SUMMARY
The molecular mechanisms underlying the axon arborization of mammalian neurons are poorly understood but are critical for the establishment of functional neural circuits. We identified a pathway defined by two kinases, LKB1 and NUAK1, required for cortical axon branching in vivo. Conditional deletion of LKB1 after axon specification or knockdown of NUAK1 drastically reduced axon branching in vivo whereas their overexpression was sufficient to increase axon branching. The LKB1-NUAK1 pathway controls mitochondria immobilization in axons. Using manipulation of Syntaphilin, a protein necessary and sufficient to arrest mitochondrial transport specifically in the axon, we demonstrate that the LKB1-NUAK1 kinase pathway regulates axon branching by promoting mitochondria immobilization. Finally, we show that LKB1 and NUAK1 are necessary and sufficient to immobilize mitochondria specifically at nascent presynaptic sites. Our results unravel a link between presynaptic mitochondrial capture and axon branching.
INTRODUCTION
The neocortex forms a complex network composed of billions of interconnected neurons arranged in six layers which are defined by molecular, morphological, electrophysiological properties and importantly by their afferent and efferent inputs. This pattern of connectivity is achieved through tightly regulated developmental processes whose impairment have profound effects on cortical function, and are linked to neurodevelopmental defects ranging from epilepsy to mental retardation and autism spectrum disorders (Walsh et al., 2008; Zoghbi and Bear, 2012). In the mouse, the development of cortical pyramidal neurons occurs in three main stages (Barnes and Polleux, 2009; Donahoo and Richards, 2009): first, from approximately (E)mbryonic day 11 to E18 neurons are born, engage radial migration and form a leading process which will eventually become the apical dendrite and a trailing process that becomes the axon. Second, during and after neuronal migration and until postnatal day (P)7 approximately, axons grow and are guided to their proper subcortical or cortical targets by axon guidance cues. Finally, during the second and third postnatal weeks, axons branch extensively upon reaching their final targets and form functional synapses with their post-synaptic partners. Important progress has been made in identifying the molecular mechanisms regulating axon growth and guidance (Huber et al., 2003; Polleux and Snider, 2010). However, relatively little is known about the molecular mechanisms controlling terminal axon branching (Gallo, 2011; Hall and Lalli, 2010; Jeanneteau et al., 2010; Kalil et al., 2000), although it is clear that in many axons, including cortical projections, axon branching is controlled by both activity-independent and activity-dependent mechanisms (Mizuno et al., 2007, 2010; Uesaka et al., 2006; Wang et al., 2007; Yu et al., 2004).
We and others have demonstrated that the Serine/Threonine kinase called LKB1 (Liver Kinase B1, also called STK11 or Par4) is critical for axon specification during neuronal polarization in vivo (Barnes et al., 2007; Shelly et al., 2007). LKB1 belongs to a family of highly conserved proteins known as the PAR (Partition defective) proteins, initially identified in C. elegans as required for the first asymmetric zygotic cell division (Kemphues et al., 1988). It has since been shown to regulate cell polarity in a wide range of cell types and animal models (Watts et al., 2000; Martin and St Johnston, 2003; Baas et al., 2004). By phosphorylating a conserved Threonine present in the T-loop of their catalytic domains, LKB1 is the ‘master’ activator of fourteen downstream kinases including AMPKα1/α2, SAD-A/B (BRSK2 and BRSK1 respectively), NUAK1/2 (ARK5 and SNARK respectively), SIK1-3, MARK1-4 and SNRK (Jaleel et al., 2005; Lizcano et al., 2004). AMPK is one of the main metabolic sensors in the cell and its activity has been linked to the regulation of metabolism and to the maintenance of polarity under stress conditions (Lee et al., 2007; Mirouse et al., 2007). However, we observed that LKB1 is not a major activator of AMPK in neurons (Barnes et al., 2007) and that AMPK is not required for cortical neuron polarization in vivo (Williams et al., 2011) although its over-activation by metabolic stress impairs axon growth in vitro (Amato et al., 2011; Williams et al., 2011). The biological functions of the other twelve AMPK-like kinases are still largely unknown in vivo (Alessi et al., 2006). LKB1 exerts its function in early axon specification through activation of SAD-A/B (Barnes et al., 2007; Kishi et al., 2005), but whether or not LKB1 and other AMPK-like kinases play any role during subsequent steps of neuronal development beyond axon specification remains unknown (Barnes and Polleux, 2009).
RESULTS
Post-mitotic deletion of LKB1 does not impair neuron polarization
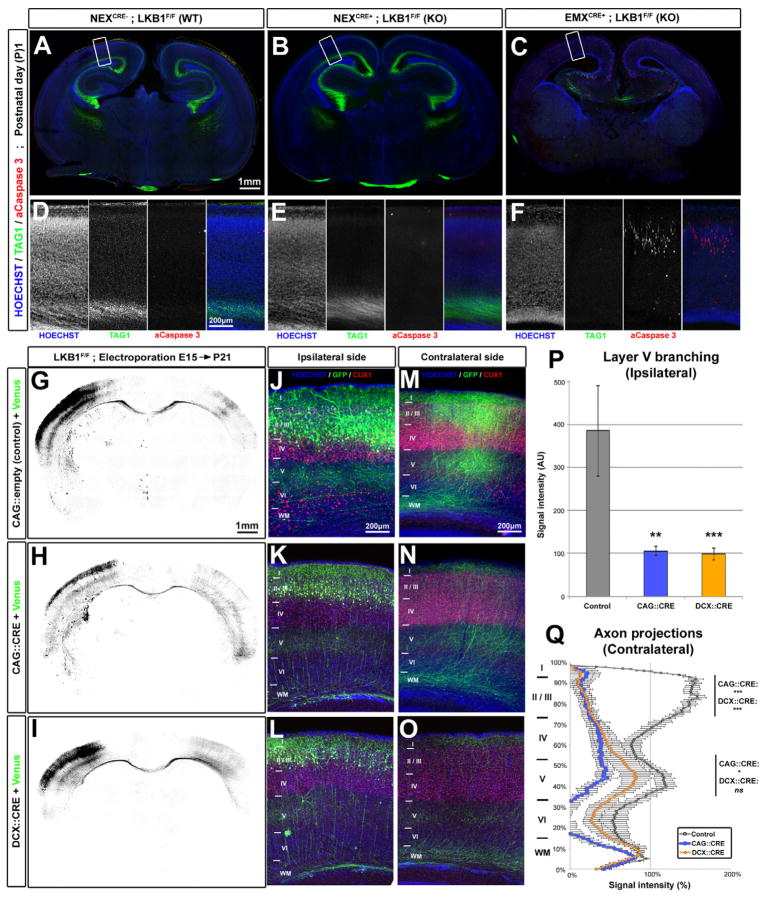
In our previous study, LKB1 conditional inactivation was performed by crossing mice harboring a floxed allele for Lkb1 (LKB1F/F) (Bardeesy et al., 2002) with a mouse line expressing the bacterial Cre-recombinase under the dorsal telencephalon-specific promoter Emx1 (Gorski et al., 2002). Recombination in Emx1Cre mice occurs as early as embryonic day (E)10 (Gorski et al., 2002) in Radial glial cells (RGC), the main neural progenitors generating all pyramidal neurons in the DT. In order to determine at what stage of neuron development LKB1 activity is required for axon specification, we used the NEXCre mouse line (Goebbels et al., 2006) which also induces recombination in dorsal telencephalic progenitors but specifically in intermediate progenitors in the sub-ventricular zone and early post-mitotic neurons, but not in RGC (Goebbels et al., 2006). Coronal slices of Postnatal (P)1 day mouse brains were stained with TAG1, a marker for corticofugal axons (green in Fig. 1A–F). TAG1 expression in NEXCre;LKB1 cKO brains was indistinguishable from WT littermate brains (Fig. 1A–B and D–E), suggesting that axon specification is not impaired in NEXCre;LKB1 cKO cortical neurons. In contrast, TAG1 immunoreactivity was entirely absent in Emx1Cre cKO brains, as reported previously (Fig. 1C and F) (Barnes et al., 2007). Moreover, deletion of LKB1 in the NEXCre line did not recapitulate the high level of neuronal apoptosis observed in Emx1Cre cKO (Fig. 1E–F) (Barnes et al., 2007), demonstrating that neuronal apoptosis in the Emx1Cre cKO is a direct consequence of not forming an axon rather than a consequence of not expressing LKB1.
Figure 1. LKB1 deletion after axon initiation does not impair axon maintenance but reduces axon branching in vivo.
(A–C) Coronal section of newborn LKB1F/F mouse brains not expressing Cre recombinase (Wildtype WT, A) or expressing Cre under the NEX (KO) (B) or EMX (KO) (C) promoters. (D–F) Higher-magnification images of the cortex region boxed in A, B and C respectively. (G–I) Low magnification images of coronal brain sections of P21 Lkb1F/F mice electroporated at E15 with plasmids expressing mVenus alone (G) or co-expressing Cre recombinase (under CAG promoter, H or Doublecortin promoter, I) and mVenus. (J–O) Higher magnification of the ipsilateral (J–L) or the contralateral side (M–O) showing reduced axon branching in both Cre-electroporated neurons (K–O) compared to control (J, M). (P–Q) Quantification of normalized Venus fluorescence in layer 5 of the ipsilateral cortex (P, ±SEM) and along the radial axis of the cortical wall in the contralateral cortex (Q, ±SEM). Statistical analysis: Mann-Whitney (P) or two-way ANOVA (Q). See also Fig. S1 and S2.
In order to examine axon morphology at a single cell resolution, we performed ex utero cortical electroporation at E15 of myristoylated-(m)Venus-expressing plasmid followed by dissociation of cKOs and littermates. After 5 days in culture (DIV), a significant fraction of Emx1Cre cKO neurons do not form an axon (defined by a neurite of length >100 μm and positive for the axonal marker SMI312; Barnes et al., 2007; Shelly et al., 2007) (Fig. S1A–B and E). On the contrary, neuronal polarization was not affected in NEXCre;LKB1 cKO neurons when compared to littermates (Fig S1C–D and E). Persistence of LKB1 expression in post-mitotic neurons could not explain this result, as we observed that LKB1 expression was largely absent in NEXCre cKO neurons cultures (Fig. S1F). Taken together, our results indicate that LKB1 is required for axon specification in dividing progenitors during or shortly after cell cycle exit and that inactivating LKB1 in post-mitotic neurons does not affect axon specification.
LKB1 is required for axonal branching in vivo
NEXCre;LKB1F/F mice were born viable and at Mendelian ratio. However, during the second postnatal week, the LKB1 cKO pups gained less weight than littermates (Fig. S1G), and during the third postnatal week showed approximately a 50% reduction in body weight even when solid or gelled food was made available in their cage bedding throughout weaning (Fig. S1I). Over 70% of the LKB1 cKO pups ultimately died before P21 (Fig. S1H), with P25 being the oldest NEXCre;LKB1F/F cKO pup we could recover. We currently do not know why the mice die around weaning but we discovered that NEXCre induces recombination in a subset of neurons from the peripheral nervous system innervating the intestine (Courchet and Polleux, unpublished observations) which might affect intestinal food absorption.
To circumvent this problem and in order to determine the cell-autonomous functions of LKB1 later during neuronal development in vivo, we performed in utero cortical electroporation (IUCE) at E15.5 of Cre-encoding plasmids in LKB1F/F mouse pups. This approach targets exclusively the progenitors of callosally projecting neurons in superficial layers 2/3 (Hand and Polleux, 2011; Mizuno et al., 2007; Wang et al., 2007). Electroporation was performed unilaterally in the somatosensory cortex, and axonal morphology of electroporated neurons was determined by expression of myristoylated (m)-Venus which allowed the (1) cell-autonomous conditional ablation of LKB1 in callosally projecting neurons and (2) quantitative assessment of axon branching (Fig 1G–Q). As shown previously (Hand and Polleux, 2011; Mizuno et al., 2007; Wang et al., 2007) (Fig. S2), callosal axons cross the cortical midline forming the Corpus Callosum (CC) around birth, then reach the contralateral cortex during the first postnatal week and branch extensively between P8 and P21 in layers 2/3, and layer 5 while avoiding branching in layers 4 and 6 both ipsilaterally (Fig. 1J, P) and contralaterally (Fig. 1M, Q). We performed IUCE with a plasmid driving Cre-recombinase expression under a CMV early enhancer/chicken β actin promoter (CAG::Cre) to induce robust recombination (Hand et al., 2005). Mice were sacrificed at P21 when callosal axons show adult-like patterns of branching (Mizuno et al., 2007; Wang et al., 2007). LKB1-deficient axons formed, grew and successfully crossed the midline (Fig. 1H) but showed significantly reduced branching ipsilaterally in layer 5 (Fig. 1K, P) and contralaterally both in layer 2/3 and 5 (Fig. 1N, Q). We also used an independent Cre driver that drives recombination in post-mitotic neurons (Doublecortin, DCX::Cre; Franco et al., 2011). Using this Cre-driver, we observed similar reduction in callosal axon branching both ipsilaterally and contralaterally (Fig 1I–Q).
These effects on axon branching upon post-mitotic deletion of LKB1 at P21 in vivo do not result from delayed axon development or axon degeneration because in both control and Cre-electroporated brains, axons crossed the midline by P4 (Fig. S2A, D and S2M–N) and reached the contralateral side by P8 (Fig. S2B, E and S2O–Q). However, between P8 and P12, callosal axons from LKB1-deficient neurons failed to form discrete cortical columns contralaterally (Fig. S2E–F and S2R) as seen in control (red arrowhead in Fig. S2B–C and S2P) and failed to branch (Fig. S2C, F and S2P–R), suggesting that LKB1-deficient neurons fail to induce terminal axon branching. The same lack of progressive branching in LKB1-deficient axons compared to control was observed ipsilaterally (Fig. S2G–L). Overall, these results uncover a function for LKB1 in the control of axon branching in vivo.
NUAK1 kinase is required for axon branching in vivo
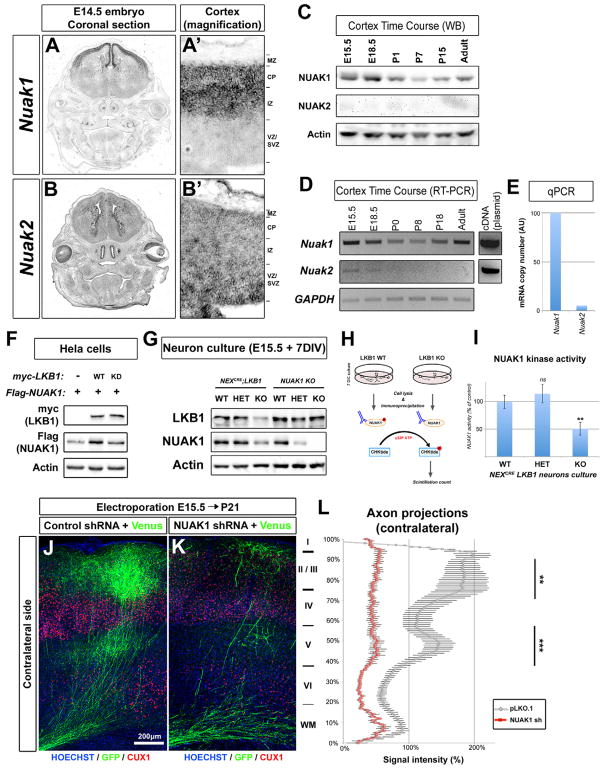
In order to identify what downstream kinase is the effector of LKB1 in mediating axon branching, we focused on two poorly studied AMPK-related kinases: NUAK1 and NUAK2. A previous report indicated that NUAK1 is highly expressed in the cortex during embryonic and postnatal development (Hirano et al., 2006), although its function during brain development is currently unknown. In situ hybridization on E14.5 wild-type mice revealed that both Nuak1 and 2 are expressed in the embryonic brain (Fig 2A–B). Nuak1 was expressed almost exclusively in the dorsal telencephalon, and restricted to the cortical plate (CP) containing post-mitotic neurons (Fig 2A–A′). Nuak2 expression was weaker and mostly restricted to the proliferative ventricular zone (VZ) of the cortex (Fig 2B–B′). We then compared NUAK1 and NUAK2 gene and protein expression in the cortex by RT-PCR and by Western-blot at various pre- and postnatal ages (Fig 2C–E). NUAK1 was expressed at all ages tested, although its expression decreased slightly at early postnatal stages but increased between P18 and adulthood in the cortex. On the contrary, Nuak2 transcript was detected weakly and only in embryonic stages, and NUAK2 protein could not be detected at those ages. Quantitative RT-PCR indicated that Nuak2 transcripts are 20 times less abundant than Nuak1 transcripts at E15.5 (Fig 2E).
Figure 2. AMPK-related kinase NUAK1 is required for axon branching in vivo.
(A–B) Expression of Nuak1 and Nuak2 genes in the head of Balb/C mouse by in situ hybridization. Magnification (A′–B′) shows that Nuak1 and Nuak2 have complementary expression patterns in the cerebral cortex. (C) Time-course of NUAK1 and NUAK2 protein expression in Balb/C mouse cortex by Western-blot. (D) Time-course of Nuak1 and Nuak2 mRNA expression in Balb/C mouse cortex by RT and PCR. (E) Quantitative PCR after RT (qRT-PCR) in E15.5 mouse cortex revealed that Nuak1 transcript is 20 times more abundant than Nuak2 transcript. (F) Overexpression of catalytically-active LKB1 increases NUAK1 protein expression. (G) Endogenous expression of LKB1 and NUAK1 proteins in LKB1 and NUAK1 knockout neurons. Western-blot analysis was performed with the indicated antibodies (H) Schema of NUAK1 kinase assay. (I) NUAK1 kinase activity is reduced in LKB1 KO neurons (±SEM). (J–K) In utero cortical electroporation of the indicated constructs revealed that loss of NUAK1 decreased axonal branching in the contralateral hemisphere similar to the loss of LKB1. (L) Quantification of EGFP normalized fluorescence along the radial axis of the cortical wall in the contralateral cortex. Statistical analysis: Mann-Whitney test (I) or two-way ANOVA (L).
To confirm that NUAK1 is a relevant target of LKB1, we co-expressed both kinases in HeLa cells that are deficient for LKB1 expression. We observed that NUAK1 protein expression was increased when co-expressed with wild-type, but not with kinase-dead LKB1 (Fig. 2F) as reported previously (Zagorska et al., 2010), suggesting that LKB1-mediated phosphorylation stabilizes NUAK1 protein. In agreement with this, we observed that NUAK1 protein expression was decreased in NEXCre;LKB1 cKO neurons by nearly 50% compared of the control condition (Fig. 2G). In order to confirm that NUAK1 catalytic activity is reduced in LKB1 cKO cortical neurons, we performed pulldown of endogenously expressed NUAK1 from cortical neurons in culture and assayed its ability to phosphorylate a NUAK1 target peptide (Fig. 2H). We found a significant reduction in NUAK1 catalytic activity in the NEXCre;LKB1F/F cKO neurons compared to both wild-type (WT) and heterozygous (HET) cortical neurons (Fig. 2I). As Nuak1 appeared to be dominant over Nuak2 we selected shRNAs against mouse Nuak1 (Fig. S3A–B) and used them to perform IUCE at E15.5. When mice were sacrificed at P21, we observed a significant reduction of callosal axon branching upon knockdown of Nuak1 compared to control condition (Fig 2J–K, quantified in 2L) and to the same extent as LKB1 loss-of-function (Fig. 1G–Q). Taken together, these results indicate that NUAK1 kinase is required cell-autonomously for cortical axon branching in vivo.
LKB1 and NUAK1 kinases are required for axon branching in vitro
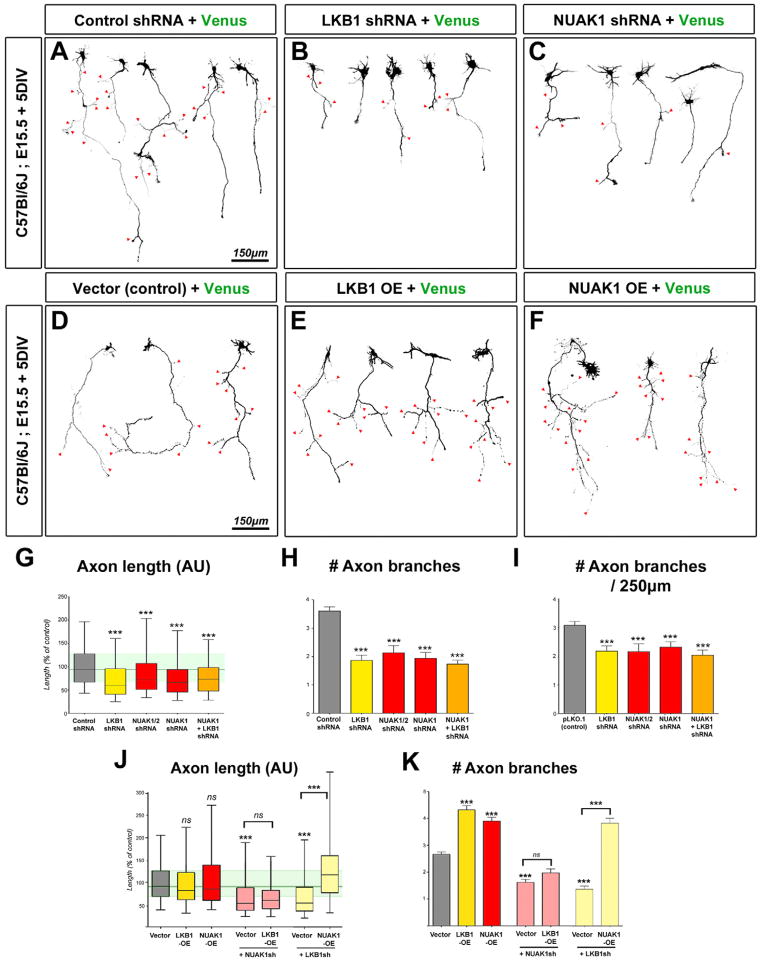
In order to quantify axon morphology at a single cell resolution, we examined the effects of Lkb1 and Nuak1 inactivation in vitro in dissociated neuronal cultures. We performed shRNA-mediated knockdown of Lkb1 and Nuak1 kinases or both Nuak1 and Nuak2 kinases using EUCE at E15.5 and observed that shRNA electroporated neurons displayed significantly shorter and less branched axons after 5DIV (Fig. 3B–C) compared to control shRNA (Fig. 3A). Quantifications confirmed that Lkb1 or Nuak1 knockdown significantly reduced the length of the axon (Fig. 3G) and also significantly reduced overall axon branching compared to control electroporation (Fig. 3H). Knocking-down Nuak2 together with Nuak1 did not show any additive effects compared to Nuak1 alone suggesting that these two kinases are not redundant with regard to axon growth and branching.
Figure 3. LKB1 regulates axon growth and branching in vitro through NUAK1.
(A–C) Representative neurons imaged after 5 day of culture in vitro (DIV) following inhibition of LKB1 (B) or NUAK1 (C) expression. (D–F) Overexpression of LKB1 (E) or NUAK1 (F) in 5 DIV cortical neurons induced the formation of supernumerary axonal branches. Red arrowheads in A–F point to axon branches. (G–I) Quantification of axon morphology shows that LKB1 or NUAK1 inhibition results in a shortened axon (G) and decreased collateral branch formation (HI). (J–K) Quantitation of axon length (J) or number of collateral branches at 5DIV (K) after overexpression of the indicated constructs. Data represent 25th, 50th and 75th percentile (G, J) or average value ±SEM (H–I, K). Statistical analysis: Mann-Whitney test. See also Fig. S3–S5.
We independently examined the consequences of complete genetic loss of Lkb1 or Nuak1 by electroporation of a Venus-encoding plasmid in NUAK1−− neurons (Fig S3D–E; (Hirano et al., 2006)) and of CAG::CRE or DCX::CRE plasmids in LKB1F/F neurons (Fig S3G–I). Quantifications confirmed that axon length and axon branching are significantly reduced in LKB1- or NUAK1-null cortical neurons when compared to the control (Fig S3F and J–K). These effects seemed largely specific to the axon since neither deleting Lkb1 nor knocking down Nuak1 had a significant effect on the number of primary MAP2+ dendrites emerging from the cell body (Fig. S3M) and that Nuak1 knockdown did not affect dendritic length at 5DIV.
LKB1 and NUAK1 regulate both axon growth and branching independently of axon specification
We tested if the decrease in axon branching could be a secondary consequence of impaired axon growth. Axon branching was still decreased in both LKB1 and NUAK1 loss-of-function experiments even when axon branch number was normalized for axon length (Fig. 3I and Fig S3L). To better visualize the dynamics of axon growth and branching, we performed long-term (15h) time-lapse imaging of neurons lacking Lkb1 or Nuak1 (Fig. S4A–F and Supplementary movie 1). We observed the axon growth rate was decreased upon loss of both Lkb1 or Nuak1 (Fig. S4G). Importantly, we also observed that loss-of-function of either Lkb1 or Nuak1 impaired axon branch initiation (for both Lkb1 and Nuak1 loss-of-function) and branch stability (for Lkb1 loss-of-function) (Fig. S4H), thus demonstrating that LKB1 and NUAK1 regulate both axon growth and axon branching.
In accordance to previous results (Barnes et al., 2007; Shelly et al., 2007), and as shown in Fig. S3H–I, both CAG::Cre and DCX::Cre mediated Lkb1 deletion results in a small but significant percentage of cortical neurons which do not form an axon under these conditions (approximately 40% with CAG::Cre and 19% with Dcx::Cre compared to 11% in control neurons). However, this is in stark contrast with genomic recombination using Emx1Cre which in the same culture conditions leads to over 80% of cortical neurons without an axon (Fig. S1D–E and H). Thus, we wanted to test unequivocally if the effect of LKB1 on axon growth and branching were indirectly linked to their effects on axon specification through a delay in axon formation. To test this, we performed Cre transfection in LKB1F/F cortical cultures (Fig. S3N–O) at 3DIV, i.e. after axon polarization and during the main phase of axon growth (Barnes et al., 2007). Our results demonstrate that at 7DIV, LKB1-deficient neurons show a decrease in axon branching (but no decrease in axon growth) compared to control (Fig. S3P–Q). Collectively, these results demonstrate that LKB1 controls both axon growth and branching but that one can operationally segregate the effects on axon branching from the effect on the main axon growth.
NUAK1 is required downstream of LKB1 for axon growth and branching
We tested whether overexpression of LKB1 or NUAK1 was sufficient to promote axon growth and/or axon branching. After 5 DIV, we observed that overexpression of LKB1 or NUAK1 was sufficient to induce a marked increase in axon branching (Fig. 3D–F and K), without a significant effect on axon length (Fig. 3J) compared to control.
We then took advantage of this overexpression-mediated increase in axon branching and tested functional epistasis between LKB1 and NUAK1. We found that knockdown of Nuak1 suppressed the increase in axon branch number induced by LKB1 overexpression (Fig 3J–K), suggesting that NUAK1 is the main downstream effector of LKB1 during axon growth and branching. Moreover, overexpression of NUAK1 was sufficient to rescue Lkb1 loss of function (Fig 3J–K) while simultaneous knockdown of Lkb1 and Nuak1 showed no additive decrease in axon length or axon branch number (Fig 3G–I), indicating that LKB1 and NUAK1 form a kinase pathway controlling axon growth and branching.
To further explore the specificity of NUAK1 in axon branching, we tested the consequences of overexpressing other LKB1-dependent AMPK-like kinases on axon branching. None of the other members of the AMPK-like kinase family we tested, including NUAK2, SAD-B and AMPKα2 had any significant effect on axon growth or branching by overexpression (Fig. S5A–F) despite comparable levels of overexpression (Fig. S5G). As SAD-A/B have been previously shown to regulate polarity in cortical neurons (Barnes et al., 2007; Kishi et al., 2005), we tested if they were required for cortical axon growth or branching in vitro. shRNA-mediated knockdown of Sad-A/B (Fig. S5L–M) had no effect on cortical axon growth or branching at 5 DIV in the fraction of neurons that successfully formed an axon (Fig. S5H–K).
Loss of LKB1 or NUAK1 increases mitochondrial motility in the axon
How do LKB1-NUAK1 kinases control axon growth and branching? MARK1-4 kinases are downstream effectors of LKB1 in some cell types and have been shown to regulate microtubule dynamics (Mian et al., 2012; Nishimura et al., 2012). Furthermore, axon growth and branching is dependent on microtubule dynamics (Gallo, 2011; Polleux and Snider, 2010). We therefore tested if LKB1 or NUAK1 affected microtubule stability and/or dynamics in developing axons. First, we found no difference in the ratio of tyrosinated and acetylated tubulin (Fig. S6H), an index of stable vs. dynamic microtubules, in Lkb1- or Nuak1-deficient neurons. To explore effects on microtubule dynamics specifically in the growing axon, we used EB3-EGFP (a marker of the polymerizing end of microtubules) in control or Nuak1 shRNA, then performed time-lapse imaging of EB3-EGFP ‘comets’ in axonal growth cones at 5DIV (Fig. S6I–L and Supplementary movie 2). We observed no significant difference in the total area of the growth cone explored by microtubules (Fig. S6M), EB3-comets velocity (Fig. S6N) or lifetime (Fig. S6O) suggesting that the effects of LKB1-NUAK1 kinases on axon growth and branching are unlikely to be due to changes in microtubule dynamics.
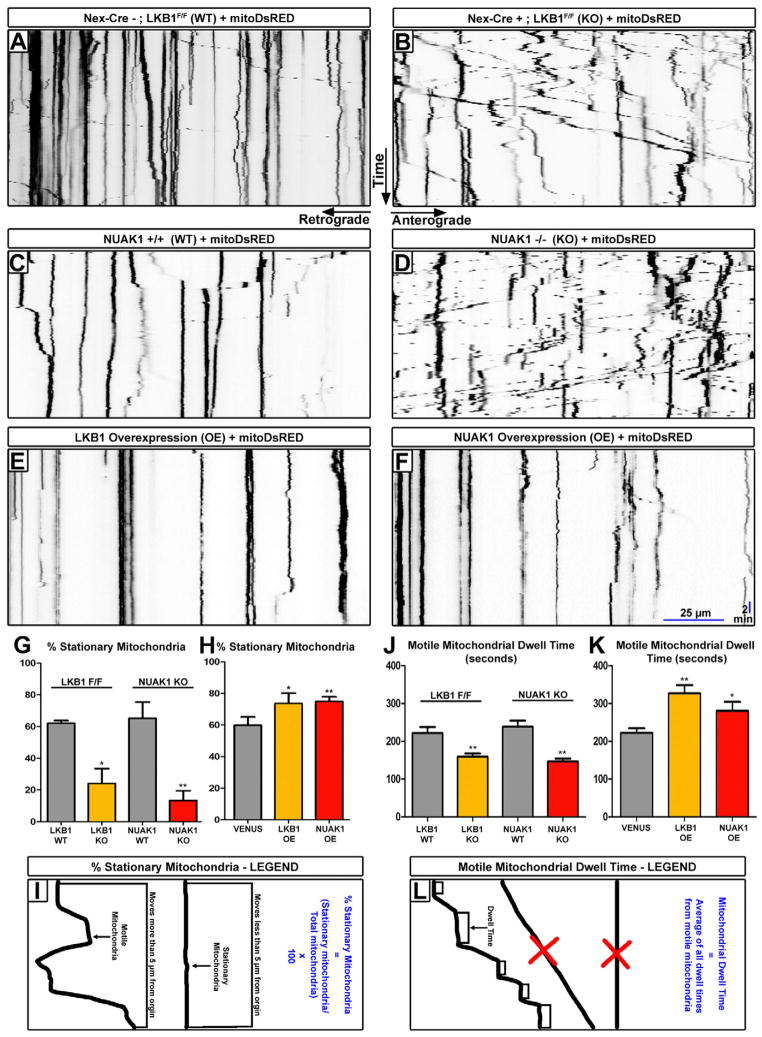
Axonal transport and targeting of presynaptic cargoes to nascent presynaptic sites has been previously implicated in both axonal growth and branch stabilization especially during activity-dependent phases of synaptic formation (Meyer and Smith, 2006; Ruthazer et al., 2006; Takeda et al., 2000). Therefore, we tested whether axonal transport was defective in LKB1 and NUAK1-deficient cortical neurons using fluorescently labeled-organelles or presynaptic vesicles coupled to time-lapse microscopy in axons. LKB1-null cortical neurons at 7DIV did not show any defect in the axonal transport of synaptic vesicle precursors (SVP) labeled with VGLUT1-Venus (Herzog et al., 2011) (Fig. S6A–D and Supplementary movie 2). On the other hand, we observed a striking change in mitochondrial axonal transport visualized using time-lapse microscopy of 5DIV cortical neurons expressing mitoDsRed (Fig. 4A–B and Supplementary movie 3). As reported previously, in wild-type growing hippocampal or cortical axons, mitochondria have a specific motility profile where approximately 65% of the mitochondrial pool is stationary while the other 35% are transported in both the anterograde and retrograde directions (see for example Brickley and Stephenson, 2011; Wang et al., 2011). Upon loss of LKB1, we observed a 3-fold decrease in the percentage of immobilized mitochondria along the axon shaft (Fig. 4A–B, G and Supplementary movie 3). Importantly, an identical phenotype was observed in Nuak1 KO cortical neurons (Fig. 4C–D, G and Supplementary movie 3). Motile mitochondria in the axon of Lkb1 and Nuak1-null cortical neurons also displayed increased maximum instantaneous velocity as well as increased maximum distance traveled, both anterogradely and retrogradely compared to controls (Fig. S6E–F). These changes in motility were not accompanied by detectable changes on mitochondria density along the axon shaft (Fig. S6G). Conversely, overexpression of either LKB1 or NUAK1 was sufficient to increase significantly (approximately from 60% to 75%) the proportion of stationary mitochondria along the axon (Fig. 4E–F, H, I). Accordingly, dwell time of motile mitochondria also decreased upon loss of LKB1 or NUAK1 (Fig. 4J, L), and increased with the overexpression of either LKB1 or NUAK1 (Fig. 4K, L).
Figure 4. LKB1 and NUAK1 are necessary and sufficient for mitochondrial immobilization in cortical axons.
(A–F) Representative kymographs of axonal mitochondria in dissociated neurons electroporated with the indicated constructs. (G) Loss of LKB1 or NUAK1 caused a significant decrease in the number of stationary mitochondria, (H) while overexpression of LKB1 or NUAK1 produced more stationary mitochondria (±SEM). (J) Loss of LKB1 or NUAK1 lead to a decrease in motile mitochondrial dwell time, (K) while overexpression of LKB1 or NUAK1 increased the dwell time of motile mitochondria (±SEM). (I) Kymograph illustration of the parameters used to determine motile and stationary mitochondria. (L) Schema illustrating the parameters used to determine mitochondrial dwell time. Motile mitochondria which did not pause for at least 60 seconds were excluded as were stationary mitochondria. All kymographs throughout the paper are oriented as shown in A–B. Statistical analysis: Mann-Whitney test. See also Fig. S6.
Syntaphilin is required for proper callosal axon branching in vitro and in vivo
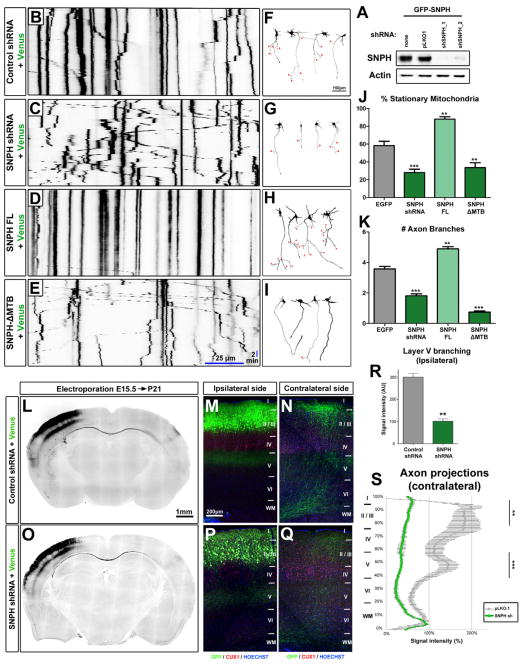
We next wanted to test if there was a causal relationship between axon branching and the ability of mitochondrial to get immobilized along the axon. To accomplish this, we needed a tool that would allow us to specifically regulate mitochondria immobilization along the axon. Syntaphilin (SNPH) is an axonally targeted, mitochondria-associated protein that also binds microtubules (Kang et al., 2008). Importantly, cortical neurons isolated from Snph knockout mice showed decreased mitochondria immobilization (Kang et al., 2008), a phenotype qualitatively and quantitatively very similar to Lkb1- and Nuak1-null cortical neurons. We first tested whether the loss of SNPH affected axonal branching, by using electroporation of shRNA against mouse Snph (Fig. 5A) in cortical progenitors at E15.5 by EUCE. We confirmed that knockdown of mouse Snph significantly decreased the percentage of immobile mitochondria in cortical axons (Fig. 5B–C, J and Supplementary movie 4) to a level comparable to that observed in Snph-null neurons (Kang et al., 2008) and Lkb1 or Nuak1-null neurons. Interestingly, knockdown of Snph in cortical neurons significantly decreased axon branching in cortical neurons in vitro (Fig. 5F–G, K). These results are phenocopied by the overexpression of a dominate-negative SNPH which is targeted to the axon and can bind mitochondria but lacks its microtubule-binding domain (SNPH-ΔMTB) (Kang et al., 2008) (Fig. 5E, I, J–K and Supplementary movie 4). Furthermore, over-expression of SNPH, which significantly increased the percentage of stationary mitochondria along cortical axons (Fig. 5D, J and Supplementary movie 4), was sufficient to increase axon branching at 5DIV (Fig. 5H, K). Finally, IUCE of shRNA against Snph revealed that SNPH in vivo is required for terminal axon branching on both the ipsilateral and contralateral hemispheres in layers 2/3 and 5 (Fig. 5O–Q) when compared to control (Fig 5L–N). Upon quantification, the reduction of axon branching upon shRNA electroporation was of similar magnitude to the reduction observed upon loss of LKB1 both ipsilaterally (Fig. 5R) and contralaterally (Fig. 5S). These results establish that SNPH is required for proper axon branching both in vitro and in vivo, and suggests that direct link between mitochondria immobilization and axon branching.
Figure 5. Syntaphilin-dependent mitochondria immobilization is necessary and sufficient for proper axon branching.
(A) Validation of shRNA targeting mouse Snph in HEK293T cells. Western-blot analysis was performed with the indicated antibodies. (B–E) Representative kymographs of axonal mitochondria in dissociated neurons electroporated with the indicated constructs. (F–I) Representative neurons at 5 DIV electroporated with the constructs indicated in B–E. Red arrowheads point to axon branches. (J–K) Loss or disruption of SNPH decreased, while overexpression of SNPH increased the number of stationary mitochondria (J) and of axonal branches (K). (L–Q) Low magnification images of coronal brain sections at P21 (L & O) and high magnification for the ipsilateral (M & P) or contralateral (N & Q) side. (R) Loss of SNPH caused a reduction in axonal branching on layer 5 of the ipsilateral hemisphere. (S) Loss of SNPH reduced axonal branching in both layers 2/3 and 5 of the contralateral hemisphere. Data represent average value ±SEM (J–K, R–S). Statistical analysis: Mann-Whitney test or two-way ANOVA (S).
LKB1 regulates axon branching by controlling mitochondrial immobilization
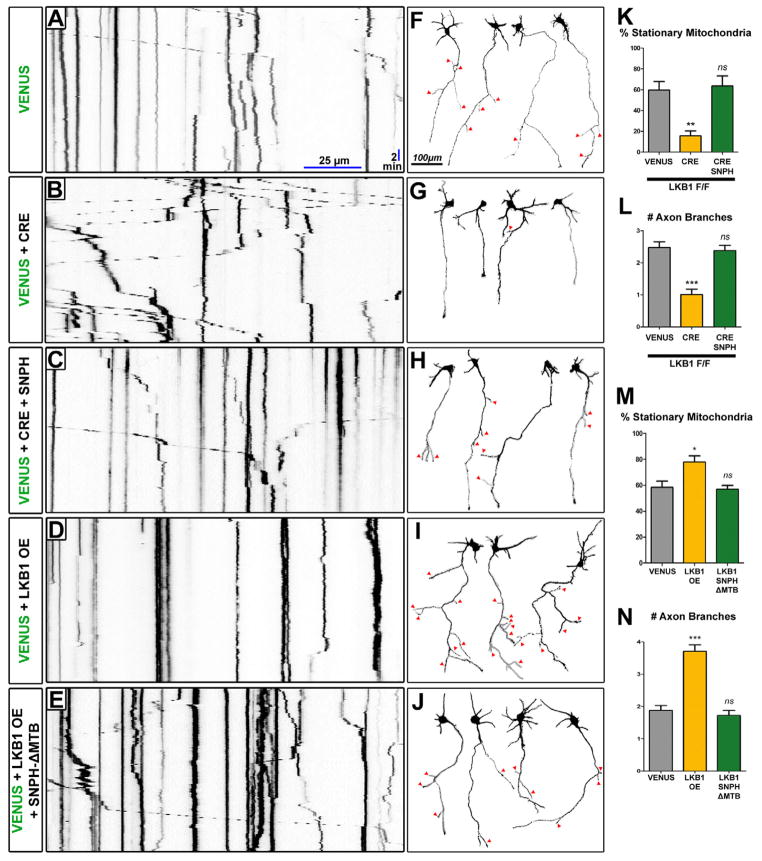
We took advantage of the fact that overexpression of full-length SNPH is sufficient to arrest mitochondria motility (Kang et al., 2008 and Fig. 5D) to determine if there was a causal relationship between mitochondria immobilization and LKB1-dependent axonal branching. We confirmed the decrease in the percentage of stationary mitochondria (Fig 6A–B, K) and the decreased axon branching in LKB1-deficient neurons compared to control neurons at 5DIV (Fig 6F–G, L). Remarkably, SNPH overexpression to levels that restored the percentage of immobile mitochondria back to control levels in Lkb1-null neurons (Fig. 6C, K and Supplementary movie 5) also rescued axonal branching to control levels (Fig. 6H, L).
Figure 6. LKB1 mediates axonal branching through mitochondria immobilization along the axon.
(A–E) Representative kymographs of axonal mitochondria in dissociated neurons electroporated with the indicated constructs. (F–J) Representative neurons at 5 DIV electroporated with the constructs indicated in A–E. Red arrowheads point to axon branches. (K) Loss of LKB1 caused a decrease in the percentage of stationary mitochondria, while overexpression of wildtype human SNPH upon loss of LKB1 returned mitochondrial transport to normal levels. (L) Loss of LKB1 decreased the number of axonal branches, while overexpression of wildtype human SNPH upon loss of LKB1 returned axonal branching to normal levels. (M) Overexpression of LKB1 increased the percentage of stationary mitochondria, while overexpression of dominate negative human SNPH-ΔMTB at the same time as LKB1 returned mitochondrial transport to normal levels. (N) Overexpression of LKB1 also increased the number of axonal branches, while overexpression of dominate negative human SNPH-ΔMTB at the same time as LKB1 returned axonal branch number to normal levels. Data represent average value ±SEM (K–N). Statistical analysis: ANOVA non-parametric test.
As shown previously, we confirmed that LKB1 overexpression increased both axonal branching and the percentage of stationary mitochondria in the same neuronal cultures when compared to control neurons (Fig 6D, I, M–N and Supplementary movie 5). Expressing SNPH-ΔMTB in LKB1-overexpressing cortical neurons restored the percentage of stationary mitochondria to approximately 60% (Fig. 6E, M) and rescued axonal branching to levels comparable to control (Fig. 6J, N). These results demonstrate that LKB1 controls axonal branching by regulating mitochondria immobilization.
LKB1 and NUAK1 induce mitochondria immobilization at nascent presynaptic sites along the axon
Based on previously published work demonstrating that presynaptic transport and synapse formation positively regulate axon branch stabilization (Meyer and Smith, 2006; Ruthazer et al., 2006), and that mitochondria are highly enriched at presynaptic sites in many axon types (Harris and Weinberg, 2012), we tested if LKB1 and NUAK1 were specifically regulating mitochondrial immobilization and/or long-term capture at nascent presynaptic sites. We performed dual-channel time-lapse imaging at 7DIV in cortical axons of dissociated neurons electroporated with VGLUT1-Venus and mito-DsRed by EUCE at E15.5. The vast majority of VGLUT1-Venus puncta overlap with three others presynaptic vesicle proteins (Synaptobrevin 2 and Synapsin1), or the presynaptic active zone scaffolding protein Bassoon (Fig. S7, (Sabo et al., 2006)), confirming that VGLUT1-Venus faithfully detects nascent presynaptic sites along developing cortical axons (Herzog et al., 2011).
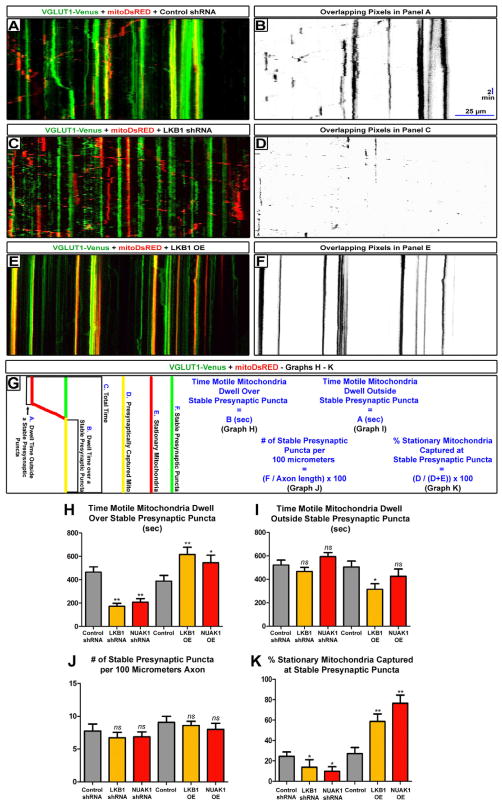
We determined that LKB1 or NUAK1 down-regulation or over-expression do not affect the density of stable (defined as stationary for 30 minutes of time-lapse) VGLUT1-Venus puncta (Fig. 7A, C, D- quantified in Fig. 7G, J and Supplementary movie 6). Using dual time-lapse microscopy of VGLUT1-Venus and mito-DsRed in wild-type axons, we observed that many motile mitochondria frequently dwelled over stable VGLUT1-Venus puncta, often before resuming movement (Fig. 7A–B and Supplementary movie 6). However, in LKB1- and NUAK1-deficient axons, we observed a significant decrease in the co-localization of motile mitochondria over stable VGLUT1 puncta (Fig. 7C–D). Strikingly, the loss of LKB1 or NUAK1 led to a 50% reduction in the average time that motile mitochondria spent dwelling over nascent presynaptic sites but importantly did not affect the average dwelling time outside presynaptic boutons (Fig. 7G–I). LKB1 or NUAK1 are also sufficient to instruct mitochondria to specifically dwell at nascent presynaptic sites (Fig. 7G–I).
Figure 7. LKB1 and NUAK1 are necessary and sufficient for mitochondrial immobilization at nascent presynaptic sites.
(A, C, E) Representative dual color kymographs of axonal VGLUT1-Venus puncta and mitochondria dynamics in cortical neurons electroporated with the indicated constructs. (B, D, F) Overlapping pixels maps of A, C, E were created in Fuji/ImageJ using the Colocalization Threshold program. (G) Schematic illustration of the parameters used to quantify the results shown in H–K. (H) Knockdown of LKB1 or NUAK1 decreased the dwell time of mitochondria over nascent presynaptic sites, while overexpression of LKB1 or NUAK1 increased it. (I) Knockdown of LKB1 or NUAK1 did not affect the dwell time of mitochondria outside nascent presynaptic sites, while overexpression of LKB1 or NUAK1 slightly decreased it. (J) Knockdown or overexpression of LKB1 and NUAK1 do not affect the linear density of stable (for 30 minutes) VGLUT1-Venus nascent presynaptic sites. (K) Loss of LKB1 or NUAK1 decreased, while overexpression of LKB1 or NUAK1 increased the percentage of mitochondria stably captured (for 30 minutes) at presynaptic sites. Data represent average value ±SEM (K–N). Statistical analysis: Mann-Whitney test. See also Fig. S7.
Finally, we found that loss of LKB1 or NUAK1 significantly reduced mitochondria long-term capture (immobilized for 30 minutes as opposed to transient dwelling; Fig. 7G) presynaptically by more than 50% while over-expression of LKB1 or NUAK1 increased by more than 2-fold the percentage of stationary mitochondria captured presynaptically (Fig. 7K). These results demonstrate that LKB1 and NUAK1 kinases are specifically involved in immobilization (dwelling or capture) of mitochondria at nascent presynaptic sites.
DISCUSSION
The polarization of cortical neurons is tightly coupled to neuron migration during which immature neurons form two molecularly and functionally distinct compartments (Barnes and Polleux, 2009): the somato-dendritic domain and the axon. LKB1 is necessary and sufficient for axon specification in vitro and in vivo (Barnes et al., 2007; Shelly et al., 2007). Our present results demonstrate that LKB1 is required in newly born neurons at the time of cell cycle exit or shortly thereafter to instruct neuron polarization.
We demonstrate here using various in vitro and in vivo strategies that LKB1 is necessary and sufficient for terminal axon branching of cortical neurons through the activation of NUAK1, an AMPK-related kinase whose function during brain development was unknown. NUAK1 seems to be the main effector of LKB1 in mediating axon growth and branching in cortical neurons. Our results do not exclude that other AMPK related kinases might have a less prominent or redundant role during axon growth and branching. We observed that NUAK1 expression is largely restricted in the dorsal telencephalon in the embryo, as previously reported (Hirano et al., 2006). Whether LKB1 is required for axon growth or branching in other types of neurons, and whether this function involves NUAK1 or other AMPK related kinases remains to be determined. The SAD kinases are required in cortical neurons to mediate LKB1-dependent polarization (Barnes et al., 2007; Kishi et al., 2005). Therefore it appears that LKB1 controls at least two kinase pathways in cortical neurons during development: SAD-A/B for axon specification and NUAK1 for axon growth and branching. How SAD-A/B and NUAK1 kinases are differentially regulated by LKB1 during development is currently unknown. Interestingly SAD-A/B kinases also play a role in presynaptic development and synaptic vesicle release (Crump et al., 2001; Inoue et al., 2006), suggesting that their function downstream of LKB1 extends beyond axon specification.
Mitochondria are critical to many functions in cells including ATP production, calcium homeostasis, and the triggering of apoptosis through release of cytochrome-c (Schon and Przedborski, 2011; Sheng and Cai, 2012). The high-energy demand of neurons requires that mitochondria be readily available throughout the large cytoplasmic volume of neurons and in particular trafficked properly along the entire length of the axon where presynaptic release consumes a large amount of ATP (Vos et al., 2010). Our results suggest that immobile mitochondria have a unique property with regard to their ability to stimulate axon branching not shared by mobile mitochondria through promotion of axon branch formation and/or branch stabilization. Further work is needed to determine the precise mechanisms by which LKB1 and NUAK1 regulate mitochondrial capture presynaptically, including the role of syntaphilin in this process. Currently we have no evidence of direct interaction or phosphorylation of SNPH by either LKB1 or NUAK1 (Courchet and Polleux, unpublished observations), however this does not exclude the involvement of other intermediate kinases or adaptor proteins regulating SNPH-mediated immobilization of mitochondria. Future investigations will determine how LKB1-NUAK1-regulates the capture of mitochondria to nascent presynaptic sites and how this capture regulates axon branching through control of ATP production and/or Ca2+ homeostasis or through other unknown mechanisms.
EXPERIMENTAL PROCEDURES
Animals
All animals were handled according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) at University of North Carolina-Chapel Hill and at The Scripps Research Institute, La Jolla. Time-pregnant females were maintained in a 12 hr light/dark cycle and obtained by overnight breeding with males of the same strain. For timed pregnant matings, noon after mating is considered embryonic day 0.5 (E0.5). Ex vivo cortical dissociation of neurons was performed on C57Bl/6J or on Balb/C timed-pregnant mice. Floxed LKB1 mice (Stk11tm1.1Rdp) and Emx1Cre transgenic mice (Emx1tm1(cre)Ito) have been used previously (Barnes et al., 2007). NEXCre mice (NeuroD6tm1(cre)Kan) were obtained from the Jackson laboratory. NUAK1 KO mice (Nuak1tm1Sia) were described previously (Hirano et al., 2006).
In utero cortical electroporation
The previously described protocol for in utero cortical electroporation (Hand and Polleux, 2011) was modified as follows. Timed pregnant hybrid F1 females were obtained by mating inbred 129/SvJ females and C57Bl/6J males and were used for shRNA electroporation. Timed pregnant LKB1F/F females were used for Cre plasmid electroporation. A mix of endotoxin-free plasmid preparation (Cre plasmid or shRNA plasmid mix -1 μg/μL; except SNPH shRNA at 0.5 μg/μL - and the reporter plasmid pSCV2 −0.5 μg/μL) was injected into one lateral hemisphere of E15.5 embryos using a picospritzer. Electroporation was performed with gold paddles to target cortical progenitors in E15.5 embryos by placing the anode (positively charged electrode) on the side of DNA injection and the cathode on the other side of the head. Four pulses of 45 V for 50 ms with 500 ms interval were used for electroporation. Animals were sacrificed 3 weeks after birth by terminal perfusion of 4% paraformaldehyde (PFA, Electron Microscopy Sciences) followed by 2 hours post-fixation in 4% PFA.
Ex utero cortical electroporation followed by dissociation and cortical neuron culture were performed as previously described (Hand et al., 2005). See Supplementary Material for details.
Primary neuronal culture, magnetofection
Cortices from E15.5 mouse embryos were dissected in Hank’s Buffered Salt Solution (HBSS) supplemented with Hepes (pH 7.4; 2.5 mM), CaCl2 (1 mM, Sigma), MgSO4 (1 mM, Sigma), NaHCO3 (4mM, Sigma) and D-glucose (30 mM, Sigma), hereafter referred to as cHBSS. Cortices were dissociated in cHBSS containing papain (Worthington) and DNAse I (100 μg/ml, Sigma) for 20 min at 37°C, washed three times and m anually triturated in cHBSS supplemented with DNAse. Cells were then plated at 5.0 × 104 cells per 35mm glass bottom dish (Matek) coated with poly-D-lysine (1 mg/ml, Sigma) and cultured for 5–7 days in Neurobasal medium supplemented with B27 (1X), N2 (1x), L-glutamine (2 mM) and penicillin (5 units/ml)-streptomycin (50 mg/ml). To transfect cultured neurons, we performed magnetofection using NeuroMag (OZ Bioscience) according to the manufacturer’s protocol. Live imaging was performed in cHBSS (see Supplementary Material for details).
Image acquisition and analysis
Images were acquired with a Nikon A1R confocal, while movies were acquired with an Andor iXon 897 CCD camera on a Nikon Ti-E microscope. Dual imaging of Mito-DsRED and VGLUT1-Venus was acquired at 1 frame per 10 seconds for 30 minutes for Figures 4–7. See Supplementary Material for details.
Western blotting
Western blotting was carried out as previously published (Williams et al, 2011). See Supplementary Material for details.
In situ hybridization
In situ hybridization was carried out using digoxigenin-labeled riboprobes at the In Situ Hybridization Core Facility from UNC-Chapel Hill. Probes were generated from the following mouse cDNA clones purchased from Open Biosystems (Huntsville, AL): NUAK1 (Genbank: CB522124, IMAGE: 6842317) and NUAK2 (Genbank: CB248251, IMAGE: 5718428).
Quantifications and statistics
Axon tracing and measurements were performed with NIS-Elements software (Nikon). Signal intensity measurements were performed using ImageJ. Statistical analyses were performed with Prism (GraphPad Software). Statistical tests performed are included in each figure legend.
Constructs, time-lapse imaging, immunocytochemistry, NUAK1 in vitro kinase assay and RT-PCR assays
See Supplemental Material for details.
Supplementary Material
The LKB1-NUAK1 kinase pathway is necessary and sufficient for mammalian axon branching in vivo. These kinases regulate axon branching through regulation of mitochondria trafficking and more specifically through presynaptic capture of mitochondria.
Acknowledgments
The authors would like to thank members of the Polleux lab and Sandra Encalada for useful discussions. We thank Kathy Spencer for excellent help with microscopy, and Trevor Sauerbrey for technical help. We would also like to thank Brendan Lilley, Ulrich Müller, Anton Maximov, Sandra Encalada for critical reading of the manuscript. We thank Joshua Sanes, Zu-Hang Shen, Ulrich Müller, Etienne Herzog, and Don Arnold for sharing reagents. This work was funded by NIH R01AG031524 (FP), ADI-Novartis funds (FP), Fondation pour la Recherche Medicale (JC), Philippe Foundation (JC), and NIH 5F32NS080464 (TL). J.C, T.L. and F.P. conceived the experiments and interpreted the results, while J.C, T.L., S.L., V.C. and D.Y.L. performed the experiments. S.A. provided the NUAK1 knockout mice. J.C., T.L., and F.P. prepared the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- Amato S, Liu X, Zheng B, Cantley L, Rakic P, Man HY. AMP-activated protein kinase regulates neuronal polarization by interfering with PI 3-kinase localization. Science. 2011;332:247–251. doi: 10.1126/science.1201678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, Loda M, Carrasco DR, DePinho RA. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annual review of neuroscience. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley K, Stephenson FA. Trafficking kinesin protein (TRAK)-mediated transport of mitochondria in axons of hippocampal neurons. The Journal of biological chemistry. 2011;286:18079–18092. doi: 10.1074/jbc.M111.236018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JG, Zhen M, Jin Y, Bargmann CI. The SAD-1 kinase regulates presynaptic vesicle clustering and axon termination. Neuron. 2001;29:115–129. doi: 10.1016/s0896-6273(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Donahoo AL, Richards LJ. Understanding the mechanisms of callosal development through the use of transgenic mouse models. Semin Pediatr Neurol. 2009;16:127–142. doi: 10.1016/j.spen.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Muller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011;69:482–497. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. The cytoskeletal and signaling mechanisms of axon collateral branching. Developmental neurobiology. 2011;71:201–220. doi: 10.1002/dneu.20852. [DOI] [PubMed] [Google Scholar]
- Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44:611–621. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harbor perspectives in biology. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R, Bortone D, Mattar P, Nguyen L, Heng JI, Guerrier S, Boutt E, Peters E, Barnes AP, Parras C, et al. Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron. 2005;48:45–62. doi: 10.1016/j.neuron.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Hand R, Polleux F. Neurogenin2 regulates the initial axon guidance of cortical pyramidal neurons projecting medially to the corpus callosum. Neural Dev. 2011;6:30. doi: 10.1186/1749-8104-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Weinberg RJ. Ultrastructure of synapses in the mammalian brain. Cold Spring Harbor perspectives in biology. 2012:4. doi: 10.1101/cshperspect.a005587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Nadrigny F, Silm K, Biesemann C, Helling I, Bersot T, Steffens H, Schwartzmann R, Nagerl UV, El Mestikawy S, et al. In vivo imaging of intersynaptic vesicle exchange using VGLUT1 Venus knock-in mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:15544–15559. doi: 10.1523/JNEUROSCI.2073-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Kiyonari H, Inoue A, Furushima K, Murata T, Suda Y, Aizawa S. A new serine/threonine protein kinase, Omphk1, essential to ventral body wall formation. Dev Dyn. 2006;235:2229–2237. doi: 10.1002/dvdy.20823. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annual review of neuroscience. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Inoue E, Mochida S, Takagi H, Higa S, Deguchi-Tawarada M, Takao-Rikitsu E, Inoue M, Yao I, Takeuchi K, Kitajima I, et al. SAD: a presynaptic kinase associated with synaptic vesicles and the active zone cytomatrix that regulates neurotransmitter release. Neuron. 2006;50:261–275. doi: 10.1016/j.neuron.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Jaleel M, McBride A, Lizcano JM, Deak M, Toth R, Morrice NA, Alessi DR. Identification of the sucrose non-fermenting related kinase SNRK, as a novel LKB1 substrate. FEBS Lett. 2005;579:1417–1423. doi: 10.1016/j.febslet.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Deinhardt K, Miyoshi G, Bennett AM, Chao MV. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nature neuroscience. 2010;13:1373–1379. doi: 10.1038/nn.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil K, Szebenyi G, Dent EW. Common mechanisms underlying growth cone guidance and axon branching. Journal of neurobiology. 2000;44:145–158. [PubMed] [Google Scholar]
- Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307:929–932. doi: 10.1126/science.1107403. [DOI] [PubMed] [Google Scholar]
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. The EMBO journal. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- Meyer MP, Smith SJ. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:3604–3614. doi: 10.1523/JNEUROSCI.0223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian I, Pierre-Louis WS, Dole N, Gilberti RM, Dodge-Kafka K, Tirnauer JS. LKB1 destabilizes microtubules in myoblasts and contributes to myoblast differentiation. PloS one. 2012;7:e31583. doi: 10.1371/journal.pone.0031583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouse V, Swick LL, Kazgan N, St Johnston D, Brenman JE. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol. 2007;177:387–392. doi: 10.1083/jcb.200702053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mizuno H, Hirano T, Tagawa Y. Evidence for activity-dependent cortical wiring: formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:6760–6770. doi: 10.1523/JNEUROSCI.1215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno H, Hirano T, Tagawa Y. Pre-synaptic and post-synaptic neuronal activity supports the axon development of callosal projection neurons during different post-natal periods in the mouse cerebral cortex. The European journal of neuroscience. 2010;31:410–424. doi: 10.1111/j.1460-9568.2009.07070.x. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Applegate K, Davidson MW, Danuser G, Waterman CM. Automated screening of microtubule growth dynamics identifies MARK2 as a regulator of leading edge microtubules downstream of Rac1 in migrating cells. PloS one. 2012;7:e41413. doi: 10.1371/journal.pone.0041413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Snider W. Initiating and growing an axon. Cold Spring Harbor perspectives in biology. 2010;2:a001925. doi: 10.1101/cshperspect.a001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Li J, Cline HT. Stabilization of axon branch dynamics by synaptic maturation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:3594–3603. doi: 10.1523/JNEUROSCI.0069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo SL, Gomes RA, McAllister AK. Formation of presynaptic terminals at predefined sites along axons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:10813–10825. doi: 10.1523/JNEUROSCI.2052-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon EA, Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Sheng ZH, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nature reviews Neuroscience. 2012;13:77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Yamazaki H, Seog DH, Kanai Y, Terada S, Hirokawa N. Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building. J Cell Biol. 2000;148:1255–1265. doi: 10.1083/jcb.148.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka N, Ruthazer ES, Yamamoto N. The role of neural activity in cortical axon branching. Neuroscientist. 2006;12:102–106. doi: 10.1177/1073858405281673. [DOI] [PubMed] [Google Scholar]
- Vos M, Lauwers E, Verstreken P. Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front Synaptic Neurosci. 2010;2:139. doi: 10.3389/fnsyn.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CA, Morrow EM, Rubenstein JL. Autism and brain development. Cell. 2008;135:396–400. doi: 10.1016/j.cell.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CL, Zhang L, Zhou Y, Zhou J, Yang XJ, Duan SM, Xiong ZQ, Ding YQ. Activity-dependent development of callosal projections in the somatosensory cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:11334–11342. doi: 10.1523/JNEUROSCI.3380-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JL, Morton DG, Bestman J, Kemphues KJ. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development. 2000;127:1467–1475. doi: 10.1242/dev.127.7.1467. [DOI] [PubMed] [Google Scholar]
- Williams T, Courchet J, Viollet B, Brenman JE, Polleux F. AMP-activated protein kinase (AMPK) activity is not required for neuronal development but regulates axogenesis during metabolic stress. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5849–5854. doi: 10.1073/pnas.1013660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CR, Power J, Barnea G, O’Donnell S, Brown HE, Osborne J, Axel R, Gogos JA. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–566. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- Zagorska A, Deak M, Campbell DG, Banerjee S, Hirano M, Aizawa S, Prescott AR, Alessi DR. New roles for the LKB1-NUAK pathway in controlling myosin phosphatase complexes and cell adhesion. Sci Signal. 2010;3:ra25. doi: 10.1126/scisignal.2000616. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, Bear MF. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harbor perspectives in biology. 2012:4. doi: 10.1101/cshperspect.a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.