Abstract
Oligodendrocyte genes and white matter tracts have been implicated in the pathophysiology of schizophrenia and may play an important etiopathogenic role in cognitive dysfunction in schizophrenia. The objective of the present study in 60 chronic schizophrenia patients individually matched to 60 healthy controls was to determine whether 1) white matter tract integrity influences cognitive performance, 2) oligodendrocyte gene variants influence white matter tract integrity and cognitive performance, and 3) effects of oligodendrocyte gene variants on cognitive performance are mediated via white matter tract integrity. We used the partial least-squares multivariate approach to ascertain relationships among oligodendrocyte gene variants, integrity of cortico-cortical and subcortico-cortical white matter tracts, and cognitive performance. Robust relationships among oligodendrocyte gene variants, white matter tract integrity, and cognitive performance were found in both patients and controls. We also showed that effects of gene variants on cognitive performance were mediated by the integrity of white matter tracts. Our results were strengthened by bioinformatic analyses of gene variant function. To our knowledge, this is the first study that has brought together these lines of investigation in the same population and highlights the importance of the oligodendrocyte/white matter pathway in schizophrenia, particularly as it pertains to cognitive function.
Keywords: cognition, DTI, genetics, oligodendrocytes, white matter
Introduction
Schizophrenia is a disease characterized by cognitive impairment across multiple domains (Heinrichs and Zakzanis 1998). Cognitive impairment in schizophrenia is heritable, largely unrelated to medication effects, and clinically important since it is a major determinant of real-world outcome (Gur et al. 2007). Though cognitive impairment is likely due to disruption in brain structure or circuitry, the genetics and neural correlates of cognitive performance in schizophrenia are poorly understood. An understanding of the relationship among genetic risk variants, neural circuitry, and cognitive performance in schizophrenia would provide new directions for the treatment of cognitive impairment in this disorder.
Schizophrenia is often conceptualized as a “disconnection syndrome” (Friston and Frith 1995). Diffusion tensor imaging (DTI) (Shergill et al. 2007; Friedman et al. 2008; Kubicki et al. 2008; Pomarol-Clotet et al. 2010; Voineskos, Lobaugh et al. 2010; Whitford et al. 2010; Camchong et al. 2011) is providing accumulating evidence for the disruption of microstructural integrity of cortico-cortical and cortico-subcortical white matter tracts in schizophrenia. This disruption can impair the fast and efficient transfer of information between brain regions and contribute to cognitive impairment (Dwork et al. 2007). Some putative relationships between white matter tract integrity and cognitive performance in schizophrenia have been identified (Nestor et al. 2008; Szeszko et al. 2008; Miyata et al. 2010). However, our current understanding of the effect of microstructural integrity of white matter on cognition arises mainly from DTI studies of healthy individuals and healthy aging (Catani et al. 2007; Sullivan et al. 2008; Voineskos, Rajji et al. 2012). To our knowledge, a multivariate examination of how white matter tract integrity may influence cognitive performance in schizophrenia has not yet been published.
Oligodendrocyte (or myelin) genes code for proteins that influence the microstructural components of white matter tracts that form the main barriers to water diffusion indexed using DTI. These genes include oligodendrocyte transcription factor-2 (OLIG2), 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP), quaking (QKI), and myelin-associated glycoprotein (MAG). They are predominantly expressed in oligodendrocytes and directly involved in their myelination (initiation, deposition, compaction, and maintenance) (Davis et al. 2003) and trophic support (Segal et al. 2007), axonal support (McTigue and Tripathi 2008), and axo-glial interactions (Pernet et al. 2008). There is replicated evidence for association between these genes and schizophrenia (Aberg et al. 2006; Georgieva et al. 2006; Voineskos, de Luca et al. 2008) and for altered regulation of these genes in the postmortem schizophrenia brain (Hakak et al. 2001; Tkachev et al. 2003). Gene variants from these genes show epistatic risk for schizophrenia with variants in the neuregulin1-tyrosine kinase receptor ErbB4 (NRG1–ErbB4) gene system and expression of these two gene systems is coordinated (Georgieva et al. 2006). NRG1 plays an important role in cortico-cortical myelination during neurodevelopment (Chen et al. 2006), and disruption of the NRG1–ErbB4 pathway in oligodendrocytes in animal models leads to alteration of the myelin sheath of major white matter tracts, reduced conduction velocity, and cognitive changes (Roy et al. 2007).
The importance of oligodendrocytes and myelin genes in schizophrenia is neuroanatomically congruent with DTI studies that implicate white matter tracts (Friedman et al. 2008; McIntosh, Maniega et al. 2008; Voineskos, Lobaugh et al. 2010) connecting the cortical regions where these genes are downregulated, and oligodendrocyte number is reduced (Haroutunian and Davis 2007). Furthermore, white matter integrity is substantially heritable (Chiang et al. 2009; Kochunov et al. 2010). Therefore, we conducted a study to examine the relationships among oligodendrocyte and NRG1–ErbB4 gene variants, white matter tract integrity, and cognitive performance in a sample of patients with chronic schizophrenia and matched controls. In order to assess relationships among gene variants, white matter tract integrity, and cognitive performance, we used the partial least-squares (PLS) multivariate approach that is well suited to combining such data and holds specific advantages over conventional univariate approaches (McIntosh and Lobaugh 2004). Our main hypotheses were: 1) There is a significant relationship between white matter tract integrity and cognitive performance; 2) oligodendrocyte and NRG1–ErbB4 gene risk variants influence white matter tract integrity and cognitive performance; 3) gene variant effects are more robustly associated with neuroimaging phenotypes than on behavioral (i.e., cognitive) measures; and 4) reliable relationships predicting cognitive function would be driven by schizophrenia patients.
Materials and Methods
Subjects
Subjects were recruited at the Centre for Addiction and Mental Health (CAMH) in Toronto, Ontario, Canada, via referrals, study registries, and advertisements. They were between the age of 20–62 years (Table 1), were administered the Structured Clinical Interview for DSM-IV Disorders (First et al. 1995), and were interviewed by a psychiatrist to enhance diagnostic accuracy. Subjects were also assessed with the Wechsler Test for Adult Reading (WTAR) to estimate their IQ, the Mini-Mental State Examination (MMSE) to characterize their gross cognitive status, the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) (Miller et al. 1992) to characterize the burden of comorbid physical illness, and the Positive and Negative Syndrome Scale (PANSS) (Kay et al. 1987) to characterize their symptoms. Medications were self-reported and verified when necessary with the patient's treating psychiatrist and chart review; antipsychotic daily doses were converted to chlorpromazine equivalent. Urine toxicology screens were performed on all subjects and those with current substance abuse or any history of substance dependence were excluded. Individuals with previous head trauma with loss of consciousness, or neurological disorders were also excluded. A history of a primary psychotic disorder in first-degree relatives was an exclusion criterion for controls. Criteria used to match controls with patients were age within 5 years, gender, handedness (Edinburgh Handedness Inventory) (Oldfield 1971), and ethnicity. Subjects were recruited for the study with matching criteria described. Although 146 matched subjects were recruited, 15 were unable to complete the study (e.g., due to failure to return for DT-MRI procedure, claustrophobia in magnetic resonance imaging scanner, request to withdraw from study) and two DTI scans were deemed unusable due to excessive artifact. From these remaining 129 individuals, 60 schizophrenia patients were matched to 60 controls and included for statistical analyses. The study was approved by the Centre for Addiction and Mental Health Research Ethics Board. All subjects provided written, informed consent.
Table 1.
Demographic and clinical characterization of subjects
| Demographic | Patients with schizophrenia (n = 60) |
Healthy controls (n = 60) |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Agea | 43 | 16 | 39 | 14 |
| Education (years)a | 13 | 2 | 16 | 2 |
| WTAR (IQ)a | 109 | 17 | 118 | 8 |
| MMSEa | 29 | 2 | 29 | 1 |
| CIRS-Ga | 6 | 3 | 1 | 2 |
| Age of onset | 24 | 7 | NA | NA |
| CPZE (mg/day) | 357 | 278 | NA | NA |
| PANSS | ||||
| Positive | 14 | 5 | NA | NA |
| Negative | 15 | 6 | NA | NA |
| General | 26 | 7 | NA | NA |
| N | N | |||
| Diagnosis | 53 SZ, 7 SA | NA | ||
| Gender | 40M, 20F | 40M, 20F | ||
| Handedness | 58 R, 2 L | 58 R, 2 L | ||
| Ethnicity | 49 C, 11 As | 49 C, 11 As | ||
| Antipsychotic generation | 5 first, 51 second | NA | ||
Note: SZ, schizophrenia; SA, schizoaffective; NA, not applicable; M, male; F, female WTAR, Wechsler test for adult reading; MMSE, mini-mental state examination; CIRS-G, cumulative illness rating scale, geriatrics; PANSS, positive and negative syndrome scale; CPZE, chlorpromazine equivalent; C, Caucasian; As, Asian (based on self-report).
aUsing two-tailed independent samples t-tests, patients and controls were compared for age, education, WTAR, MMSE, and CIRS-G. Significant differences (P < 0.05) were present only for education (t94 = 4.9, P < 0.001), WTAR (t94 = 2.7, P = 0.008), and CIRS-G (t94 = 9.6, P < 0.001).
Cognitive Testing
All subjects underwent a battery of cognitive tests administered over approximately 1.5 h. This battery included tasks that assess a wide range of cognitive domains in which impairment has been reported in schizophrenia (Snitz et al. 2006; Rajji et al. 2009), namely executive function, working memory, attention, verbal fluency, verbal memory, visual memory, set-shifting, response inhibition, mental flexibility, spatial ability, and sensorimotor function (Table 2).
Table 2.
Cognitive performance
| Test | Mean ± SD (patients with schizophrenia) | Mean ± SD (healthy controls) | Test, P-value |
|---|---|---|---|
| EXITa | 5.2 ± 3.6 | 2.5 ± 2.2 | F = 22.0, P < 0.001 |
| Letter number span | 12.1 ± 4.1 | 16.5 ± 3.4 | F = 39.1, P < 0.001 |
| Stroop ratioa | 2.4 ± 0.7 | 2.1 ± 0.6 | F = 6.2, P = 0.01 |
| Letter cancellationa | 80.0 ± 29.1 | 57.4 ± 14.7 | F = 25.3, P < 0.001 |
| Finger taps (DH) | 40.5 ± 11.3 | 48.4 ± 9.4 | F = 13.3, P < 0.001 |
| Finger taps (NDH) | 37.4 ± 9.3 | 43.3 ± 9.1 | F = 8.8, P = 0.004 |
| Grooved pegboard (DH)a | 93.7 ± 26.7 | 69.7 ± 14.7 | F = 35.4, P < 0.001 |
| Grooved pegboard (NDH)a | 107.5 ± 35.8 | 77.5 ± 18.9 | F = 29.4, P < 0.001 |
| RBANS | |||
| List learning | 25.9 ± 5.7 | 30.5 ± 4.0 | F = 23.3, P < 0.001 |
| Figure copy | 16.4 ± 3.5 | 17.6 ± 2.4 | F = 3.3, P = 0.07 |
| Line orientation | 15.3 ± 4.6 | 17.9 ± 2.4 | F = 12.9, P < 0.001 |
| Category fluency | 39.8 ± 16.1 | 55.5 ± 22.3 | F = 17.5, P < 0.001 |
| F + A + S | 34.4 ± 11.8 | 48.8 ± 13.4 | F = 35.3, P < 0.001 |
| Digit span | 10.0 ± 2.8 | 12.1 ± 2.5 | F = 19.6, P < 0.001 |
| Coding | 40.6 ± 11.0 | 53.6 ± 12.4 | F = 31.8, P < 0.001 |
| List recall | 5.2 ± 2.3 | 7.3 ± 1.7 | F = 27.3, P < 0.001 |
| Story memory | 15.7 ± 4.2 | 19.6 ± 3.2 | F = 32.9, P < 0.001 |
| Story recall | 8.3 ± 2.7 | 10.4 ± 1.4 | F = 27.1, P < 0.001 |
| Figure recall | 10.7 ± 5.0 | 12.9 ± 4.4 | F = 4.7, P = 0.03 |
| TrailsB/TrailsAa | 2.6 ± 1.3 | 2.3 ± 0.7 | F = 2.2, P = 0.14 |
Note: DH, dominant hand; NDH, nondominant hand; F + A + S, total words starting with F, A, and S; EXIT, Executive Interview; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status.
aHigher scores are indicative of poorer performance.
Neuroimaging
DTI Acquisition
Images were acquired using an eight-channel head coil on a 1.5 T GE Echospeed system (General Electric Medical Systems, Milwaukee, WI, USA), which permits maximum gradient amplitudes of 40 mT/m. A single shot spin echo planar sequence was used with diffusion gradients applied in 23 noncollinear directions and b = 1000 s/mm2. Two b = 0 images were obtained. Fifty-seven slices were acquired for whole-brain coverage oblique to the axial plane obtained parallel to the plane passing through the anterior and posterior commissures (i.e., AC-PC aligned). Slice thickness was 2.6 mm, and voxels were isotropic. The field of view was 330 mm and the size of the acquisition matrix was 128 × 128 mm, with time echo = 85.5 ms, time repetition = 15 000 ms. The entire sequence was repeated three times to improve the signal-to-noise ratio.
Image Analysis and Tractography
The three repetitions were coregistered to the first b = 0 image in the first repetition using FSL (v. 4.0) www.fmrib.ox.ac.uk to produce a new averaged image, with gradients reoriented according to the registration transformation. A final diffusion tensor was then estimated based on all 75 aligned volumes using a weighted least-squares approach. Registration corrects eddy current distortions and subject motion, and averaging improves the signal-to-noise ratio. A brain “mask” was then generated. Points were seeded throughout each voxel of the brain. Whole-brain tractography was performed with a deterministic (streamline) approach (the Runge–Kutta order-two tractography with a fixed step size of 0.5 mm). Detailed descriptions of our tractography approach and our clustering segmentation algorithm have been published (O'Donnell et al. 2006; Voineskos et al. 2009). In brief, threshold parameters for tractography were based on the linear anisotropy measure CL, where CL = (λ1−λ2)/λ1 and λ1 and λ2, the two largest eigenvalues of the diffusion tensor sorted in the descending order. Thresholds were based on the CL rather than on fractional anisotropy (FA), to ensure that seeding in planar regions is minimized (Ennis and Kindlmann 2006). Furthermore, for the clustering segmentation approach that we use, seeding according to CL is helpful because it lessens the effect of planar partial-volume regions where a fiber may jump from one structure to another. By somewhat reducing partial-volume tractography errors, the use of CL improves the ability of the clustering algorithm to separate different white matter tracts. Tractography and creation of white matter fiber tracts were performed using 3D Slicer (www.slicer.org) and Matlab 7.0 (www.mathworks.com).
Once the whole-brain cluster model was produced, a trained investigator (A.N.V.) combined the clusters that correspond to the following fiber tract: Left and right uncinate fasciculus (UF), inferior occipitofrontal fasciculus (IFOF), cingulum bundle (CB), inferior longitudinal fasciculus (ILF), arcuate fasciculus (AF), and genu and splenium (parietal, temporal, occipital fibers) of the corpus callosum (see Supplementary Figure 1). As reported elsewhere (Voineskos et al. 2009), the entire clustering procedure was performed on 10 patients with schizophrenia and 10 healthy controls and achieved excellent spatial and quantitative reliability (i.e., both voxel overlap and scalar measures of the tensor showed high agreement). Matlab (v. 7.0) was used to calculate FA (Basser and Pierpaoli 1996); the mean values along the selected tracts are presented.
We chose FA as our main diffusion-based measure of white matter tract microstructural integrity. FA can be robustly measured with the image acquisition parameters that we employed (Jones 2004). Furthermore, there are several studies demonstrating FA reductions in white matter tracts in schizophrenia, providing biological rationale to combine FA measures of white matter tracts with genetic variation in oligodendrocyte genes and cognitive performance. Changes in FA are not due to any one tissue or cellular substrate, but rather are primarily due to changes in axonal membranes or density, myelin sheath or fiber number, or indirect effects of interactions between axons and myelin (Beaulieu 2002). Such tissue substrates of FA align with our chosen genes of interest that play key roles in axonal development, myelin development, trophic support of axons and myelin, and axo-glial interactions, as well as the likelihood that integrity of these structures is required for effective cognitive performance.
Genetics
Genes and Single-Nucleotide Polymorphism Selection
Oligodendrocyte gene variants were selected for study if they met the following strong a priori rationale: Replicated genetic associations in independent samples with schizophrenia, and replicated evidence from independent samples indicating significant downregulation of these genes in schizophrenia postmortem brain. Four genes met these criteria: QKI, MAG, CNP, and OLIG2. Single-nucleotide polymorphisms (SNPs) from these genes with a priori rationale for effects on gene expression were then selected, such as the exonic CNP SNP, rs2070106, and the 3′-untranslated region (UTR) OLIG2 SNP rs1059004. These variants have been associated with schizophrenia and with reduced expression of the respective gene in the schizophrenia postmortem brain (Peirce et al. 2006). The expression of several oligodendrocyte genes is tightly coordinated primarily by QKI (Aberg et al. 2006). A mutation in the 5′-UTR of QKI directs its own alternative splicing, leading to the QKI-7b splice variant, which directs the regulation of several oligodendrocyte genes by binding to their 3′-UTR, thus providing a plausible mechanistic explanation of oligodendrocyte gene downregulation in schizophrenia (Aberg et al. 2006). QKI has been demonstrated to regulate both MAG and CNP expression. The MAG SNP rs756796 lies within or very near the putative QKI-binding site, which directs alternative splicing of the MAG gene (Aberg et al. 2006). Therefore, the SNPs selected include: The 5′-UTR SNP of QKI (rs2784865), the MAG SNP rs756596 just downstream of the MAG 3′-UTR, the previously identified MAG schizophrenia risk SNPs rs720308, rs720309, and rs2301600, CNP rs2070106, and OLIG2 rs1059004 and rs9653711. In addition, we genotyped SNPs that belong to the NRG1–ErbB4 system and that have recently shown association with white matter integrity using DTI: 1) SNP8NRG243177, which leads to differential expression of a neuregulin transcript, particularly in schizophrenia patients (Law et al. 2006); 2) SNP8NRG221533, which gave the strongest association with schizophrenia in original reports (Stefansson et al. 2002); and 3) ErbB4 rs839523, which was associated with elevated expression of ErbB4 splice variants in schizophrenia (Law et al. 2007).
Genotyping Protocol
Genotyping of SNPs was performed using a standard ABI 5′-nuclease Taqman® assay-on-demand protocol in a total volume of 10 µL. Postamplification products were analyzed on the AB 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, United States of America) and genotype calls were performed manually. Results were verified independently by two laboratory personnel blind to demographic or diagnostic information. Ten percent of the sample was genotyped at each SNP for a second time for quality control analysis.
Statistical Analysis
Demographic and Group Differences
All SNPs were tested for the Hardy–Weinberg equilibrium. Demographic characteristics, such as age, IQ, and education were compared between the schizophrenia and control groups using two-tailed independent samples t-tests.
Although not a focus of this study, potential associations between genotypes and diagnostic group were tested using χ2. For potential white matter tract differences and cognitive differences between patients and controls, univariate ANCOVAs with age as a covariate were used. The Bonferroni correction for multiple comparisons was applied in each of these series of tests.
Multivariate Data Analysis
Multivariate analysis was performed using PLS, an approach that can assess a large covariance matrix in multivariate neuroimaging (McIntosh et al. 1996; McIntosh and Lobaugh 2004) and genetics data (Raadsma et al. 2008; Opiyo and Moriyama 2009). PLS has several advantages over conventional univariate approaches (McIntosh and Lobaugh 2004), including: 1) Greater power; 2) the capability to deal with datasets where the dependent measures within a block are highly correlated; and 3) the capability to evaluate the reliability of the findings over and above tests of significance. The use of resampling algorithms to evaluate reliability enables a degree of certainty in the analysis that conventional parametric statistics cannot provide. Therefore, we used PLS (McIntosh et al. 1996; McIntosh and Lobaugh 2004) to examine the relationship among gene variants, white matter tract integrity, and cognitive performance in patients and controls. For analyses with genetic data, risk allele carriers were grouped together and compared with nonrisk allele carriers (based on previously published reports), except for the ErbB4 rs839523 variant where G allele homozygotes have been previously identified as the risk genotype.
For each analysis, the two diagnostic groups were analyzed together to characterize correlation patterns common to both groups, and then each group was examined separately to characterize correlation patterns that are specific to each group. Age and antipsychotic exposure were residualized from brain measures and cognitive performance scores. Correlation matrices were constructed by stacking the between-subject correlations of two blocks of data (e.g., allelic variants with microstructural integrity of white matter tracts). The correlation matrices were constructed separately for each diagnostic category. The matrices were analyzed with singular value (SV) decomposition to produce mutually orthogonal latent variables (LVs), each comprised a singular “independent variable image” (e.g., in the first analysis consisting of DTI measures that reliably contributed to the LV) and a singular “dependent variable image” (e.g., in the first analysis a composite of cognitive test scores). Each LV also has an SV, which is the covariance between the independent and dependent variable image. The cross-block covariance (CC) is the percent of total covariance explained by the LV between the two data matrices. In the first analysis, the weights within the independent variable image are the linear combinations of FA of the white matter tracts that covaried with the dependent cognitive performance measures. In the second analysis, the weights within the independent variable image are the linear combinations of the allelic variants that covary with white matter tract FA. For independent measures, for example, in the case of allelic variation, the weightings reflect the contribution of individual SNPs to the LV. In the third analysis, weighted linear combinations of allelic variation that covary with cognitive performance were shown. Finally, in the fourth analysis, weighted linear combinations of white matter tract FA that covary with genetic variants and cognitive performance were examined. This final analysis was designed to model relationships among these three sets of variables to test the assumption that the effects of genes on cognitive performance are mediated by their effects on white matter integrity.
The significance of the SV (i.e., whether the LV accounts for an amount of covariance that is unlikely to have arisen by chance) is determined by permutation sampling that involves randomly reassigning subjects across groups. Here, we used 1000 permutations. The stability of these results is then determined by the bootstrap resampling (done 500 times), which involves sampling the dataset with replacements to derive estimates of standard errors (SE) for the weights of the independent variables, and 95% confidence intervals for the obtained correlations. The ratio of weight to SE >2.0 corresponds to approximately 95% confidence limits and was used to establish the reliability of each independent variable. If the correlation confidence interval did not include zero, the correlation was considered to be stable. In parallel, correlation confidence intervals that did include zero were considered unstable. Thus, when gene variants were examined in relation to DTI phenotypes and cognitive scores, the bootstrap ratios for the gene variants reflect the consistency with which a specific gene variant–DTI or gene variant–cognition relationship is manifested reliably across subjects.
Bioinformatic Analysis: In Silico SNP Function Prediction
In silico methods were used to predict potential function of the SNPs investigated in this study. Depending on location, SNPs were assessed for alteration in transcription factor binding using MatInspector (Genomatix; promoter and intron 1). The presence of splicing enhancers, repressors, or intronic regulatory elements (intronic and exonic, synonymous, and nonsynonymous SNPs) were determined using F-SNP (http://compbio.cs/queensu.ca/F-SNP/) and Human Splicing Finder (http://www.umd.be/HSF/). The F-SNP also includes prediction for the possible damaging effect of the amino acid change using PolyPhen/SIFT, etc. We also assessed whether exonic SNPs leading to synonymous amino acid substitution causes codon usage bias, that is, the codon changes from a frequently used to a rarely used codon. In addition, exonic and 3′-UTR SNPs were examined with CentroidFold predictive software to determine their effects on the structure of RNA (Hamada et al. 2009); 3′-UTR SNPs were also assessed for alteration in microRNA-binding sites (http://www.mirbase.org/search.shtml).
Results
Subjects' characteristics are shown in Table 1. All SNPs were genotyped at a 100% success rate and were in Hardy–Weinberg equilibrium (see Supplementary Table 1). No SNP was significantly associated with schizophrenia following multiple comparison correction. For DTI measures, patients had lower FA at all white matter tracts examined, but only the left UF (F1,117 = 9.6, P = 0.002) met our Bonferroni corrected threshold of P = 0.0042 (corresponding to 12 tracts analyzed). Nearly all the cognitive tests demonstrated significant differences between schizophrenia patients and healthy controls (Table 2).
Relationship Between White Matter Tract Integrity and Cognitive Performance
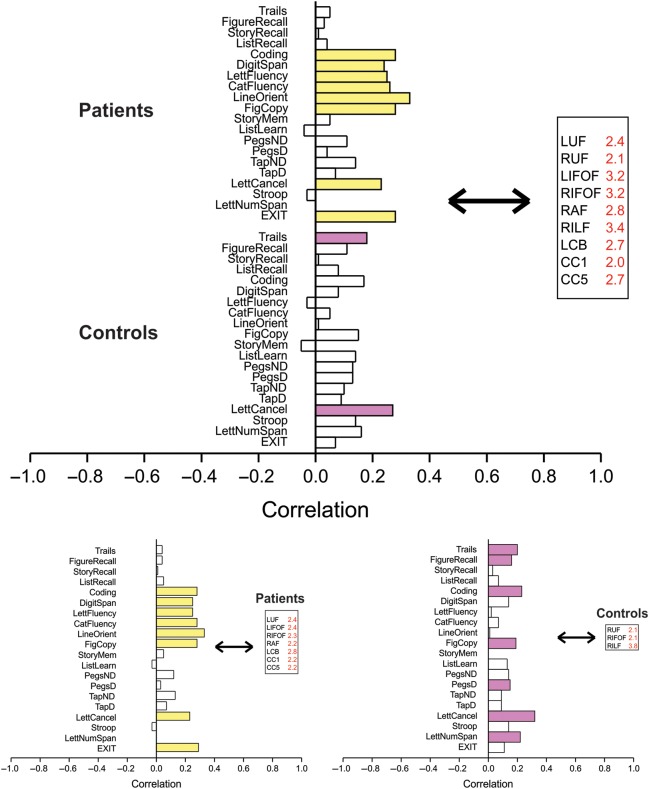
In relating brain circuitry to cognitive performance in the two-group analysis, we found (SV = 2.3, CC = 56%, P = 0.01) (Fig. 1, upper panel) that the bilateral UF and IFOF, left CB, right AF and ILF, and genu and splenium of the corpus callosum were reliably associated with performance on executive function, language, visuospatial ability, and attention in schizophrenia patients and with attentional measures in controls. Follow-up analysis in each diagnostic group showed that reliable white matter tract–cognition relationships in schizophrenia patients were driven by an almost identical network of white matter tracts as seen in the two-group analysis: Left UF and CB, bilateral IFOF, and genu and splenium of the corpus callosum reliably influenced cognitive tests that measured visual attention, processing speed, visuospatial ability, language, and executive function (SV = 2.0, CC = 67%, P < 0.001) (Fig. 1, lower left panel). In healthy controls, right UF, IFOF, and ILF reliably predicted cognitive performance in tasks of working memory, visuospatial ability, visual attention and memory, and motor speed (SV = 1.4, CC = 52%, P = 0.02) (Fig. 1, lower right panel).
Figure 1.
White matter tract integrity covarying with cognitive performance. Upper panel: Patients with schizophrenia and healthy controls analyzed together. A significant LV was found with SV = 2.3, cross-block covariance (cc) = 56.4%, P < 0.001. Predictors are those white matter tracts whose integrity reliably predicts cognitive performance (i.e., SE to salience ratio >2.0). Cognitive measures reliably correlated with predictors are strongly colored (patients: Upper stack, yellow; controls: Lower stack, purple). This LV was driven by the relationship of white matter tract integrity of left (L) and right (R) UF, IFOF, right AF, right ILF, left CB, and genu (CC1) and splenium (CC5) of the corpus callosum on coding and digit span, letter fluency and category fluency, line orientation and figure copy, letter cancellation task, and the executive interview in schizophrenia patients, and trails ratio score and letter cancellation task performance in controls. Lower panels: Patients (left panel) and controls (right panel) are analyzed separately. Significant LVs were found in both patients and controls. Predictors are those white matter tracts whose integrity reliably predicts cognitive performance (i.e., SE to salience ratio of >2.0). Cognitive measures reliably correlated with predictors are colored. In patients (yellow), FA of left uncinate, left and right inferior occipitofrontal, right arcuate, left cingulum, and genu and splenium of the corpus callosum reliably predicted performance on coding, digit span, letter and category fluency, line orientation and figure copy, letter cancellation task, and executive interview: SV = 2.0, CC = 67%, P < 0.001. In controls (purple), FA of right uncinate, right inferior occipitofrontal, and right ILF reliably predicted performance on the trails tests, figure recall, coding, figure copy, grooved pegboard, letter cancellation, and letter number sequence tasks: SV = 1.4, CC = 52%, P = 0.02.
Relationship Between Gene Variants and White Matter Tract Integrity
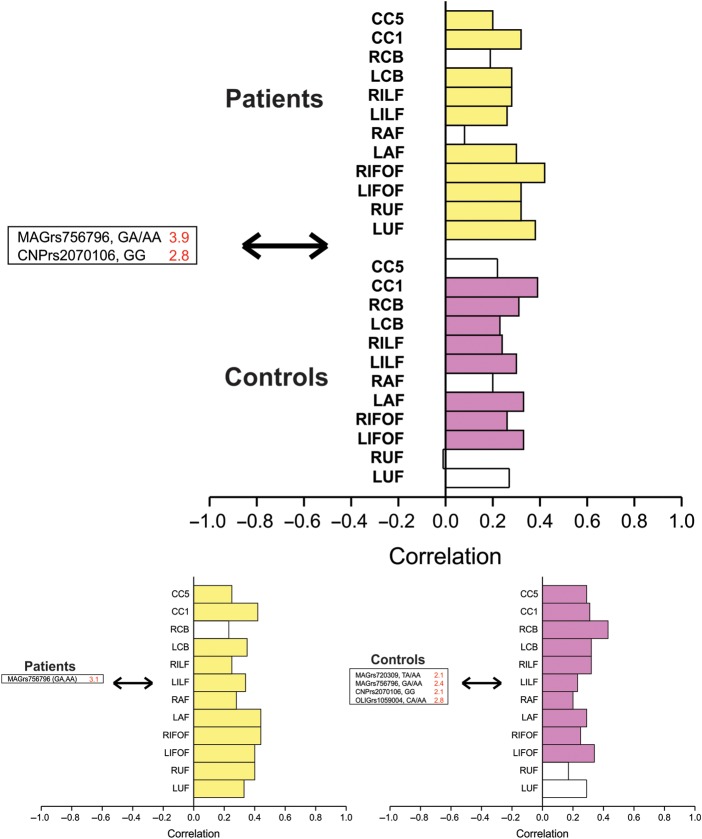
In the two-group analysis, a significant LV demonstrated the influence of MAG rs756796 and CNP rs2070106 on microstructural integrity of all white matter tracts (SV = 1.6, CC = 44%, P < 0.001) (Fig. 2, upper panel) in both groups except for right CB and AF in schizophrenia patients and bilateral UF and splenium of the corpus callosum in controls. In the schizophrenia within group analysis, MAG rs756796 predicted microstructural integrity of all white matter tracts except right CB (SV = 1.4, CC = 64%, P = 0.03) (Fig. 2, lower left panel). In controls, MAG rs756796 and rs720309, CNP rs2070106 and OLIG2 rs1059004 predicted microstructural integrity of all white matter tracts except for bilateral UF (SV = 1.3, CC = 67%, P = 0.01) (Fig. 2, lower right panel).
Figure 2.
Gene variants covarying with white matter tract integrity. Upper panel: Patients with schizophrenia and healthy controls analyzed together demonstrating a significant LV of gene variants that reliably predict white matter tract integrity (i.e., SE to salience ratio >2.0). White matter tract integrity reliably correlated as part of this significant LV is strongly colored (schizophrenia: Upper stack, yellow; healthy controls: Lower stack, purple): SV = 1.6, CC = 44%, P < 0.001. Gene variants in MAG and CNP reliably predicted FA of all white matter tracts except for right arcuate and cingulum in schizophrenia patients and bilateral UF, right arcuate and splenium of the corpus callosum in controls. Lower panels: Patients (left) and controls (right) analyzed separately. Significant LVs were found in each group where gene variants reliably predict white matter tract integrity (i.e., SE to salience ratio >2.0). White matter tract integrity reliably correlated with genetic predictors is strongly colored. In patients (yellow), the MAG rs756796 SNP predicted integrity of all white matter tracts examined, except for right CB: SV = 1.4, CC = 64%, P = 0.03, while in healthy controls (purple), MAG, CNP, and OLIG2 SNPs predicted integrity at all white matter tracts except left and right UF: SV = 1.3, CC = 67%, P = 0.01.
Relationship Between Gene Variants and Cognitive Performance
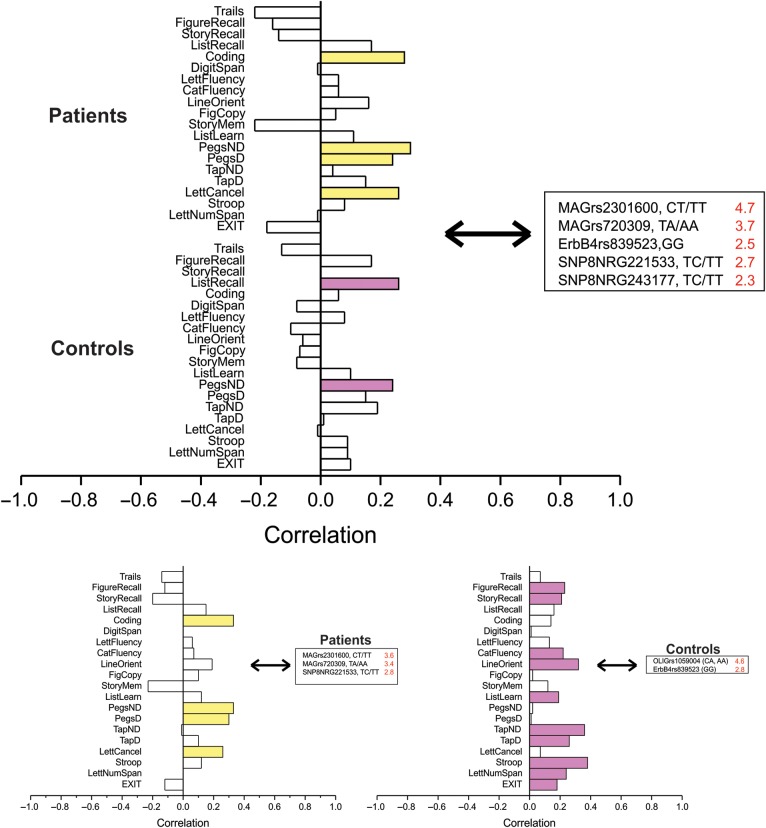
In the two-group analysis, a significant LV reliably demonstrated the influence of MAG rs2301600 and rs720309, SNP8NRG221533 and SNP8NRG243177, and ErbB4 rs839523 on processing speed, visuomotor speed and attention in schizophrenia patients, and visuomotor speed and verbal memory in controls (SV = 1.3, CC = 25%, P = 0.02) (Fig. 3, upper panel). The examination by the diagnostic group shows that in schizophrenia patients, the same two MAG SNPs and SNP8NRG221533 reliably predicted performance on the exact same tasks as in the two-group analysis (SV = 1.1, CC = 32%, P = 0.01) (Fig. 3, lower left panel). By contrast, in healthy controls OLIG2 rs1059004 and ErbB4 rs839253 reliably predicted performance on tests of memory, language/executive function, visuospatial ability, visuomotor speed and dexterity, and working memory (SV = 1.0, CC = 36%, P = 0.05) (Fig. 3, lower right panel).
Figure 3.
Gene variants covarying with cognitive performance. Upper panel: Patients with schizophrenia and healthy controls analyzed together. A significant LV was found where gene variants reliably predict cognitive task performance (i.e., SE to salience ratio >2.0) (patients: Upper stack, yellow; healthy controls: Lower stack, purple). Cognitive tasks reliably correlated with genetic predictors are strongly colored: SV = 1.3, CC = 25%, P = 0.02. Here, the pattern was driven by the relationship of MAG, NRG1, and ErbB4 SNPs reliably predicting performance on the coding, grooved pegboard, and letter cancellation tasks in patients, and list recall and grooved pegboard in controls. Lower panels: Patients (left) and controls (right) analyzed separately. Significant LV were found in each group patients, where gene variants reliably predict cognitive task performance (i.e., SE to salience ratio >2.0). Cognitive tasks reliably correlated with genetic predictors are strongly colored. In schizophrenia patients (yellow), MAG and NRG1 SNPs reliably predicted performance on the coding, grooved pegboard, and letter cancellation tasks: SV = 1.1, CC = 32%, P = 0.01. In healthy individuals, OLIG2 and ErbB4 SNPs reliably predicted performance on memory, language, visuospatial ability, visuomotor speed, working memory, and executive function: SV = 1.0, CC = 36%, P = 0.05.
Relationship Between Gene Variants, White Matter Tract Integrity, and Cognitive Performance
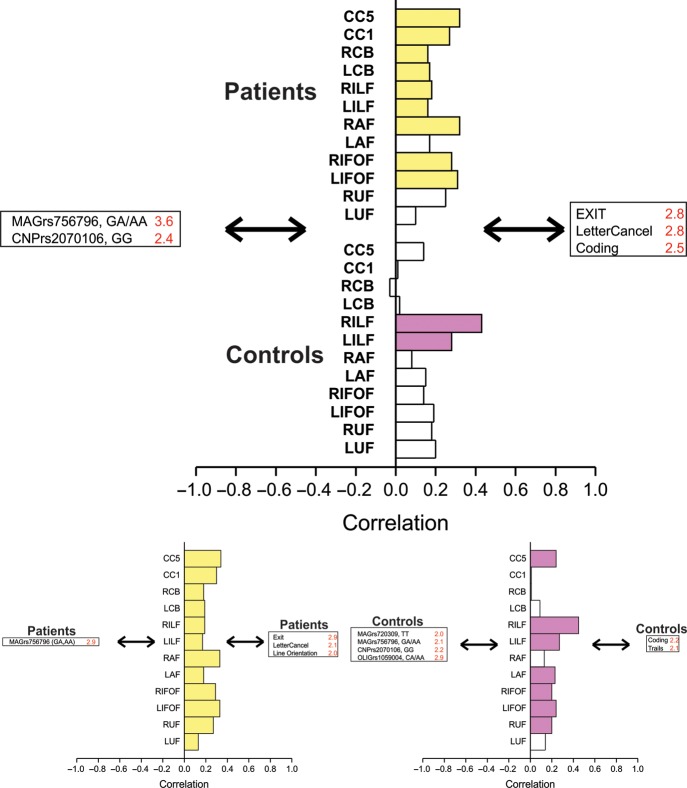
The final PLS analysis modeled the effects of gene variants on white matter integrity and the resultant effects on cognition. The effects of the MAG rs756796 and CNP rs2070106 variants on executive function, attention, and processing speed were reliably mediated by nearly all major white matter tracts in schizophrenia patients and by bilateral ILF in healthy controls (SV = 2.6, CC = 43%, P < 0.001) (Fig. 4, upper panel). When the groups were examined separately, the effects of the MAG rs756796 on executive function and attention in schizophrenia patients were reliably mediated by integrity of all white matter tracts (except left UF) (SV = 2.3, CC = 63%, P = 0.05) (Fig. 4, lower left panel). In healthy controls, effects of MAG rs756796 and rs720309 along with CNP rs2070106 and OLIG2 rs1059004 on visual attention and processing speed were mediated by bilateral ILF and IFOF, along with right UF, AF and splenium of the corpus callosum (SV = 2.0, CC = 53%, P = 0.02) (Fig. 4, lower right panel). A summary of all of the PLS analyses is provided in Figure 5.
Figure 4.
Integrity of white matter tracts mediating relationships between gene variants and cognitive performance. Upper panel: Schizophrenia patients and healthy controls analyzed together. A significant LV was found where microstructural integrity of white matter tracts reliably mediate relationships between gene variants and cognitive task performance (i.e., SE to salience ratio of >2.0). Gene variants, white matter tracts, and cognitive tasks reliably correlated (95% confidence interval) are strongly colored: SV = 2.6, CC = 43%, P < 0.001. Here, nearly all white matter tracts in schizophrenia patients: Upper stack, yellow, and right and left ILF in healthy controls: Lower stack, purple, mediated the relationship of MAG and CNP SNPs with cognitive performance on coding, letter cancellation task, and the executive interview. Lower panels: Schizophrenia patients and healthy controls analyzed separately. A significant LV was found in schizophrenia patients (yellow), where microstructural integrity of all white matter tracts mediated the relationships between the MAG rs756796 SNP with performance on visuospatial and visuomotor speed tasks, and executive function: SV = 2.3, CC = 63%, P = 0.05. In healthy controls, bilateral ILF and IFOF, left AF and UF, and splenium of CC mediated effects of MAG, CNP, and OLIG2 SNPs on coding and trials BA ratio scores: SV = 2.0, CC = 53%, P = 0.02.
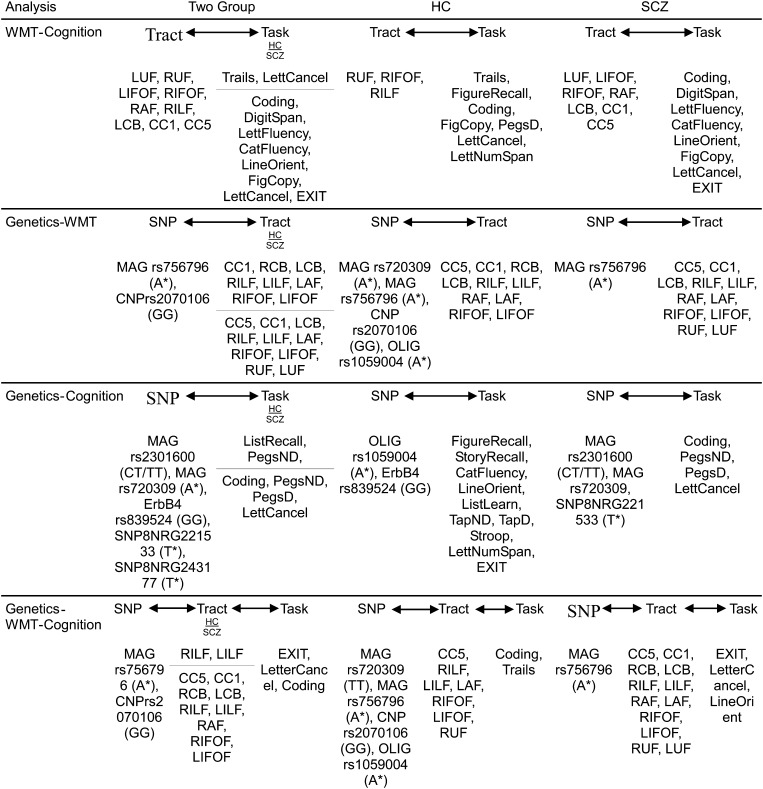
Figure 5.
Summary of significant correlations from PLS analysis. HC, healthy controls; SCZ, schizophrenic patients; WMT, white matter tracts; SNP, single-nucleotide polymorphism; LUF/RUF, left/right uncinate fasciculus; LIFOF/RIFOF, left/right inferior fronto-occipital fasciculus; LAF/RAF, left/right arcuate fasciculus; LILF/RILF, left/right inferior longitudinal fasciculus; LCB/RCB, left/right cingulum bundle; CC1, genu of corpus callosum; CC5, splenium of corpus callosum. *Allele carrier (either homozygote or heterozygote).
In Silico SNP Function Prediction
In silico predictions for the OLIG2 rs1059004, in the 3′-UTR, demonstrated that the presence of the C-allele, but not the A-allele, predicted transcription factor binding for two factors, zinc-binding protein factors (ZNF219) and EGR/nerve growth factor induced protein C and related factors (WT1). The presence of the A- allele predicted a binding site for microRNA hsa-miR-323-5p and hsa-miR-608. The synonymous exonic CNP SNP rs2070106 was associated with alteration in free energy of the predicted local mRNA structure (100 bp oligonucleotide, CentroidFold). The free energy of the A-allele carrying mRNA was −16.70 kcal/mol compared with the G-allele carrying mRNA −26.40 kcal/mol. Alteration in an exon splicing enhancer was predicted at MAG rs2301600 (http://www.umd.be/HSF/). Functional information for NRG1 SNPs and the ErbB4 SNP agreed with previous descriptions and analyses (Law et al. 2006, 2007) (see Supplementary Table 2).
Discussion
We conducted a study in 60 patients with chronic schizophrenia and 60 matched healthy controls examining relationships among oligodendrocyte and NRG1–ErbB4 gene system variants, microstructural integrity of cortico-cortical and cortico-subcortical white matter tracts, and cognitive performance using the multivariate PLS approach, followed by bioinformatic analyses of gene variant function. We identified significant and reliable LVs predicting the influence of white matter tract integrity on cognitive performance both in controls and patients, but driven overall by the patient group. Combined genetics and neuroimaging data showed that variants from the MAG, OLIG2, and CNP genes influenced white matter tract integrity and cognitive performance. Finally, our data demonstrated that effects of oligodendrocyte gene variants on cognitive performance were mediated via the integrity of white matter tracts in both controls and patients, but again driven by the patient group. As hypothesized, gene variants played a more prominent role in influencing brain phenotypes (i.e., white matter integrity) than cognitive phenotypes (i.e., cognitive performance), and in certain analyses, particularly robust covariance structures were elicited in schizophrenia patients. Taken together, our data support the importance of the oligodendrocyte/white matter pathway of disease in schizophrenia (Bartzokis 2002; Bartzokis et al. 2003; Davis et al. 2003; Bartzokis and Altshuler 2005; Kubicki et al. 2005, 2007; Szeszko et al. 2005; McIntosh, Maniega et al. 2008) by identifying key relationships among oligodendrocyte risk variants, white matter tract integrity, and cognitive performance in this disorder.
White Matter Integrity and Cognitive Performance
Our PLS analysis provides a “big-picture” of the relationship between microstructural integrity of several major white matter tracts and performance on a battery of cognitive tests in healthy controls and patients with schizophrenia, while controlling for correlations among dependent variables. In patients, microstructural integrity of the left UF, CB, bilateral IFOF, right AF and ILF, and corpus callosum (Kubicki et al. 2007; Friedman et al. 2008) predicted cognitive performance in domains impaired in schizophrenia, namely executive function, visual attention, visuospatial ability, language, and processing speed (Bokat and Goldberg 2003; Gur et al. 2007). Although others have identified relationships between white matter tract integrity and cognitive performance in schizophrenia (Fitzsimmons et al. 2009; Perez-Iglesias et al. 2010), our finding provides a novel window into the complexity of these relationships. Our findings are congruent with functional imaging data and they suggest that compared with controls, patients with schizophrenia have a broader networks of white matter tracts reliably associated with cognitive task performance (Tan et al. 2006; Ursu et al. 2011). The relationships discovered in the two-group analysis for schizophrenia patients remained almost entirely consistent for the within-group analysis in patients, unlike the controls, suggesting that the covariance structure in the two-group analysis was driven by the schizophrenia patients. Therefore, the white matter tracts that we examined likely play an especially important role in cognitive function in schizophrenia patients. Such findings suggest that a subtle perturbation in vulnerable white matter tracts may disproportionately impact cognitive performance in schizophrenia, possibly due to decreased cognitive reserve present in this disorder (Dwork et al. 2007).
Gene Variants, White Matter Integrity, and Cognitive Performance
By combining genetics, brain imaging, and cognitive data, we found novel evidence for the effects of oligodendrocyte gene variants on white matter tract integrity and cognitive function in both healthy individuals and in patients with schizophrenia. Variants in the MAG, OLIG2, and CNP genes influenced both microstructural integrity of white matter tracts and cognitive performance. To our knowledge, these are the first reported findings associating variants in these genes with white matter tracts and cognitive performance. Using PLS, we were able to show that these variants influence a network of white matter tracts, rather than any one tract alone. Our data also provide evidence that these gene variants' effect on cognition in schizophrenia is mediated by subtle, but neuroanatomically widespread, disconnectivity in white matter circuitry.
Among the genes that we studied, OLIG2 is a basic helix–loop–helix transcription factor, a master regulator of oligodendrocyte lineages, necessary for their genesis and myelination (Jakovcevski and Zecevic 2005; Georgieva et al. 2006; Nicolay et al. 2007). Its effect on brain circuitry and cognitive performance may occur by influencing expression of other oligodendrocyte genes across development as OLIG2 is expressed in both precursor and fully matured oligodendrocytes. The MAG gene is also important for oligodendrocytes and the myelin sheath, where it is critical for axo-glial interactions (Li et al. 1994; Schachner and Bartsch 2000) and is a key component of the myelin-mediated complex that inhibits axonal growth (Domeniconi et al. 2002). Although the putative function of individual MAG SNPs that we found associated with microstructural integrity of white matter is not yet clear, the MAG variants that we examined have been previously associated with schizophrenia, and downregulation of MAG in postmortem brain is a well-replicated finding in this disorder (Voineskos, Lang et al. 2008). Reduction in MAG expression can disrupt the exquisite balance of the myelin-mediated complex of inhibition of axonal growth (consisting of Nogo-A, oligodendrocyte myelin glycoprotein, tyrosine-kinase receptor ErBb3, and the Nogo-66 receptor). In turn, this would lead to disorganized axonal sprouting and influence microstructural integrity of white matter and cognitive performance (Voineskos 2009). This protein complex may be a worthwhile target of treatment intervention for cognitive deficits in schizophrenia and is currently a site of intensive research in the central nervous system injury field (Budel et al. 2008). Given recent convergent evidence for a role for the Nogo-66 receptor gene in schizophrenia (Voineskos 2009), an examination of potential epistasis between this 22q11 region gene and its binding partner (MAG) would also be of interest. The MAG gene has correlated expression with the CNP gene and may be regulated by similar “upstream” factors (McCullumsmith et al. 2007).
NRG1 variants were not reliably associated with microstructural integrity of white matter, but were associated with cognitive performance in patients with schizophrenia. Others have reported effects of NRG1 variants on white matter integrity in healthy controls (McIntosh, Moorhead et al. 2008; Winterer et al. 2008) and in schizophrenia patients (Wang et al. 2009). There is substantial evidence for NRG1 as a risk gene for schizophrenia (Li et al. 2006; Munafo et al. 2006), and NRG1 plays key roles in proliferation and survival of oligodendrocyte precursors, oligodendrocyte differentiation, and CNS myelination (Chen et al. 2006; Nave and Salzer 2006; Taveggia et al. 2008). However, genetic association studies of NRG1 in schizophrenia have not always been consistent. One possible explanation for the lack of reliable association with NRG1 and white matter integrity in our study is that NRG1 variants may influence risk for specific subgroups of patients, rather than for the disorder as a whole (Georgieva et al. 2008). This is supported by the fact that conventional univariate statistical analysis reveals significant effects of this variant on white matter tract integrity (data not shown) in our sample combined with the fact that this effect NRG1 variation on white matter tract integrity did not quite reach our reliability threshold (the salience to SE ratio was 1.98). The fact that this significant effect is not reliable demonstrates the value of using reliability/bootstrapping measures to ensure that demonstrated effects are not driven by a subsample of the data. NRG1's effects on cognitive performance might also be mediated via white matter tracts that we did not study, such as the anterior thalamic radiation. Finally, it is possible that risk for NRG1's effects on cognitive performance may occur via effects on oligodendrocytes and other cellular substrates in gray matter rather than white matter, possibly due to upregulation of NRG1 isoforms in postmortem schizophrenia brain (Law et al. 2006). The gene variant located in NRG1's binding partner, ErbB4, did not influence white matter phenotypes, but, like NRG1, was associated with cognitive performance. ErbB4 demonstrates coordinated expression with CNP and OLIG2 in postmortem brain (Georgieva et al. 2006), and the SNP we examined was associated with integrity of the UF in a recent study (Konrad et al. 2009). Given the considerable genetic heterogeneity of schizophrenia, ErbB4 (and QKI—which was not associated with phenotypes in our study), like NRG1, may be important only in certain subsets of patients. Alternatively, there may be smaller effects of these gene variants on imaging and cognitive phenotypes that could not be detected in our sample.
Genes to Brain to Behavior Model
In an attempt to mirror the underlying biological processes (i.e., genes to brain to behavior), our final PLS analysis modeled white matter tract integrity as a group of variables mediating the effects of genetic variants on cognitive performance. This analysis highlighted three patterns in our data. First, relationships among gene variants, white matter tract integrity, and cognitive performance were found across all subjects. Second, the strongest relationships occurred between genetic variants and white matter integrity. Gene effects were more pronounced at the proximal level of the brain than at the distal level of cognition, supporting the imaging genetics paradigm. Finally, rather than influencing any single white matter tract, gene variants affected the system of white matter tracts we examined, supporting a broad effect of oligodendrocyte genes on cortico-cortical connectivity in white matter. The same system of white matter tracts predicted cognitive performance in patients providing biological continuity from genetic variants to brain circuitry to cognitive performance. These results also support that genetic variation is an important determinant of heterogeneity of brain structure and cognitive performance in both healthy individuals and in patients with schizophrenia.
Overall, there are several advantages to using a multivariate type approach (we used PLS, but others are using independent components analysis [Calhoun et al. 2008] for instance). In general, as the field moves toward embracing biological complexity, particularly in light of technology that can provide information on multiple gene variants and multiple brain measures, multivariate approaches are become increasingly necessary in order to ascertain relationships among all the data (Tura et al. 2008). Furthermore, mediational relationships can be demonstrated that are biologically meaningful. Another benefit of such an approach is that the “P-value chase” that plagues our field and leads to many false-positive findings, especially in genetics, is bypassed using these approaches (Ioannidis 2005). In addition, the debate about how to set an appropriate P-value (i.e., via Bonferonni correction, false discovery, or other types of corrections) is also obviated (Sullivan 2007; Meyer-Lindenberg et al. 2008). Finally, the combination of permutation and bootstrapping analyses, while not necessarily providing conventional tests of “differences” between groups, supplies quantitative data that speak significance of LVs and simultaneously, presence versus absence of reliable relationships within each LV. The permutation test assesses whether the effect represented in a given LV is sufficiently strong, in a statistical sense, to be different from random noise. While permutation tests indicate whether a signal can be differentiated from noise, they do not index signal reliability. Although detection and reliability are strongly related, they are not mutually exclusive (McIntosh and Lobaugh 2004). The bootstrap adds an important complement to the overall assessment of significance in PLS, in that the technique allows one to de-emphasize signals that are not reliable.
Limitations
A limitation of our study is that other gene systems—such as the glutamatergic, oxidative stress, and autoimmune/cytokine systems—may influence the oligodendrocyte/white matter pathway in schizophrenia (Salter and Fern 2005). Future investigations should examine the collective role of variants from these gene systems and their relationship with white matter integrity in schizophrenia. However, we focused on two gene systems and carefully selected each variant based on strong a priori evidence. It is also possible that other gene variants in linkage disequilibrium with the variants we examined may be the true causative variants influencing our phenotypic measures. Similarly, we did not examine all major white matter tracts. Our DTI acquisition sequence was not cardiac gated (Jones et al. 2002), and thus, we were at somewhat higher risk for introducing artifact; however, all scans were checked for major artifacts prior to inclusion for analysis. It has been shown that experiential circumstances (Scholz et al. 2009) can influence brain structural measures, including FA, and although we attempted to limit the effects of substance use in our sample, the effects on brain structure from other types of experiences cannot be ruled out. Finally, we residualized antipsychotic exposure from dependent variable measures and recent evidence does not support medication effects as a confound on white matter tract microstructure (Zhang et al. 2008; Perez-Iglesias et al. 2010). However, like with experience, the confounding effects of medication on white matter and cognition in our schizophrenia group cannot be ruled out.
Conclusion
In summary, taken together, our genetics, neuroimaging, and cognitive findings provide novel evidence that support a key role for the oligodendrocyte/white matter pathway in cognitive function in both healthy individuals and schizophrenia patients. We have provided novel evidence linking the effects of oligodendrocyte gene variants on white matter tract integrity and cognitive performance. Our findings highlight the need for therapeutic strategies aimed at this pathway that may help ameliorate cognitive impairment in schizophrenia.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the Canadian Institutes of Health Research Clinician Scientist Award (A.N.V.), APA/APIRE Astra-Zeneca Young Minds in Psychiatry Award (A.N.V.), NARSAD (A.N.V., A.K.T., and T.K.R.), K05 MH070047 and R01 MH 50740 (for M.E.S.) from the National Institutes of Health, and by the Centre for Addiction and Mental Health. We also wish to acknowledge support through the CAMH Foundation from the Michael and Sonja Koerner Foundation (Koerner New Scientist Program), the Kimel Family, and the Paul E Garfinkel New Investigator Catalyst Award.
Supplementary Material
Notes
The authors would also like to acknowledge the support of the CAMH Foundation (Koerner New Scientist Program and Paul Garfinkel New Investigator Catalyst Fund). A.N.V. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. No funder/sponsor had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Conflict of Interest: B.G.P. receives research support from the National Institute of Health and the Canadian Institutes of Health Research. Within the past 5 years, he has been a member of the advisory board of Lundbeck Canada (final meeting was May 2009) and Forest Laboratories (final meeting was March 2008). He has served one time as a consultant for Wyeth (October 2008) and Takeda (July 2007). He was also a faculty member of the Lundbeck International Neuroscience Foundation (LINF) (final meeting was April 2010). B.H.M. currently receives research support from the US National Institute of Mental Health, the Canadian Institutes for Health Research, Bristol-Myers Squibb, and Wyeth. During the past 5 years, he has also received research support or honoraria from Astra-Zeneca, Eli Lilly, Forest Laboratories, GlaxoSmithKline, Janssen, Lundbeck, and Pfizer. J.L.K. received one time speaker fees from Eli Lilly in 2010.
References
- Aberg K, Saetre P, Jareborg N, Jazin E. Human QKI, a potential regulator of mRNA expression of human oligodendrocyte-related genes involved in schizophrenia. Proc Natl Acad Sci USA. 2006;103:7482–7487. doi: 10.1073/pnas.0601213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Schizophrenia: Breakdown in the well-regulated lifelong process of brain development and maturation. Neuropsychopharmacology. 2002;27:672–683. doi: 10.1016/S0893-133X(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Altshuler L. Reduced intracortical myelination in schizophrenia. Am J Psychiatry. 2005;162:1229–1230. doi: 10.1176/appi.ajp.162.6.1229-a. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Nuechterlein KH, Lu PH, Gitlin M, Rogers S, Mintz J. Dysregulated brain development in adult men with schizophrenia: A magnetic resonance imaging study. Biol Psychiatry. 2003;53:412–421. doi: 10.1016/s0006-3223(02)01835-8. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bokat CE, Goldberg TE. Letter and category fluency in schizophrenic patients: A meta-analysis. Schizophr Res. 2003;64:73–78. doi: 10.1016/s0920-9964(02)00282-7. [DOI] [PubMed] [Google Scholar]
- Budel S, Padukkavidana T, Liu BP, Feng Z, Hu F, Johnson S, Lauren J, Park JH, McGee AW, Liao J, et al. Genetic variants of Nogo-66 receptor with possible association to schizophrenia block myelin inhibition of axon growth. J Neurosci. 2008;28:13161–13172. doi: 10.1523/JNEUROSCI.3828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, MacDonald AW, 3rd, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37:640–650. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA. 2007;104:17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Velardez MO, Warot X, Yu ZX, Miller SJ, Cros D, Corfas G. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;26:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, et al. Genetics of brain fiber architecture and intellectual performance. J Neurosci. 2009;29:2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: Evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Dwork AJ, Mancevski B, Rosoklija G. White matter and cognitive function in schizophrenia. Int J Neuropsychopharmacol. 2007;10:513–536. doi: 10.1017/S1461145707007638. [DOI] [PubMed] [Google Scholar]
- Ennis DB, Kindlmann G. Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. Magn Reson Med. 2006;55:136–146. doi: 10.1002/mrm.20741. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York: Biometrics Research; 1995. [Google Scholar]
- Fitzsimmons J, Kubicki M, Smith K, Bushell G, Estepar RS, Westin CF, Nestor PG, Niznikiewicz MA, Kikinis R, McCarley RW, et al. Diffusion tractography of the fornix in schizophrenia. Schizophr Res. 2009;107:39–46. doi: 10.1016/j.schres.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, Golembo S, Kanellopoulou I, Ng J, Hof PR, et al. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165:1024–1032. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: A disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Georgieva L, Dimitrova A, Ivanov D, Nikolov I, Williams NM, Grozeva D, Zaharieva I, Toncheva D, Owen MJ, Kirov G, et al. Support for neuregulin 1 as a susceptibility gene for bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:419–427. doi: 10.1016/j.biopsych.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Georgieva L, Moskvina V, Peirce T, Norton N, Bray NJ, Jones L, Holmans P, Macgregor S, Zammit S, Wilkinson J, et al. Convergent evidence that oligodendrocyte lineage transcription factor 2 (OLIG2) and interacting genes influence susceptibility to schizophrenia. Proc Natl Acad Sci USA. 2006;103:12469–12474. doi: 10.1073/pnas.0603029103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, Kanes S, Blangero J, Gur RC. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164:813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Kiryu H, Sato K, Mituyama T, Asai K. Prediction of RNA secondary structure using generalized centroid estimators. Bioinformatics. 2009;25:465–473. doi: 10.1093/bioinformatics/btn601. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Davis KL. Introduction to the special section: Myelin and oligodendrocyte abnormalities in schizophrenia. Int J Neuropsychopharmacol. 2007;10:499–502. doi: 10.1017/S1461145706007449. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N. Olig transcription factors are expressed in oligodendrocyte and neuronal cells in human fetal CNS. J Neurosci. 2005;25:10064–10073. doi: 10.1523/JNEUROSCI.2324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: A Monte Carlo study. Magn Reson Med. 2004;51:807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- Jones DK, Williams SC, Gasston D, Horsfield MA, Simmons A, Howard R. Isotropic resolution diffusion tensor imaging with whole brain acquisition in a clinically acceptable time. Hum Brain Mapp. 2002;15:216–230. doi: 10.1002/hbm.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Glahn DC, Lancaster JL, Winkler AM, Smith S, Thompson PM, Almasy L, Duggirala R, Fox PT, Blangero J. Genetics of microstructure of cerebral white matter using diffusion tensor imaging. Neuroimage. 2010;53:1109–1116. doi: 10.1016/j.neuroimage.2010.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad A, Vucurevic G, Musso F, Stoeter P, Dahmen N, Winterer G. ErbB4 genotype predicts left frontotemporal structural connectivity in human brain. Neuropsychopharmacology. 2009;34:641–650. doi: 10.1038/npp.2008.112. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley RW, Shenton ME. Evidence for white matter abnormalities in schizophrenia. Curr Opin Psychiatry. 2005;18:121–134. doi: 10.1097/00001504-200503000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Styner M, Bouix S, Gerig G, Markant D, Smith K, Kikinis R, McCarley RW, Shenton ME. Reduced interhemispheric connectivity in schizophrenia-tractography based segmentation of the corpus callosum. Schizophr Res. 2008;106:125–131. doi: 10.1016/j.schres.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci USA. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Tropak MB, Gerlai R, Clapoff S, Abramow-Newerly W, Trapp B, Peterson A, Roder J. Myelination in the absence of myelin-associated glycoprotein. Nature. 1994;369:747–750. doi: 10.1038/369747a0. [DOI] [PubMed] [Google Scholar]
- Li D, Collier DA, He L. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet. 2006;15:1995–2002. doi: 10.1093/hmg/ddl122. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Gupta D, Beneyto M, Kreger E, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of transcripts for myelination-related genes in the anterior cingulate cortex in schizophrenia. Schizophr Res. 2007;90:15–27. doi: 10.1016/j.schres.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Maniega SM, Lymer GK, McKirdy J, Hall J, Sussmann JE, Bastin ME, Clayden JD, Johnstone EC, Lawrie SM. White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Moorhead TW, Job D, Lymer GK, Munoz Maniega S, McKirdy J, Sussmann JE, Baig BJ, Bastin ME, Porteous D, et al. The effects of a neuregulin 1 variant on white matter density and integrity. Mol Psychiatry. 2008;13:1054–1059. doi: 10.1038/sj.mp.4002103. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: Applications and advances. Neuroimage. 2004;23(Suppl 1):S250–263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, Weinberger DR. False positives in imaging genetics. Neuroimage. 2008;40:655–661. doi: 10.1016/j.neuroimage.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF., 3rd. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Miyata J, Yamada M, Namiki C, Hirao K, Saze T, Fujiwara H, Shimizu M, Kawada R, Fukuyama H, Sawamoto N, et al. Reduced white matter integrity as a neural correlate of social cognition deficits in schizophrenia. Schizophr Res. 2010;119:232–239. doi: 10.1016/j.schres.2009.12.038. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Thiselton DL, Clark TG, Flint J. Association of the NRG1 gene and schizophrenia: A meta-analysis. Mol Psychiatry. 2006;11:539–546. doi: 10.1038/sj.mp.4001817. [DOI] [PubMed] [Google Scholar]
- Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Niznikiewicz M, Gurrera RJ, McCarley RW, Shenton ME. Neuropsychological disturbance in schizophrenia: A diffusion tensor imaging study. Neuropsychology. 2008;22:246–254. doi: 10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolay DJ, Doucette JR, Nazarali AJ. Transcriptional control of oligodendrogenesis. Glia. 2007;55:1287–1299. doi: 10.1002/glia.20540. [DOI] [PubMed] [Google Scholar]
- O'Donnell LJ, Kubicki M, Shenton ME, Dreusicke MH, Grimson WE, Westin CF. A method for clustering white matter fiber tracts. Am J Neuroradiol. 2006;27:1032–1036. [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Opiyo SO, Moriyama EN. Mining the Arabidopsis and rice genomes for cyclophilin protein families. Int J Bioinform Res Appl. 2009;5:295–309. doi: 10.1504/IJBRA.2009.026421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce TR, Bray NJ, Williams NM, Norton N, Moskvina V, Preece A, Haroutunian V, Buxbaum JD, Owen MJ, O'Donovan MC. Convergent evidence for 2′,3′-cyclic nucleotide 3′-phosphodiesterase as a possible susceptibility gene for schizophrenia. Arch Gen Psychiatry. 2006;63:18–24. doi: 10.1001/archpsyc.63.1.18. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, Barker GJ, Roiz-Santianez R, Mata I, de Lucas EM, Rodriguez-Sanchez JM, Ayesa-Arriola R, Vazquez-Barquero JL, et al. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010;167:451–458. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- Pernet V, Joly S, Christ F, Dimou L, Schwab ME. Nogo-A and myelin-associated glycoprotein differently regulate oligodendrocyte maturation and myelin formation. J Neurosci. 2008;28:7435–7444. doi: 10.1523/JNEUROSCI.0727-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Canales-Rodriguez EJ, Salvador R, Sarro S, Gomar JJ, Vila F, Ortiz-Gil J, Iturria-Medina Y, Capdevila A, McKenna PJ. Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Mol Psychiatry. 2010;15:823–830. doi: 10.1038/mp.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raadsma HW, Moser G, Crump RE, Khatkar MS, Zenger KR, Cavanagh JA, Hawken RJ, Hobbs M, Barris W, Solkner J, et al. Predicting genetic merit for mastitis and fertility in dairy cattle using genome wide selection and high density SNP screens. Dev Biol (Basel) 2008;132:219–223. doi: 10.1159/000317163. [DOI] [PubMed] [Google Scholar]
- Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: Meta-analysis. Br J Psychiatry. 2009;195:286–293. doi: 10.1192/bjp.bp.108.060723. [DOI] [PubMed] [Google Scholar]
- Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, Benoit-Marand M, Chen C, Moore H, O'Donnell P, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci USA. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Schachner M, Bartsch U. Multiple functions of the myelin-associated glycoprotein MAG (siglec-4a) in formation and maintenance of myelin. Glia. 2000;29:154–165. doi: 10.1002/(sici)1098-1136(20000115)29:2<154::aid-glia9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D, Koschnick JR, Slegers LH, Hof PR. Oligodendrocyte pathophysiology: A new view of schizophrenia. Int J Neuropsychopharmacol. 2007;10:503–511. doi: 10.1017/S146114570600722X. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Kanaan RA, Chitnis XA, O'Daly O, Jones DK, Frangou S, Williams SC, Howard RJ, Barker GJ, Murray RM, et al. A diffusion tensor imaging study of fasciculi in schizophrenia. Am J Psychiatry. 2007;164:467–473. doi: 10.1176/ajp.2007.164.3.467. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: A meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiol Aging. 2008;31:464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF. Spurious genetic associations. Biol Psychiatry. 2007;61:1121–1126. doi: 10.1016/j.biopsych.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, Gunduz-Bruce H, Malhotra AK, Kane JM, Bilder RM, et al. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: A diffusion tensor imaging study. Am J Psychiatry. 2005;162:602–605. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S, Ardekani BA, Lencz T, Malhotra AK, McCormack J, et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33:976–984. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer-Lindenberg A, Egan MF, Weinberger DR, Callicott JH. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163:1969–1977. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, Salzer JL. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56:284–293. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Tura E, Turner JA, Fallon JH, Kennedy JL, Potkin SG. Multivariate analyses suggest genetic impacts on neurocircuitry in schizophrenia. Neuroreport. 2008;19:603–607. doi: 10.1097/WNR.0b013e3282fa6d8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu S, Kring AM, Gard MG, Minzenberg MJ, Yoon JH, Ragland JD, Solomon M, Carter CS. Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. Am J Psychiatry. 2011;168:276–285. doi: 10.1176/appi.ajp.2010.09081215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos A, Lang D, Zai G, Bulgin N, Shaikh S, Su W, Kopala L, MacEwan G, Thornton A, Smith J, et al. MAG gene variation and cortical gray matter volume in first episode schizophrenia. Brain Imaging Behav. 2008;2:117–122. [Google Scholar]
- Voineskos AN. Converging evidence for the Nogo-66 receptor gene in schizophrenia. J Neurosci. 2009;29:5045–5047. doi: 10.1523/JNEUROSCI.0477-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, de Luca V, Bulgin NL, van Adrichem Q, Shaikh S, Lang DJ, Honer WG, Kennedy JL. A family-based association study of the myelin-associated glycoprotein and 2′,3′-cyclic nucleotide 3′-phosphodiesterase genes with schizophrenia. Psychiatr Genet. 2008;18:143–146. doi: 10.1097/YPG.0b013e3282fa1874. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Lobaugh NJ, Bouix S, Rajji TK, Miranda D, Kennedy JL, Mulsant BH, Pollock BG, Shenton ME. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain. 2010;133:1494–1504. doi: 10.1093/brain/awq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, O'Donnell LJ, Lobaugh NJ, Markant D, Ameis SH, Niethammer M, Mulsant BH, Pollock BG, Kennedy JL, Westin CF, et al. Quantitative examination of a novel clustering method using magnetic resonance diffusion tensor tractography. Neuroimage. 2009;45:370–376. doi: 10.1016/j.neuroimage.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, Pollock BG, Mulsant BH. Age-related decline in white matter tract integrity and cognitive performance: A DTI tractography and structural equation modeling study. Neurobiol Aging. 2012;33:21–34. doi: 10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Jiang T, Sun Z, Teng SL, Luo X, Zhu Z, Zang Y, Zhang H, Yue W, Qu M, et al. Neuregulin 1 genetic variation and anterior cingulum integrity in patients with schizophrenia and healthy controls. J Psychiatry Neurosci. 2009;34:181–186. [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneiderman JS, O'Donnell LJ, King R, Alvarado JL, Khan U, Markant D, Nestor PG, Niznikiewicz M, et al. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biol Psychiatry. 2010;68:70–77. doi: 10.1016/j.biopsych.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Konrad A, Vucurevic G, Musso F, Stoeter P, Dahmen N. Association of 5′ end neuregulin-1 (NRG1) gene variation with subcortical medial frontal microstructure in humans. Neuroimage. 2008;40:712–718. doi: 10.1016/j.neuroimage.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu H, Jiang W, Xiao L, Yan B, He J, Wang Y, Bi X, Li X, Kong J, et al. Quetiapine alleviates the cuprizone-induced white matter pathology in the brain of C57BL/6 mouse. Schizophr Res. 2008;106:182–191. doi: 10.1016/j.schres.2008.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.