Abstract
Background & Aims
While the majority of HCV-infected patients progress to chronic hepatitis, a small fraction of individuals are able to clear the virus. Resolution of infection occurs within the first few weeks to months of infection, suggesting that innate immune functions may be critical for early control. Epidemiologic data support a role for particular NK cell receptor bearing populations in this control, yet the mechanism by which NK cells respond to HCV early in infection is unknown.
Methods
Changes in the phenotype and function of NK cells were investigated in a cohort of 43 individuals identified during various stages of HCV infection with different clinical outcomes.
Results
Acute, chronic, and resolved HCV infections were characterized by an expansion of CD56neg NK cells. Furthermore, increased levels of HLA-C-binding KIR+ NK cells were observed in HCV resolvers, while all stages of HCV infection were associated with reduced percentages of NKG2D+, NKp30+, and NKp46+ NK cells, and a slight increase in the ability of NK cells to respond to target cells bearing the ligands for these receptors. In contrast, NKG2A+ and CD94+ NK cells were elevated in acute and chronic HCV infection, but not in resolved infection. Most importantly, in acute infection, lower frequencies of NKp30+, NKp46+, CD161+, and NKG2D+ NK cells were observed in patients who were subsequently able to clear HCV infection than in those becoming chronically infected.
Conclusions
These data implicate particular populations of NK cells in the early control and clearance of HCV infection.
Keywords: Hepatitis C virus, Natural killer cells, Innate immunity, CD161, NK-p30, NKp46, Resolution of HCV infection, Acute infection
Introduction
Roughly 170 million people worldwide suffer from chronic hepatitis C virus (HCV) infection, a condition that results in liver cirrhosis or hepatocellular carcinoma in approximately 10–20% and 1–5% of cases, respectively [1]. One-fifth of de novo HCV infections are cleared during the acute phase; thus progression to chronic disease occurs in the majority of infected individuals [2]. Viral clearance is thought to rely largely on a broad, potent, and prolonged host cellular immune response [3–5]. Accordingly, defective T cell immunity is strongly associated with viral persistence [6].
In individuals that are able to clear HCV infection, viral control occurs within the first few months of infection, at a time when the adaptive immune response is just developing. Before the onset of the adaptive immune response, it is thought that innate immune effector cells, such as natural killer (NK) and NKT cells release interferon-gamma (IFN-γ), which is directly responsible for the non-cytopathic inhibition of HCV replication [7]. Besides producing inflammatory cytokines with antiviral activity, NK cells are also capable of eliminating infected cells without the need for prior antigen sensitization. Furthermore, epidemiological data suggest that particular NK cell receptor– ligand combinations are associated with the clearance of HCV infection, directly implicating these cells in the early control of HCV infection [8].
NK cell activation is tightly regulated by a balancing act of activating and inhibitory signals that are integrated in a complex network of receptors expressed on the cell surface. NK receptors include members of (i) the killer cell immunoglobulin-like receptor (KIR) superfamily that primarily recognizes certain allotypes of human leukocyte antigens (HLA)-A, -B, -C, and -G, (ii) the C-type lectin superfamily including the lectin-like heterodimers CD94–NKG2 recognizing the non-classical major histocompatibility complex class I (MHC-I) molecule HLA-E and the activating NKG2D recognizing the MHC-I-related molecules MICA and MICB [9], and (iii) the natural cytotoxicity receptors (NCRs) that interact with particular viral proteins but whose cellular ligands remain largely undefined [10–12]. In addition, nearly 90% of peripheral NK cells also express the FcγRIIIa (CD16) receptor, involved in the recognition and lysis of antibody-coated cells [13]. NK cells can also modulate the quality of the adaptive immune response, mainly via their interaction with dendritic cells (DCs) [14]. Thus, NK cells play a critical role during the acute response to infection, including the early direct containment of viral replication and the initiation and maintenance of an effective adaptive immune response.
Given the pivotal role of NK cells in the host’s immune response to viral infections and the fact that these cells are dramatically enriched in the liver compared to other tissues [15], numerous studies have investigated their importance in chronic HCV infection. One mechanism by which HCV establishes chronicity could involve the alteration of some important functions of NK cells very early in the course of the infection. This hypothesis is supported by mounting evidence demonstrating that patients with chronic HCV infection have altered NK cell subset distribution and/or NK cell receptor expression [16–25]; it is nevertheless less well understood whether altered NK cell phenotypic changes correlate with impaired NK cell function [16–27]. However, chronic HCV infection is associated with an increased number of NK cells bearing the inhibitory receptor CD94/NKG2A, a feature that has been proposed to result in NK cell dysfunction and impaired DC activation, potentially leading to viral persistence [20, 23, 25]. Whether alterations occur in the frequency of NCR-bearing NK cells in the context of chronic HCV infection is still a matter of debate [16, 23].
Given that control of the infection occurs within the first weeks to months of infection, we hypothesized that distinct cellular immune responses are elicited during acute infection in individuals that go on to clear the infection. While the phenotypic and functional changes of NKT cells in acute HCV infection have been studied in detail [25], little is known regarding changes in the NK cell receptor expression profiles in the acute phase of the infection. In order to assess the importance of NK cell activation in acute HCV infection and to determine if altered patterns of NK cell receptor expression in the early stages of the infection is associated with the outcome of the disease, we performed a comprehensive phenotypic and functional analysis, including the extensive characterization of the repertoire of NK cell receptors in the context of acute, chronic, and resolved HCV infections. Our data show that acute HCV infection is associated with an expansion of NK cells with an altered phenotype but preserved cytotoxic function, and suggest that an early expansion of NK cells expressing low levels of NKp46, NKp30, CD161, and NKG2D may predict viral clearance.
Materials and methods
Study subjects
A total of 57 subjects were included in this study, including 20 patients in the acute phase of HCV infection, 11 presenting with chronic hepatitis C infection, 12 who naturally resolved HCV infection and 14 HCV negative controls (Table 1). None of the patients had received antiviral therapy at the time of entry into the study. Twenty patients were infected for 1–8 months and were thus considered in the acute phase of HCV infection. Acute HCV infection was diagnosed based on HCV antibody seroconversion in subjects who tested negative in the previous 6 months or HCV RNA positivity with HCV antibody negativity. Follow-up of these subjects showed that nine of them resolved the infection while eight became persistently infected. No information on disease outcome was available for another three patients identified in the acute phase. Eleven patients had been infected for more than 1 year and were in the chronic phase of the disease, as shown by the persistence of HCV RNA detected by RT-qPCR. Twelve HCV antibody positive subjects had previously cleared HCV spontaneously and tested repeatedly negative for HCV RNA by RT-qPCR (lower level of detection 50 IU/ml). Negative controls comprised four males and 10 females. The study was approved by the MGH Institutional Review Board and each subject gave written informed consent for participation in the study.
Table 1.
Characteristics of HCV patients and healthy controls.
| HCV stage | N | Age | Sex (M:F) | ALT | Viral load IU/ml | GT | Days PI | Risk | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| A | 25 | F | 148 | 16726 | 1b | 65 | surgery | R | |
| A | 58 | M | 53 | UD | ND | 80 | surgery | R | |
| A | 44 | M | 22 | UD | 3 (sero) | 230 | sex | R | |
| A | 40 | F | 14 | UD | 1 (sero) | 115 | sex | R | |
| A | 13 | M | 125 | 5067 | 1a | 70 | ND | R | |
| A | 26 | M | 144 | 7553 | 1 (sero) | 90 | IDU | R | |
| A | 38 | M | 30 | < 615 | ND | 60 | intranasal | R | |
| A | 29 | F | 175 | UD | 1 | 120 | ND | R | |
| A | 31 | M | 1621 | 500000 | 1a | ND | IDU | R | |
| A | 30 | M | 336 | 363000 | 1a | 200 | IDU | C | |
| A | 34 | F | 39 | 2745 | 1 | 55 | sex | C | |
| A | 54 | M | 1446 | > 700000 | 1 | 30 | stick | C | |
| A | 20 | M | 39 | 280 | 1a | 130 | IDU | C | |
| A | 20 | M | 32 | 11500 | 1b | 150 | IDU | C | |
| A | 26 | M | 441 | 335000 | 1b | 100 | ND | C | |
| A | 62 | F | 91 | 850001 | 1a | 60 | Transfusion | C | |
| A | 54 | F | 733 | 5050 | 2 | 90 | stick | C | |
| A | 38 | M | 253 | 130111 | 4 | ND | IDU | C | |
| A | 26 | M | 957 | 627000 | 4a | ND | Shared razor | C | |
| A | 28 | F | 82 | 2680000 | 3 | 60 | ND | C | |
| Acute Median (range) | 20 |
31 (13–62) |
13:7 |
135 (14–1621) |
9527 (0–2680000) |
90 (30–230) |
|||
| Resolved Median (range) | 12 |
46 (37–55) |
4:5 |
22 (8–94) |
0 |
||||
| Chronic Median (range) | 11 |
38 (24–76) |
7:4 |
44 (15–79) |
500000 (0–2900000) |
||||
| Negative Median (range) | 14 |
26 (22–57) |
4:10 |
ND |
0 |
A, acute; R, resolved; C, chronic; N, number of patients; M, male; F, female; ALT, alanine aminotransferase; IU, international units; UD, undetectable; GT, genotype; PI, postinfection; ND, not determined; IDU, injection drug user.
Flow cytometric analysis of NK cells numbers and surface receptors expression
PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation (Sigma) and cryopreserved. The phenotype and frequency of NK cell subpopulations were determined by staining 1 million thawed PBMCs for each panel. NK cell populations were defined as lymphocytes that were CD3-negative and were further defined by their expression of CD56 and/or CD16 as described elsewhere [28] using CD3-Pacific Blue, CD56-A700, and CD16-APC-CY7 (BD Biosciences). Analysis of NK cell receptors was performed by adding the following antibodies in three different panels: To study NCR expression, PBMCs were stained with NKp44-A647, NKp30-PE, and NKp46-PE (BD Biosciences). Changes in NKG2s expression were monitored using CD94-FITC, NKG2D-APC (BD Biosciences), and NKG2A-PE (Beckman Coulter). To study KIR expression, PBMCs were first stained with unconjugated CD158a (KIR2DL1/2DS1) and CD158b (KIR2DL2/2DL3/2DS2) (BD Biosciences), followed by anti-mouse IgG-APC (BioLegend), and finally stained with CD3-Pacific Blue, CD161-PE-CY5, CD56-PE-CY7, CD16-APC-Cy7, CD8-A700, DX9-FITC (BD Biosciences), and z27-PE (Beckman Coulter). Fixed cells were analyzed on an LSRII system using FacsDiva version 8.8.3 (BD Biosciences). The frequency and phenotypes of NK cells were analyzed using FlowJo version 7.2.4. (Treestar).
Flow cytometric analysis of NK cell function
NK cell activation was quantified after stimulation of PBMCs with MHC–devoid K562 cells and 221 cells (ATCC) at an effector-to-target cell ratio of 10:1 as previously described [29]. To measure NK-cell mediated antibody-dependent cellular cytotoxicity (ADCC) function, p815 cells (a mouse leukemic cell line) were cocultured with 1 mg/ml p815-specific antibody (Abcam) for 1 h and then washed twice. PBMCs were then stimulated with p815 cells or p815 cells plus p815-specific antibodies at an effector-to-target ratio of 10:1. PBMCs were stimulated for 6 h in the presence of 20 μl/ml CD107a-PECY5 antibody (BD Biosciences), 5 μg/ml brefeldin A (Sigma) and 0.3 μg/ml monensin (Golgi block; BD Biosciences). Medium alone served as the negative control, and PMA/ionomycin (2.5 and 0.5 mg/ml, respectively) served as the positive control. PBMCs were then stained with CD56-PE-CY7, CD16-APC-CY7 (BD Biosciences), and CD3-Pac Blue (BD Biosciences), fixed, permeabilized (Caltag) and finally stained for intracellular interferon using IFN-γ-FITC (BD Biosciences). Multiparameter flow cytometric analysis was performed on an LSRII instrument (BD Biosciences). A response was considered positive if the frequency of CD107a+ or cytokine-secreting cells after stimulation was at least 3-fold greater than that of unstimulated controls.
Statistical analyses
Analyses were performed using GraphPad Prism 5. The non-parametric Kruskal–Wallis test was used to compare phenotype frequencies and functional differences among patient groups when more than two groups were involved. The Wilcoxon rank-sum test was used to compare phenotype frequencies and functional differences between two groups. The p values were two-sided and were adjusted for multiple comparisons using the Holm’s method. The Wilcoxon signed-rank test was used to assess differences in NKp30 and NKp46 expression on NK cells pre and post stimulation with 221 or K562 target cells. Spearman’s rank correlation was used for correlation analysis. p values of < 0.05 were considered significant.
Results
Alteration in NK cell numbers but not in the distribution of NK cell subsets in HCV infection
Human NK cells have been classically defined as CD3−CD56+ lymphocytes which can be further subdivided into CD56bright NK cells that lack the expression of CD16 and KIR and that preferentially secrete cytokines [13], and CD56dim NK cells which express CD16 and KIR and have strong cytotoxic activity [13]. In addition, a third population of anergic NK cells has been described that is defined as CD56−CD16+ and KIR+ (CD56neg) [28, 30]. To assess the impact of HCV replication on the number and subset composition of NK cells, we compared the distribution of the different NK cell subpopulations in the peripheral blood of patients with acute, chronic, or resolved HCV infections with that of HCV-negative individuals (Table 1 and Fig. 1). The absolute percentage of NK cells in the peripheral circulation was significantly elevated in the acute phase of HCV infection compared to HCV-negative individuals (14.1 (6.6–26.1) vs. 7.5 (3.7–13); p = 0.001) (Fig. 1A), yet importantly no significant change in the distribution of NK cell subsets was detectable among HCV-infected groups (Fig. 1B). However, CD56dim NK cells were significantly reduced in acute (62.9 (6.9–89.1); p = 0.02), chronic (64.5 (40.4–73.6); p = 0.0006) and in resolved (66.8 (31.1–85.1); p = 0.01) HCV infections compared to HCV-negative controls (79.6 (72.5– 93.3)), whereas, interestingly, CD56neg NK cells were significantly expanded in all phases of the infection despite a remarkable variability among patients (15.9 (6.6–52.2), 23.8 (10.5–52.2) and 19.8 (8.3–63.9) vs. 9.3 (3.5–14.6); p = 0.008, 0.002 and 0.001, respectively) (Fig. 1B). These data demonstrate that a skewing occurs within the NK cell compartment during HCV infection resulting in a significant expansion of anergic CD56neg NK cells at the expense of a loss of the cytolytic CD56dim NK cells.
Fig. 1. NK cell subset distribution in acute, chronic, and resolved HCV infection.

The dot plots represent the percentages of total NK cells (A) or the proportions of CD56bright (CD3−CD56+ CD16−), CD56dim (CD3−CD56+ CD16+) and CD56neg (CD3−CD56−CD16+) NK cells (B) from 13 patients with acute, 11 patients with chronic and 12 individuals with resolved HCV infection and 14 HCV-negative subjects. (▲, Acute HCV; red N, acute HCV with subsequent progression to chronic infection; blue ▲, acute HCV with subsequent resolution of infection; △, chronic HCV; ▼, HCV resolvers; and ○, HCV-negative controls). Horizontal lines indicate the median percentages. p values < 0.05 after adjustment for multiple comparisons are indicated. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
Increased frequency of NK cells expressing HLA-C binding KIRs in resolvers
In particular, KIR receptors along with their HLA ligands have been implicated in resistance in a number of different viral infection models [31]. Similarly, the KIR/HLA genotype KIR2DL3/HLA-C1 has been associated with HCV clearance [8]. To determine whether particular expansions or contractions of the KIR+ NK cell populations corresponded to differential HCV disease outcome, we analyzed the frequency of HLA-B and HLA-C-binding KIR+ NK cells in the peripheral circulation of patients with acute, resolved and chronic HCV infections. To do so, we used the NKB1 antibody, which recognizes the HLA-B-binding KIR3DL1 receptor, or an equal mixture of the CD158a and CD158b antibodies that bind to the HLA-C binding KIR2DS1, -2DS2, -2DL1, -2DL2, and -2DL3. There was a trend toward a reduced frequency of NK cells expressing HLA-B-binding KIRs among the chronically infected (8.4 (2.4–24.5); p = 0.4) and the HCV resolvers (9.1 (3.4–19.6); p = 0.2) compared to the HCV-negative individuals (14.2 (0.5–24.1)), while normal levels of NKB1+ NK cells were detected in patients with acute HCV infection (Fig. 2A). In contrast, the expression of KIRs recognizing HLA-C molecules was significantly upregulated in individuals who cleared the virus (39.3 (20.7–57.6); p = 0.01) compared to the other groups and particularly HCV-negative controls (27.6 (3.2–39.9)) (Fig. 2B). In accordance with previous reports, these results suggest that elevated frequencies of NK cells expressing HLA-C-binding KIRs, and potentially the inhibitory KIR2DL3, may be associated with HCV clearance.
Fig. 2. Increased proportions of HLA-C binding KIR+ (CD158+) NK cells in resolvers.
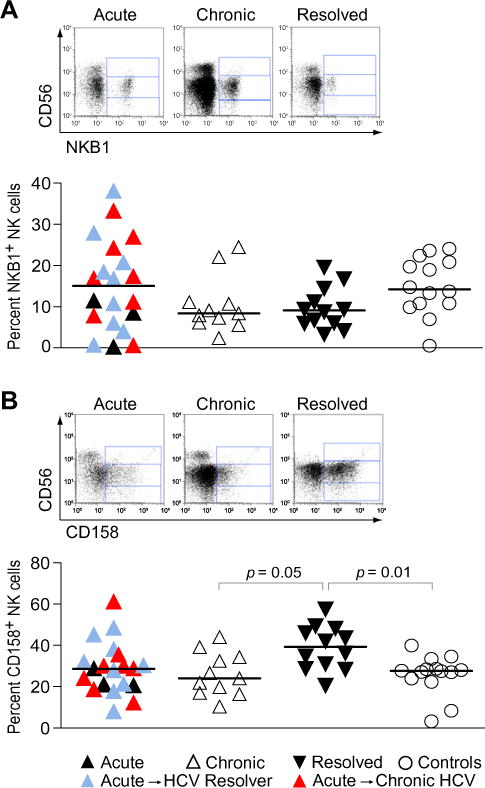
The dot plots show the overall amounts of (A) HLA-B and (B) HLA-C binding KIR+ NK cells using the NKB1 (KIR3DL1) and the CD158a and CD158b (KIR2DL1/2DS1 and KIR2DL2/2DL3/2DS2) antibodies, in patients with acute (▲), chronic (△) and resolved (▼) HCV infection compared to uninfected controls. Red ▲, acute HCV with subsequent progression to chronic infection; blue ▲, acute HCV with subsequent resolution of infection. Horizontal lines indicate the median percentages. p values < 0.05 after adjustment for multiple comparisons are indicated. Representative primary flow panels show percentages of HLA-B (A) and HLA-C (B) binding KIR+ CD56bright, CD56dim, and CD56neg NK cells for an acute, a chronic and a resolved HCV infection. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
Alteration in C-type lectin expression in acute, chronic, and resolved HCV infection
Increased expression of the inhibitory heterodimeric receptor NKG2A/CD94 on NK cells from chronically infected HCV patients has been previously associated with reduced cytolytic activity [20, 23, 25]. To examine changes in the frequency of NKG2A/CD94+ NK cells in individuals at different stages of HCV infection, we compared the expression of these molecules on NK cells from HCV-negative individuals with that on NK cells from subjects with acute, chronic, or resolved HCV infection. As previously described, we observed an increased proportion of NK cells expressing the inhibitory receptors NKG2A and CD94 in patients with chronic infection as compared to resolvers and HCV-negative controls, although this difference was not statistically significant (Fig. 3). In contrast, NK cells expressing the activating receptor NKG2D were significantly decreased in patients with resolved HCV infection (43 (25–93) vs. 81.3 (60.1–100) and 88.7 (80.4–92.9); p = 0.004 and 0.002) and to a lower extent in subjects suffering from a persistent infection (67 (34.8–100) vs. 81.3 (60.1–100) and 88.7 (80.4–92.9); p = 0.3 and 0.2) as compared to the acutely infected and control groups (Fig. 3). Interestingly, similar to persistent infection, acute HCV infection in a subset of individuals was associated with elevated levels of NKG2A+ and decreased proportions of NKG2D+ NK cells. Taken together, these data demonstrate diverging responses by NKG2A and NKG2D, where NKG2D levels decline in chronic and resolved infection while NKG2A-bearing NK cells expand during acute infection and persist through the chronic phase of the disease.
Fig. 3. Changes in C-type lectin expression at different stages of HCV infection.

Each dot plot compares the overall amount of NK cells expressing relevant NKG2 family members including the inhibitory heterodimer NKG2A/CD94 and the activating NKG2D receptors in HCV-negative (○) individuals with that of patients with acute (▲), chronic (△) and resolved (▼) HCV infection. Red ▲, acute HCV with subsequent progression to chronic infection; blue ▲, acute HCV with subsequent resolution of infection. Horizontal lines indicate the median percentages. p values < 0.05 after adjustment for multiple comparisons are indicated. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
Decreased frequencies of NKp30- and NKp46-expressing NK cells in HCV infection
While the cellular ligands for the NCRs remain largely undefined [10–12], these receptors have been implicated in NK cell mediated recognition and the control of several different viral infections, including influenza and vaccinia [10–12]. Chronic HIV-1 infection is associated with significantly reduced NKp30 and NKp46 expression and concomitantly reduced NK cell cytolytic activity [16], whereas chronic HCV infection has been associated with similar reductions in NCRs but no alteration in cytolytic function [23]. However, others have reported conserved NK cell functionality in persistent hepatitis, but in the context of increased numbers of NCR+ NK cells [16]. When compared to that of the control group, flow cytometric analysis of NCRs expression in our cohort revealed a substantial decline in the proportions of NKp30- and NKp46-expressing NK cells in subjects with acute (54.7 (5.9–87.7) vs. 80 (64.6–88.4) and 47.1 (1.4–84) vs. 78.8 (65.3–84); p = 0.03 and 0.004, respectively), chronic (57.2 (22.9– 86.3) vs. 80 (64.6–88.4) and 39.7 (7–68.1) vs. 78.8 (65.3–84); p = 0.005 and 0.002, respectively), and resolved (64.8 (47.6– 92.6) vs. 80 (64.6–88.4) and 42.3 (20.7–80.4) vs. 78.8 (65.3–84); p = 0.04 and 0.003, respectively) HCV infection, while levels of NKp44 expression on NK cells were similar between all groups (Fig. 4). The observed decrease in NKp46- and NKp30-expressing NK cells reveals that the expression of these activating receptors is strongly affected by HCV infection and suggests these surface markers may play a role in the control of the virus.
Fig. 4. HCV infection is associated with a loss of NK cells expressing NKp46 and NKp30.

Each dot plot compares the overall amount of NK cells expressing NKp30, NKp46, and NKp44 in HCV-negative individuals (○) with that of patients with acute (▲), chronic (△), and resolved (▼) HCV infection. Red ▲, acute HCV with subsequent progression to chronic infection; blue ▲, acute HCV with subsequent resolution of infection. Horizontal lines indicate the median percentages. p values < 0.05 after adjustment for multiple comparisons are indicated. Representative primary flow panels show percentages of NKp46+ and NKp30+ CD56bright, CD56dim, and CD56neg NK cells for an acute, a chronic, and a resolved HCV infection. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
Reduced NKp46 and NKp30 expression in acute HCV infection predicts resolution of HCV infection
Longitudinal clinical information was available for a subset of 17 individuals with acute HCV infection, of whom nine resolved the infection and eight developed chronic infection. These data provided a unique opportunity to investigate whether preferential expansion of NK cells expressing particular receptors is associated with the outcome of acute HCV infection. Based on our analyses of a large panel of NK cell receptors, at the time of acute infection the percentages of NK cells expressing NKp30 (80.2 (25.3–87.7) vs. 36.4 (5.9–65.5); p = 0.006), NKp46 (69 (39.5–84) vs. 36.1 (1.4–65.7); p = 0.002), NKG2D (89.5 (68–100) vs. 70.3 (60.1–99); p = 0.04) and CD161 (67.4 (29.6–85.6) vs. 45.3 (31.4– 53.7); p = 0.03) were significantly higher in patients who became chronically infected than in HCV resolvers; potentially reflecting the fact that the early activation of NK cells may be critical for the early control of viral replication (Fig. 5A). Moreover, subjects that went on to resolve infection exhibited significantly lower frequencies of double NKp30+ NKp46+ NK cells (14.7 (1.5–48) vs. 58.6 (24.5–70.4); p = 0.006) (Fig. 5B). Interestingly, the inhibitory receptor CD161 was expressed at lower levels on NK cells from subjects that went on to clear the infection, while significant differences in the proportions of CD161+ NK cells were observable between HCV-negative individuals (77.8 (60.8–81.4)) and subjects with acute (52.5 (29.6–85.6); p = 0.002) and chronic (57 (38.6–70); p = 0.002) but not resolved (62.8 (44.1–83.8); p = 0.2) HCV infection (Fig. 5A and C and data not shown). These data show that early NK cell activation marked by a loss of NCRs, CD161, and NKG2D may predict spontaneous HCV clearance, and strongly implicates early changes in NK cells in control and clearance of HCV infection.
Fig. 5. Resolution of HCV infection can be predicted by decreased proportions of NKp46+ NKp30+ and CD161+ NK cells at the time of acute infection.

The dot plots compare the proportions of NK cells expressing the indicated surface receptors (A) or both NKp30 and NKp46 (B) between study subjects identified during acute HCV infection who subsequently developed a chronic disease (■) and subjects who subsequently resolved the infection (■). Horizontal lines indicate the median percentages. Differences where p < 0.05 are indicated. (C) Representative primary flow panels show percentages of CD161+ CD56bright, CD161+ CD56dim and CD161+ CD56neg NK cells for an HCV acute infection which subsequently became chronic, an HCV acute infection which was subsequently cleared, a chronic, and a resolved HCV infection.
NK cells from individuals with acute HCV infection respond more robustly to K562 cells
Contradictory data has been reported on the changes in NK cell activity in patients with persistent HCV infection [16–27]. Thus, to determine whether NK cell activity becomes compromised during HCV infection, we compared the functional capacity of NK cells at various stages of the disease. We found that during acute HCV infection, NK cells exhibited an increased capacity to degranulate in response to K562 cells (27.3 (12.2–34.5) vs. 12.5 (6.6–39.6); p = 0.06) (Fig. 6A). K562 cells are devoid of MHC-I molecules at their surface but express NKG2D ligands. Accordingly, the potency of the NK cell response to K562 cells correlated with the proportion of NK cells expressing NKG2D at different stages of HCV infection (r2 = 0.5; p = 0.0007) (Fig. 6B). Furthermore, four out of the five subjects that went on to clear HCV infection exhibited reduced responses to K562 cells at the time of acute infection compared to three of the individuals that developed persistent infection (Fig. 6C). All stages of HCV infection and particularly the acute phase were associated with NK cells with a higher potential to degranulate in response to a second MHC-I-negative cell line, 221 cells, but these differences did not reach statistical significance (Fig. 6A). Unexpectedly, we observed an inverse correlation between the percentage of NK cells expressing NKp46 and the response to 221 cells that bear NCR ligands (r2 = 0.2; p = 0.08) (Fig. 6D). Finally, ADCC activity was slightly increased in resolvers compared to the other patient groups, and Fc-mediated NK cell degranulation correlated with the level of NK cells expressing FcγR3A (r2 = 0.4; p = 0.06) (Fig. 6A and E). Regarding IFN-γ production, there were no significant differences in the responses to 221, K562, and p815-coated target cells between HCV-infected groups when considering the bulk NK cell response (data not shown), yet CD56dim NK cells from chronically infected individuals produced less IFN-γ than CD56dim NK cells from resolvers upon exposure to K562 cells (2.7 (0.4–5.5) vs. 5.2 (0.8–9.5); p = 0.06) (Fig. 6F). As HCV infection was associated with an increase in CD56neg NK cells, we examined CD107a upregulation and IFN-γ production by this NK cell subset in response to the various target cells and, as has been previously shown [30], found that this particular population lacks the capacity to produce CD107a or IFN-γ upon stimulation in control and HCV-infected groups (Fig. 6F and G). Overall, these data demonstrate either a preserved or an increased NK cell function during acute HCV infection, suggesting that an early impairment of peripheral NK cell cytotoxic activity does not play a major role in the development of viral persistence. Moreover, NK cells that have lost NKp46 expression present a more potent response to the 221 target cells than NKp46high NK cells, further suggesting that the NKp46low NK cells are activated following HCV infection, possibly resulting from a lower inhibition status due to decreased expression levels of CD161.
Fig. 6. The response to K562 MHC-I-devoid target cells is increased in patients with acute HCV infection.

(A) Dot plots represent the percent of NK cells that produced CD107a following a 6 h incubation with 221 cells, K562 cells and p815 cells covered with p815 antibody at an E:T ratio of 10:1 for each individual divided among. ▲, acute HCV; △, chronic HCV; ▼, HCV resolvers; and ○, HCV negative controls. Horizontal lines indicate the median percentages. Representative primary flow panels show percentages of CD107a+ CD56bright, CD107a+ CD56dim and CD107a+ CD56negNK cells in response to 221, K562, and p815-coated target cells. (B) The functional capacity of NK cells against K562 target cells correlates with the proportion of NK cells expressing NKG2D. (C) The dot plots compare the proportions of CD107a+ NK cells between study subjects identified during acute HCV infection who subsequently developed a chronic disease (■) and subjects who subsequently resolved the infection (■). Horizontal lines indicate the median percentages. (D) Inverse correlation between the intensity of the NK cell response against 221 target cells and the proportion of NK cells expressing NKp46. (E)The ability of NK cells to perform ADCC positively correlates with the proportion of NK cells expressing theFcγRCD16. (F) and (G) The dot plots compare the percentages of CD56bright, CD56dim, and CD56neg NK cells that produced IFN-γ (F) or CD107a (G) following a 6 h incubation with 221, K562, and p815 cells covered with p815 antibody at an E:T ratio of 10:1 for each individual divided among. ▲, Acute HCV; △, chronic HCV; ▼, HCV resolvers; and ○, HCV negative controls. Horizontal lines indicate the median percentages. Representative primary flow panels show percentages of IFN-γ + CD56bright, IFN-γ + CD56dim, and IFN-γ + CD56neg NK cells in response to 221, K562 and p815-coated target cells.
Stimulation results in NCR downregulation
Following activation, many activating NK cell receptors are downregulated from the cell surface to prevent activation induced cell death [32]. While in some instances this downregulation may be due to direct engagement of the receptor, some receptors may also be lost in a non-specific manner. To determine whether NCR downregulation in HCV pertains directly to NCR stimulation, we stimulated NK cells from healthy donors with MHC-I-negative target cells bearing NCR-ligands (221 cells) or not (K562 cells). The percentages of both NKp46+ (85.4 (84.7– 92) vs. 94.3 (90–96.9); p = 0.02) and NKp30+ (89.6 (62.4–91.7) vs. 91.8 (70.6–94.3); p = 0.02) NK cells declined significantly following stimulation with 221 cells (Fig. 7, panels A and C). Interestingly, NCRs declined following stimulation with K562 cells as well (90.8 (79.2–95) vs. 94.3 (90–96.9) and 87.3 (53.4–90.6) vs. 91.8 (70.6–94.3); p = 0.02 and 0.02 for NKp46 and NKp30, respectively). Of note, the mean fluorescence intensity of NKp46 and NKp30 also significantly decreased on NK cells in response to 221 and K562 target cells, suggesting that stimulation results in the downregulation of the NCRs rather than in a contraction of the NKp30+ and NKp46+ NK cell populations (Fig. 7, panels B and D) Thus NCR downregulation occurs both following direct and indirect activation. This suggests that the loss of NCRs does not directly implicate these receptors in the early response to HCV, but rather indicates that early activation of NK cells, as measured by a loss of NCRs, is critical for the early containment and eventual clearance of the infection.
Fig. 7. NCRs downregulation occurs following both direct and indirect-NCR activation of NK cells.

The whisker box plots depict changes in both NKp46 (A and B) and NKp30 (C and D) expression on NK cells following direct stimulation with the NCR-ligand expressing cell line 221, or the non-NCR-ligand expressing cell line K562. (A and C) The percentages of NKp46+ and NKp30+ NK cells; (B and D) The mean fluorescence intensity of each NCR on NK cells differences where p < 0.05 is indicated.
Discussion
In this study, we aimed to define whether early alterations in the phenotype or function of peripheral NK cells are associated with containment of HCV infection and disease outcome. To do so, we characterized NK cells from individuals during acute, chronic, and resolved HCV infection and found that reduced proportions of NK cells expressing the two activating receptors NKp46 and NKp30 and/or the inhibitory CD161 and/or the activating NKG2D receptor in the acute phase of HCV infection correlated with HCV clearance (Fig. 5). Thus peripheral NK cells in subjects controlling acute infection express lower levels of NKp30/NKp46, CD161, and NKG2D, revealing the expansion or accumulation of a phenotypically unique population of NK cells that may be involved in the early recognition and control of HCV infection. In addition, HLA-C-binding KIR receptors may also play a role in control of HCV infection, as NK cells bearing these KIR receptors appear to preferentially expand in individuals that have resolved the infection. Finally, we show for the first time that phenotypic alterations of NK cells, including elevated proportions of NKG2A+ and CD94+ NK cells and decreased numbers of NKG2D-expressing NK cells are already observable at early stages of the infection and are maintained throughout chronic HCV infection.
We first investigated changes in NK cells numbers and subset distributions and showed that acute HCV infection is associated with an expansion of NK cells, a phenomenon that has been previously described in the context of other acute viral infections [33, 34]. To define whether specific populations of NK cells expanded preferentially in acute HCV infection, we dissected changes in the NK cell compartment at different stages of HCV infection. Importantly, we found that the distribution of NK cells subsets in resolvers was comparable to that seen in patients with acute or chronic infection, suggesting that the percentages of CD56bright, CD56dim, and CD56neg in the bulk NK cell population might not influence the outcome of the infection. However, upon comparison with HCV-negative subjects, we detected a decrease in the proportion of CD56dim NK cells at all stages of the disease. As the phenotype of hepatic NK cells could not be investigated, we cannot rule out that this observation reflects an accumulation of CD56dim NK cells in the liver rather than a contraction of this subset. Furthermore, in accordance with recently published data showing an increase in the proportion of CD56neg NK cells in some patients with chronic HCV infection [35], we report a consistent expansion of CD56neg NK cells in all groups of HCV-infected subjects, with a relative heterogeneity in the level of increase in this particular subset among studied individuals. As previously described in the context of HIV, this subset also exhibited signs of functional exhaustion in HCV infection [28, 30]. Finally, it is not excluded that infections with other viruses, including cytomegalovirus, GB virus type C and parvovirus 4, may contribute to the observed differences in the percentages of NK cell subsets between resolvers and healthy individuals, as susceptibility to these pathogens is increased in patients with a resolved HCV infection. These results suggest that acute HCV infection is associated with a rapid expansion of bulk NK cells but does not lead to major changes in the distribution of NK cell subsets throughout the course of the disease. However, there is a rapid and sustained expansion of CD56neg NK cells and a decrease in CD56dim NK cells in HCV-infected subjects compared to healthy individuals that may reflect a contraction in these cytolytic effector cells or the potential recruitment of these cells to the liver.
Using large flow cytometric panels to analyze changes in the expression of NK cell receptors, we found that an increased frequency of HLA-C-binding KIR+ NK cells, but not HLA-B-binding KIR+ NK cells, was associated with resolved HCV infection. Previous epidemiologic studies showed that individuals homozygous for KIR2DL3 as well as for its ligand HLA-C group 1 alleles were more likely to clear HCV infection [8]. However, the underlying mechanism for this clearance is not understood. It is plausible that the expansion of HLA-C-binding KIRs may be restricted to the KIR2DL3+ NK cells, which may be intimately involved in the control of viral replication early in infection. Further investigations are warranted to elucidate the specific clonal populations of KIR+ NK cells that may be expanding during acute HCV infection, and to determine whether it is through the rapid deployment of KIR2DL3+ NK cells in early HCV infection that individuals with the protective KIR/HLA genotype are able to control and clear the infection.
While further investigating changes in NK cell phenotype in HCV infected individuals, we detected a decrease in the frequency of NK cells bearing the activating receptors NKp30 and NKp46. In parallel, we observed an increase in the frequency of NKG2A+ and CD94+ NK cells in individuals that are persistently infected by HCV, in line with previous reports [20, 23, 25]. Importantly, we found that these phenotypic alterations were observable as early as the acute phase of the infection. Expression of NKG2D in acutely infected individuals showed great variability, with decreased NKG2D+ NK cell numbers in some but not all subjects, whereas a significant loss of receptor expression was observed in resolved HCV infection. These phenotypic differences were not influenced by discrepancies in gender and age between HCV infected patients and healthy individuals as these parameters do not significantly affect the expression of NK cell surface markers that are relevant for HCV infection (data not shown). Altogether, these observations reveal that the expression of NK cell receptors is altered early in HCV infection, probably resulting in the accumulation of a predominantly inhibited NK cell population that may contribute to the subsequent establishment of persistent disease in subjects that go on to become chronically infected.
As previously reported, we did not observe any change in NK cell function during HCV infection [26]. Unexpectedly, the reported phenotypic alterations did not significantly affect NK cell cytotoxic activity or ADCC functions, as has been demonstrated by others [16], suggesting that these changes may impair the manner in which NK cells see HCV-infected cells directly rather than their overall functionality in response to generic target cells. Of note, NK cells from acute infected patients showed an increased potential to degranulate in the presence of K562 target cells, positively correlating with the proportions of the cells expressing NKG2D (Fig. 6). However, NK cells from acutely infected individuals who subsequently cleared the virus showed a weaker response to K562 target cells than NK cells from individuals who developed a chronic disease, in line with decreased proportions of NKG2D+ NK cells in resolvers. These differences in responsiveness to K562 cells may reflect a downregulation of NKG2D as a consequence of NK cell activation through this receptor and thus, a subsequent inability of NK cells to respond to these target cells upon ex vivo stimulation. However, further experiments will be required to confirm this hypothesis. In contrast, we observed a negative correlation between the response against 221 target cells and the expression of NKp46+ NK cells, suggesting that an early loss of these receptors, potentially due to the direct involvement of these molecules in early control of the infection, results in diminished responsiveness to target cells encoding their ligands, as expected.
In order to identify NK cell-related immunological correlates of viral clearance, we compared the phenotypic profiles of NK cells at the time of acute infection between patients who developed chronic disease and those who went on to clear the virus. Interestingly, lower frequencies of NKp46- and NKp30-expressing NK cells were associated with HCV clearance. In contrast, previous work has shown elevated levels of NKp30 and NKp46 on NKT cells in acute HCV, particularly in the setting of spontaneous resolution of the infection [25], suggesting that regulation of NCRs is governed by different mechanisms in NK and NKT cells. Furthermore, high levels of NKp30 expression were recently associated with protection against HCV infection in exposed uninfected individuals [36], potentially reflecting NK cells with a higher capacity to respond through this NCR upon HCV exposure. While we found that high percentages of NKp30+ NK cells in acute infection were associated with a reduced likelihood to clear infection, this discrepancy in NKp30-associated protection may potentially reflect incomplete NK cell activation in individuals which do not control and clear infection. In the context of NK cells, it is plausible that, similar to other activating receptors, NKp46 and NKp30 may be internalized following both direct and indirect stimulation [32]. Activation of NK cells with NCR-ligand expressing or non-expressing target cells demonstrated that NCRs are downregulated following both direct and indirect activation, indicating that the loss of these receptors in early HCV infection does not directly implicate NCRs in the response to viral replication. Thus, the diminished percentage of NK cells expressing activating NCRs in patients who subsequently resolve the infection may rather reflect that early NK cell activation is critical and results in the onset of an effective innate immune response that participates in viral clearance.
Reduced proportions of NK cells expressing the activating receptor NKG2D were also observed in acutely infected individuals who became HCV resolvers. It is not excluded that NK cells bearing NKp46 and NKp30 and/or NKG2D may be efficiently recruited to the liver in order to eliminate infected cells. Thus, in individuals who fail to clear infection, the presence of NKp46, NKp30, or NKG2D positive NK cells in the peripheral blood would reveal a failure to activate and/or recruit these effector cells and to provide efficient antiviral pressure at the site of infection, enhancing the probability to establish chronic disease. However, little is known about the particular NK cell populations found in the liver during HCV infection, and further work on NK cells in the liver may provide critical insights into NK subpopulations and functions that may be decisive in both clearance and disease-associated immunopathogenesis. Finally, lower proportions of CD161+ NK cells were also a hallmark of the capacity to clear HCV infection. CD161 inhibits NK cell activation upon binding with its ligand, LLT1, which is expressed on activated dendritic cells and B cells [37]. Although it is unknown how CD161 may be involved in the specific recognition of HCV-infected cells, CD161 levels did not decline following stimulation in vitro with generic target cells (data not shown). However the loss of CD161, either due to the recruitment of CD161+ NK cells to the liver or to its potential interaction with its ligand early in infection, may result in the accumulation of a population of NK cells with a lower activation threshold that may be more easily triggered, suggesting that an aggressive early innate NK cell response may be responsible for the containment of HCV replication and the induction of a qualitatively superior adaptive immune response that is then able to effectively clear the infection. It was recently demonstrated that NK cell degranulation during acute infection correlates positively with the magnitude of HCV-specific adaptive T cell responses [38]. Thus further studies exploring potential correlations between the NK cell functional responses or the levels of NKp30, NKp46, NKG2D, and CD161 expression on NK cells in acute infection and the magnitude of HCV-specific CD8 T cell responses are warranted.
Overall, chronic HCV infection is associated with changes in NK cell receptor expression that occur very early in the infection and result in the accumulation of predominantly inhibited NCR−CD94+ NKG2A+ NKG2D− NK cells in the peripheral circulation. In contrast, resolved infection was associated with the accumulation of a population of NK cells expressing HLA-C-binding KIR, which may be potentially critical for clearing HCV infection. Furthermore, NK cells from both chronic and resolved infection exhibited normal functional profiles, suggesting that an altered capacity to respond to target cells rather than direct NK cell dysfunction may contribute to an inability to control and clear the infection. Finally, most interestingly, decreased frequencies of NKp30+ NKp46+, CD161+, and NKG2D+ NK cells during early infection were strongly associated with enhanced control and eventual clearance of infection, suggesting that these unique populations of NK cells may play a central role in the control of the infection and that the phenotypic profile of NK cells in the earliest phase of HCV infection can predict disease outcome. Further investigation into the specific expansions and contractions of NK cells in the liver during acute infection may further provide insights into the specific populations of NK cells that may be involved in early control. Defining the role of NK cells in early control of HCV may offer novel opportunities to manipulate these potential therapeutic targets in order to improve the immune response to HCV and increase the rates of HCV clearance.
Acknowledgments
We would like to thank Dr. Raymond Chung, Dr. Arthur Kim and Dr. Lia Lewis-Ximenez for their help with obtaining patient samples.
Abbreviations
- HCV
hepatitis C virus
- NK
natural killer
- IFN-γ
interferon-gamma
- KIR
killer cell immunoglobulin-like receptor
- HLA
human leukocyte antigen
- MHC
major histocompatibility complex
- NCR
natural cytotoxicity receptor
- DC
dendritic cell
- RT-qPCR
quantitative reverse transcription polymerase chain reaction
- PBMC
peripheral blood mononuclear cell
- ADCC
antibody-dependent cellular cytotoxicity
Footnotes
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- 1.WHO. Hepatitis C fact sheet. [Google Scholar]
- 2.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 3.Day CL, Lauer GM, Robbins GK, et al. Broad specificity of virus-specific CD4+ T-helper-cell responses in resolved hepatitis C virus infection. J Virol. 2002;76:12584–12595. doi: 10.1128/JVI.76.24.12584-12595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauer GM, Ouchi K, Chung RT, et al. Comprehensive analysis of CD8(+)-T-cell responses against hepatitis C virus reveals multiple unpredicted specificities. J Virol. 2002;76:6104–6113. doi: 10.1128/JVI.76.12.6104-6113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grakoui A, Shoukry NH, Woollard DJ, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 6.Ulsenheimer A, Gerlach JT, Gruener NH, et al. Detection of functionally altered hepatitis C virus-specific CD4 T cells in acute and chronic hepatitis C. Hepatology. 2003;37:1189–1198. doi: 10.1053/jhep.2003.50194. [DOI] [PubMed] [Google Scholar]
- 7.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 8.Khakoo SI, Thio CL, Martin MP, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 9.Lanier LL. NKG2D in innate and adaptive immunity. Adv Exp Med Biol. 2005;560:51–56. doi: 10.1007/0-387-24180-9_7. [DOI] [PubMed] [Google Scholar]
- 10.Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31:2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::aid-immu2680>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Gazit R, Gruda R, Elboim M, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 12.Mandelboim O, Lieberman N, Lev M, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 13.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 14.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Doherty DG, Norris S, Madrigal-Estebas L, et al. The human liver contains multiple populations of NK cells, T cells, and CD3+ CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–2321. [PubMed] [Google Scholar]
- 16.De Maria A, Fogli M, Mazza S, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–455. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 17.Kawarabayashi N, Seki S, Hatsuse K, et al. Decrease of CD56(+)T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32:962–969. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–49. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crotta S, Stilla A, Wack A, et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195:35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jinushi M, Takehara T, Tatsumi T, et al. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173:6072–6081. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 21.Bonavita MS, Franco A, Paroli M, et al. Normalization of depressed natural killer activity after interferon-alpha therapy is associated with a low frequency of relapse in patients with chronic hepatitis C. Int J Tissue React. 1993;15:11–16. [PubMed] [Google Scholar]
- 22.Corado J, Toro F, Rivera H, Bianco NE, Deibis L, De Sanctis JB. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin Exp Immunol. 1997;109:451–457. doi: 10.1046/j.1365-2249.1997.4581355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–877. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morishima C, Paschal DM, Wang CC, et al. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43:573–580. doi: 10.1002/hep.21073. [DOI] [PubMed] [Google Scholar]
- 25.Golden-Mason L, Kelly AM, Doherty DG, et al. Hepatic interleukin 15 (IL-15) expression: implications for local NK/NKT cell homeostasis and development. Clin Exp Immunol. 2004;138:94–101. doi: 10.1111/j.1365-2249.2004.02586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duesberg U, Schneiders AM, Flieger D, Inchauspe G, Sauerbruch T, Spengler U. Natural cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) is not impaired in patients suffering from chronic hepatitis C. J Hepatol. 2001;35:650–657. doi: 10.1016/s0168-8278(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 27.Yoon JC, Shiina M, Ahlenstiel G, Rehermann B. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009;49:12–21. doi: 10.1002/hep.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–3369. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 29.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Mavilio D, Lombardo G, Benjamin J, et al. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci USA. 2005;102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bashirova AA, Martin MP, McVicar DW, Carrington M. The killer immunoglobulin-like receptor gene cluster: tuning the genome for defense. Annu Rev Genomics Hum Genet. 2006;7:277–300. doi: 10.1146/annurev.genom.7.080505.115726. [DOI] [PubMed] [Google Scholar]
- 32.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alter G, Rihn S, Walter K, et al. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J Virol. 2009;83:6798–6805. doi: 10.1128/JVI.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez VD, Falconer K, Michaelsson J, et al. Expansion of CD56-NK cells in chronic HCV/HIV-1 co-infection: reversion by antiviral treatment with pegylated IFNalpha and ribavirin. Clin Immunol. 2008;128:46–56. doi: 10.1016/j.clim.2008.03.521. [DOI] [PubMed] [Google Scholar]
- 36.Golden-Mason L, Cox AL, Randall JA, Cheng L, Rosen HR. Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in vitro. Hepatology. 2010;52:1581–1589. doi: 10.1002/hep.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen DB, Cao W, Avery DT, et al. Functional consequences of interactions between human NKR-P1A and its ligand LLT1 expressed on activated dendritic cells and B cells. J Immunol. 2008;180:6508–6517. doi: 10.4049/jimmunol.180.10.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelletier S, Drouin C, Bedard N, Khakoo SI, Bruneau J, Shoukry NH. Increased degranulation of natural killer cells during acute HCV correlates with the magnitude of virus-specific T cell responses. J Hepatol. 2010;53:805–816. doi: 10.1016/j.jhep.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


