Abstract
Recent findings from developmental neuroimaging studies suggest that the enhancement of cognitive processes during development may be the result of a fine-tuning of the structural and functional organization of brain with maturation. However, the details regarding the developmental trajectory of large-scale structural brain networks are not yet understood. Here, we used graph theory to examine developmental changes in the organization of structural brain networks in 203 normally growing children and adolescents. Structural brain networks were constructed using interregional correlations in cortical thickness for 4 age groups (early childhood: 4.8–8.4 year; late childhood: 8.5–11.3 year; early adolescence: 11.4–14.7 year; late adolescence: 14.8–18.3 year). Late childhood showed prominent changes in topological properties, specifically a significant reduction in local efficiency, modularity, and increased global efficiency, suggesting a shift of topological organization toward a more random configuration. An increase in number and span of distribution of connector hubs was found in this age group. Finally, inter-regional connectivity analysis and graph-theoretic measures indicated early maturation of primary sensorimotor regions and protracted development of higher order association and paralimbic regions. Our finding reveals a time window of plasticity occurring during late childhood which may accommodate crucial changes during puberty and the new developmental tasks that an adolescent faces.
Keywords: adolescence, connectivity, connector hub, cortical thickness, maturation
Introduction
Converging evidence from behavioral, imaging, and post-mortem studies on human and non-human primates have demonstrated dramatic structural and functional brain changes during development from infancy to adulthood that extend till the third decade of life (Huttenlocher and Dabholkar 1997; Giedd et al. 1999; Sowell et al. 2003; Casey et al. 2010; Lebel and Beaulieu 2011; Petanjek et al. 2011). Longitudinal magnetic resonance (MR) scans of typically developing children and adolescents have demonstrated increasing white matter (WM) volumes and inverted U-shaped trajectories of gray matter (GM) volumes with increasing age (Giedd et al. 1999; Gogtay et al. 2004). This process of structural brain maturation extends beyond adolescence to adulthood, as evident from human imaging studies (Sowell et al. 2003; Lebel and Beaulieu 2011) and circuitry reorganization in post-mortem tissue (Petanjek et al. 2011).
Several recent studies have postulated that the measurable enhancement of cognitive skills occurring with typical development may be the result of a process of fine-tuning of brain structure and function, which may underlie brain changes as described above. A fine-tuning may involve progression from an immature and less organized brain in early life toward a mature organizational structure during development (Changeux and Danchin 1976; Casey et al. 1997; Brown et al. 2005; Durston et al. 2006). A process of fine-tuning is supported by post-mortem studies on humans and non-human primates (Rakic et al. 1994) as well as imaging studies (Giedd et al. 1999; Paus 2005; Kuhn 2006; Fair et al. 2008, 2009; Dosenbach et al. 2010; Tamnes et al. 2010). A synaptic fine-tuning process was first proposed by Changeux and Danchin (1976) as a mechanism of synaptic stabilization during development. The basic assumption of this hypothesis was that though connections were genetically programmed, neuronal activity determined the final wiring pattern by refining those connections through synaptic elimination. Structural and functional imaging studies have also suggested circuitry reorganization during development. Structural imaging studies have indicated GM loss occurring earliest at lower order primary sensorimotor areas and later in higher order association areas in typical development, suggesting a progressive refinement of neural connections through ongoing neural regressive events (such as pruning and elimination; Paus 2005; Kuhn 2006). During the same developmental time period, there is increasing WM volume, presumably reflecting ongoing myelination of axons by oligodendrocytes that would contribute to enhanced conduction (Giedd et al. 1999; Tamnes et al. 2010). Consistent with structural imaging findings, functional MR imaging (fMRI) studies have shown weakening of short-range and strengthening of long-range functional connections during development that contribute to brain maturity (Fair et al. 2008, 2009; Dosenbach et al. 2010). Therefore, collectively, current evidence indicates that structural and functional changes observed with typical brain development may reflect a process of fine-tuning that supports enhanced cognitive function with increased brain maturation (Amso and Casey 2006).
Up to now, evidence from structural imaging studies for the fine-tuning hypothesis of brain development has been based on examining structure at the regional level, though recent evidence also implicates the presence of structural changes occurring at the global (large-scale) level during maturation (Zielinski et al. 2010; Raznahan et al. 2011). As yet, large-scale analysis of structural brain networks that would provide us with an improved conceptual understanding of developmental fine-tuning has not been undertaken. Graph-theoretic tools are ideal for such an analysis as they have been extensively used for investigating large-scale brain networks in healthy and diseased populations (Bullmore and Sporns 2009; He and Evans 2010). The principal goal of this study was therefore to assess the maturational changes in the organization of structural brain networks using graph theory as well as inter-regional connectivity analyses. We hypothesized that graph theory metrics would support a process of fine-tuning in typical structural brain development. Specifically, we predicted that structural brain networks inferred from cortical thickness, a measure of GM, would show age-related developmental trajectories consistent with previous regionally based analyses, whereby lower order primary sensorimotor networks would show maturation by early childhood, while higher order association areas and their corresponding pathways subserving higher cognitive abilities would show the most protracted maturation.
Materials and Methods
Subject Sampling and Recruitment
Data were obtained from the Pediatric MRI Data Repository (database release 2.0) created for the NIH MRI Study of Normal Brain Development. Details are available in Evans (2006). Single scans of 203 subjects were used for the study; the demographics of the subjects are given in Table 1.
Table 1.
Demographics of subjects
| Groups | Early childhood | Late childhood | Early adolescence | Late adolescence |
|---|---|---|---|---|
| N | 51 | 51 | 51 | 50 |
| Age (year) | 4.8–8.4 | 8.5–11.3 | 11.4–14.7 | 14.8–18.3 |
| Gender (M/F) | 25/26 | 24/27 | 25/26 | 23/27 |
Note: The population sample consisted of 203 subjects (one scan per subject) and was classified into 4 age groups. Since structural brain networks were constructed by calculating the inter-regional cortical thickness across subjects, we selected the same number of subjects (n = 51) for each group, except late adolescence (n = 50).
Cortical Thickness Measurements
Using a 9-parameter linear transformation (Collins et al. 1995), native MRI images were corrected for non-uniformity artifacts using the N3 algorithm (Sled et al. 1998) and registered into stereotaxic space (Talairach and Tournoux 1988). Using an advanced neural net classifier (Zijdenbos et al. 2002), the registered and corrected images were further segmented into GM, WM, cerebrospinal fluid, and background. From each MR volume, the inner and outer GM surfaces were then automatically extracted using the Constrained Laplacian-based Automated Segmentation with Proximities (CLASP) algorithm (MacDonald et al. 2000; Kim et al. 2005). Cortical thickness was then measured in native space using the linked distance between the 2 surfaces at 81 924 vertices throughout the cortex (Shaw et al. 2006). Validation of the CLASP cortical thickness algorithm has been done using both manual measurements (Kabani et al. 2001) and simulation approaches (Lerch and Evans 2005; Lee et al. 2006).
Cortical Parcellations
We used the automatic anatomical labeling (AAL) atlas that is widely used for brain parcellation (Tzourio-Mazoyer et al. 2002). Since our analysis is based upon a cortical surface model, we have included only those 78 AAL regions that are defined for the neocortex. Cortical thickness for each brain region was calculated as the average thickness of all vertices in that region (He et al. 2007).
Construction of Structural Cortical Networks
Network Edges
Since our structural brain networks are constructed on the basis of cortical thickness covariance in a “group” of subjects (see below), we divided our population sample into 4 age groups (early childhood: Group-I: n = 51, age = 4.8–8.4 years; late childhood: Group-II: n = 51, age = 8.5–11.3 years; early adolescence: Group-III: n = 51, age = 11.4–14.7 years; late adolescence: Group-IV: n = 50, age = 14.8–18.3 years; Table 1).
Structural connectivity was defined as the statistical similarity between 2 brain regions, with respect to cortical thickness, a method which has been described previously (He et al. 2007). The presence of an (undirected) edge connecting 2 regions is determined by obtaining the Pearson correlation coefficient for their respective mean cortical thicknesses, across a group of subjects. This produces a correlation matrix Cij where i, j = 1, 2, … N, here N = 78. Prior to this correlation analysis, a linear regression was performed at every region to remove the effects of age, gender, and mean regional cortical thickness. The correlation matrix for each group was thresholded into a binarized matrix Bij = [bij], where bij is 1 if the absolute value of the correlation matrix Cij between regions i and j is larger than a given correlation threshold, and 0 otherwise.
Sparsity Threshold
In general, the binary graph G(N,E) is represented by the binary matrix Bij, where N is the number of nodes and E is the number of edges. The nodes and edges represent cortical regions and undirected links between them, which correspond to the nonzero elements in Bij. We denote such a graph based upon cortical thickness as GCT(N,E). As in earlier studies (He et al. 2008), a range of sparsity thresholds was used to characterize the topological differences between the groups of networks. Sparsity refers to the connectedness of a graph and is here defined as the total number of edges E in a graph divided by the maximum possible number of edges N (N − 1)/2. A fixed sparsity threshold (e.g. x% threshold implies x% of the topmost connections preserved) ensures that the graphs for different groups have the same number of edges (Achard and Bullmore 2007). A range of sparsity thresholds 5% ≤ Sτ ≤ 25% were used, as in earlier studies (Bassett et al. 2008).
Network Analysis
To investigate the global topological properties of the networks obtained for the different age-related groups, we used a number of network parameters: Global efficiency, local efficiency, and modularity. Each of these network parameters can provide insights into the global topological properties of network groups.
The global efficiency EGlobal of a graph G(N,E) is defined as (Latora and Marchiori 2001)
where dij is the shortest path length between node i and node j in G.
Small-world behavior for the graph G is assessed according to the following criteria:
1. EGlobal(Gregular) < EGlobal(G) < EGlobal(Grandom)
2. ELocal(Grandom) < ELocal(G) < ELocal(Gregular)
where EGlobal(Gregular), EGlobal(Grandom), ELocal(Gregular), and ELocal(Grandom) are the global and local efficiency values of node- and degree-matched regular and random networks.
For a graph G, a module is defined as the subset of nodes that are more densely connected to each other in the same module. Given a configuration of modular organization m with nm modules, the modularity Q(m) is defined as (Newman and Girvan 2004):
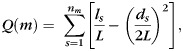 |
where L is the total number of edges of G, ls is the total number of edges in module s and ds is the sum of the degrees of the nodes in module s.
To have a summary metric and a comparative measure that can be used for all the networks groups, we used the normalized integrals of the network parameters, specifically:
where a and b are the lower and upper limits of sparsity (here, a = 5 and b = 25).
In addition to the EGlobal, ELocal, and Q parameters, we also derived metrics for the intra- and inter-modular connectivity. A fast modularity algorithm (Clauset et al. 2004) was used to find the optimal configuration of modules, after which the intra-modular and inter-modular connectivity patterns can be computed using the normalized intra-modular degree, which determines how densely a node i connects to other nodes in the same module, and the participation index P(i), which determines how densely a node i connects to nodes in other modules (Guimera et al. 2005). These parameters are defined as:
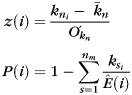 |
where 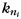 is the number of edges connecting the ith node to other nodes in its module, referred to as intra-modular node degree;
is the number of edges connecting the ith node to other nodes in its module, referred to as intra-modular node degree; 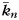 and
and 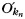 are the mean and standard variance of intra-modular node degrees of all nodes in the nth module;
are the mean and standard variance of intra-modular node degrees of all nodes in the nth module; 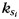 is the number of edges connecting the ith node to module si, and
is the number of edges connecting the ith node to module si, and 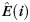 is the number of edges that connect node i to all other nodes. Nodes with z(i) > 2.5 are classified as modular hubs and are otherwise classified as non-hubs. The nodes with P(i) > 0.62 are classified as connector hubs (Guimera et al. 2005).
is the number of edges that connect node i to all other nodes. Nodes with z(i) > 2.5 are classified as modular hubs and are otherwise classified as non-hubs. The nodes with P(i) > 0.62 are classified as connector hubs (Guimera et al. 2005).
Statistical Analysis
The effect of age on cortical thickness was analyzed for the whole population using a vertex-wise general linear model in which age and gender were taken into account. T-statistic was used to test the main effect of age on cortical thickness correcting for gender using SurfStat (http://www.math.mcgill.ca/keith/surfstat/). Random field theory was used for correction of multiple comparisons of the vertex data (Taylor and Worsley 2007).
For group comparison of network parameters, we generated 1000 bootstrap samples (with replacement) from each age group (Efron and Tibshirani 1993) and computed a thickness correlation matrix for each sample. Global efficiency, local efficiency, and modularity were computed from the correlation matrix of each bootstrap sample, over a range of sparsity thresholds (5% ≤ Sτ ≤ 25%) and their summary metrics, hereafter, denoted as
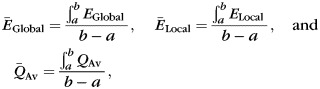 |
with a = 5, b = 25, then used to compare the age groups. The distributions of the 1000 summary graph metrics were checked for normality and a false discovery rate (FDR) correction (q = 0.05) for the 4 age groups was first done (Genovese et al. 2002). Then Student's t-test (for normal distribution) and Kolmogorov–Smirnov test (for non-normal distribution) were used to examine the significant difference of a summary graph metric between 2 groups.
Analysis of Significant Correlations
An FDR of q = 0.05 was applied to all correlation matrices and then the significant correlations for all age groups were computed. The number of significant correlations for each of the correlation matrix corresponding to the bootstrap samples was counted, and the distribution was used for statistical comparisons. We also computed the ratio of the number of significant positive correlations to the number of significant negative correlations. A positive correlation between 2 cortical regions indicates both cortical regions to either get thicker or thinner together; while a negative correlation indicates one cortical region to get thicker and the other to get thinner.
Analysis of Interregional Topological Properties and Structural Connectivity
To investigate the interregional changes in topological properties and connectivity, we used the functional brain divisions described by Mesulam (1998). The 78 brain regions were grouped into 3 major divisions: Association, paralimbic, and primary sensorimotor. Association division consisted of 46 brain regions, paralimbic had 24, and primary sensorimotor had 8 brain regions (Supplementary Table S1). First, we calculated the regional efficiency for the specified sparsity range for each brain region (5–25%) from which we computed the summary metric of regional efficiency,
Next, we combined the summary metrics for all the brain regions that belong to a major division and, the mean and standard error were calculated. Group comparisons were then made for each of the 4 major divisions across age groups using one-way ANOVA for normal distributions (Kruskal–Wallis for non-normal distributions), and significant difference between 2 groups was checked using Student's t-test (Kolmogorov–Smirnov test for non-normal distributions) after doing an FDR correction for the 4 age groups.
We investigated the developmental changes in interregional connectivity for the 3 major brain divisions. We refer to connectivity changes as the change in interregional correlations in mean cortical thickness for the brain divisions. For 2 age groups (early and late childhood), the correlation coefficients were first z-transformed, and interregional pairs belonging to 2 brain divisions (primary sensorimotor and association) were compared for significant difference in correlation (Liang et al. 2006; Supekar et al. 2009). If there was a significant decrease in correlation for an inter-regional pair, one −1 was counted; if there was a significant increase, one +1 was counted. This step was done for all the possible inter-regional pairs. The sum total of counts (increased and decreased counts) was normalized by the number of possible interregional pairs for the 2 major brain divisions under study.
Results
Cortical Thickness Analysis at Vertex-Wise Level
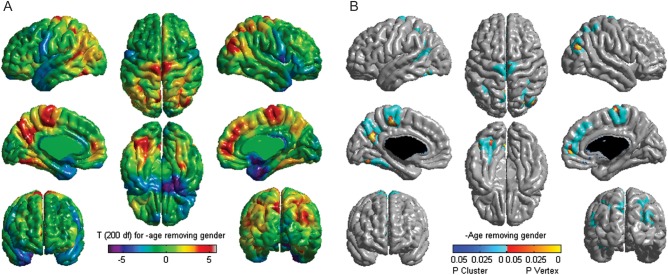
The effect of age on cortical thickness is shown in Figure 1, as t-values on the average cortical surface model of the whole sample. The most significant age-related cortical thinning with age is observed in parietal and frontal regions, while cortical thickening is seen in temporal regions, consistent with earlier studies (Sowell et al. 2003, 2004; Gogtay et al. 2004; Shaw et al. 2008).
Figure 1.
Effect of age on cortical thickness. (A) Analysis of the effect of age on cortical thickness for the whole population (n = 203) using a vertex-wise general linear model in which age and gender were taken into account. T-statistic was used to test the main effect of age on cortical thickness. (B) Correction of multiple comparisons of the vertex data using random field theory.
Interregional Correlations of Cortical Thickness and Development
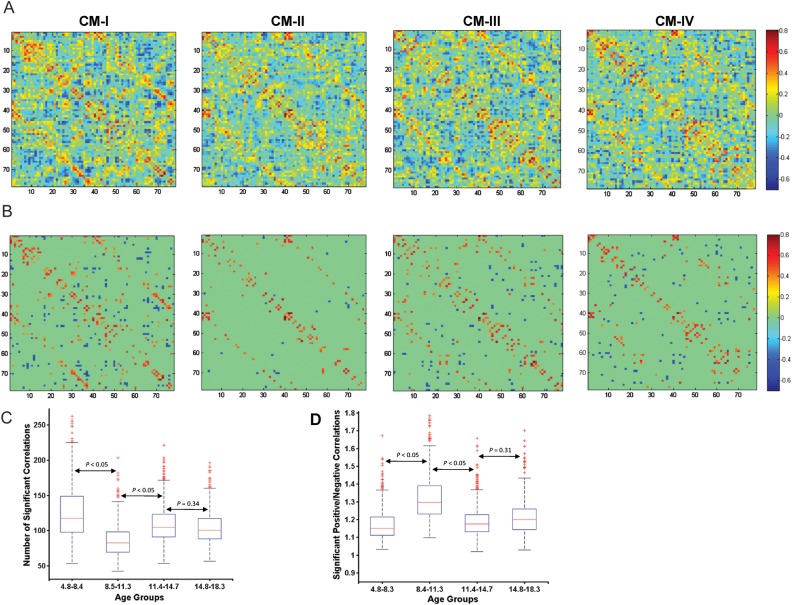
Correlation matrices were calculated for each age group, based upon mean cortical thickness values from 78 anatomical regions (Fig. 2A). The correlation matrices CM-I, CM-II, CM-III, and CM-IV refer to that of early childhood, late childhood, early adolescence, and late adolescence, respectively. The correlation patterns exhibited several common features between age groups. High correlations were observed between contralateral homologous regions and neighboring ipsilateral regions (Supplementary Table S2A–D), consistent with earlier reported studies (Mechelli et al. 2005; He et al. 2007).
Figure 2.
Correlation matrix for age groups. (A) A matrix of Pearson correlation coefficients between inter-regional cortical thickness across subjects after removing for age, gender and mean thickness, denoted as CM-I for early childhood, CM-II for late childhood, CM-III for early adolescence, and CM-IV for late adolescence. (B) The correlation matrices after an FDR threshold of q = 0.05. Major changes in correlation in CM-II can be observed. (C) Statistical comparison of the number of significant correlations between the age groups using 1000 bootstrap samples of subjects. (D) Ratio of the number of significantly positive correlations to the number of significantly negative correlations. For more details, please see Materials and Methods.
Figure 2B shows the significant correlations for each age group after correction for multiple comparisons (FDR at q = 0.05). A notable decrease in the number of significant correlations, specifically in the number of significantly negative correlations, can be observed in the matrix CM-II corresponding to the late childhood (8.4–11.3 years). Using 1000 bootstrap samples of the age groups, the number of significant correlations was compared to confirm the above qualitative finding. A significant decrease (P < 0.05, FDR corrected) in the number of significant correlations were observed for the late childhood group (Fig. 2C), indicating that the strength of correlations is much less in this age group. More specifically, the ratio of the number of significantly positive to negative correlations is higher (P < 0.05, FDR corrected) in late childhood compared with the other age groups (Fig. 2D), suggesting that there is a major reduction in the number of negative correlations during this age range.
Small-World Efficient Brain Networks
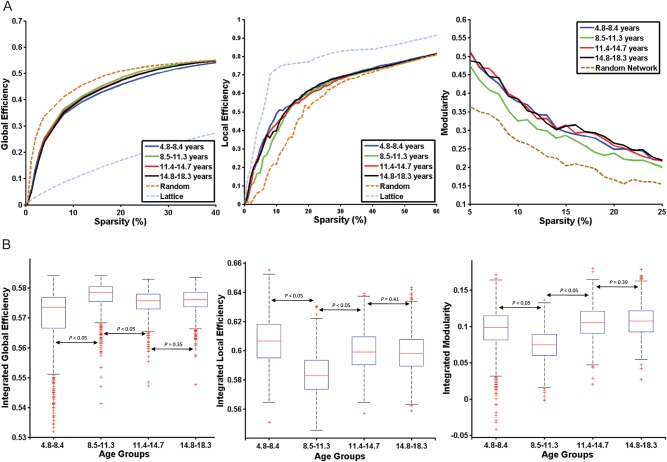
The small-world efficient behavior of all the real networks was analyzed by global and local efficiency for a range of sparsity values. They were compared with the corresponding degree-matched random and regular networks for the same range of sparsity values. All the networks derived in this study showed small-world efficiency in the sparsity range of 5–25%, as assessed by the criteria explained given in the section Materials and Methods (Fig. 3A).
Figure 3.
Developmental changes in global topological parameters. (A) Global efficiency, local efficiency, and modularity for the 4 age groups as a function of sparsity. (B) Statistical comparisons of the graph metrics namely, integrated global efficiency, integrated local efficiency, and integrated modularity for sparsity range (5–25%) using 1000 bootstrap samples. The distributions of the 1000 summary graph metrics were checked for normality, and Student's t-test (for normal distribution) and Kolmogorov–Smirnov test (for non-normal distribution) were used to examine the significant difference of a summary graph metric between the 2 groups.
Global Topological Properties and Age-Related Development
The summary metrics of global network topological properties (namely, global efficiency, local efficiency, and modularity) showed considerable changes with increasing age, indicating developmental changes in the networks (Fig. 3B). There was a significant decrease with age in integrated local efficiency (P < 0.05, FDR corrected) and a significant increase in integrated global efficiency (P < 0.05, FDR corrected) from early to late childhood. We also found that the integrated local efficiency increased significantly (P < 0.05, FDR corrected) and integrated global efficiency decreased significantly (P < 0.05, FDR corrected) from late childhood to early adolescence, and to late adolescence. There was no significant difference in the integrated global and local efficiency between early and late adolescence (P = 0.35 and 0.41, respectively). The integrated modularity showed a similar developmental trajectory to that of local efficiency. The late childhood group showed significantly lower (P < 0.05, FDR corrected) modularity than that of the other age groups.
Regional Differences in Network Topology with Development
To investigate the functional relevance of our observed network changes, we partitioned the AAL parcellation into 3 major subdivisions (primary sensorimotor, paralimbic, and association) as defined in Mesulam (1998). The mean integrated regional efficiency values for each of the subdivision and each age group are shown in Figure 4.
Figure 4.
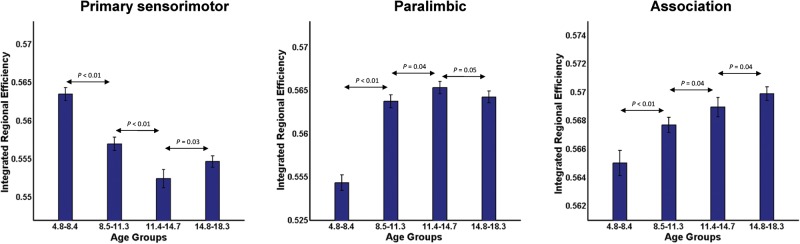
Regional efficiency of brain divisions. The integrated regional efficiencies of all cortical regions belonging to a functional brain division are aggregated and the same is used as a metric to compare between age groups. The standard error is represented by the bar and P-values were calculated using Student's t-test.
In the primary sensorimotor division, the efficiency values are significantly higher (P < 0.05, FDR corrected) in early and late childhood when compared with later age groups. There was no significant difference in the efficiency values in the later age groups. For the paralimbic division, there was a significant increase in efficiency (P < 0.05, FDR corrected) from early to late childhood. There was also a significant increase in efficiency (P < 0.05, FDR corrected) in the association division from early to late childhood. Taken together, primary sensorimotor regions showed higher values of regional efficiency in early childhood which decrease with development, while the paralimbic and association regions displayed a general pattern of increasing regional efficiency during development.
Developmental Changes in Regional and Inter-regional Structural Connectivity
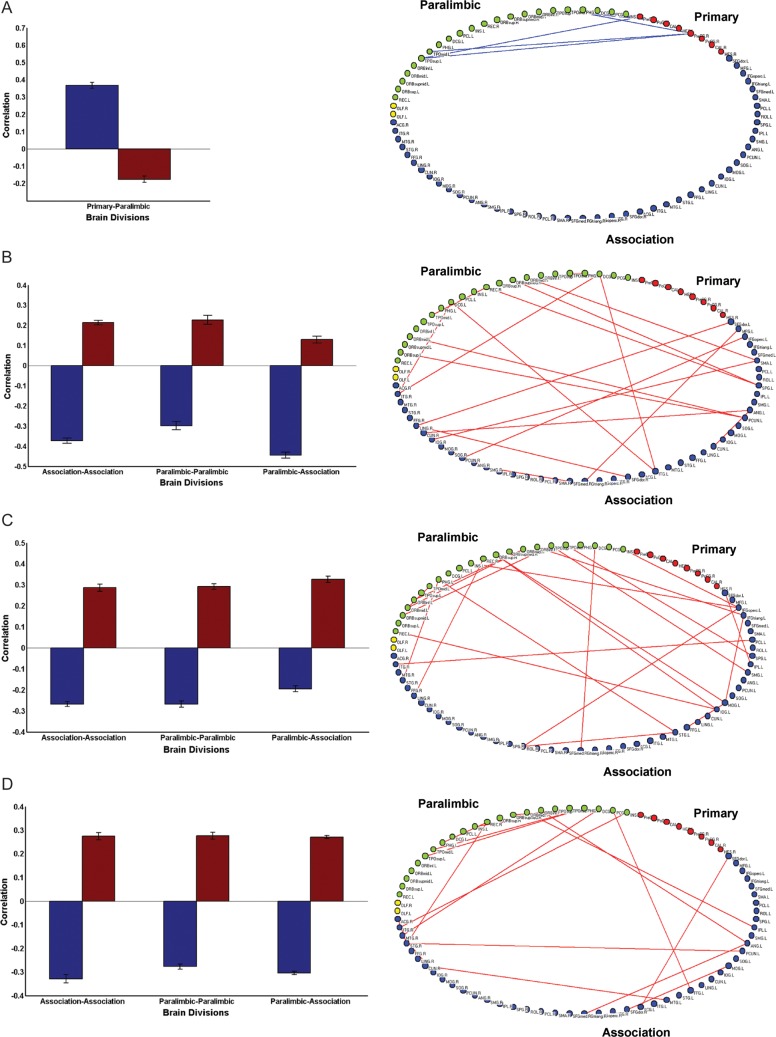
The age-related connectivity changes within and between the 3 major subdivisions were assessed by determining the change in correlation coefficients across age groups (Fig. 5). Primary sensorimotor regions were already well-connected with paralimbic regions by early age of development (early childhood), and this connectivity was decreased in late childhood (P < 0.01, FDR corrected; Fig. 5A). There was an increase from early to late childhood in connectivity within both paralimbic and association regions, as well as an increase between paralimbic and association regions (P < 0.01, FDR corrected; Fig. 5B). Similar increases in connectivity were observed within paralimbic and association regions, and between paralimbic and association regions, in the later ages of development (early and late adolescence; Fig. 5C,D). The age-related changes in inter-regional connectivity are shown on a 2-dimensional brain layout (Fig. 5).
Figure 5.
Developmental to in inter-regional connectivity. (A) Changes in connectivity from early to late childhood are computed by comparing the mean correlation of cortical regions belonging 2 brain divisions (for details, see Materials and Methods). Decreased connectivity is observed between primary sensorimotor and paralimbic regions and visualized on a 2D brain layout. (B) Increased connectivity is observed between association and paralimbic, and within association, paralimbic regions from early to late childhood. (C) From late childhood to early adolescence, increased connectivity is observed between association and paralimbic, and within paralimbic and association regions. (D) From early to late adolescence, increased connectivity is observed between association and paralimbic, and within paralimbic, and association regions.
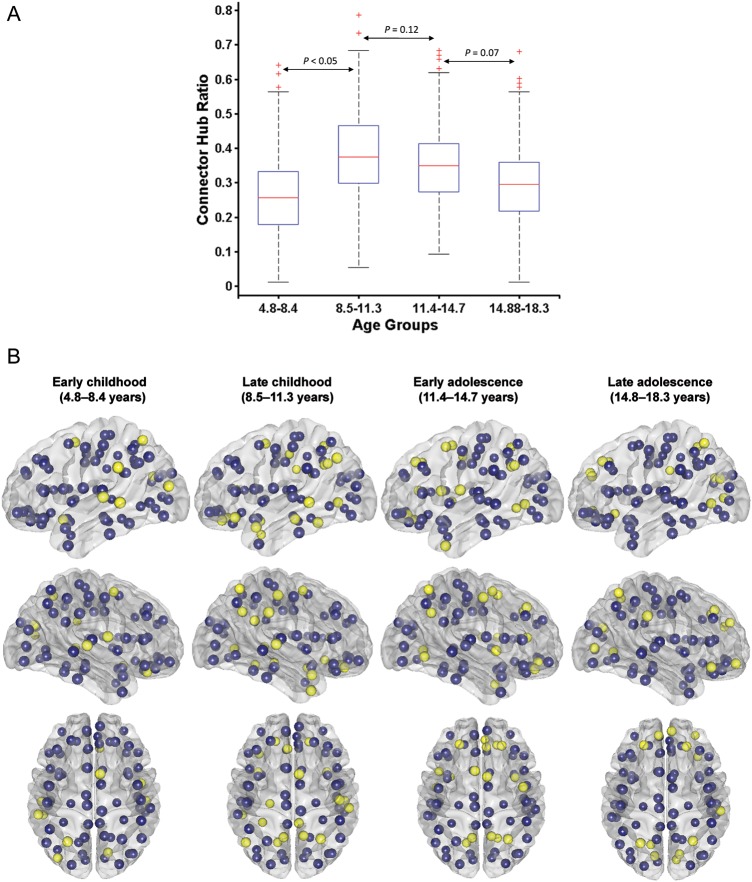
Connector Hubs and Age-Related Development
The localization and distribution of connector hubs in the different age groups (at sparsity = 13%) are shown in Figure 6B and Table 2. Eleven connector hubs were identified in the early childhood groups (Table 2A) that include bilateral superior temporal gyrus, middle temporal gyrus, middle occipital gyrus, cuneus and few frontal regions, rolandic operculum, rectus gyrus, and olfactory cortex. There was an increase in the number of connector hubs from early to late childhood (Table 2B; Fig. 6A for statistical comparisons); 21 hubs were identified across the entire cortex. The extensive distribution of connector hubs was also found in early adolescence, albeit with lesser cingulate regions and emergence of prefrontal regions; most prominently, bilateral dorso-lateral superior frontal gyri (Table 2C). In the late adolescence group, connector hubs are again reduced (15 in total), and localized, to multi-modal association areas; namely, bilateral medial superior frontal gyri, bilateral dorso-lateral superior frontal gyri, right orbital frontal, bilateral superior parietal gyri, bilateral cuneus, and lingual gyrus (Table 2D).
Figure 6.
Developmental changes in connector hub distribution. (A) Statistical comparison of connector hubs for the age groups using 1000 bootstrap samples. Student's t-test is used for checking significance between 2 age groups. (B) Connector hub distribution for the age groups. Yellow ones represent cortical regions that are identified as connector hubs (with participation index, P > 0.62; for details, see Materials and Methods), while blue ones are the rest of the cortical regions.
Table 2.
Connector hubs with development
| S. No. | ROI | Participation coefficient (P) |
|---|---|---|
| (A) Early childhood | ||
| 1 | SMA.R | 0.72 |
| 2 | STG.R | 0.72 |
| 3 | ROL.R | 0.71 |
| 4 | STG.L | 0.69 |
| 5 | OLF.L | 0.68 |
| 6 | SPG.L | 0.67 |
| 7 | MTG.L | 0.64 |
| 8 | REC.R | 0.64 |
| 9 | MOG.L | 0.64 |
| 10 | CUN.R | 0.62 |
| 11 | SMG.L | 0.62 |
| (B) Late childhood | ||
| 1 | SPG.R | 0.77 |
| 2 | PCUN.R | 0.76 |
| 3 | MTG.L | 0.72 |
| 4 | ANG.L | 0.72 |
| 5 | LING.L | 0.72 |
| 6 | FFG.L | 0.71 |
| 7 | ORBsup.L | 0.69 |
| 8 | SMG.R | 0.68 |
| 9 | PCUN.L | 0.68 |
| 10 | TPOsup.L | 0.67 |
| 11 | STG.R | 0.67 |
| 13 | PoCG.R | 0.65 |
| 14 | ORBinf.L | 0.65 |
| 15 | TPOmid.R | 0.63 |
| 16 | PHG.L | 0.63 |
| 17 | PCG.R | 0.62 |
| 18 | SMA.R | 0.62 |
| 19 | TPOsup.R | 0.62 |
| 20 | ACG.L | 0.62 |
| 21 | ORBsup.R | 0.62 |
| (C) Early adolescence | ||
| 1 | ORBsup.R | 0.74 |
| 3 | TPOmid.L | 0.72 |
| 4 | PCUN.L | 0.70 |
| 5 | SMA.L | 0.69 |
| 6 | LING.R | 0.68 |
| 7 | LING.L | 0.68 |
| 8 | PCUN.R | 0.67 |
| 9 | IFGtriang.L | 0.67 |
| 10 | ACG.R | 0.66 |
| 11 | SMA.R | 0.65 |
| 13 | ORBsupmed.R | 0.65 |
| 14 | ROL.L | 0.64 |
| 15 | SFGdor.L | 0.63 |
| 16 | SFGdor.R | 0.63 |
| 17 | INS.R | 0.62 |
| 18 | SPG.R | 0.62 |
| (D) Late adolescence | ||
| 1 | ORBinf.R | 0.73 |
| 3 | ORBmid.R | 0.72 |
| 4 | SFGdor.R | 0.70 |
| 5 | SFGmed.L | 0.69 |
| 6 | LING.L | 0.69 |
| 7 | SMG.R | 0.68 |
| 8 | SFGdor.L | 0.67 |
| 9 | CUN.R | 0.66 |
| 10 | SPG.R | 0.66 |
| 11 | SPG.L | 0.65 |
| 13 | SFGmed.R | 0.65 |
| 14 | CAL.R | 0.63 |
| 15 | CUN.L | 0.62 |
Discussion
In the present study, graph theory was applied to cortical thickness measurements from structural MRI to investigate global topological properties of structural brain maturation in a large population of typically developing children within 4 developmental age groups. Our work reveals: 1) Changes in inter-regional correlation with age, including prominent decreases in negative correlations in late childhood, 2) changes in topological properties of brain networks in late childhood, such as reduced local efficiency, modularity, and increased global efficiency indicating a shift of topological organization toward a more random configuration, 3) variation in the distribution of connector hubs during development, and 4) evolution of connectivity patterns whereby early connectivity is focused on sensorimotor areas, followed by greater connectivity in association with the areas in later development. Taken together, these findings indicate significant developmental changes in organization occurring at the large-scale structural brain network level that is consistent with a process of fine-tuning of immature brain system into a mature one.
Inter-regional Correlations
Our work highlights prominent changes in inter-regional correlations with development. Earlier studies have shown that interregional correlations in cortical thickness are associated with neuroanatomical pathways in the human brain (Lerch et al. 2006; He et al. 2007). Though the exact neurobiological processes behind coordinated interregional variation are not known, it has been attributed to mutually tropic effects or environment-related plasticity (Andrews et al. 1997; Mechelli et al. 2005; Bohbot et al. 2007). Similarly, negative correlations are believed to arise from weakened interregional inhibitory relationships among cortical regions (Mechelli et al. 2005; He et al. 2007). Comparison of the inter-regional correlations in cortical thickness for our 4 age groups revealed a reduction in the number of significant correlations in the late childhood group, specifically, a major decrease in the number of significantly negative correlations. Interestingly, significant negative correlations emerge again in early and late adolescence age groups. Earlier studies have shown that most cortical regions attain peak cortical thickness around this late childhood period (Gogtay et al. 2004; Shaw et al. 2008). Since most cortical regions grow together toward peak thickness, one can speculate that the negative correlations would be predominantly weaker during this age range. The observed reduction in interregional negative correlations in late childhood might also arise from weakened inhibitory influences.
Global Topological Properties
A surge of recent graph-theoretic studies have shown that structural and functional brain networks are small-world efficient using topological parameters like clustering coefficient, path length, and global and local efficiency (Bullmore and Sporns 2009; He and Evans 2010). Moreover, disruptions in small-world efficiency in several disease conditions (Bassett et al. 2008; He et al. 2008) and aging (Gong et al. 2009; Wu et al. 2012) have also been shown. Graph-theoretic studies of development have mainly focused on functional brain networks (Fair et al. 2008, 2009; Supekar et al. 2009; for a detail review, see Power et al. 2010), with very few studies on anatomical networks (Hagmann et al. 2010; Fan et al. 2011). One of the several conclusions that can be drawn from these studies is that the graph structures of anatomical and functional networks for children and adults, consistently showed small-world efficient behavior with high global and local efficiency and modular organization. Our results using cortical thickness also showed small-world behavior for all age groups suggesting an efficient organization of brain from early development; and are consistent with the small-world behavior seen in the previous anatomical and functional network studies during development.
The functional network studies have consistently shown no statistical difference in global topological properties between children and adults (Fair et al. 2008, 2009; Supekar et al. 2009), while graph-theoretic studies using gray matter volume (Fan et al. 2011) and diffusion MRI tractography (Hagmann et al. 2010) have shown age-dependent changes in the global topological properties. Our results of global topological properties showed a non-linear age-dependent developmental trajectory of the global topological properties, with significantly decreased local efficiency and modularity and increased global efficiency found in the late childhood group. The inconsistency in the developmental trajectory of topological properties might be explained as follows. First, the findings from functional studies must be interpreted cautiously as most of these functional studies have been done with limited ages and also with few regions of interest (ROIs) limited to select brain regions (Fair et al. 2008, 2009). Also, extensive statistical comparisons in global topological properties have not been done in these functional studies, thus detailed investigations into the differences in topological properties are not available (for a detail review, see Power et al. 2010). Another speculation for the different developmental trajectories of global topological properties is that these graph theoretic studies on development are from different imaging modalities that capture different tissue types and brain structures.
Global efficiency mainly reflects long-range connections that enable rapid information transfer between remote brain regions and are believed to form the integrative basis of many cognitive processes. In contrast, local efficiency predominantly reflects short-range connections between neighboring brain regions that facilitate localized information-processing (Latora and Marchiori 2001). Our findings of increased global efficiency and decreased local efficiency in late childhood indicate that structural brain networks may take on a more random configuration during this developmental period, whereas increased local efficiency and decreased global efficiency in adolescence may point to a return to a more optimal configuration with further development.
Distribution of Connector Hubs
We found changes in the location of structural connector hubs between age groups that correspond to regions that subserve major cognitive developmental milestones. The early and late childhood ages between 5 and 11 years are times of major advances in cognitive and language development (Friederici 2006). It is within these age groups that we found prominent hubs in the language-related temporal, parietal, and inferior frontal regions (Price 2010). These hubs are less pronounced in our early adolescent group and almost disappear in the late adolescent group. At the same time, large hubs appear in the frontal lobes in the early adolescent group (i.e. during the time of puberty) and remain prominent in late adolescence. An impact of puberty on frontal lobe development is implicated from studies on monkey and also human brain development, with respect to syntaptogenesis (Huttenlocher 1979; Bourgeois et al. 1994), GM and WM changes (Giedd et al. 1999). These processes of restructuring in the pubertal brain are argued to be driven by hormonal changes (Sisk and Foster 2004; Sisk and Zehr 2005; Schulz et al. 2009) and to carry functional consequences on higher order brain functions such as executive functions and social cognition (Blakemore and Choudhury 2006; Blakemore et al. 2010). It may be mentioned here that recent studies have indicated changes in dopaminergic innervation to have more influence on circuitry development during adolescence (Rosenberg and Lewis 1995; Weickert et al. 2007; Wahlstrom et al. 2010). Interestingly, this evolving localization of connector hubs between childhood and adolescence also compliments well-known models of cognitive development, such as that outlined by Piaget (1999). The dominance of connector hubs within language regions during childhood age groups is consistent with Piaget's preoperational stage of cognitive development (between ages 2–7), where children master the use of language for symbolic representation of the external world. Similarly, progression to connector hubs in frontal regions in later development corresponds well with maturation from concrete operational thinking in late childhood to formal operations in adolescence, and the acquisition of abstract reasoning.
Inter-regional Connectivity Patterns
Finally, our inter-regional connectivity analysis and graph-theoretic measure (specifically regional efficiency) demonstrate distinct developmental trajectories of different cortical divisions (primary sensorimotor, paralimbic, and association).
However, it is important to note that there is ongoing debate about the developmental trajectory of primary sensorimotor, paralimbic, and association areas. Investigations of synaptogenesis in primary sensorimotor and association areas in macaque monkey have indicated a synchronous maturation, specifically in the ascending phase of the course of synaptogenesis (Rakic et al. 1994). Contrary to this viewpoint is the assumption of a hierarchical sequence of structural and functional developments from sensory to motor and, finally, to association cortex, supported by several findings from human and non-human primates (Greenfield 1991; Huttenlocher and Dabholkar 1997; Travis et al. 2005; Elston et al. 2009). Several imaging studies, however, have shown findings that are indicative of the hierarchical sequence of maturation (Gogtay et al. 2004; Nagy et al. 2004; Paus 2005). However, such a hierarchical sequence of cortical maturation has not yet been shown from the perspective of large-scale structural brain networks. Our study utilized whole-brain connectivity level analysis using cortical thickness measures over all cortical regions. As such, our results add to a network-level understanding of the debatable issue of differential maturation of primary sensory-motor, paralimbic, and association connectivity during development.
Our results indicating that primary sensorimotor connectivity is well-established by early childhood, but increased connectivity in association regions occurs with age, are consistent with findings from several earlier studies. Diffusion tensor imaging (DTI) studies have demonstrated increasing connectivity among higher order association areas from childhood to adolescence that have functional consequences. For example, increasing prefrontal-parietal connectivity was shown to correlate positively with working memory capacity (Nagy et al. 2004), while increasing fronto-striatal connectivity has been correlated positively with inhibitory control (Liston et al. 2003). Analytical methods using a combination of DTI and fMRI have also demonstrated a positive correlation between the maturation of prefrontal-parietal connectivity and performance on working memory tasks (Olesen et al. 2003). Recent studies have also shown layer-specific dendritic development in layer IIIC pyramidal neurons in the human prefrontal cortex (specifically in Broca region) which is coincident with preoperational stage of cognitive development (Judas and Cepanec 2007; Petanjek et al. 2008).
Methodological Issues
One limitation of our study is the grouping of subjects to obtain the correlation matrix. For each age group, the structural brain networks were determined by computing the correlations of regional cortical thickness across subjects. Because of this, the relation of topological properties and age could not be investigated on an individual level. However, graph theory has been applied to developmental fMRI (Fair et al. 2009) and DTI (Hagmann et al. 2010) studies. A combination of such multi-modal imaging techniques will provide a comprehensive understanding of the developmental changes in organization of brain networks. Another notable point is the choice of brain parcellation. The regional cortical thickness measurements and the 3 broad brain divisions (primary sensorimotor, paralimbic, and association) were based on the AAL template in which a priori anatomical classifications were well defined. The choice of parcellation may have some effect (Wang et al. 2009) and the use of an alternative with higher resolution which is not constrained by anatomical landmarks is needed in future. Further, here we used cross-sectional data to infer developmental changes. Analysis of cross-sectional data might be limited with respect to detection of specific developmental changes considering the large variability in brain structure between individuals during development (Kraemer et al. 2000; Gogtay et al. 2004; Sowell et al. 2004).
Conclusions and Future Directions
The main finding of the paper is that during late childhood there is significant reduction in local efficiency, modularity, and increased global efficiency, suggesting a shift of topological organization toward a more random configuration. This observation of major changes in structural organization during late childhood may seem paradoxical to findings from several studies performed on post-mortem human and non-human primate tissues that have shown no prominent structural changes in terms of neuronal-synaptic organization during this period (Rakic et al. 1994; Huttenlocher and Dabholkar 1997; Petanjek et al. 2011). Histological studies have shown that most of the dendritic growth and synaptogenesis occur during infancy and are completed by the beginning of childhood. Synaptic elimination in human and non-human primates has shown to start with puberty only. Thus, histological studies suggest late childhood to be a developmental stage with little reorganization in structural circuitry.
One possible explanation for the inconsistency between our finding and histological observation is that this developmental phase is a period of massive developmental synaptic fine-tuning. Changeux and Danchin (1976) first proposed the synaptic fine-tuning hypothesis as a mechanism of synaptic stabilization during development. According to this hypothesis, during the period of overproduction of synapses, neuronal activity shapes the neural network by retaining certain synapses and eliminating others. The fine-tuning hypothesis is supported by findings from several human and non-human primate studies that have shown an initial overproduction of synapses and massive pruning later on (Huttenlocher 1979; Rakic et al. 1986; Bourgeois et al. 1994; Huttenlocher and Dabholkar 1997; Petanjek et al. 2011). This fine-tuning precedes and determines synaptic pruning. And the most intensive fine-tuning is occuring during the period of synaptic overproduction, which is the childhood period. Therefore, our findings of a more random topological organization during late childhood may indicate a fine-tuning process that precedes the reorganization of structural brain networks during development.
A speculation, which arises from our main findings, is that there is a time window of greater plasticity (reorganization) during normal brain development that has several potential implications. Since fine-tuning occurring during this time period is influenced by environment, one can speculate the role of experience (e.g. social interactions, education strategies, etc.) on the consequent formation of cortical circuitry. Experience in childhood has shown to influence long-term developmental paths (Champagne 2010). In one such study, young adults who attended a preschool project were shown to do better in several life indicators than their peers who did not attend the project (Schweinart et al. 2005). The authors proposed that the early intervention improved neurological development, resulting to a higher degree of academic success. Our findings of a critical time window of plasticity during late childhood lend support to the need for early intervention (e.g. education programmes) which might have potential effects that extend not only to brain development but to intellectual values (Kuhn and Park 2005; Barnett 2011). The findings also emphasize a developmental phase of vulnerability during development that necessitates future investigations of neuropsychopathology to look at structural reorganization during this time period (Andersen 2003).
Further, developmental changes at the regional level, as demonstrated in our findings, reveal distinct developmental trajectories of specific cortical divisions which might relate to the observed organizational changes that we found at the global level with respect to the evolving topological properties of structural neural networks observed between childhood and adolescence. More specifically, our findings of early maturation of primary sensorimotor and protracted maturation of higher order association connectivity are consistent with structural and functional brain imaging studies that reflect a fine-tuning of an immature to a more mature brain organization (Changeux and Danchin 1976; Casey et al. 1997; Brown et al. 2005; Durston et al. 2006). Interestingly, our regional findings including changes with respect to distribution and location of connector hubs are in line with well-known theories of typical cognitive development. Stages of considerable network re-configuration may relate to non-linear enhancement of certain cognitive skills, as indicated by findings that highlight periods of stagnation or even regression prior to periods of further cognitive advancement (Carey et al. 1980; Anderson et al. 2001; Strenziok et al. 2011). Future works examining associations between structural network properties and behavior are needed to further elucidate the behavioral correlates of our findings. This work represents the first examination of the topological properties of large-scale structural brain networks in children and adolescents. The results of our study provide new details, hitherto unknown, regarding maturational changes in structural brain networks, possibly pointing to a time window of plasticity that can accommodate the important changes during puberty.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This work was supported in whole or in part with Federal funds from the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract #s N01-HD02-3343, N01-MH9-0002, and N01-NS-9-2314, -2315, -2316, -2317, -2319 and -2320). Special thanks to the NIH contracting officers for their support.
Supplementary Material
Notes
We also acknowledge the important contribution and remarkable spirit of John Haselgrove, PhD (deceased). B.S.K. is supported by a PDF Fellowship from FRSQ. We thank the anonymous reviewers for their invaluable comments. Conflict of Interest: This manuscript reflects the views of the authors and may not reflect the opinions or views of all Study Investigators or the NIH.
Appendix
Brain Development Cooperative Group
Key personnel from the 6 pediatric study centers are as follows: Children's Hospital Medical Center of Cincinnati: Principal Investigator William S. Ball, MD; Investigators Anna Weber Byars, PhD; Mark Schapiro, MD; Wendy Bommer, RN; April Carr, BS; April German, BA; Scott Dunn, RT; Children's Hospital Boston: Principal Investigator Michael J. Rivkin, MD; Investigators Deborah Waber, PhD; Robert Mulkern, PhD; Sridhar Vajapeyam, PhD; Abigail Chiverton, BA; Peter Davis, BS; Julie Koo, BS; Jacki Marmor, MA; Christine Mrakotsky, PhD, MA; Richard Robertson, MD; Gloria McAnulty, PhD; University of Texas Health Science Center at Houston: Principal Investigators Michael E. Brandt, PhD; Jack M. Fletcher, PhD; Larry A. Kramer, MD; Investigators Grace Yang, MEd; Cara McCormack, BS; Kathleen M. Hebert, MA; Hilda Volero, MD; Washington University in St. Louis: Principal Investigators Kelly Botteron, MD; Robert C. McKinstry, MD, PhD; Investigators William Warren, Tomoyuki Nishino, MS; C. Robert Almli, PhD; Richard Todd, PhD, MD; John Constantino, MD; University of California Los Angeles: Principal Investigator James T. McCracken, MD; Investigators Jennifer Levitt, MD; Jeffrey Alger, PhD; Joseph O'Neil, PhD; Arthur Toga, PhD; Robert Asarnow, PhD; David Fadale, BA; Laura Heinichen, BA; Cedric Ireland, BA; Children's Hospital of Philadelphia: Principal Investigators Dah-Jyuu Wang, PhD and Edward Moss, PhD; Investigators Robert A. Zimmerman, MD, and Research Staff Brooke Bintliff, BS; Ruth Bradford, Janice Newman, MBA. The Principal Investigator of the data coordinating center at McGill University is Alan C. Evans, PhD; Investigators Rozalia Arnaoutelis, BS; G. Bruce Pike, PhD; D. Louis Collins, PhD; Gabriel Leonard, PhD; Tomas Paus, MD; Alex Zijdenbos, PhD; and Research Staff Samir Das, BS; Vladimir Fonov, PhD; Luke Fu, BS; Jonathan Harlap, Ilana Leppert, BE; Denise Milovan, MA; Dario Vins, BC; and at Georgetown University: Thomas Zeffiro, MD, PhD and John Van Meter, PhD. Investigators at the Neurostatistics Laboratory, Harvard University/McLean Hospital: Nicholas Lange, ScD and Michael P. Froimowitz, MS, work with data coordinating center staff and all other team members on biostatistical study design and data analyses. The Principal Investigator of the Clinical Coordinating Center at Washington University is Kelly Botteron, MD; Investigators C. Robert Almli, PhD; Cheryl Rainey, BS; Stan Henderson, MS; Tomoyuki Nishino, MS; William Warren, Jennifer L. Edwards, MSW; Diane Dubois, RN; Karla Smith, Tish Singer, and Aaron A. Wilber, MS. The Principal Investigator of the Diffusion Tensor Processing Center at the National Institutes of Health is Carlo Pierpaoli, MD, PhD; Investigators Peter J. Basser, PhD; Lin-Ching Chang, ScD; Chen Guan Koay, PhD and Lindsay Walker, MS. The Principal Collaborators at the National Institutes of Health are Lisa Freund, PhD (NICHD); Judith Rumsey, PhD (NIMH); Lauren Baskir, PhD (NIMH); Laurence Stanford, PhD (NIDA); Karen Sirocco, PhD (NIDA); and from NINDS, Katrina Gwinn-Hardy, MD; and Giovanna Spinella, MD. The Principal Investigator of the Spectroscopy Processing Center at the University of California Los Angeles is James T. McCracken, MD; Investigators Jeffry R. Alger, PhD; Jennifer Levitt, MD; Joseph O'Neill, PhD.
References
- Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. doi:10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amso D, Casey BJ. Beyond what develops when: neuroimaging may inform how cognition changes with development. Curr Dir Psychol Sci. 2006;15:24–29. doi:10.1111/j.0963-7214.2006.00400.x. [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. doi:10.1016/S0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Dev Neuropsychol. 2001;20:385–406. doi: 10.1207/S15326942DN2001_5. doi:10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Halpern SD, Purves D. Correlated size variations in human visual cortex, lateral geniculate nucleus, and optic tract. J Neurosci. 1997;17:2859–2868. doi: 10.1523/JNEUROSCI.17-08-02859.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett WS. Effectiveness of early educational intervention. Science. 2011;333:975–978. doi: 10.1126/science.1204534. doi:10.1126/science.1204534. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. doi:10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. doi:10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. doi:10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Lerch J, Thorndycraft B, Iaria G, Zijdenbos AP. Gray matter differences correlate with spontaneous strategies in a human virtual navigation task. J Neurosci. 2007;27:10078–10083. doi: 10.1523/JNEUROSCI.1763-07.2007. doi:10.1523/JNEUROSCI.1763-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. doi:10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. doi:10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. doi:10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Carey S, Diamond R, Woods B. Development of face recognition: a maturational component? Dev Psychol. 1980;16:257–269. doi:10.1037/0012-1649.16.4.257. [Google Scholar]
- Casey BJ, Duhoux S, Malter Cohen M. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67:749–760. doi: 10.1016/j.neuron.2010.08.033. doi:10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orenddi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos X, Haxby JV, Noll DC, Cohen JD, et al. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. doi:10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic influence of social experiences across the lifespan. Dev Psychobiol. 2010;52:299–311. doi: 10.1002/dev.20436. doi:10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. doi:10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Clauset A, Newman M, Moore C. Finding community structure in very large networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70:066111. doi: 10.1103/PhysRevE.70.066111. doi:10.1103/PhysRevE.70.066111. [DOI] [PubMed] [Google Scholar]
- Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3-D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208. doi:10.1002/hbm.460030304. [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. doi:10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. doi:10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. An introduction to bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- Elston GN, Oga T, Fujita I. Spinogenesis and pruning scales across functional hierarchies. J Neurosci. 2009;29:3271–3275. doi: 10.1523/JNEUROSCI.5216-08.2009. doi:10.1523/JNEUROSCI.5216-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. doi:10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain's default network. Proc Natl Acad Sci USA. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. doi:10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. doi:10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Shi F, Smith JK, Lin W, Gilmore JH, Shen D. Brain anatomical networks in early human brain development. Neuroimage. 2011;54:1862–1871. doi: 10.1016/j.neuroimage.2010.07.025. doi:10.1016/j.neuroimage.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. The neural basis of language development and its impairment. Neuron. 2006;52:941–952. doi: 10.1016/j.neuron.2006.12.002. doi:10.1016/j.neuron.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. doi:10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos AP, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. doi:10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. doi:10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Rosa-Neto P, Carbonell F, Chen ZJ, He Y, Evans AC. Age- and gender-related differences in the cortical anatomical network. J Neurosci. 2009;29:15684–15693. doi: 10.1523/JNEUROSCI.2308-09.2009. doi:10.1523/JNEUROSCI.2308-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield PM. Language, tools and brain: the ontogeny and phylogeny of heirarchically organized sequential behaviour. Behav Brain Sci. 1991;14:531–595. doi:10.1017/S0140525X00071235. [Google Scholar]
- Guimera R, Mossa S, Turtschi A, Amaral LA. The worldwide air transportation network: anomalous centrality, community structure, and cities’ global roles. Proc Natl Acad Sci USA. 2005;102:7794–7799. doi: 10.1073/pnas.0407994102. doi:10.1073/pnas.0407994102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Sporns O, Madan N, Cammoun L, Pienaar R, Wedeen VJ, Meuli R, Thiran JP, Grant PE. White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci USA. 2010;107:19067–19072. doi: 10.1073/pnas.1009073107. doi:10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex. 2007;17:2407–2419. doi: 10.1093/cercor/bhl149. doi:10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer's disease. J Neurosci. 2008;28:4756–4766. doi: 10.1523/JNEUROSCI.0141-08.2008. doi:10.1523/JNEUROSCI.0141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Evans A. Graph theoretical modeling of brain connectivity. Curr Opin Neurol. 2010;23:341–350. doi: 10.1097/WCO.0b013e32833aa567. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex – developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. doi:10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. doi:10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Judas M, Cepanec M. Adult structure and development of the human fronto-opercular cerebral cortex (Broca's region) Clin Linguist Phon. 2007;21:975–989. doi: 10.1080/02699200701617175. doi:10.1080/02699200701617175. [DOI] [PubMed] [Google Scholar]
- Kabani N, Le Goualher G, MacDonald D, Evans AC. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage. 2001;13:375–380. doi: 10.1006/nimg.2000.0652. doi:10.1006/nimg.2000.0652. [DOI] [PubMed] [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab'bagh Y, MacDonald D, Lee JM, Kim SI, Evans AC. Automated 3-D extraction and evaluation of of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. doi:10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. doi:10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Kuhn D. Do cognitive changes accompany developments in the adolescent brain? Perspect Psychol Sci. 2006;1:59–67. doi: 10.1111/j.1745-6924.2006.t01-2-.x. doi:10.1111/j.1745-6924.2006.t01-2-.x. [DOI] [PubMed] [Google Scholar]
- Kuhn D, Park S-H. Epistemological understanding and the development of intellectual values. Int J Educ Res. 2005;43:111–124. doi:10.1016/j.ijer.2006.05.003. [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. doi:10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. doi:10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Lee JM, Kim JS, Kim IY, Evans AC, Kim SI. A novel quantitative cross-validation of different cortical surface reconstruction algorithms using MRI phantom. Neuroimage. 2006;31:572–584. doi: 10.1016/j.neuroimage.2005.12.044. doi:10.1016/j.neuroimage.2005.12.044. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. doi:10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31:993–1003. doi: 10.1016/j.neuroimage.2006.01.042. doi:10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. doi:10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug A, Casey BJ. Developmental differences in diffusion measures of cortical fibers. J Cogn Neurosci. 2003;15:S57–S58. doi:10.1162/089892903321107828. [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. doi:10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. J Neurosci. 2005;25:8303–8310. doi: 10.1523/JNEUROSCI.0357-05.2005. doi:10.1523/JNEUROSCI.0357-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. doi:10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. doi:10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Newman ME, Girvan M. Finding and evaluating community structure in networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;69:026113. doi: 10.1103/PhysRevE.69.026113. doi:10.1103/PhysRevE.69.026113. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Res Cogn Brain Res. 2003;18:48–57. doi: 10.1016/j.cogbrainres.2003.09.003. doi:10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. doi:10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Kostovic I, Uylings HB. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. doi:10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. doi:10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. The stages of the intellectual development of the child. In: Slater A, editor. The Blackwell reader in developmental psychology. Malden: Blackwell Publishing Ltd; 1999. pp. 35–42. [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. doi:10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. doi:10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. doi:10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. doi:10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lerch JP, Lee N, Greenstein D, Wallace GL, Stockman M, Clasen L, Shaw PW, Giedd JN. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72:873–884. doi: 10.1016/j.neuron.2011.09.028. doi:10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. doi:10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: the organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. doi:10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinart LJ, Montie J, Xiang Z, Barnett WS, Belfield CR, Nores M. Lifetime effects: the High/Scope Perry Preschool study through age 40. Ypsilanti (MI): High/Scope Press; 2005. [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. doi:10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. doi:10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. doi:10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. doi:10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. doi:10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. doi:10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. doi:10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenziok M, Krueger F, Heinecke A, Lenroot RK, Knutson KM, van der Meer E, Grafman J. Developmental effects of aggressive behavior in male adolescents assessed with structural and functional brain imaging. Soc Cogn Affect Neurosci. 2011;6:2–11. doi: 10.1093/scan/nsp036. doi:10.1093/scan/nsp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. doi:10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Tamnes CK, Ostby Y, Walhovd KB, Westlye LT, Due-Tonnessen P, Fjell AM. Intellectual abilities and white matter microstructure in development: a diffusion tensor imaging study. Hum Brain Mapp. 2010;31:1609–1625. doi: 10.1002/hbm.20962. doi:10.1002/hbm.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JE, Worsley KJ. Detecting sparse signals in random fields, with an application to brain mapping. J Am Stat Assoc. 2007;102:913–928. doi:10.1198/016214507000000815. [Google Scholar]
- Travis K, Ford K, Jacobs B. Regional dendritic variation in neonatal human cortex: a quantitative Golgi study. Dev Neurosci. 2005;27:277–287. doi: 10.1159/000086707. doi:10.1159/000086707. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. doi:10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. doi:10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang L, Zang Y, Yang H, Tang H, Gong Q, Chen Z, Zhu C, He Y. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum Brain Mapp. 2009;30:1511–1523. doi: 10.1002/hbm.20623. doi:10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Webster MJ, Gondipalli P, Rothmond D, Fatula RJ, Herman MM, Kleinman JE, Akil M. Postnatal alterations in dopaminergic markers in the human prefrontal cortex. Neuroscience. 2007;144:1109–1119. doi: 10.1016/j.neuroscience.2006.10.009. doi:10.1016/j.neuroscience.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Kinomura S, Goto R, Okada K, Kawashima R, He Y, Evans AC, Fukuda H. Age-related changes in topological organization of structural brain networks in healthy individuals. Hum Brain Mapp. 2012;33:552–568. doi: 10.1002/hbm.21232. doi:10.1002/hbm.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. Proc Natl Acad Sci USA. 2010;107:18191–18196. doi: 10.1073/pnas.1003109107. doi:10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. doi:10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.