevisiae promotes exchange of guanine nucleotides bound to ras. Mol Cell Biol. 1991 May;11(5):2641–2646. doi: 10.1128/mcb.11.5.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback D. B., Feldberg L. R. Saccharomyces cerevisiae exhibits a sporulation-specific temporal pattern of transcript accumulation. Mol Cell Biol. 1985 Apr;5(4):751–761. doi: 10.1128/mcb.5.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Broek D., Wigler M. DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell. 1985 Dec;43(2 Pt 1):493–505. doi: 10.1016/0092-8674(85)90179-5. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., Cameron S., Fasano O., Goldfarb M., Broach J., Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985 Jan;40(1):19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., McGill C., Fasano O., Strathern J., Broach J., Wigler M. Genetic analysis of yeast RAS1 and RAS2 genes. Cell. 1984 Jun;37(2):437–445. doi: 10.1016/0092-8674(84)90374-x. [DOI] [PubMed] [Google Scholar]
- Kearsey S., Kipling D. Recombination and RNA processing: a common strand? Trends Cell Biol. 1991 Nov;1(5):110–112. doi: 10.1016/0962-8924(91)90101-e. [DOI] [PubMed] [Google Scholar]
- Kim J., Ljungdahl P. O., Fink G. R. kem mutations affect nuclear fusion in Saccharomyces cerevisiae. Genetics. 1990 Dec;126(4):799–812. doi: 10.1093/genetics/126.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipling D., Tambini C., Kearsey S. E. rar mutations which increase artificial chromosome stability in Saccharomyces cerevisiae identify transcription and recombination proteins. Nucleic Acids Res. 1991 Apr 11;19(7):1385–1391. doi: 10.1093/nar/19.7.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R., Evans D. H., Morrison P. T. Purification and characterization of an activity from Saccharomyces cerevisiae that catalyzes homologous pairing and strand exchange. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5560–5564. doi: 10.1073/pnas.84.16.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas R. Misconceptions about the energy metabolism of Saccharomyces cerevisiae. Yeast. 1986 Dec;2(4):221–228. doi: 10.1002/yea.320020403. [DOI] [PubMed] [Google Scholar]
- Larimer F. W., Stevens A. Disruption of the gene XRN1, coding for a 5'----3' exoribonuclease, restricts yeast cell growth. Gene. 1990 Oct 30;95(1):85–90. doi: 10.1016/0378-1119(90)90417-p. [DOI] [PubMed] [Google Scholar]
- Lee D. H., Tamura T., Chung C. H., Tanaka K., Ichihara A. Molecular cloning of the yeast proteasome PRS2 gene identical to the suppressor gene scl1+. Biochem Int. 1991 Mar;23(4):689–696. [PubMed] [Google Scholar]
- Lee F. J., Lin L. W., Smith J. A. A glucose-repressible gene encodes acetyl-CoA hydrolase from Saccharomyces cerevisiae. J Biol Chem. 1990 May 5;265(13):7413–7418. [PubMed] [Google Scholar]
- Lee F. J., Lin L. W., Smith J. A. N alpha acetylation is required for normal growth and mating of Saccharomyces cerevisiae. J Bacteriol. 1989 Nov;171(11):5795–5802. doi: 10.1128/jb.171.11.5795-5802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. J., Lin L. W., Smith J. A. Purification and characterization of an N alpha-acetyltransferase from Saccharomyces cerevisiae. J Biol Chem. 1988 Oct 15;263(29):14948–14955. [PubMed] [Google Scholar]
- Legrain P., Chapon C., Galisson F. Proteins involved in mitosis, RNA synthesis and premRNA splicing share a common repeating motif. Nucleic Acids Res. 1991 May 11;19(9):2509–2510. doi: 10.1093/nar/19.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie S. H., Pringle J. R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980 Sep;143(3):1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P., Lenzen G., Jacquemin J. M., Danchin A. Yeast adenylate cyclase catalytic domain is carboxy terminal. Curr Genet. 1986;10(5):343–352. doi: 10.1007/BF00418405. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Oshima Y., Ishikawa T. Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2355–2359. doi: 10.1073/pnas.79.7.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister L., Holland M. J. Differential expression of the three yeast glyceraldehyde-3-phosphate dehydrogenase genes. J Biol Chem. 1985 Dec 5;260(28):15019–15027. [PubMed] [Google Scholar]
- McAlister L., Holland M. J. Targeted deletion of a yeast enolase structural gene. Identification and isolation of yeast enolase isozymes. J Biol Chem. 1982 Jun 25;257(12):7181–7188. [PubMed] [Google Scholar]
- McCusker J. H., Haber J. E. Cycloheximide-resistant temperature-sensitive lethal mutations of Saccharomyces cerevisiae. Genetics. 1988 Jun;119(2):303–315. doi: 10.1093/genetics/119.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker J. H., Haber J. E. crl mutants of Saccharomyces cerevisiae resemble both mutants affecting general control of amino acid biosynthesis and omnipotent translational suppressor mutants. Genetics. 1988 Jun;119(2):317–327. doi: 10.1093/genetics/119.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw P., Henry S. A. Mutations in the Saccharomyces cerevisiae opi3 gene: effects on phospholipid methylation, growth and cross-pathway regulation of inositol synthesis. Genetics. 1989 Jun;122(2):317–330. doi: 10.1093/genetics/122.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C. S., Magee P. T., Hartwell L. H. Role of isoleucyl-transfer ribonucleic acid synthetase in ribonucleic acid synthesis and enzyme repression in yeast. J Bacteriol. 1969 Nov;100(2):579–584. doi: 10.1128/jb.100.2.579-584.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C. S., Warner J. R., Edmonds M., Nakazato H., Vaughan M. H. Polyadenylic acid sequences in yeast messenger ribonucleic acid. J Biol Chem. 1973 Feb 25;248(4):1466–1471. [PubMed] [Google Scholar]
- Mendenhall M. D., Jones C. A., Reed S. I. Dual regulation of the yeast CDC28-p40 protein kinase complex: cell cycle, pheromone, and nutrient limitation effects. Cell. 1987 Sep 11;50(6):927–935. doi: 10.1016/0092-8674(87)90519-8. [DOI] [PubMed] [Google Scholar]
- Meussdoerffer F., Fink G. R. Structure and expression of two aminoacyl-tRNA synthetase genes from Saccharomyces cerevisiae. J Biol Chem. 1983 May 25;258(10):6293–6299. [PubMed] [Google Scholar]
- Mitts M. R., Bradshaw-Rouse J., Heideman W. Interactions between adenylate cyclase and the yeast GTPase-activating protein IRA1. Mol Cell Biol. 1991 Sep;11(9):4591–4598. doi: 10.1128/mcb.11.9.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitts M. R., Grant D. B., Heideman W. Adenylate cyclase in Saccharomyces cerevisiae is a peripheral membrane protein. Mol Cell Biol. 1990 Aug;10(8):3873–3883. doi: 10.1128/mcb.10.8.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle C. M., Aynardi M. W., Kolodny M. R., Park F. J., Jones E. W. Protease B of Saccharomyces cerevisiae: isolation and regulation of the PRB1 structural gene. Genetics. 1987 Feb;115(2):255–263. doi: 10.1093/genetics/115.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle C. M., Dixon C. K., Jones E. W. Processing pathway for protease B of Saccharomyces cerevisiae. J Cell Biol. 1989 Feb;108(2):309–325. doi: 10.1083/jcb.108.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle C. M., Jones E. W. Consequences of growth media, gene copy number, and regulatory mutations on the expression of the PRB1 gene of Saccharomyces cerevisiae. Genetics. 1990 Jan;124(1):39–55. doi: 10.1093/genetics/124.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita T., Uno I. A dominant interfering mutation (CYR3) of the Saccharomyces cerevisiae RAS2 gene. J Bacteriol. 1991 Jul;173(14):4533–4536. doi: 10.1128/jb.173.14.4533-4536.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountain H. A., Sudbery P. E. Regulation of the Saccharomyces cerevisiae WHI2 gene. J Gen Microbiol. 1990 Apr;136(4):727–732. doi: 10.1099/00221287-136-4-727. [DOI] [PubMed] [Google Scholar]
- Mountain H. A., Sudbery P. E. The relationship of growth rate and catabolite repression with WHI2 expression and cell size in Saccharomyces cerevisiae. J Gen Microbiol. 1990 Apr;136(4):733–737. doi: 10.1099/00221287-136-4-733. [DOI] [PubMed] [Google Scholar]
- Mullen J. R., Kayne P. S., Moerschell R. P., Tsunasawa S., Gribskov M., Colavito-Shepanski M., Grunstein M., Sherman F., Sternglanz R. Identification and characterization of genes and mutants for an N-terminal acetyltransferase from yeast. EMBO J. 1989 Jul;8(7):2067–2075. doi: 10.1002/j.1460-2075.1989.tb03615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neigeborn L., Carlson M. Genes affecting the regulation of SUC2 gene expression by glucose repression in Saccharomyces cerevisiae. Genetics. 1984 Dec;108(4):845–858. doi: 10.1093/genetics/108.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolet C. M., Craig E. A. Isolation and characterization of STI1, a stress-inducible gene from Saccharomyces cerevisiae. Mol Cell Biol. 1989 Sep;9(9):3638–3646. doi: 10.1128/mcb.9.9.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederberger P., Miozzari G., Hütter R. Biological role of the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Jul;1(7):584–593. doi: 10.1128/mcb.1.7.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa J., Sass P., Wigler M. Cloning and characterization of the low-affinity cyclic AMP phosphodiesterase gene of Saccharomyces cerevisiae. Mol Cell Biol. 1987 Oct;7(10):3629–3636. doi: 10.1128/mcb.7.10.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E., Finley D., Solomon M. J., Varshavsky A. The yeast ubiquitin genes: a family of natural gene fusions. EMBO J. 1987 May;6(5):1429–1439. doi: 10.1002/j.1460-2075.1987.tb02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E. C., Szostak J. W. ARD1 and NAT1 proteins form a complex that has N-terminal acetyltransferase activity. EMBO J. 1992 Jun;11(6):2087–2093. doi: 10.1002/j.1460-2075.1992.tb05267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell D. A., Sanchez Y., Stitzel J. D., Lindquist S. Hsp104 is a highly conserved protein with two essential nucleotide-binding sites. Nature. 1991 Sep 19;353(6341):270–273. doi: 10.1038/353270a0. [DOI] [PubMed] [Google Scholar]
- Pavlović B., Hörz W. The chromatin structure at the promoter of a glyceraldehyde phosphate dehydrogenase gene from Saccharomyces cerevisiae reflects its functional state. Mol Cell Biol. 1988 Dec;8(12):5513–5520. doi: 10.1128/mcb.8.12.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petko L., Lindquist S. Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell. 1986 Jun 20;45(6):885–894. doi: 10.1016/0092-8674(86)90563-5. [DOI] [PubMed] [Google Scholar]
- Phillips S. L., Tse C., Serventi I., Hynes N. Structure of polyadenylic acid in the ribonucleic acid of Saccharomyces cerevisiae. J Bacteriol. 1979 May;138(2):542–551. doi: 10.1128/jb.138.2.542-551.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillar T. M., Bradshaw R. E. Heat shock and stationary phase induce transcription of the Saccharomyces cerevisiae iso-2 cytochrome c gene. Curr Genet. 1991 Aug;20(3):185–188. doi: 10.1007/BF00326230. [DOI] [PubMed] [Google Scholar]
- Piñon R. A probe into nuclear events during the cell cycle of Saccharomyces cerevisiae: studies of folded chromosomes in cdc mutants which arrest in G1. Chromosoma. 1979 Jan 31;70(3):337–352. doi: 10.1007/BF00328771. [DOI] [PubMed] [Google Scholar]
- Piñon R. Folded chromosomes in meiotic yeast cells: analysis of early meiotic events. J Mol Biol. 1979 Apr 15;129(3):433–447. doi: 10.1016/0022-2836(79)90505-9. [DOI] [PubMed] [Google Scholar]
- Piñon R. Folded chromosomes in non-cycling yeast cells: evidence for a characteristic g0 form. Chromosoma. 1978 Jul 31;67(3):263–274. doi: 10.1007/BF02569039. [DOI] [PubMed] [Google Scholar]
- Plesset J., Ludwig J. R., Cox B. S., McLaughlin C. S. Effect of cell cycle position on thermotolerance in Saccharomyces cerevisiae. J Bacteriol. 1987 Feb;169(2):779–784. doi: 10.1128/jb.169.2.779-784.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praekelt U. M., Meacock P. A. HSP12, a new small heat shock gene of Saccharomyces cerevisiae: analysis of structure, regulation and function. Mol Gen Genet. 1990 Aug;223(1):97–106. doi: 10.1007/BF00315801. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. Ubiquitin-mediated pathways for intracellular proteolysis. Annu Rev Cell Biol. 1987;3:1–30. doi: 10.1146/annurev.cb.03.110187.000245. [DOI] [PubMed] [Google Scholar]
- Reed S. I. G1-specific cyclins: in search of an S-phase-promoting factor. Trends Genet. 1991 Mar;7(3):95–99. doi: 10.1016/0168-9525(91)90279-Y. [DOI] [PubMed] [Google Scholar]
- Reed S. I. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics. 1980 Jul;95(3):561–577. doi: 10.1093/genetics/95.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads R. E. Cap recognition and the entry of mRNA into the protein synthesis initiation cycle. Trends Biochem Sci. 1988 Feb;13(2):52–56. doi: 10.1016/0968-0004(88)90028-x. [DOI] [PubMed] [Google Scholar]
- Robinson L. C., Gibbs J. B., Marshall M. S., Sigal I. S., Tatchell K. CDC25: a component of the RAS-adenylate cyclase pathway in Saccharomyces cerevisiae. Science. 1987 Mar 6;235(4793):1218–1221. doi: 10.1126/science.3547648. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Fink G. R. KAR1, a gene required for function of both intranuclear and extranuclear microtubules in yeast. Cell. 1987 Mar 27;48(6):1047–1060. doi: 10.1016/0092-8674(87)90712-4. [DOI] [PubMed] [Google Scholar]
- Rudolph H. K., Antebi A., Fink G. R., Buckley C. M., Dorman T. E., LeVitre J., Davidow L. S., Mao J. I., Moir D. T. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989 Jul 14;58(1):133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- Sakai A., Shimizu Y., Kondou S., Chibazakura T., Hishinuma F. Structure and molecular analysis of RGR1, a gene required for glucose repression of Saccharomyces cerevisiae. Mol Cell Biol. 1990 Aug;10(8):4130–4138. doi: 10.1128/mcb.10.8.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y., Lindquist S. L. HSP104 required for induced thermotolerance. Science. 1990 Jun 1;248(4959):1112–1115. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- Sanchez Y., Taulien J., Borkovich K. A., Lindquist S. Hsp104 is required for tolerance to many forms of stress. EMBO J. 1992 Jun;11(6):2357–2364. doi: 10.1002/j.1460-2075.1992.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass P., Field J., Nikawa J., Toda T., Wigler M. Cloning and characterization of the high-affinity cAMP phosphodiesterase of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9303–9307. doi: 10.1073/pnas.83.24.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt H. D., Puzicha M., Gallwitz D. Study of a temperature-sensitive mutant of the ras-related YPT1 gene product in yeast suggests a role in the regulation of intracellular calcium. Cell. 1988 May 20;53(4):635–647. doi: 10.1016/0092-8674(88)90579-x. [DOI] [PubMed] [Google Scholar]
- Schmitt H. D., Wagner P., Pfaff E., Gallwitz D. The ras-related YPT1 gene product in yeast: a GTP-binding protein that might be involved in microtubule organization. Cell. 1986 Nov 7;47(3):401–412. doi: 10.1016/0092-8674(86)90597-0. [DOI] [PubMed] [Google Scholar]
- Segev N., Botstein D. The ras-like yeast YPT1 gene is itself essential for growth, sporulation, and starvation response. Mol Cell Biol. 1987 Jul;7(7):2367–2377. doi: 10.1128/mcb.7.7.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988 Mar 25;52(6):915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Sentandreu R., Herrero E., Martínez-García J. P., Larriba G. Biogenesis of the yeast cell wall. Subcell Biochem. 1984;10:193–235. doi: 10.1007/978-1-4613-2709-7_3. [DOI] [PubMed] [Google Scholar]
- Seufert W., Jentsch S. In vivo function of the proteasome in the ubiquitin pathway. EMBO J. 1992 Aug;11(8):3077–3080. doi: 10.1002/j.1460-2075.1992.tb05379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert W., Jentsch S. Ubiquitin-conjugating enzymes UBC4 and UBC5 mediate selective degradation of short-lived and abnormal proteins. EMBO J. 1990 Feb;9(2):543–550. doi: 10.1002/j.1460-2075.1990.tb08141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert W., McGrath J. P., Jentsch S. UBC1 encodes a novel member of an essential subfamily of yeast ubiquitin-conjugating enzymes involved in protein degradation. EMBO J. 1990 Dec;9(13):4535–4541. doi: 10.1002/j.1460-2075.1990.tb07905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Adam G., Rapatz W., Spevak W., Ruis H. The Saccharomyces cerevisiae ADR1 gene is a positive regulator of transcription of genes encoding peroxisomal proteins. Mol Cell Biol. 1991 Feb;11(2):699–704. doi: 10.1128/mcb.11.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. The SPA2 protein of yeast localizes to sites of cell growth. J Cell Biol. 1989 Apr;108(4):1419–1429. doi: 10.1083/jcb.108.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin S. J., Saunders C. A. Fluctuation in polyadenylate size and content in exponential- and stationary-phase cells of Saccharomyces cerevisiae. J Bacteriol. 1980 Oct;144(1):74–81. doi: 10.1128/jb.144.1.74-81.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spevak W., Hartig A., Meindl P., Ruis H. Heme control region of the catalase T gene of the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1986 Apr;203(1):73–78. doi: 10.1007/BF00330386. [DOI] [PubMed] [Google Scholar]
- Stevens A., Maupin M. K. A 5'----3' exoribonuclease of Saccharomyces cerevisiae: size and novel substrate specificity. Arch Biochem Biophys. 1987 Feb 1;252(2):339–347. doi: 10.1016/0003-9861(87)90040-3. [DOI] [PubMed] [Google Scholar]
- Stevens A. Purification and characterization of a Saccharomyces cerevisiae exoribonuclease which yields 5'-mononucleotides by a 5' leads to 3' mode of hydrolysis. J Biol Chem. 1980 Apr 10;255(7):3080–3085. [PubMed] [Google Scholar]
- Sudbery P. E., Goodey A. R., Carter B. L. Genes which control cell proliferation in the yeast Saccharomyces cerevisiae. Nature. 1980 Nov 27;288(5789):401–404. doi: 10.1038/288401a0. [DOI] [PubMed] [Google Scholar]
- Susek R. E., Lindquist S. Transcriptional derepression of the Saccharomyces cerevisiae HSP26 gene during heat shock. Mol Cell Biol. 1990 Dec;10(12):6362–6373. doi: 10.1128/mcb.10.12.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Immanuel D., Arndt K. T. The SIT4 protein phosphatase functions in late G1 for progression into S phase. Mol Cell Biol. 1991 Apr;11(4):2133–2148. doi: 10.1128/mcb.11.4.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Lin B. K., Wood D. R., Tamanoi F. IRA2, an upstream negative regulator of RAS in yeast, is a RAS GTPase-activating protein. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):468–472. doi: 10.1073/pnas.88.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Matsumoto K., Toh-E A. IRA1, an inhibitory regulator of the RAS-cyclic AMP pathway in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Feb;9(2):757–768. doi: 10.1128/mcb.9.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Matsumoto K., Toh-e A. Dual regulation of the expression of the polyubiquitin gene by cyclic AMP and heat shock in yeast. EMBO J. 1988 Feb;7(2):495–502. doi: 10.1002/j.1460-2075.1988.tb02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Nakafuku M., Satoh T., Marshall M. S., Gibbs J. B., Matsumoto K., Kaziro Y., Toh-e A. S. cerevisiae genes IRA1 and IRA2 encode proteins that may be functionally equivalent to mammalian ras GTPase activating protein. Cell. 1990 Mar 9;60(5):803–807. doi: 10.1016/0092-8674(90)90094-u. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Nakafuku M., Tamanoi F., Kaziro Y., Matsumoto K., Toh-e A. IRA2, a second gene of Saccharomyces cerevisiae that encodes a protein with a domain homologous to mammalian ras GTPase-activating protein. Mol Cell Biol. 1990 Aug;10(8):4303–4313. doi: 10.1128/mcb.10.8.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell K. RAS genes and growth control in Saccharomyces cerevisiae. J Bacteriol. 1986 May;166(2):364–367. doi: 10.1128/jb.166.2.364-367.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell K., Robinson L. C., Breitenbach M. RAS2 of Saccharomyces cerevisiae is required for gluconeogenic growth and proper response to nutrient limitation. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3785–3789. doi: 10.1073/pnas.82.11.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor F. R., Parks L. W. Triaglycerol metabolism in Saccharomyces cerevisiae. Relation to phospholipid synthesis. Biochim Biophys Acta. 1979 Nov 21;575(2):204–214. doi: 10.1016/0005-2760(79)90022-5. [DOI] [PubMed] [Google Scholar]
- Taylor S. S., Buechler J. A., Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Thevelein J. M. Regulation of trehalose mobilization in fungi. Microbiol Rev. 1984 Mar;48(1):42–59. doi: 10.1128/mr.48.1.42-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Jaeger S., François J., Gaughran J. P., Tatchell K. Deletion of SNF1 affects the nutrient response of yeast and resembles mutations which activate the adenylate cyclase pathway. Genetics. 1991 Nov;129(3):697–706. doi: 10.1093/genetics/129.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff D. X., Johnson A. W., Kolodner R. D. Molecular and genetic analysis of the gene encoding the Saccharomyces cerevisiae strand exchange protein Sep1. Mol Cell Biol. 1991 May;11(5):2593–2608. doi: 10.1128/mcb.11.5.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Scott J. D., McMullen B., Hurwitz M., Krebs E. G., Wigler M. Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Apr;7(4):1371–1377. doi: 10.1128/mcb.7.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Wigler M. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell. 1987 Jul 17;50(2):277–287. doi: 10.1016/0092-8674(87)90223-6. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Trumbly R. J. Glucose repression in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1992 Jan;6(1):15–21. doi: 10.1111/j.1365-2958.1992.tb00832.x. [DOI] [PubMed] [Google Scholar]
- Uno I., Mitsuzawa H., Tanaka K., Oshima T., Ishikawa T. Identification of the domain of Saccharomyces cerevisiae adenylate cyclase associated with the regulatory function of RAS products. Mol Gen Genet. 1987 Dec;210(2):187–194. doi: 10.1007/BF00325683. [DOI] [PubMed] [Google Scholar]
- Valentín E., Herrero E., Rico H., Miragall F., Sentandreu R. Cell wall mannoproteins during the population growth phases in Saccharomyces cerevisiae. Arch Microbiol. 1987 Jul;148(2):88–94. doi: 10.1007/BF00425354. [DOI] [PubMed] [Google Scholar]
- Vallari R. C., Cook W. J., Audino D. C., Morgan M. J., Jensen D. E., Laudano A. P., Denis C. L. Glucose repression of the yeast ADH2 gene occurs through multiple mechanisms, including control of the protein synthesis of its transcriptional activator, ADR1. Mol Cell Biol. 1992 Apr;12(4):1663–1673. doi: 10.1128/mcb.12.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier J. M., Camonis J. H., Jacquet M. Cloning of CDC33: a gene essential for growth and sporulation which does not interfere with cAMP production in Saccharomyces cerevisiae. Yeast. 1989 Mar-Apr;5(2):79–90. doi: 10.1002/yea.320050203. [DOI] [PubMed] [Google Scholar]
- Vojtek A., Haarer B., Field J., Gerst J., Pollard T. D., Brown S., Wigler M. Evidence for a functional link between profilin and CAP in the yeast S. cerevisiae. Cell. 1991 Aug 9;66(3):497–505. doi: 10.1016/0092-8674(81)90013-1. [DOI] [PubMed] [Google Scholar]
- Wek R. C., Jackson B. M., Hinnebusch A. G. Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4579–4583. doi: 10.1073/pnas.86.12.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Washburne M., Becker J., Kosic-Smithers J., Craig E. A. Yeast Hsp70 RNA levels vary in response to the physiological status of the cell. J Bacteriol. 1989 May;171(5):2680–2688. doi: 10.1128/jb.171.5.2680-2688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Washburne M., Brown D., Braun E. Bcy1, the regulatory subunit of cAMP-dependent protein kinase in yeast, is differentially modified in response to the physiological status of the cell. J Biol Chem. 1991 Oct 15;266(29):19704–19709. [PubMed] [Google Scholar]
- Whiteway M., Freedman R., Van Arsdell S., Szostak J. W., Thorner J. The yeast ARD1 gene product is required for repression of cryptic mating-type information at the HML locus. Mol Cell Biol. 1987 Oct;7(10):3713–3722. doi: 10.1128/mcb.7.10.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteway M., Szostak J. W. The ARD1 gene of yeast functions in the switch between the mitotic cell cycle and alternative developmental pathways. Cell. 1985 Dec;43(2 Pt 1):483–492. doi: 10.1016/0092-8674(85)90178-3. [DOI] [PubMed] [Google Scholar]
- Wiemken A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Van Leeuwenhoek. 1990 Oct;58(3):209–217. doi: 10.1007/BF00548935. [DOI] [PubMed] [Google Scholar]
- Williams N. P., Hinnebusch A. G., Donahue T. F. Mutations in the structural genes for eukaryotic initiation factors 2 alpha and 2 beta of Saccharomyces cerevisiae disrupt translational control of GCN4 mRNA. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7515–7519. doi: 10.1073/pnas.86.19.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills C. Regulation of sugar and ethanol metabolism in Saccharomyces cerevisiae. Crit Rev Biochem Mol Biol. 1990;25(4):245–280. doi: 10.3109/10409239009090611. [DOI] [PubMed] [Google Scholar]
- Wilson R. B., Brenner A. A., White T. B., Engler M. J., Gaughran J. P., Tatchell K. The Saccharomyces cerevisiae SRK1 gene, a suppressor of bcy1 and ins1, may be involved in protein phosphatase function. Mol Cell Biol. 1991 Jun;11(6):3369–3373. doi: 10.1128/mcb.11.6.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. B., Tatchell K. SRA5 encodes the low-Km cyclic AMP phosphodiesterase of Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jan;8(1):505–510. doi: 10.1128/mcb.8.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H., Adam G., Mattes E., Schanz M., Hartig A., Ruis H. Co-ordinate control of synthesis of mitochondrial and non-mitochondrial hemoproteins: a binding site for the HAP1 (CYP1) protein in the UAS region of the yeast catalase T gene (CTT1). EMBO J. 1988 Jun;7(6):1799–1804. doi: 10.1002/j.1460-2075.1988.tb03011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg C., Sugimoto K., Reed S. I. G1-specific cyclins of S. cerevisiae: cell cycle periodicity, regulation by mating pheromone, and association with the p34CDC28 protein kinase. Cell. 1990 Jul 27;62(2):225–237. doi: 10.1016/0092-8674(90)90361-h. [DOI] [PubMed] [Google Scholar]
- Wolfner M., Yep D., Messenguy F., Fink G. R. Integration of amino acid biosynthesis into the cell cycle of Saccharomyces cerevisiae. J Mol Biol. 1975 Aug 5;96(2):273–290. doi: 10.1016/0022-2836(75)90348-4. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Kaufmann I., Rasenberger H., Haubetamann P. Genetics of carbon catabolite repression in Saccharomycess cerevisiae: genes involved in the derepression process. Mol Gen Genet. 1977 Feb 28;151(1):95–103. doi: 10.1007/BF00446918. [DOI] [PubMed] [Google Scholar]
- Zlotnik H., Fernandez M. P., Bowers B., Cabib E. Saccharomyces cerevisiae mannoproteins form an external cell wall layer that determines wall porosity. J Bacteriol. 1984 Sep;159(3):1018–1026. doi: 10.1128/jb.159.3.1018-1026.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nobel J. G., Klis F. M., Priem J., Munnik T., van den Ende H. The glucanase-soluble mannoproteins limit cell wall porosity in Saccharomyces cerevisiae. Yeast. 1990 Nov-Dec;6(6):491–499. doi: 10.1002/yea.320060606. [DOI] [PubMed] [Google Scholar]


