Abstract
Bupropion is indicated to promote smoking cessation. Animal studies suggest that bupropion’s major metabolite hydroxybupropion can mediate bupropion’s pharmacologic activity. We measured plasma bupropion and metabolite levels in a double-blind, placebo controlled, randomized smoking cessation trial. Among the treatment adherent individuals, higher hydroxybupropion concentrations (per µg/mL) resulted in better smoking cessation outcomes (Week 3, 7 and 26 OR=2.82, 2.96 and 2.37, P=0.005–0.040), this was not observed with bupropion levels (OR=1.00–1.03, P=0.59–0.90). Genetic variation in CYP2B6, the enzyme that metabolizes bupropion to hydroxybupropion, was identified as a significant source of variability in hydroxybupropion formation. Our data indicate that hydroxybupropion contributes to the pharmacologic effects of bupropion for smoking cessation, and that variability in response to bupropion treatment is related to variability in CYP2B6-mediated hydroxybupropion formation. These findings suggest dosing bupropion to achieve a hydroxybupropion level of 0.7 µg/ml or increasing bupropion dose for CYP2B6 slow metabolizers, could improve bupropion’s cessation outcomes.
Introduction
Smoking is a major cause of preventable death with multiple morbidities associated including lung cancer, chronic obstructive pulmonary disease, cardiovascular disease and diabetes. While the prevalence of smoking has declined since the 1960s it has plateaued at ≈20% over the last decade. There are currently 45 million smokers in American, 40% percent of these smokers attempt to quit each year, yet fewer than 10% of these successfully quit.1 Thus, there is a pressing need for efficacious smoking cessation treatments. In addition, smoking cessation research has been predominantly conducted in Caucasian heavy smokers, and relatively little information is available about the efficacy of smoking cessation therapy in African American smokers despite the disproportionally higher risks of smoking related morbidities and mortalities.2,3 The lack of research in African Americans is especially notable in African American light smokers, who smoke less than 10 cigarettes per day and make up more than 50% of African American smokers. Only two studies have evaluated the efficacy of smoking cessation treatment in African American light smokers.2,4
Bupropion is a non-nicotine treatment for smoking cessation.5 In humans, bupropion is metabolized by the genetically polymorphic enzyme CYP2B6 to hydroxybupropion.6–8 Hydroxybupropion may contribute to bupropion’s pharmacologic activity. Pharmacodynamically, hydroxybupropion demonstrates similar or greater inhibition effects (IC50) compared to bupropion in blocking dopamine, norepinephrine transporters and the α4β2 nicotinic receptor in vitro as well as blocking the development of nicotine induced condition place preference and reducing the somatic signs of withdrawal in animal models.9,10 Pharmacokinetically, hydroxybupropion has a ten times higher free plasma concentration at steady state and a slightly higher unbound fraction compared to bupropion, resulting in considerably higher hydroxybupropion drug exposure than bupropion at steady state.11–17 Together this suggests that hydroxybupropion may play an important role in mediating the smoking cessation effects of bupropion.
The objective of our study was to understand the contribution of hydroxybupropion to bupropion’s ability in promoting smoking cessation, and the sources of variability in hydroxybupropion levels. We hypothesize hydroxybupropion is a major determinant of bupropion’s ability to promote smoking cessation, and that greater hydroxybupropion formation, mediated by faster CYP2B6 activities, will increase bupropion’s treatment outcomes. A better characterization of hydroxybupropion’s contribution to bupropion’s ability to promote smoking cessation will result in a better understanding of the mechanism of action and variability in bupropion’s treatment outcomes leading to more efficacious treatment strategies. Our study focused on African American light smokers due to their disproportionally higher risks of smoking related morbidities and mortalities, as well as the fact that there are few clinical trials which investigated smoking cessation in light smokers despite their growing prevalence in the general population of smokers.
Results
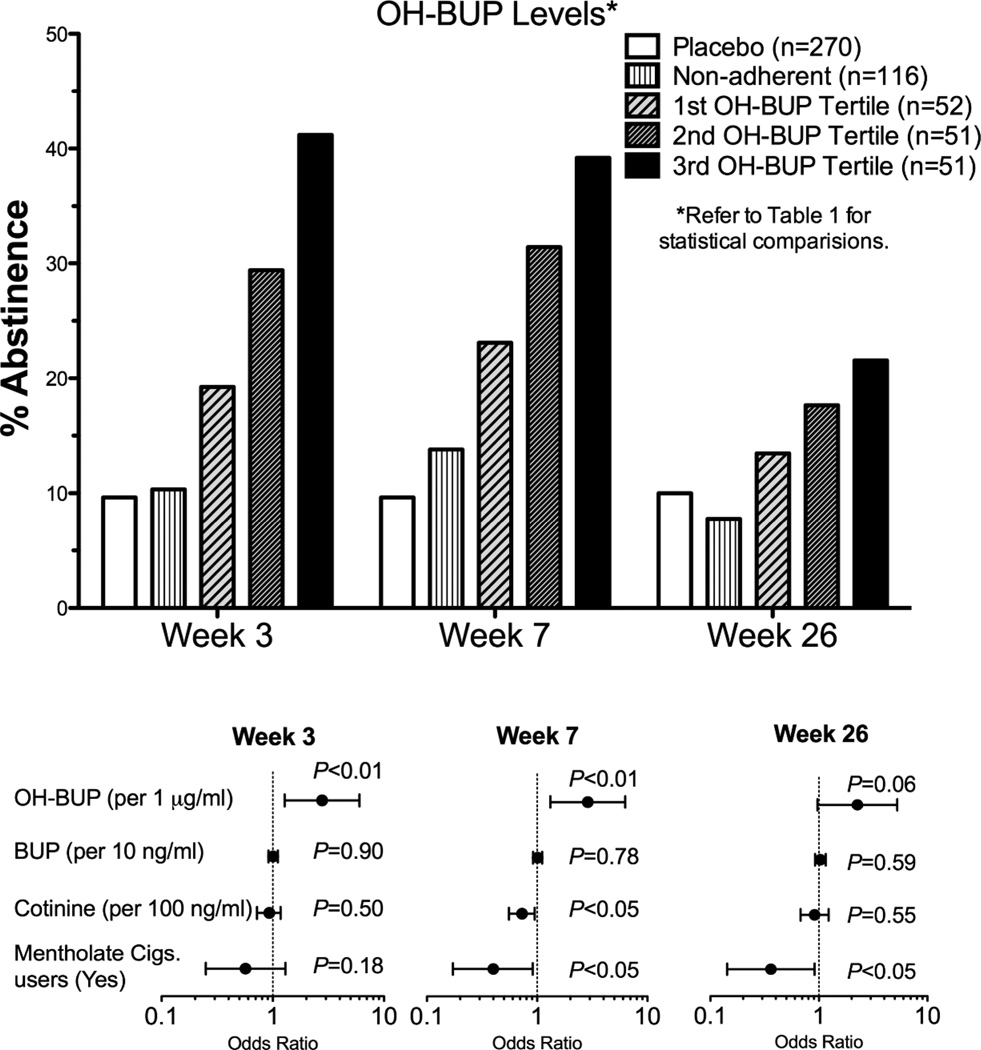
Among the 270 subjects assigned to bupropion treatment, some subjects did not have any detectable bupropion in their plasma (n=60) or did not give plasma samples (n=5), or did not attend the Week 3 visit (n=51). These subjects were classified as treatment non-adherent (n=116). The remaining 154 individuals with detectable bupropion were classified as bupropion adherent. We found that the non-adherent group had similar smoking cessation rates to the placebo group (Fig. 1 top, Week 26: 8% in the non-adherent group vs. 10% in the placebo group), and that individuals who had detectable bupropion levels (implying at least partial adherence to bupropion treatment, n=154) had significantly higher cessation rates compared to the placebo group (n=270) at Week 26 (18% vs. 10% respectively, P=0.025). Furthermore, as illustrated by Fig. 1 top and Table 1, bupropion adherent individuals with higher hydroxybupropion levels were more likely to be abstinent at Weeks 3, 7 and 26 compared to individuals with lower hydroxybupropion levels (OR=2.82, 2.96 and 2.37 respectively; P <0.05, Fig. 1 top and Table 1). Lastly, bupropion levels did not predict cessation outcomes either alone or after controlling for hydroxybupropion levels (bupropion alone without hydroxybupropion: P=0.39, 0.33 and 0.31 respectively for week 3, week 7 and week 26; in combination with hydroxybupropion levels: P=0.90, 0.78 and 0.59 respectively for week 3, week 7 and week 26, Fig. 1 Bottom), and were not included in subsequent analyses. Predictors that failed to reach statistical significance and were not included in the final models included gender (95%CI =0.45 −2.03), age (95%CI =0.97 – 1.04), BMI (95%CI =0.98 – 1.06) and having an adverse event (95%CI =0.43 −2.31). In addition to hydroxybupropion levels, baseline cotinine level (reflecting heaviness of smoking) and smoking mentholated cigarettes also significantly predicted cessation outcomes as reported previously (Fig. 1 bottom and Table 1).18,19 Removing baseline COT levels and/or mentholated cigarettes from the logistic regression did not alter hydroxybupropion’s odds ratio or P-value (i.e. hydroxybupropion’s odds ratios ranged from 2.67–2.82 at Week 3, P-values ranged: 0.007–0.009).
Figure 1. Hydroxybupropion level is a strong predictor smoking cessation outcomes.
Top: The impact of increasing levels of hydroxybupropion on abstinence. Bottom: The predictors of abstinence at Week 3, 7 and 26 among the bupropion adherent individuals. The odds ratio refers to the variable listed in the brackets beside each predictor. OH-BUP=hydroxybupropion, and BUP=Bupropion. The cotinine variable refers to baseline cotinine levels.
Table 1.
Logistic regression of hydroxybupropion levels as a predictor of abstinence rates.
| Predictor | Week 3 | Week 7 | Week 26 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% Cl | P | Odds ratio | 95% Cl | P | Odds ratio | 95% Cl | P | |
| Bupropion adherent only (n=154) | |||||||||
| OH-BUP levels (per 1 µg /mL) | 2.82 | 1.34 – 5.95 | 0.007 | 2.96 | 1.38 – 6.34 | 0.005 | 2.37 | 1.04 – 5.41 | 0.040 |
| Baseline COT (per 100 ng/mL) | 0.92 | 0.72 – 1.18 | 0.506 | 0.73 | 0.56 – 0.96 | 0.022 | 0.92 | 0.68 – 1.24 | 0.580 |
| Mentholated cigarettes (Yes) | 0.57 | 0.25 – 1.30 | 0.180 | 0.40 | 0.17 – 0.91 | 0.030 | 0.36 | 0.14 – 0.92 | 0.032 |
The odds ratio provided refers to the variables listed in parentheses beside each categorical predictor. OH-BUP=hydroxybupropion.
Bupropion is metabolized to hydroxybupropion primarily by CYP2B6,6,7 therefore we examined the contribution of genetic variation in the CYP2B6 gene to the variability in bupropion and hydroxybupropion levels among bupropion adherent individuals (n=154). The CYP2B6 allele frequencies are shown in Supplementary Table 3. Consistent with CYP genetic analyses with multiple low prevalence alleles,20,21 individuals were categorized into CYP2B6 genotype groups. The mean hydroxybupropion/bupropion ratios (an indicator of CYP2B6 activity6) in CYP2B6 intermediate and slow metabolizers were 54% and 34% of that observed in the CYP2B6 normal metabolizers (P<0.05, Fig. 2 left). The CYP2B6 intermediate metabolizers and slow metabolizers exhibited 20% (non-significant) and 40% (P<0.05) lower steady state hydroxybupropion levels, respectively, compared to the CYP2B6 normal metabolizers group (Fig. 2 middle), while no differences in bupropion levels were observed across genotype groups (Table 2, Supplementary Figure 1). In addition, no differences in erythrohydrobupropion and threohydrobupropion levels, alternative bupropion metabolites not mediated by CYP2B6, were observed among the CYP2B6 genotype groups (Supplementary Figure 1).
Figure 2. CYP2B6 genotype groups alter hydroxybupropion formation.
Association of CYP2B6 genotype groups with left: hydroxybupropion/bupropion ratios and middle: hydroxybupropion levels in bupropion adherent individuals (n=154, slow: n=31, intermediate: n=64, normal: n=58). Right: CYP2B6 genotype-based predicted dose adjustment to 700 ng/ml hydroxybupropion. OH-BUP=hydroxybupropion, and BUP=Bupropion.
Table 2.
Regression analyses of the predictors of the hydroxybupropion/bupropion ratio, hydroxybupropion levels, and bupropion levels.
| Predictors | OH-BUP/BUP ratio R2=0.055 P=0.02 |
OH-BUP levels (ng/ml) R2=0.11 P=0.001 |
BUP levels (ng/ml) R2=0.03 P=0.21 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | β | 95% CI | P | B | β | 95% CI | P | B | β | 95% CI | P | |
| Bupropion adherent only (n=154) | ||||||||||||
| CYP2B6 groups (Intermediate vs. normal) | −16.9 | −0.20 | −31.8 to −1.9 | 0.027 | −162.3 | −0.17 | −325.8 to 1.3 | 0.052 | 13.5 | 0.17 | −0.3 to 27.4 | 0.056 |
| CYP2B6 groups (Slow vs. normal) | −24.3 | −0.23 | −42.7 to −6.0 | 0.010 | −333.6 | −0.28 | −534.3 to −132.9 | 0.001 | 9.0 | 0.09 | −8.0 to 26.0 | 0.297 |
| Age (Increasing) | 0.2 | 0.04 | −0.4 to 0.8 | 0.580 | 9.1 | 0.22 | 2.7 to 15.6 | 0.006 | 0.2 | 0.07 | −0.3 to 0.8 | 0.366 |
B is the unstandardized coefficient. β is the standardized coefficient (i.e. standardized to a variance of 1). The B and β provided refers to the variables listed in parentheses beside each categorical predictor. OH-BUP=hydroxybupropion. Total n=154 (slow: n=31, intermediate: n=64, normal: n=58).
Regression analyses among bupropion adherent subjects identified CYP2B6 genotype groups as the significant source of variation in both the hydroxybupropion/bupropion ratio and the hydroxybupropion levels (Table 2). Steady state hydroxybupropion levels were also positively correlated with age (Table 2), but age was not associated with the hydroxybupropion/bupropion ratio suggesting an effect on adherence rather than on metabolism. Predictors that failed to reach statistical significance, gender and body mass index, were not included in these models; including either in the model did not alter the significance of the other predictors.
Slow metabolizers were under-represented in the fastest tertile as expected; in the highest hydroxybupropion tertile (3rd tertile, good response) there were over 6 times more normal than slow metabolizers (51% and 8% respectively) while there were approximately equal numbers of normal and slow metabolizers (24% and 28% respectively) in the lowest tertile of plasma hydroxybupropion concentration (1st, poor response) (P=0.002, Fisher’s exact test). There was no significant difference in abstinence between slow metabolizers (20% abstinent) and normal metabolizers (24% abstinent) at Week 3 where we expect the genotype effect would be strongest. Overall, the CYP2B6 genotype groups did not differ in their prevalence among placebo, the bupropion non-adherent group and bupropion adherent group (Supplementary Figure 2), nor did the genotype groups differ in their cessation rates among the total group (both arms) or within the placebo arm only.22
Discussion
We present three novel findings. First, while African American smokers typically have lower rates of abstinence following smoking cessation treatment,2 our results suggest that bupropion is an effective long-term smoking cessation treatment in adherent African American light smokers, especially those with greater CYP2B6 activity. Our pharmacokinetic analyses suggest that non-adherence to bupropion was substantial, probably contributing to the lack of long-term therapeutic benefit of bupropion in this cessation trial.23 Improving adherence to the treatment paradigm would be an important way to increase the effectiveness of smoking cessation treatment.
Secondly, we demonstrate a positive metabolite-response relationship between hydroxybupropion levels and treatment efficacy among participants who were adherent to bupropion treatment, but such a relationship was absent for bupropion. This is likely due to the accumulation of high levels of hydroxybupropion at steady state and its potent pharmacodynamic effects as demonstrated by in vitro studies.12,13,15,24,25 Our results are further supported by animal studies showing that hydroxybupropion blocks the development of nicotine-induced condition place preference (indicative of the rewarding effect of nicotine) and reduces the somatic signs of withdrawal in nicotine dependent mice.9,10,26,27 It is possible that variation in hydroxybupropion formation, and CYP2B6 pharmacogenetics, influences the antidepressant effects of bupropion as well, supported by the fact that hydroxybupropion has been investigated by GlaxoSmithKline as a novel antidepressant (Radafaxine). Overall, our results suggest that hydroxybupropion contributes, at least to a considerable extent, bupropion’s effect to promote cessation.
Thirdly, we demonstrated that CYP2B6 genotype is a significant contributor to the variability in hydroxybupropion levels. CYP2B6 genetic polymorphism significantly alters bupropion hydroxylation activity,28–30 consistent with our observations that individuals with one or two copies of reduced function allele(s) have lower hydroxybupropion levels and hydroxybupropion/bupropion ratios. CYP2B6 slow metabolizers were more prevalent in the lowest tertile of plasma hydroxybupropion concentration compared to the highest tertile of plasma hydroxybupropion concentration, further supporting the idea that the lower production of hydroxybupropion, due to higher frequencies of CYP2B6 slow metabolizers, leads to poorer bupropion treatment response. This has important clinical implications as around 38% of Caucasians and more than 48% of African Americans are CYP2B6 intermediate metabolizers and 6% of Caucasians and 16% of African Americans are CYP2B6 slow metabolizers.30,31 We speculate that poorer bupropion response in African Americans compared to Caucasians is partially due to the high prevalence of CYP2B6 reduced function alleles in African Americans.32 We did not observe a direct association between CYP2B6 genotype and smoking cessation outcomes in this study which is likely due to the poor adherence reducing the effect CYP2B6 genotype on hydroxybupropion levels (relative to taking the drug). In a clinical pharmacokinetic study in which the time since last bupropion dose, and adherence to bupropion treatment were controlled for, roughly 50% of the variation in steady state hydroxybupropion AUC was accounted for by CYP2B6 genotypes (i.e. R2≈0.5, Benowitz et al., unpublished observation). Thus, the relatively low amount of variation in hydroxybupropion levels accounted by CYP2B6 genotypes (R2=0.11) in the present study was likely due to the variable adherence to bupropion treatment. We anticipant, in a study where bupropion adherence was high, the CYP2B6 genotype would have a substantial impact on hydroxybupropion levels and treatment outcomes. Recently a study with a larger sample size observed the association between a single nucleotide polymorphisms (i.e. rs1808682 of unknown functional consequence) in CYP2B6 and bupropion’s treatment outcomes.33 A similar analysis of the role of hydroxybupropion, and CYP2B6 genotype, in the antidepressant action of bupropion is warranted.
We propose two potential approaches to enhance bupropion’s treatment effectiveness. The first is therapeutic drug monitoring with a steady state hydroxybupropion target of a minimum 700 ng/ml, which represents the minimal concentration within the good bupropion response tertile (3rd and highest hydroxybupropion tertile, Fig. 1 top). About 40% of the patients with hydroxybupropion levels of 700 ng/ml or higher were abstinent at Week 7 and 25% were abstinent at Week 26 (compared to 20% and 11% in those who were in the bupropion arm, but had levels below 700 ng/ml). Therapeutic hydroxybupropion monitoring would assess both drug adherence and individual variation in rate of hydroxybupropion formation. However, it remains to be determined whether the 700 ng/mL target is generalizable to all populations. The second approach is a prospective genotype-based bupropion dose adjustment from the currently used 300mg daily dose. Theoretically, the CYP2B6 intermediate metabolizers and slow metabolizers need a daily bupropion dose of at least 320 mg and 420 mg, respectively, to reach the 700 ng/ml hydroxybupropion concentrations (Fig. 2 right). The seizure rate associated with 300 mg per day sustained-release bupropion is roughly one in a thousand. However, this rate increases with doses above 450 mg per day.34 Rodent experiments suggested that hydroxybupropion induces seizure more potently than bupropion.35 Since the adjustments result in hydroxybupropion levels equivalent to the normal metabolizers’ during standard dosing, it is unlikely that the seizure risk will increase beyond what is currently observed in the normal metabolizers. The effect of dose adjustment on bupropion-related toxicities will require clarification, but neither our study nor a recent bupropion pharmacogenetic study observed any association between CYP2B6 genotype and adverse events associated with bupropion treatment.33 It may not be currently practical, with existing formulations, to do genotype-based dose adjustments. However, if doses other than the currently available dose formulation are proven to improve outcome, either the manufacture could develop new formulations or prescribers could combine different dose formulations for a particular patient. Alternatively, a different treatment could be proposed for the CYP2B6 slow metabolizers.
Our study is the first human study, to the best of our knowledge, that evaluated the hypothesis that hydroxybupropion is the major determinant of bupropion’s ability to promote smoking cessation. It is also the first study to measure bupropion and its metabolites levels in a large-scale clinical trial. We recognize several limitations of our study. Foremost with respect to the generalizability of our study is our focus on African American light smokers, which can be both a strength and a limitation. Due the high prevalence of CYP2B6 reduced function alleles in African Americans, we were able to study the relationship between hydroxybupropion levels and smoking cessation in a relatively small study. Our focus on African American light smokers also revealed much-needed information regarding to the adherence and efficacy of bupropion in this population, which warrants replication in other populations. Secondly, like most outpatient clinical trials, we had limited ability to assess the level of daily treatment adherence in the patients with detectable levels of bupropion. Thus, human laboratory studies, in which adherence is more easily controlled, are required to extend our findings. Overall, the strong agreement between our data and hydroxybupropion experiments in animal models as well as the strikingly higher hydroxybupropion levels compared to bupropion levels at steady state support our contention that hydroxybupropion is mediating bupropion’s ability to promote smoking cessation.
In conclusion, our results suggest that hydroxybupropion contributes to, at least to a considerable extent, bupropion’s smoking cessation effect. Drug adherence and variation in CYP2B6 mediated hydroxybupropion formation are important determinants of bupropion’s efficacy. Targeting a minimum 700 ng/ml steady state hydroxybupropion concentration by therapeutic drug monitoring or genotype based dose adjustment might be useful to improve bupropion’s effectiveness in promoting smoking cessation.
Methods and Materials
Study design
A detailed description of the clinical trial design and participant recruitment is published elsewhere.23 In brief, 540 self-identified African American light smokers (≤10 cigarettes per day) were enrolled in a randomized, double blind, placebo-controlled study in Kansas City, MO. This study consisted of a bupropion treatment arm (n=270; 150 mg/day for 3 days, then 150 mg twice daily to 7 weeks) and a placebo treatment arm (n=270; placebo twice daily). Inclusion criteria included being ≥18 years of age, having smoked an average of 10 or fewer cigarettes per day for at least 6 months prior to enrollment, and having smoked on at least 25 of the last 30 days. Exclusion criteria were consistent with contraindications for bupropion use.23
The participants were asked to quit smoking one week after starting the 7-week drug treatment. Participants were followed for a total of 26 weeks. The research protocol was approved and monitored by the University of California San Francisco human subjects committee, the University of Kansas human subjects committee and the University of Toronto ethics review office.
Measurements
Demographic information, baseline-smoking behaviors such as the severity of nicotine dependence and smoking history were recorded at the time of randomization (Week 0, Supplementary Table 1).36 Plasma samples for measurement of bupropion and metabolite levels were taken during the week 3 follow-up visits and took place throughout the day. Bupropion and metabolite levels were measured by methods previously described.37 Since bupropion and its metabolites have half-lives ranging from 10 to 40 hours,13,28 the Week 3 plasma levels were considered to be at steady state. Biochemically verified smoking abstinence (salivary cotinine ≤15 ng/ml) was assessed at Week 3 (steady state plasma drug sampling), Week 7 (end of treatment) and Week 26 (6 month follow-up).
CYP2B6 genotyping
Blood samples for genotyping were available for 534 out of the 540 participants. DNA from blood samples was isolated by Genelute column purification kit from Sigma (Sigma Canada). Common CYP2B6 alleles (*4 K262R, *5 R487C, *6 K262R & Q172H, and *9 Q172H) were genotyped using two-step allele-specific PCR assay as previously described.38 In addition, new genotyping assays were developed for CYP2B6*16, *18 and *22. In order to avoid the amplification of CYP2B7 pseudogene, each assay included a gene-specific amplification followed by an allele specific amplification. A list of primers, reagent concentrations and cycling conditions can be found in Supplementary Table 3. The assays were verified by sequencing (ACGT Corporation, Toronto ON, Canada).
CYP2B6 genotype groupings
Briefly, individuals with one copy of a reduced function allele (e.g. *6 and *18), alleles associated with reduced protein expression and in vivo pharmacokinetics,30,39,40 were grouped into the intermediate metabolizer group (e.g. heterozygous). Individuals with two copies of reduced function alleles (e.g. homozygous) were grouped as slow metabolizers. The estimation of the relative impact of these alleles was based on the previous published in vivo pharmacokinetics data, hydroxybupropion/bupropion ratio (Supplementary Table 3), as well as assessments in human liver tissues.29,38,40 CYP2B6*5’s functional impact is inconsistent in vivo,8 while CYP2B6*4 and *22 were previously associated with increased CYP2B6 activity.8 However, as individuals with *4, *5 and *22 alleles were not significantly different from the normal metabolizers group in hydroxybupropion/bupropion ratio (Supplementary Table 3) and there were only 10 out of the 270 individuals in the bupropion arm, they were grouped as part of the normal metabolizers group. Namingly, individuals with *1/*1, *1/*4, *1/*5, *1/*22 and *22/*22 genotype were considered as normal metabolizers.
Statistical analyses
Logistic regression models were used to assess the association between hydroxybupropion levels and bupropion levels with quit rates at Week 3, Week 7 and Week 26. Hydroxybupropion levels and baseline cotinine levels were fitted as continuous variables. Univariate analysis of variance (ANOVA) was used to determine the differences in bupropion levels, hydroxybupropion levels and the hydroxybupropion/bupropion ratio between CYP2B6 genotype groups with Bonferroni tests used for post-hoc analyses. For the ANOVA analyses, the bupropion levels, hydroxybupropion levels and the hydroxybupropion/bupropion ratios were log-transformed. Chi2 tests were used to test for Hardy-Weinberg equilibrium and Fisher’s exact test were used to test the prevalence of CYP2B6 normal metabolizers and slow metabolizers in the hydroxybupropion tertiles. Linear regression models were used to evaluate the associations between CYP2B6 genotype groups, gender, age, BMI and bupropion or hydroxybupropion levels. To enable better interpretation of the regression results, the hydroxybupropion to bupropion ratio, hydroxybupropion, and bupropion levels were not normalized. The residuals of the regression model were normally distributed, and normalization by log transformation did not change the significances of the finding. All statistical analyses of treatment effects were performed on an intent-to-treatment basis, and subjects lost during follow-up were considered smokers. Statistical analyses were performed using Stata 11 (StataCorp, College Station, TX)
Supplementary Material
Study highlights.
What is the current knowledge on the topic?
Bupropion is a widely used to promote smoking cessation and treat depression.
What question this study addressed?
While animal studies suggest that bupropion’s major metabolite, hydroxybupropion, is pharmacologically active, the relative pharmacological importance of bupropion versus hydroxybupropion is not known.
What this study adds to our knowledge?
This study demonstrated, in a real-world out-patient clinical trial, that increasing hydroxybupropion levels, but not bupropion, increased smoking cessation rates. Genetic variation in CYP2B6, the enzyme mediates bupropion hydroxylation, was identified as a significant source variation in the hydroxybupropion levels.
How this might change clinical pharmacology and therapeutics?
This study reports an important advance in our understanding of bupropion’s mechanism of action and identified major sources of variation in metabolite levels and resulting response. This study has clinical implications not only for smoking cessation, but which may extend to bupropion’s antidepressant actions as well. The findings indicate that personalized/stratified bupropion therapy based on hydroxybupropion levels, or CYP2B6 genotype, may substantially improve bupropion’s therapeutic efficacy.
Acknowledgements
We thank Lisa Yu and Olivia Yturralde for performing the bupropion analytical chemistry, Peyton Jacob III for assay development, and Ewa Hoffmann and Qian Zhou for assisting DNA sample preparation. We acknowledge the support of NIH grant CA091912 to fund this study, National Center for Minority Health Disparities grant 1P60MD003422 (JSA), National Cancer Institute grant 1U54CA153603 (KSO), the National Heart, Lung, and Blood Institute grant 1HLR01081522 (KSO). A CRC (RFT), CIHR grant MOP86471 (RFT), Ontario Graduate Scholarship (AZZ), CAMH and the CAMH foundation (RFT), NIH grants DA020830 (RFT, NB) and DA012353 (NB), the Canada Foundation for Innovation (#20289 and #16014) and the Ontario Ministry of Research and Innovation (RFT).
Funding: NIH grant CA091912 and CIHR grant MOP86471
Footnotes
Conflict of Interest
KSO has an active smoking cessation research grant funded by Pfizer, Inc., a company that makes smoking cessation drugs. NLB has been a paid consultant to pharmaceutical companies that market medications for smoking cessation treatment, and has served as a paid expert witness in litigation against tobacco companies. RFT has consulted for Novartis and McNeil. No conflicts of interest were declared by AZZ, BF, JSA, LSC, and NN.
Reference
- 1.Benowitz NL. Nicotine addiction. The New England journal of medicine. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahluwalia JS, et al. The effects of nicotine gum and counseling among African American light smokers: a 2×2 factorial design. Addiction. 2006;101:883–891. doi: 10.1111/j.1360-0443.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- 3.Gariti P, et al. Comparing smoking treatment programs for lighter smokers with and without a history of heavier smoking. J Subst Abuse Treat. 2009;37:247–255. doi: 10.1016/j.jsat.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King G, Polednak A, Bendel RB, Vilsaint MC, Nahata SB. Disparities in smoking cessation between African Americans and Whites: 1990–2000. American journal of public health. 2004;94:1965–1971. doi: 10.2105/ajph.94.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siu EC, Tyndale RF. Non-nicotinic therapies for smoking cessation. Annu Rev Pharmacol Toxicol. 2007;47:541–564. doi: 10.1146/annurev.pharmtox.47.120505.105354. [DOI] [PubMed] [Google Scholar]
- 6.Faucette SR, et al. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos. 2000;28:1222–1230. [PubMed] [Google Scholar]
- 7.Hesse LM, et al. CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos. 2000;28:1176–1183. [PubMed] [Google Scholar]
- 8.Zanger UM, et al. Polymorphic CYP2B6: molecular mechanisms and emerging clinical significance. Pharmacogenomics. 2007;8:743–759. doi: 10.2217/14622416.8.7.743. [DOI] [PubMed] [Google Scholar]
- 9.Damaj MI, et al. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004;66:675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- 10.Damaj MI, et al. Effects of Hydroxymetabolites of Bupropion on Nicotine Dependence Behavior in Mice. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.110.166850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jefferson JW, Pradko JF, Muir KT. Bupropion for major depressive disorder: Pharmacokinetic and formulation considerations. Clin Ther. 2005;27:1685–1695. doi: 10.1016/j.clinthera.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Laizure SC, DeVane CL. Stability of bupropion and its major metabolites in human plasma. Ther Drug Monit. 1985;7:447–450. doi: 10.1097/00007691-198512000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Hsyu PH, et al. Pharmacokinetics of bupropion and its metabolites in cigarette smokers versus nonsmokers. J Clin Pharmacol. 1997;37:737–743. doi: 10.1002/j.1552-4604.1997.tb04361.x. [DOI] [PubMed] [Google Scholar]
- 14.DeVane CL, et al. Disposition of bupropion in healthy volunteers and subjects with alcoholic liver disease. J Clin Psychopharmacol. 1990;10:328–332. [PubMed] [Google Scholar]
- 15.Lai AA, Schroeder DH. Clinical pharmacokinetics of bupropion: a review. J Clin Psychiatry. 1983;44:82–84. [PubMed] [Google Scholar]
- 16.Findlay JW, et al. Pharmacokinetics of bupropion, a novel antidepressant agent, following oral administration to healthy subjects. Eur J Clin Pharmacol. 1981;21:127–135. doi: 10.1007/BF00637513. [DOI] [PubMed] [Google Scholar]
- 17.Johnston AJ, et al. Pharmacokinetic optimisation of sustained-release bupropion for smoking cessation. Drugs. 2002;62(Suppl 2):11–24. doi: 10.2165/00003495-200262002-00002. [DOI] [PubMed] [Google Scholar]
- 18.Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007;102:1979–1986. doi: 10.1111/j.1360-0443.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 19.Harris KJ, et al. Predictors of smoking cessation among African-Americans enrolled in a randomized controlled trial of bupropion. Preventive medicine. 2004;38:498–502. doi: 10.1016/j.ypmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Ho MK, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85:635–643. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaedigk A, et al. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clinical pharmacology and therapeutics. 2008;83:234–242. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]
- 22.Lee AM, et al. CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biol Psychiatry. 2007;62:635–641. doi: 10.1016/j.biopsych.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Cox LS, et al. Bupropion for smoking cessation in African American light smokers: A randomized controlled trial. Journal of the National Cancer Institute. 2012;104:1–9. doi: 10.1093/jnci/djr513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweet RA, et al. Pharmacokinetics of single-and multiple-dose bupropion in elderly patients with depression. J Clin Pharmacol. 1995;35:876–884. doi: 10.1002/j.1552-4604.1995.tb04132.x. [DOI] [PubMed] [Google Scholar]
- 25.Stewart JJ, et al. Single-dose pharmacokinetics of bupropion in adolescents: effects of smoking status and gender. J Clin Pharmacol. 2001;41:770–778. doi: 10.1177/00912700122010564. [DOI] [PubMed] [Google Scholar]
- 26.Lukas RJ, et al. Synthesis and characterization of in vitro and in vivo profiles of hydroxybupropion analogues: aids to smoking cessation. J Med Chem. 2010;53:4731–4748. doi: 10.1021/jm1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bondarev ML, Bondareva TS, Young R, Glennon RA. Behavioral and biochemical investigations of bupropion metabolites. Eur J Pharmacol. 2003;474:85–93. doi: 10.1016/s0014-2999(03)02010-7. [DOI] [PubMed] [Google Scholar]
- 28.Kirchheiner J, et al. Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics. 2003;13:619–626. doi: 10.1097/00008571-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann MH, et al. Aberrant splicing caused by single nucleotide polymorphism c.516G>T [Q172H], a marker of CYP2B6*6, is responsible for decreased expression and activity of CYP2B6 in liver. J Pharmacol Exp Ther. 2008;325:284–292. doi: 10.1124/jpet.107.133306. [DOI] [PubMed] [Google Scholar]
- 30.Klein K, et al. Genetic variability of CYP2B6 in populations of African and Asian origin: allele frequencies, novel functional variants, and possible implications for anti-HIV therapy with efavirenz. Pharmacogenet Genomics. 2005;15:861–873. doi: 10.1097/01213011-200512000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Zanger UM, Turpeinen M, Klein K, Schwab M. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal Bioanal Chem. 2008;392:1093–1108. doi: 10.1007/s00216-008-2291-6. [DOI] [PubMed] [Google Scholar]
- 32.Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA. 2002;288:468–474. doi: 10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- 33.King DP, et al. Smoking Cessation Pharmacogenetics: Analysis of Varenicline and Bupropion in Placebo-Controlled Clinical Trials. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zyban® (bupropion hydrochloride) sustained-release tablets: product information. US: GlaxoSmithKline. 2001 [Google Scholar]
- 35.Silverstone PH, Williams R, McMahon L, Fleming R, Fogarty S. Convulsive liability of bupropion hydrochloride metabolites in Swiss albino mice. Annals of general psychiatry. 2008;7:19. doi: 10.1186/1744-859X-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 37.Haas JS, Kaplan CP, Barenboim D, Jacob P, 3rd, Benowitz NL. Bupropion in breast milk: an exposure assessment for potential treatment to prevent post-partum tobacco use. Tobacco control. 2004;13:52–56. doi: 10.1136/tc.2003.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al Koudsi N, Tyndale RF. Hepatic CYP2B6 is altered by genetic, physiologic, and environmental factors but plays little role in nicotine metabolism. Xenobiotica. 2010;40:381–392. doi: 10.3109/00498251003713958. [DOI] [PubMed] [Google Scholar]
- 39.Wyen C, et al. Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. J Antimicrob Chemother. 2008;61:914–918. doi: 10.1093/jac/dkn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arab-Alameddine M, et al. Pharmacogenetics-based population pharmacokinetic analysis of efavirenz in HIV-1-infected individuals. Clin Pharmacol Ther. 2009;85:485–494. doi: 10.1038/clpt.2008.271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.