Abstract
The parietal cortex is central to numerical cognition. The right parietal region is primarily involved in basic quantity processing, while the left parietal region is additionally involved in precise number processing and numerical operations. Little is known about how the 2 regions interact during numerical cognition. We hypothesized that functional connectivity between the right and left parietal cortex is critical for numerical processing that engages both basic number representation and learned numerical operations. To test this hypothesis, we estimated neural activity using functional magnetic resonance imaging in participants performing numerical and arithmetic processing on dot arrays. We first found task-based functional connectivity between a right parietal seed and the left sensorimotor cortex in all task conditions. As we hypothesized, we found enhanced functional connectivity between this right parietal seed and both the left parietal cortex and neighboring right parietal cortex, particularly during subtraction. The degree of functional connectivity also correlated with behavioral performance across individual participants, while activity within each region did not. These results highlight the role of parietal functional connectivity in numerical processing. They suggest that arithmetic processing depends on crosstalk between and within the parietal cortex and that this crosstalk contributes to one's numerical competence.
Keywords: arithmetic processing, functional connectivity, intraparietal sulcus, numerical cognition
Introduction
The parietal cortex plays a central role in the representation and processing of numbers. Functional neuroimaging studies have shown that regions in and around the bilateral intraparietal sulcus (IPS) are robustly activated during number comparison and arithmetic tasks (Chochon et al. 1999; Dehaene et al. 1999; Pinel et al. 2001; Ansari and Dhital 2006; Castelli et al. 2006; Prado et al. 2011) as well as during tasks involving mere detection of symbolic numerals (Eger et al. 2003) or release of adaptation from a specific numerosity (Piazza et al. 2004, 2007; Cohen Kadosh et al. 2007). Combined with neuropsychological studies that demonstrate selective deficits in number processing in patients with parietal lesions (Warrington and James 1967; Dehaene and Cohen 1991; Polk et al. 2001; Lemer et al. 2003), these findings provide evidence that the parietal cortex plays a central role in numerical cognition.
While number processing tasks, in general, activate bilateral parietal cortex, recent studies are beginning to suggest that there are interesting distinctions between the left and the right parietal regions. Activation in the right intraparietal area is most frequently shown in tasks that require basic understanding of numerosity such as an approximate magnitude comparison task typically using nonsymbolic numbers (Dehaene et al. 1999; Piazza et al. 2006; Holloway et al. 2010; Prado et al. 2011). These tasks have been hypothesized to engage a basic number sense, that is, an intuitive understanding of numbers and their relationships (Dehaene 1999). The finding that such primitive numerical judgment tasks induce primarily right parietal activation suggests that the right parietal region might be the cortical root for innate quantitative competencies. Supporting this argument, functional magnetic resonance imaging (fMRI) adaptation studies have shown that number-related activation in the right parietal cortex is notation and presentation-mode independent (Piazza et al. 2004, 2007; Dormal et al. 2010, 2012). Even more convincing are neuroimaging studies of infants and children who are presumably equipped with basic quantitative competencies but not yet acquainted with knowledge of more precise number, counting, and arithmetic competencies. When presented with a numerosity adaptation task, infants and children show predominantly right parietal activation (Cantlon et al. 2006; Izard et al. 2008; Hyde et al. 2010) suggesting that the right parietal cortex may be innately organized to be a neural locus of basic quantitative competencies that exist before the acquisition of the precise number system available to educated adults.
When tasks require more precise representation and processing of number, such as exact arithmetic tasks, or symbolic comparison tasks, the left parietal cortex is engaged in addition to the right parietal cortex (Dehaene et al. 1996; Chochon et al. 1999; Pinel et al. 2001). These tasks engage not only the primitive number sense but also precise numerical values represented in symbols and learned arithmetic facts and operations. Activation in the left parietal region in response to numerosity and numerical operations increases over the course of development and as a function of behavioral performance in mathematics (Rivera et al. 2005; Cantlon et al. 2006; Grabner et al. 2007). The left angular gyrus, in particular, has been associated with exact calculation, arithmetical fact retrieval, and the passive perception of Arabic numerals (Gruber et al. 2001; Grabner et al. 2009; Price and Ansari 2011), and the left supramarginal gyrus has been associated with Arabic numeral processing (Polk et al. 2001; Roux et al. 2008). These findings suggest that learning of numerals and arithmetic may give rise to the specialization of enculturated numerical representations and operations in the left parietal region.
While these findings suggest important roles of the left and right parietal regions for numerical cognition, little attention has been given to how the 2 regions work together when representing and processing numbers. In the present study, we address this issue by using the psychophysiological interaction (PPI) approach to estimate task-based functional connectivity arising from the right parietal cortex during numerical cognition in a fMRI experiment. Specifically, we hypothesized that during numerical cognition there would be enhanced functional connectivity between the right parietal region involved in a more primitive representation of numerical information and the left parietal regions involved in more precise representation and processing of number, and that this functional connectivity would be modulated by the type of numerical processing, ranging from number comparison to arithmetic. Furthermore, if such functional connectivity subserves numerical cognition, then we would expect that individual differences in connectivity would predict individual differences in task performance.
Materials and Methods
Participants
Twenty-seven healthy adults (ages 18–29 with mean of 23.0; 10 males) participated in this fMRI study. All participants were screened through self-report questionnaires to ensure they were right-handed, psychologically and physically healthy, and free of any other MRI safety contraindications. All study procedures were reviewed and approved by the Institutional Review Boards at the University of Texas at Dallas, the University of Texas Southwestern, and the University of Michigan. All participants provided detailed written consent prior to their involvement in the study.
Stimulus Materials
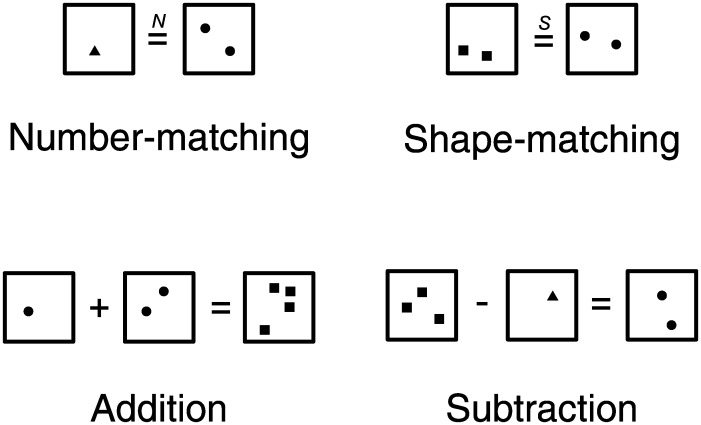
To elicit brain activations related to number processing, a number-matching condition and a shape-matching (control) condition were devised. In both conditions, the stimuli were comprised of 2 arrays—one on the left and the other on the right of an equal sign. The items within the 2 arrays varied in numerosity (from 1 to 4) and shape (triangles, squares, or circles). In the number-matching condition, participants were asked to make a numerosity judgment, determining whether the number of items in the left array matched the number in the right (regardless of shape). In the shape-matching condition, the same stimuli were used and participants judged whether the left and right arrays contained the same shapes (regardless of numerosity). The only difference between shape and numerosity stimuli was that an “S” appeared above the equal sign for the shape-matching trial and an “N” appeared above the equal sign for the number-matching trial.
Two other experimental conditions (addition and subtraction) that make additional demands on arithmetic processing were devised using similar stimuli. In the addition condition, participants viewed stimuli consisting of 3 arrays. On the left were 2 arrays with a plus sign in the middle, followed by an equal sign, and a third array on the right. Participants judged whether the numerosity of stimuli on the right of the equal sign was the same as the sum of the numerosities on the left. The subtraction condition was similar except that the plus sign was replaced with a minus sign and participants judged whether the numerosity of stimuli on the right was the same as the difference of the numerosities on the left.
Stimulus arrays were restricted to small numerosities within the subitizing range (4 or fewer). This was to ensure that enumeration of these sets of items was done very fast and with little effort and error (Kaufman et al. 1949; Revkin et al. 2008) so that more primitive numerical operations (i.e. number-matching) and more complex numerical operations (i.e. addition and subtraction) could be done using the same set of non-symbolic stimuli. Item size was roughly identical in all arrays. As enumeration of small set of items is fast and effortless, non-numerical visual parameters such as cumulative surface area were thought to have a little influence in these numerical judgments. In another set of behavioral pilot studies, participants (N= 12) underwent the number-matching task in which half of the trials were equated in item size and the other half were equated in the total surface area. They performed the task equally well in both conditions (accuracy t(11) = −0.170, P= 0.566; median reaction time (RT) t(11) = 0.299, P= 0.615). Furthermore, we also asked another set of participants (N= 10) to match the total surface area instead of the numerosity, using the same set of stimuli. While these subjects performed above chance in making this judgment (mean accuracy 63.7%, t(9) = 3.679, P= 0.003), the accuracy was substantially worse than the accuracy in the case of number-matching (t(20) = 8.00, P< 0.001). Examples of the stimuli are shown in Figure 1.
Figure 1.
Examples of stimuli used in the experiment. Participants performed simple numerical and arithmetic tasks on dot arrays differing in numerosity and in shape. They judged whether the numerical operation was correct or not (for number-matching, addition, and subtraction) and whether the shapes matched or not (for shape-matching).
Procedure
The task consisted of five 5-min runs with twenty 16-s blocks, pseudorandomly ordered (4 for each of the 4 conditions in addition to 4 fixation blocks). Each block was preceded by a 2-s instruction screen in which the name of the task was displayed on the screen so that the participants could prepare for the upcoming block. The task was self-paced within each block, and participants were asked to solve as many problems as possible within each block. The stimuli were on the screen until subjects responded, and the next stimuli were displayed after a 500 ms inter-trial interval of the blank screen. This was to ensure that there was no dead time in each block caused by differences in task difficulties and to make sure that time-on-task was matched across conditions (because the participants were engaged throughout the duration of each block regardless of the task). The correct answer was “yes” in half of all the trials. In all the trials with incorrect numerical statements, the incorrect answer differed from the correct answer by one. All visual stimuli were presented via E-prime (Psychology Software Tools, Pittsburgh, PA, United States of America) and displayed by a back-projection system. Participants made responses using buttons under the right index and middle fingers (Lumina response pad; Cedrus, San Pedro, CA, United States of America).
Data Acquisition
Brain images were acquired with a Philips Achieva 3T whole-body scanner at UT Southwestern using the Philips SENSE parallel acquisition technique. Functional scans were acquired as axial slices, with a voxel size of 3.4 × 3.4 × 3.5 mm. At each of 246 blood oxygen level-dependent (BOLD) acquisitions per run, 33 axial slices were acquired (field of view = 220 mm, matrix size = 64 × 64, repetition time (TR) = 1.5 s, echo time (TE) = 25 ms, flip angle = 60°). A high-resolution axial T1 MPRAGE was also acquired (voxel size 1 mm isotropic; TR = 8.27 ms, TE = 3.82 ms, flip angle = 12°).
Data Analysis
Functional data were processed using SPM8 (Wellcome Department of Cognitive Neurology, London, United Kingdom, http://www.fil.ion.ucl.ac.uk). Functional images underwent slice-timing correction and realignment to the mean volume. Each participant's T1 anatomical image was coregistered with the functional images and then segmented into gray matter, white matter, and cerebral spinal fluid. The gray matter was normalized into the default gray matter probability template in standard MNI (Montreal Neurological Institute) space, and the acquired normalization parameters were used to normalize the functional images for each individual with a spatial resolution of 3 × 3 × 3 mm.
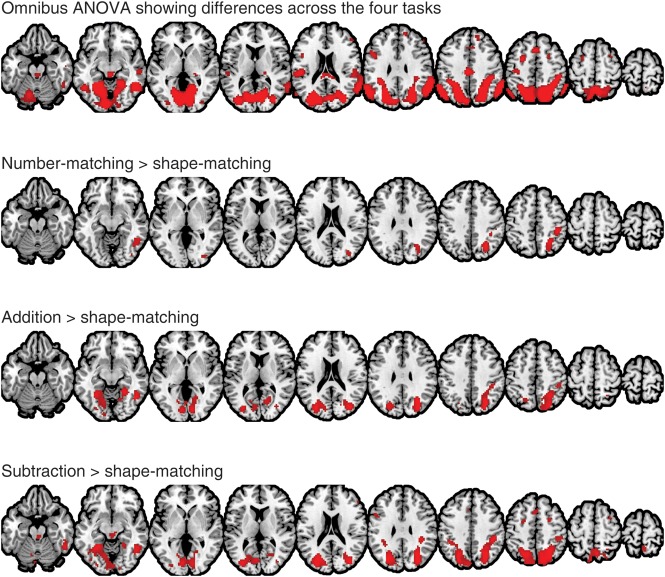
The functional data were then modeled using a general linear model, which included separate regressors for each of the experimental conditions convolved with a canonical hemodynamic response function, as well as 24 head motion regressors as nuisance covariates, including the linear, squared, time-shifted, and squared time-shifted transformations of the 6 rigid-body movement parameters. An additional covariate was included to model the 2-s instructions at the beginning of each experimental block. As a first pass, we confirmed that a number of regions show activation differences across the 4 conditions by using an omnibus test in a repeated-measures analysis of variance (ANOVA) at the group level (top of Fig. 2). Then, the contrast maps for numerical processing (i.e. number-matching vs. shape-matching, addition vs. shape-matching, and subtraction vs. shape-matching) from each subject were entered into a second-level random effects group analysis at a very conservative threshold (family-wise error P < 0.05; Fig. 2). For simplicity, these 3 contrasts are henceforth referred to as the number-matching contrast, addition contrast, and subtraction contrast, respectively.
Figure 2.
A neural activation map for the omnibus repeated-measures analysis of variance and 3-independent contrasts with a stringent threshold, P < 0.05 (family-wise error corrected) and extent >10 voxels (group-level random effects map with N = 27). Brain maps are shown in axial slices from z = −20 to z = 70 in increments of 10 mm.
Task-based functional connectivity between a seed region and the rest of the brain as a function of numerical operations was tested using a PPI analysis (Friston et al. 1997). First, a conjunction mask was constructed by intersecting the number-matching, addition, and subtraction maps from Figure 2 to identify the voxels that showed significant activation in all 3 at the group level. This mask identified 2 contiguous regions: One in the right parietal cortex (411 voxels) and the other in the right posterior inferior temporal cortex (54 voxels). The right parietal mask was selected to be the candidate region for the seed region of interest (ROI) in the PPI analysis (Fig. 3).
Figure 3.
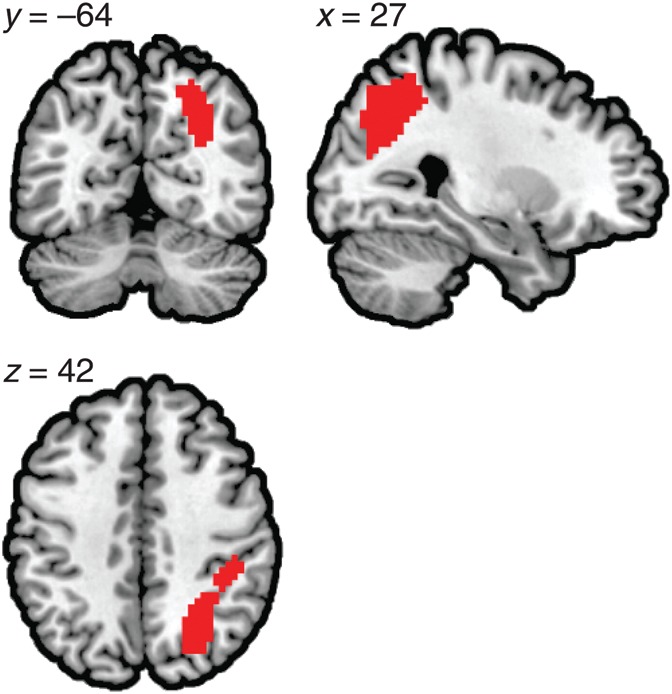
The conjunction of the 3 contrasts which served as a candidate region for the individual seed for the subsequent PPI connectivity analyses.
Secondly, the seed region was defined in each participant individually. The peak activation voxel in the number-matching contrast within the right parietal conjunction mask was selected in each participant. Then a spherical ROI was defined around the peak activation voxel to include all the voxels (81 voxels) within 8 mm of the peak voxel. The BOLD time course (i.e. first eigenvariate) within this ROI was extracted. The time course was adjusted using the F-contrast of all the task regressors. This procedure removes confounds in the BOLD signal (such as motion) that cannot be explained by the effects of interest. This time course served as the physiological variable.
Thirdly, 3 separate PPI models were constructed for each of the 3 main contrasts: Number-matching, addition, and subtraction contrasts. Then, the PPI term was constructed by taking the product of the deconvolved physiological variable and each psychological variable. The interaction term in each model therefore tests whether the BOLD signal coherence between the seed region and a target region is modulated by the psychological variable. Note that the shape-matching condition served as the control condition in all models. Thus, the modulation of the BOLD signal coherence may be interpreted as the degree of coherence in each experimental condition over and above the degree of coherence in the shape-matching condition. The parameter estimate of the PPI variable, which represents the difference (or contrast) in this degree of coherence, is henceforth referred to as the PPI parameter estimate. The individual contrast images for the PPI parameter estimates were entered into a second-level random effects analysis for inference at the group level. Regions surviving a threshold of P < 0.05 (voxel-wise false discovery rate corrected) and extent >10 voxels were considered statistically significant. As recent work shows that the parietal cortex is cytoarchitectonically heterogeneous (Scheperjans et al. 2008; Caspers et al. 2012), we further used a probabilistic cytoarchitectonic map toolbox (Eickhoff et al. 2005) to report resulting activation and PPI maps at a finer anatomical level.
Results
Behavioral Performance
Reaction time and accuracy in each condition are listed in Table 1. While there was little difference in accuracy between the number-matching and shape-matching tasks (t(26) = 0.414, P = 0.682), the reaction time was faster in the shape-matching task (t(26) = 9.426, P < 0.001). The addition task was performed less accurately (t(26) = 2.860, P = 0.008) and more slowly (t(26) = 14.789, P < 0.001) than the number-matching task. Likewise, the subtraction task was less accurate (t(26) = 3.649, P = 0.002) and slower (t(26) = 14.967, P < 0.001) than the number-matching task.
Table 1.
Behavioral measures of task performance in the scanner
| Number-matching | Addition | Subtraction | Shape-matching | |
|---|---|---|---|---|
| Accuracy (%) | 96.2 (0.024) | 94.8 (0.034) | 93.5 (0.052) | 96.4 (0.030) |
| Reaction time (ms) | 792.5 (96.36) | 1039.1 (153.72) | 1208.7 (156.99) | 678.9 (82.78) |
Note: Mean accuracy and median reaction time for correct trials were computed for each participant. The mean (standard deviations) of these scores across all participants (N = 27) is reported.
Number-Selective Activation in the Right Parietal Cortex as the Seed Region
Our primary hypothesis was that the right parietal cortex, the primary locus of number representation, is functionally connected to the left parietal cortex during numerical tasks. As a first step to test this hypothesis, we identified number-selective brain regions derived from number-matching, addition, and subtraction contrasts (Fig. 2). The number-matching contrast elicited activation predominantly in the right parietal cortex along the the IPS. Paired t-test between the response magnitude in the right parietal peak [27 −64 40] and a local maximum point in the left parietal cortex [−18 −64 46] identified by lowering the overall threshold showed that the right parietal activity was significantly greater than the activity in the left parietal region (t(26) = 5.396, P < 0.001). These results are consistent with previous studies showing predominantly right parietal involvement in basic and primitive form of number processing (Cantlon et al. 2006; Piazza et al. 2006; Izard et al. 2008; Holloway et al. 2010; Hyde et al. 2010; Prado et al. 2011). The addition and subtraction contrasts showed additional neural recruitment in the precuneus and the left parietal cortex. The conjunction of these 3 contrast maps identified a large region in the right parietal cortex (Fig. 3) over IPS (hIP3), superior parietal lobule (7A, 7PC, 7P), and Area 2. This region served as the candidate seed region for the subsequent PPI analysis.
Right Parietal Functional Connectivity to the Left
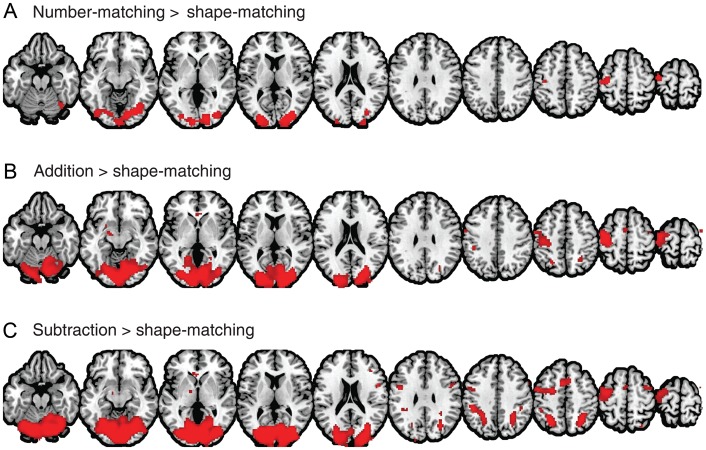
We then tested our primary hypothesis using the PPI analysis. In 3 separate PPI models, regions showing significant task-based increases in functional connectivity from the right parietal seed were identified as shown in Figure 4 with peak voxels listed in Tables 2–4. As we hypothesized, robust PPI parameter estimates were identified in the left parietal cortex, but importantly, only in the addition (in and around 7A and hIP3; Fig. 4B) and the subtraction conditions (in and around 7A, hIP1, hIP2, and hIP3; Fig. 4C). These 2 contrasts also showed significant PPI parameter estimates in additional right parietal regions (hIP3, 7PC, and 7A in the addition contrast; hIP1, hIP3, 7A, 7PC, and 7P in the subtraction contrast).
Figure 4.
PPI maps of number-matching (A), addition (B), and subtraction (C) contrasts. Each of these maps shows regions with a greater time-course correlation with the number-selective right parietal seed region during the experimental condition compared with the control (shape-matching) condition.
Table 2.
Clusters identified from the PPI analysis with the number-matching contrast
| Region | Cluster size (voxels) | Z-score | Peak coordinate |
|---|---|---|---|
| Bilateral middle and inferior occipital cortex | 1367 | 5.41 | 21 −88 10 |
| 5.07 | 24 −79 −11 | ||
| 4.87 | 30 −85 7 | ||
| Left precentral gyrus | 165 | 4.50 | −39 −13 70 |
| 4.48 | −48 −22 67 | ||
| 4.35 | −36 −19 55 | ||
| Right anterior cerebellum | 15 | 3.92 | 18 −52 −26 |
Note: MNI coordinates of the local maxima >8 mm apart are reported. This table corresponds to the statistical parametric map shown in Figure 4A.
Table 3.
Clusters identified from the PPI analysis with the addition contrast
| Region | Cluster size (voxels) | Z-score | Peak coordinate |
|---|---|---|---|
| Bilateral middle and inferior occipital cortex | 4161 | 6.07 | 21 −91 10 |
| 5.49 | 9 −82 −8 | ||
| 5.32 | −18 −94 7 | ||
| Left precentral gyrus | 616 | 4.69 | −39 −22 55 |
| 4.61 | −39 −13 70 | ||
| 4.46 | −45 −7 61 | ||
| Left thalamus/globus pallidus | 60 | 4.3 | −21 −7 −8 |
| Left IPS | 25 | 3.65 | −24 −61 49 |
| Supplementary motor area | 29 | 3.61 | −3 2 58 |
| Right IPS | 31 | 3.43 | 27 −52 46 |
| Vermis | 11 | 3.26 | 3 −55 −38 |
| Right superior frontal gyrus | 12 | 3.25 | 39 −4 70 |
Note: MNI coordinates of the local maxima >8 mm apart are reported. This table corresponds to the statistical parametric map shown in Figure 4B.
Table 4.
Clusters identified from the PPI analysis with the subtraction contrast
| Region | Cluster size (voxels) | Z-score | Peak coordinate |
|---|---|---|---|
| Occipital cortex extending to IPS and angular gyrus | 6484 | 6.82 | 24 −88 10 |
| 6.55 | −9 −91 −8 | ||
| 6.47 | 0 −88 −8 | ||
| Right inferior frontal area | 48 | 4.47 | 51 8 25 |
| Supplementary motor area | 142 | 4.38 | −6 8 49 |
| 3.59 | 9 14 49 | ||
| Left precentral gyrus | 594 | 4.26 | −39 −10 67 |
| 4.24 | −24 −4 49 | ||
| 4.2 | −45 2 28 | ||
| Left thalamus/globus pallidus | 38 | 3.87 | −15 −7 −8 |
| Undefined | 16 | 3.74 | −3 26 1 |
| Right middle frontal gyrus | 15 | 3.46 | 39 35 22 |
| Right dorsal middle frontal gyrus | 44 | 3.45 | 36 2 64 |
Note: MNI coordinates of the local maxima >8 mm apart are reported. This table corresponds to the statistical parametric map shown in Figure 4C.
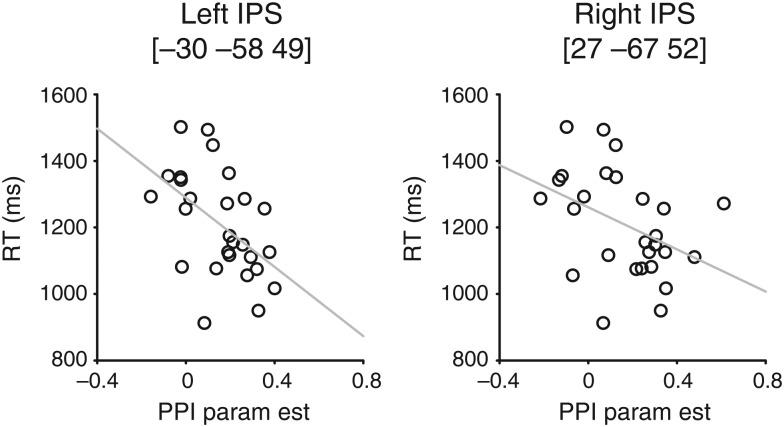
We then examined the magnitude of PPI parameter estimates in these regions across the different conditions. Spherical ROIs with radius of 8-mm were constructed around the peak voxels in the left IPS [−30 −58 49] and the right IPS [27 −67 52] identified from the subtraction condition. As shown in Figure 5, PPI parameter estimates were significantly greater in the subtraction condition compared with the addition condition (left IPS, t(26) = 4.01, P < 0.001; right IPS, t(26) = 2.76, P = 0.011), but the addition and the shape-matching conditions were not significantly different (left IPS, t(26) = 1.13, P = 0.287; right IPS, t(26) = 0.56, P = 0.580). These results are not due to the ROI selection bias. Even when the ROIs were constructed around the peak voxels identified from the addition condition, PPI parameter estimates were greater in the subtraction condition compared with the addition condition (left IPS, t(26) = 3.61, P = 0.001; right IPS, t(26) = 1.99, P = 0.057), but the addition and the shape-matching conditions were not significantly different (left IPS, t(26) = 1.5, P = 0.146; right IPS, t(26) = 0.8, P = 0.431). These results suggest that inter- and intra-parietal functional connectivity is critical in these arithmetic tasks, particularly in the subtraction task, and that the functional connectivity reflects greater demands on numerical processing required by the mental process for subtraction.
Figure 5.
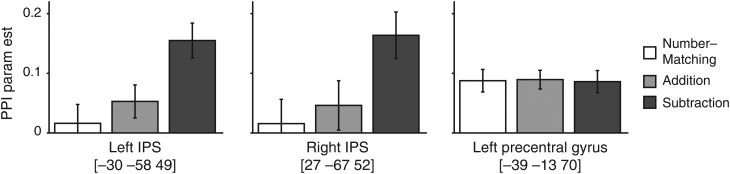
PPI parameter estimates in the left IPS [−30 −58 49], right IPS [27 −67 52], and left sensorimotor cortex [−39 −13 70]. Spherical ROI's were constructed (radius = 8 mm) around the peak coordinates, and the PPI parameter estimates from the 3 contrasts were extracted from each participant. This bar graph shows the mean (±standard error) PPI parameter estimates of the 3 contrasts in the 3 ROI's.
In theory, a positive PPI effect could also arise if the BOLD time-course correlation was “negative” in the shape-matching condition and either less negative or positive in the number-matching condition. This was not the case, however. For example, the positive PPI estimates in the left IPS stemmed from greater “positive” time-course correlation between the left IPS and the right parietal seed region during the subtraction condition (r = 0.846) compared with the shape-matching condition (r = 0.763).
One might wonder whether greater PPI parameter estimates in the IPS in the subtraction condition compared with the other conditions could be due to greater BOLD response in the subtraction condition compared with the others (see Fig. 2). In the PPI analysis procedure, however, the psychological variable—representing the BOLD response difference between conditions—is controlled for when estimating the PPI term. In fact, the estimates for the psychological variable in the bilateral IPS were significantly greater in the subtraction condition than in the other conditions (Supplementary Fig. S2). These results indicate that greater PPI estimates in the subtraction condition are not simply due to greater BOLD response.
One might also consider whether the enhanced functional connectivity between the seed region and the left and right IPS could be due to more frequent eye movements in the subtraction condition than in the other conditions, as the parietal cortex is recruited when making saccades (Simon et al. 2002; Medendorp et al. 2005). We examined this alternative possibility by conducting an eye-tracking experiment with an independent group of subjects (Supplementary Materials) that allowed us to quantify the eye movement in each of the 4 conditions (shape-matching, number-matching, addition, and subtraction). The results showed comparable eye movements across the 4 conditions (proportion of time spent on making saccades was 32.5% in the shape-matching condition, 32.0% in the number-matching condition, 33.6% in the addition condition, and 32.7% in the subtraction condition), suggesting that it is unlikely that the current PPI results are due to eye movements.
Right Parietal Functional Connectivity to the Left Sensorimotor Area
In addition to the inter- and intra-parietal functional connectivity results, we also found significant PPI parameter estimates in the left sensorimotor area in all 3 contrasts (Fig. 4). The magnitude of these PPI estimates was comparable across the 3 different conditions (Greenhouse–Geisser corrected repeated-measures ANOVA, F1.949, 50.667= 0.018, P = 0.981).
We considered whether these enhanced PPI parameter estimates in the left sensorimotor cortex could be related to button presses during the task. For example, subjects might press the button harder during the number-matching condition than during the shape-matching condition. If so, the activation parameter estimates in this region would be greater in the number-matching condition. However, we report the opposite finding: The mean activation estimate of the number-matching contrast in the 8-mm spherical ROI defined at the left sensorimotor peak [−36 −13 70] was −0.087 (one-sample t-test, t(26) = −1.194, P = 0.243), suggesting a tendency for greater BOLD in the shape-matching condition compared with the number-matching condition. Note that the task was self-paced within each block, so a task with longer reaction time (Table 1) showing smaller activation estimates is plausible in the context of our study. This is further corroborated by even smaller BOLD response during the addition (mean activation estimate = −0.303, one-sample t-test, t(26) = −2.999, P = 0.006) and subtraction (mean activation estimate = −0.369, one-sample t-test, t(26) = −3.496, P = 0.002) contrasts in this left sensorimotor area (Supplementary Fig. S1). Thus, the observed PPI parameter estimates in the left sensorimotor cortex cannot be explained by differences in button press activity across conditions.
Functional Connectivity and Behavioral Performance
The results so far suggest that arithmetic tasks, particularly subtraction, make greater demands on effective communication between the 2 parietal regions as well as within the right parietal cortex. We further tested whether the observed functional connectivity has implications for behavior. If such functional connectivity subserves the cognitive processes required for subtraction, then individuals with greater functional connectivity should exhibit better behavioral task performance.
To test this hypothesis, we correlated individual subject PPI parameter estimates for the subtraction contrast in the left IPS with individual subject RT measures during the subtraction condition (Fig. 6). We found a strong and significant negative correlation between the RT and the PPI estimates in the left IPS (r = −0.505, P = 0.007) and in the right IPS (r = −0.409, P = 0.034). Interestingly, RT could not be reliably predicted from the activation estimates in only the left IPS (r = −0.089, P = 0.658) or in only the right IPS (r = −0.160, P = 0.426). Similarly, RT could not be significantly predicted from the activation estimates in the right parietal seed region (r = −0.089, P = 0.658). Thus, functional connectivity between the right parietal seed and the left/right IPS is a better predictor of behavioral performance than activation in any of these regions. This significant correlation between RT and the PPI parameter estimates was only evident in the subtraction condition (see Supplementary Fig. S3), a point to which we will return in the Discussion.
Figure 6.
The relationship between the PPI parameter estimates and RT during the subtraction task in the left and right IPS. The gray line indicates the best linear fit.
This strong negative relationship between RT and the PPI parameter estimates also suggests that enhanced PPI parameter estimates in the subtraction task compared with the other 2 tasks (Fig. 4) cannot be explained by the differences in RT across the conditions. If greater PPI parameter estimates in the left IPS, for example, are due to greater task difficulty or time on task, then participants who are slower at performing the task should show greater PPI parameter estimates, which is inconsistent with the current result (Fig. 6). As a way to quantitatively address this potential issue we compared the PPI parameter estimates for the three conditions while removing the linear effects of RT. There was still a significant difference between the subtraction and the addition conditions both in the left (t(26) = 22.5, P < 0.001) and right (t(26) = 14.4, P < 0.001) IPS, but little difference between the addition and the number-matching conditions (left, t(26) = 1.09, P = 0.287; right, t(26) = 0.426, P = 0.673).
Discussion
In this study, we tested the hypothesis that numerical processing would be supported by functional connectivity between the left and right parietal cortex. We estimated task-based functional connectivity using a PPI analysis of fMRI data from participants performing simple numerical and arithmetic tasks. Consistent with our primary hypothesis, we found significant and robust functional connectivity between a number-selective right parietal seed region and the left IPS extending to the angular gyrus, particularly in the subtraction contrast. Additionally, a right IPS region also showed enhanced PPI parameter estimates, suggesting that functional connectivity among neighboring regions in the right parietal cortex is also critical.
As discussed in the Introduction, the involvement of the left parietal cortex in numerical processing is well established. In particular, the left supramarginal gyrus and angular gyrus have been repeatedly found to be activated during arithmetic problem solving and exact calculation (Dehaene et al. 1999; Delazer et al. 2003; Rivera et al. 2005; Ischebeck et al. 2006; Grabner et al. 2007) and are often hypothesized to support the automatic retrieval of arithmetic facts. Our results extend these previous findings and suggest that arithmetic problem solving, specifically subtraction, requires not only activation in the left parietal cortex but also effective neural communication between the right and left parietal cortex and within the right parietal cortex itself.
Moreover, we found that individual participants who were faster in performing the subtraction task tended to have stronger functional connectivity between bilateral parietal regions and the seed region. These results provide additional support for the hypothesis that effective neural communication between and within the parietal cortex is important for arithmetic processing. It is worth noting that such a strong negative correlation between RT and the functional connectivity measure is unique to the IPS region. For example, RT during the subtraction task was not predicted by PPI estimates in the left sensorimotor cortex [−39 −10 67] (r = −0.010, P = 0.962) or visual cortex [0 −88 −8] (r = −0.193, P = 0.335) despite the significant connectivity effects in those regions. Moreover, this correlation was unique to the subtraction condition, suggesting that the results cannot be attributed to task-general factors such as attention or motivation because otherwise we should expect similar negative correlations in the addition and number-matching conditions as well.
The brain maps from our PPI analyses show that the addition contrast also yields weak but significant PPI effects in the bilateral IPS (Fig. 4). These results suggest that addition also makes demands on functional connectivity between and within and the parietal cortex over and above shape-matching. However, there was little difference between the PPI parameter estimates for the number-matching and addition contrasts, while the subtraction contrast showed significantly greater PPI parameter estimates compared with the other 2 contrasts (Fig. 5). Moreover, strong negative correlations between RT and the PPI parameter estimates were only evident in the subtraction contrast (Fig. 6). These results suggest that the subtraction task may be qualitatively different from the other tasks.
One possibility is that the subtraction task requires mental manipulation of numbers to a much greater extent than the other 2 tasks. The number-matching task does not require any such mental arithmetic. While the addition task requires such mental arithmetic, given the simultaneous presentation of dot arrays in the present study, some subjects could have conceivably performed the addition task simply by estimating the 2 dot arrays collectively without an explicit addition process. Alternatively, the simple addition required in our study may have been facilitated by automatic summation processes to a greater degree than was subtraction (Zbrodoff and Logan 1986; Lefevre et al. 1988). In either case, the addition task put significantly fewer demands on number operations than the subtraction task. According to this interpretation, inter- and intra-parietal functional connectivity is critical in arithmetic processing that requires active mental operations on numbers. Future studies could explore different types of arithmetic processing (perhaps an addition task that does require substantial mental operations) and their relationship to interhemispheric and regional parietal functional connectivity.
Future studies could also explore parietal functional connectivity patterns during approximate arithmetic using large non-symbolic numbers. We used dot arrays with small numerosities to enable both more primitive and more complex numerical operations such as exact addition and subtraction in the same experiment. However, the literature suggests that small (4 or less) and large numbers may be processed in qualitatively different ways (for a review see Feigenson et al. 2004). Large numbers are hypothesized to be processed by an approximate number system (Dehaene 2001) that enables approximate estimation and manipulation of the numerical magnitude of a large set of objects, while small numbers are processed by a parallel individuation system (Carey 2001) that enables precise representation of a small set of objects. In this context, the parietal functional connectivity we observed suggests that people were recruiting the parallel individuation system. However, it would be interesting to test whether similar functional connectivity is necessary for mental manipulations of numbers based on the approximate number system.
In addition to the results arising from our primary hypotheses, several additional points are worth mentioning. First, in all tasks, we found robust functional connectivity between the number-selective right parietal seed region and a large extent of the visual cortex. This connectivity suggests that extraction of numerical information from the visual stimuli depends on neural communication between the visual cortex and the right parietal cortex. Next and more interestingly, we found significant increases in functional connectivity between the number-selective right parietal seed and the left sensorimotor cortex in all 3 contrasts (Figs. 4 and 5). These findings provide further evidence for the recruitment of motor and premotor areas during numerical processing. Previous studies have shown left motor cortex involvement in counting (Zago et al. 2001; Piazza et al. 2006; Andres et al. 2007; Tschentscher et al. 2012), and these results have often been tentatively interpreted as subvocalization during the task or as a manifestation of finger counting. It should also be noted that there was no significant neural activation in this region during the number-matching task compared with the shape-matching task (Fig. 2 and Supplementary Fig. S1), implying that the involvement of the left sensorimotor cortex is only evident through its functional connectivity to the right parietal region. Thus, these results highlight the importance of examining functional connectivity between regions, which may provide important insight not available from an examination of activation in the 2 regions.
In summary, our findings indicate that effective communication between and within the parietal cortex is particularly important during subtraction, possibly reflecting a mental process related to manipulation of numerical values. More interestingly, the degree of parietal connectivity predicted behavioral performance on this task across participants, suggesting a strong functional role of this parietal connectivity. Critically, neural activation measures in each of the 2 regions did not predict behavioral performance. These results suggest that mental operations on numerical quantities depend on crosstalk between and within the parietal cortex and that the effectiveness of this crosstalk influences one's competence in such mental operations.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This study was supported by grant 3R37AG006265 from the National Institute on Aging to D.C.P.
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Andres M, Seron X, Olivier E. Contribution of hand motor circuits to counting. J Cogn Neurosci. 2007;19:563–576. doi: 10.1162/jocn.2007.19.4.563. [DOI] [PubMed] [Google Scholar]
- Ansari D, Dhital B. Age-related changes in the activation of the intraparietal sulcus during nonsymbolic magnitude processing: an event-related functional magnetic resonance imaging study. J Cogn Neurosci. 2006;18:1820–1828. doi: 10.1162/jocn.2006.18.11.1820. [DOI] [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM, Carter EJ, Pelphrey KA. Functional imaging of numerical processing in adults and 4-y-old children. PLoS Biol. 2006;4:e125. doi: 10.1371/journal.pbio.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey S. Cognitive foundations of arithmetic: evolution and ontogenisis. Mind Lang. 2001;16:37–55. [Google Scholar]
- Caspers S, Schleicher A, Bacha-Trams M, Palomero-Gallagher N, Amunts K, Zilles K. Organization of the human inferior parietal lobule based on receptor architectonics. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs048. doi:10.1093/cercor/bhs048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Glaser DE, Butterworth B. Discrete and analogue quantity processing in the parietal lobe: a functional MRI study. Proc Natl Acad Sci USA. 2006;103:4693–4698. doi: 10.1073/pnas.0600444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chochon F, Cohen L, van de Moortele PF, Dehaene S. Differential contributions of the left and right inferior parietal lobules to number processing. J Cogn Neurosci. 1999;11:617–630. doi: 10.1162/089892999563689. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R, Cohen Kadosh K, Kaas A, Henik A, Goebel R. Notation-dependent and -independent representations of numbers in the parietal lobes. Neuron. 2007;53:307–314. doi: 10.1016/j.neuron.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Dehaene S. 1999 The number sense: how the mind creates mathematics. Oxford: Oxford University Press. [Google Scholar]
- Dehaene S. Précis of the number sense. Mind Lang. 2001;16:16–36. [Google Scholar]
- Dehaene S, Cohen L. Two mental calculation systems: a case study of severe acalculia with preserved approximation. Neuropsychologia. 1991;29:1045–1054. doi: 10.1016/0028-3932(91)90076-k. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Tzourio N, Frak V, Raynaud L, Cohen L, Mehler J, Mazoyer B. Cerebral activations during number multiplication and comparison: a PET study. Neuropsychologia. 1996;34:1097–1106. doi: 10.1016/0028-3932(96)00027-9. [DOI] [PubMed] [Google Scholar]
- Delazer M, Domahs F, Bartha L, Brenneis C, Lochy A, Trieb T, Benke T. Learning complex arithmetic–an fMRI study. Brain Res Cogn Brain Res. 2003;18:76–88. doi: 10.1016/j.cogbrainres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Dormal V, Andres M, Dormal G, Pesenti M. Mode-dependent and mode-independent representations of numerosity in the right intraparietal sulcus. Neuroimage. 2010;52:1677–1686. doi: 10.1016/j.neuroimage.2010.04.254. [DOI] [PubMed] [Google Scholar]
- Dormal V, Dormal G, Joassin F, Pesenti M. A common right fronto-parietal network for numerosity and duration processing: an fMRI study. Hum Brain Mapp. 2012;33:1490–1501. doi: 10.1002/hbm.21300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger E, Sterzer P, Russ MO, Giraud AL, Kleinschmidt A. A supramodal number representation in human intraparietal cortex. Neuron. 2003;37:719–725. doi: 10.1016/s0896-6273(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Feigenson L, Dehaene S, Spelke E. Core systems of number. Trends Cogn Sci. 2004;8:307–314. doi: 10.1016/j.tics.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Grabner RH, Ansari D, Koschutnig K, Reishofer G, Ebner F, Neuper C. To retrieve or to calculate? Left angular gyrus mediates the retrieval of arithmetic facts during problem solving. Neuropsychologia. 2009;47:604–608. doi: 10.1016/j.neuropsychologia.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Grabner RH, Ansari D, Reishofer G, Stern E, Ebner F, Neuper C. Individual differences in mathematical competence predict parietal brain activation during mental calculation. Neuroimage. 2007;38:346–356. doi: 10.1016/j.neuroimage.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Gruber O, Indefrey P, Steinmetz H, Kleinschmidt A. Dissociating neural correlates of cognitive components in mental calculation. Cereb Cortex. 2001;11:350–359. doi: 10.1093/cercor/11.4.350. [DOI] [PubMed] [Google Scholar]
- Holloway ID, Price GR, Ansari D. Common and segregated neural pathways for the processing of symbolic and nonsymbolic numerical magnitude: an fMRI study. Neuroimage. 2010;49:1006–1017. doi: 10.1016/j.neuroimage.2009.07.071. [DOI] [PubMed] [Google Scholar]
- Hyde DC, Boas DA, Blair C, Carey S. Near-infrared spectroscopy shows right parietal specialization for number in pre-verbal infants. Neuroimage. 2010;53:647–652. doi: 10.1016/j.neuroimage.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck A, Zamarian L, Siedentopf C, Koppelstatter F, Benke T, Felber S, Delazer M. How specifically do we learn? Imaging the learning of multiplication and subtraction. Neuroimage. 2006;30:1365–1375. doi: 10.1016/j.neuroimage.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Izard V, Dehaene-Lambertz G, Dehaene S. Distinct cerebral pathways for object identity and number in human infants. PLoS Biol. 2008;6:e11. doi: 10.1371/journal.pbio.0060011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman EL, Lord MW, Reese TW, Volkmann J. The discrimination of visual number. Am J Psychol. 1949;62:498–525. [PubMed] [Google Scholar]
- Lefevre JA, Bisanz J, Mrkonjic L. Cognitive arithmetic – evidence for obligatory activation of arithmetic facts. Memory Cogn. 1988;16:45–53. doi: 10.3758/bf03197744. [DOI] [PubMed] [Google Scholar]
- Lemer C, Dehaene S, Spelke E, Cohen L. Approximate quantities and exact number words: dissociable systems. Neuropsychologia. 2003;41:1942–1958. doi: 10.1016/s0028-3932(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Vilis T. Remapping the remembered target location for anti-saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:734–740. doi: 10.1152/jn.01331.2004. [DOI] [PubMed] [Google Scholar]
- Piazza M, Izard V, Pinel P, Le Bihan D, Dehaene S. Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron. 2004;44:547–555. doi: 10.1016/j.neuron.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Piazza M, Mechelli A, Price CJ, Butterworth B. Exact and approximate judgements of visual and auditory numerosity: an fMRI study. Brain Res. 2006;1106:177–188. doi: 10.1016/j.brainres.2006.05.104. [DOI] [PubMed] [Google Scholar]
- Piazza M, Pinel P, Le Bihan D, Dehaene S. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron. 2007;53:293–305. doi: 10.1016/j.neuron.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Pinel P, Dehaene S, Riviere D, LeBihan D. Modulation of parietal activation by semantic distance in a number comparison task. Neuroimage. 2001;14:1013–1026. doi: 10.1006/nimg.2001.0913. [DOI] [PubMed] [Google Scholar]
- Polk TA, Reed CL, Keenan JM, Hogarth P, Anderson CA. A dissociation between symbolic number knowledge and analogue magnitude information. Brain Cogn. 2001;47:545–563. doi: 10.1006/brcg.2001.1486. [DOI] [PubMed] [Google Scholar]
- Prado J, Mutreja R, Zhang H, Mehta R, Desroches AS, Minas JE, Booth JR. Distinct representations of subtraction and multiplication in the neural systems for numerosity and language. Human Brain Mapp. 2011;32:1932–1947. doi: 10.1002/hbm.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GR, Ansari D. Symbol processing in the left angular gyrus: evidence from passive perception of digits. Neuroimage. 2011;57:1205–1211. doi: 10.1016/j.neuroimage.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Revkin SK, Piazza M, Izard VR, Cohen L, Dehaene S. Does subitizing reflect numerical estimation? Psychol Sci. 2008;19:607–614. doi: 10.1111/j.1467-9280.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- Rivera SM, Reiss AL, Eckert MA, Menon V. Developmental changes in mental arithmetic: evidence for increased functional specialization in the left inferior parietal cortex. Cereb Cortex. 2005;15:1779–1790. doi: 10.1093/cercor/bhi055. [DOI] [PubMed] [Google Scholar]
- Roux FE, Lubrano V, Lauwers-Cances V, Giussani C, Demonet JF. Cortical areas involved in Arabic number reading. Neurology. 2008;70:210–217. doi: 10.1212/01.wnl.0000297194.14452.a0. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex. 2008;18:846–867. doi: 10.1093/cercor/bhm116. [DOI] [PubMed] [Google Scholar]
- Simon O, Mangin J-Fo, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002;33:475–487. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- Tschentscher N, Hauk O, Fischer MH, Pulvermuller F. You can count on the motor cortex: finger counting habits modulate motor cortex activation evoked by numbers. Neuroimage. 2012;59:3139–3148. doi: 10.1016/j.neuroimage.2011.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, James M. Tachistoscopic number estimation in patients with unilateral cerebral lesions. J Neurol Neurosurg Psychiatry. 1967;30:468–474. doi: 10.1136/jnnp.30.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago L, Pesenti M, Mellet E, Crivello F, Mazoyer B, Tzourio-Mazoyer N. Neural correlates of simple and complex mental calculation. Neuroimage. 2001;13:314–327. doi: 10.1006/nimg.2000.0697. [DOI] [PubMed] [Google Scholar]
- Zbrodoff NJ, Logan GD. On the autonomy of mental processes – a case-study of arithmetic. J Exp Psychol Gen. 1986;115:118–130. doi: 10.1037//0096-3445.115.2.118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.