Abstract
Structure activity relationships for the A-region in a series of N-4-t-butylbenzyl 2-(4-methylsulfonylaminophenyl) propanamides as TRPV1 antagonists have been investigated. Among them, the 3-fluoro analogue 54 showed high binding affinity and potent antagonism for both rTRPV1 and hTRPV1 in CHO cells. Its stereospecific activity was demonstrated with marked selectivity for the (S)-configuration (54S versus 54R). A docking study of 54S with our hTRPV1 homology model highlighted crucial hydrogen bonds between the ligand and the receptor contributing to its potency.
Keywords: TRPV1 antagonists, analgesic, molecular modeling, capsaicin, resiniferatoxin
1. Introduction
The transient receptor potential V1 (TRPV1) receptor1 is a molecular integrator of nociceptive stimuli, including protons,2 heat,3 inflammatory mediators such as anandamide4 and lipoxygenase products,5 and vanilloids such as capsaicin (CAP)6 and resiniferatoxin (RTX)7. The receptor functions as a non-selective cation channel with high Ca2+ permeability and its activation leads to an increase in intracellular Ca2+ that results in excitation of primary sensory neurons and ultimately the central perception of pain.
TRPV1 antagonists are promising drug candidates. Therapeutic applications include inhibiting the transmission of nociceptive signaling from the periphery to the CNS as well as blocking other pathological states associated with this receptor. TRPV1 antagonists have thus emerged as novel and promising analgesic and antiinflammatory agents, particularly for chronic pain and inflammatory hyperalgesia.8 The number of antagonists reported continues to increase and their clincal development has been extensively reviewed.9–13
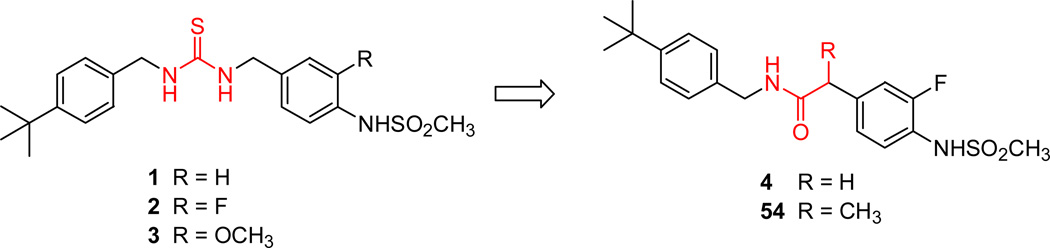
Previously, we have reported that a series of N-4-(methylsulfonylaminobenzyl) thiourea analogues were effective antagonists of the action of capsaicin on rat TRPV1 (Figure 1).14–17 A prototype antagonist (1) showed high binding affinity and potent antagonism (Ki = 63 nM and Ki(ant) = 54 nM in rTRPV1/CHO).14 We further found that 3-substitutents of the 4-(methylsulfonylamino)phenyl group in the A-region affected the extent of agonism/antagonism. Thus, the 3-fluoro derivative 2 (Ki = 53.5 nM, Ki(ant) = 9.2 nM for rTRPV1/CHO) was a potent antagonist not only of capsaicin stimulation of rTRPV1 but also of stimulation by temperature and pH.14,16 Conversely, the 3-methoxy derivative 3 showed a shift to partial agonism (Ki = 51 nM, 17% agonism and 84% antagonism for rTRPV1/CHO) while the binding affinity remained unaffected.15
Figure 1.
In order to further optimize the antagonistic activities of N-4-(methylsulfonylaminobenzyl) thioureas and avoid the potential toxicity associated with the thiourea functionality, we explored the amide B-region surrogates of the above parent thiourea antagonists in a series of simplified RTX derivatives and concluded that the propanamide B-region surrogates showed stereospecific high binding affinities and potent antagonism.18
As a continuation of our effort to optimize the 4-methylsulfonamide TRPV1 antagonists, we have investigated a series of 2-(4-methylsulfonylaminophenyl) propanamide analogues as TRPV1 antagonists. In this paper, we report the structure activity relationships of the A-region in a series of N-4-t-butylbenzyl 2-(4-methylsulfonylaminophenyl) propanamide TRPV1 antagonists. Further biological characterization and molecular modeling of a key antagonist from this series will also be described.
2. Chemistry
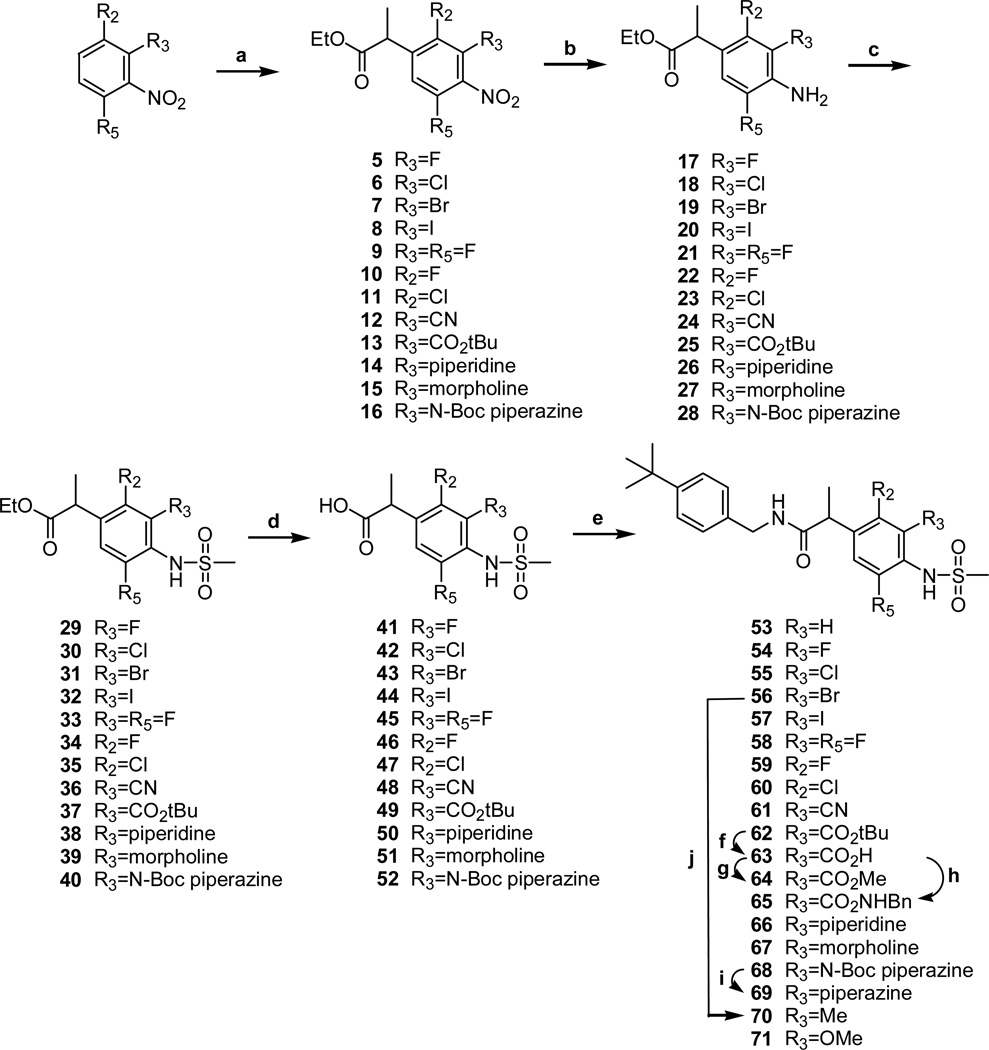
The syntheses of substituted N-(4-tert-butylbenzyl)-2-[4-(methylsulfonylamino) phenyl] propionamide derivatives are represented in Scheme 1.
Figure 3.
Reagents and Conditions: (a) t-BuOK, ethyl 2-chloropropionate, DMF; (b) Method A: H2, Pd-C, MeOH, Method B: Fe, AcOH; (c) MsCl, pyridine; (d) LiOH, H2O-THF; (e) 4-t-butylbenzylamine, EDC, CH2Cl2; (f) CF3CO2H, CH2Cl2(2:1); (g) TMS-diazomethane, MeOH; (h) BnNH2, EDC, CH2Cl2; (i) CF3CO2H, CH2Cl2(1:2); (j) Sn(CH3)4, (PPh3)4Pd, toluene
Ethyl 2-(4-nitrophenyl)propionates (5–16) were prepared from the corresponding 2-halonitrobenzenes by the reaction of Makosza’s vicarious nucleophilic substitution.19 The 4-nitro groups of 5–16 were reduced to amines 17–28 and then mesylated to give the corresponding 4-methylsulfonylamino compounds 29–40, respectively. The esters of 29–40 were hydrolyzed to afford the acids 41–52 and then coupled with 4-t-butylbenzylamine to provide the final propionamides.
The t-butyl ester 62 was hydrolyzed to acid 63 under acidic conditions and was converted to methyl ester 64 and benzyl amide 65, respectively. The 3-piperazinyl 69 was obtained from 68 by acidic hydrolysis. 3-Methyl 70 was synthesized from 3-bromo 56 using tetramethyltin with palladium catalyst. The compounds 53 and 71 were prepared from the corresponding acids previously reported18 by the coupling reaction.
The two enantiomers of 54, 54S and 54R, were synthesized from the corresponding optically pure acids, prepared by the resolution method using L-phenylalanol,18 by the above coupling method.
3. Result and Discussion
3.1. Biological Activity
The binding affinities and potencies as agonists/antagonists of the synthesized TRPV1 ligands were assessed in vitro by a binding competition assay with [3H]RTX and by a functional 45Ca2+ uptake assay using rat and human TRPV1 heterologously expressed in Chinese hamster ovary (CHO) cells, as previously described.16 The results are summarized in Tables 1 and 2, together with the potencies of the previously reported thiourea antagonists 1–3.14–16
Table 1.
rTRPV1 activities of N-(4-tert-butylbenzyl)-2-[4-(methylsulfonylamino)phenyl] propionamide ligands
 | ||||||
|---|---|---|---|---|---|---|
| R3 | R2 | R5 | Ki (nM) Binding Affinityc |
EC50 (nM) Agonismc |
Ki (nM) Antagonismc |
|
| 1 | 63.0 | NE | 54 | |||
| 2 | 53.5 | NE | 9.2 | |||
| 3 | 51.0 | (17%)a | (84%)b | |||
| 4 | 470 (±130) | NE | 118 (±35) | |||
| 53 | H | H | H | 106 (±34) | NE | 17.5 (±1.6) |
| 54 | F | H | H | 46.2 (±3.0) | NE | 7.6 (±1.6) |
| 55 | Cl | H | H | 30.7 (±7.4) | NE | 29.5 (±8.5) |
| 56 | Br | H | H | 7.4 (±1.5) | NE | 25.0 (±7.4) |
| 57 | I | H | H | 23.3 (±7.1) | NE | 30.0 (±2.2) |
| 58 | F | H | F | 19.9 (±6.1) | NE | 7.4 (±2.1) |
| 59 | H | F | H | 360 (±110) | NE | 120 (±27) |
| 60 | H | Cl | H | 1420 (±280) | NE | 4500 (±1000) |
| 61 | CN | H | H | 344 (±99) | NE | 467 (±60) |
| 62 | CO2tBu | H | H | 6700 (±1200) | NE | (16%)b |
| 63 | CO2H | H | H | NE | NE | NE |
| 64 | CO2CH3 | H | H | 1606 (±53) | NE | 952 (±120) |
| 65 | CONHBn | H | H | 3710 (±470) | NE | (23%)b |
| 66 | H | H | WE | NE | (37%)b | |
| 67 | H | H | WE | NE | (34%)b | |
| 68 | H | H | 7000 (±1600) | NE | (13%)b | |
| 69 | H | H | NE | NE | NE | |
| 70 | CH3 | H | H | 32.8 (±6.1) | NE | 18.9 (±8.3) |
| 71 | OCH3 | H | H | 540 (±130) | NE | 232 (±71) |
only fractional calcium uptake compared with that induced by 300 nM capsaicin
only fractional antagonism
Values represent mean ± SEM from 3 or more experiments
NE, no effect. WE, weak effect (quantitation of fractional agonism/antagonism is from 1 – 3 experiments).
Table 2.
TRPV1 activities of the two chiral isomers of 54
 | |||||
|---|---|---|---|---|---|
| rTRPV1 | hTRPV1 | ||||
| Ki (nM) Binding Affinity |
Ki (nM) [CAP] Antagonism |
Ki (nM) Binding Affinity |
Ki (nM) (CAP) Antagonism |
Ki (nM) (pH) Antagonism |
|
| 54 | 46.2 (±3.0) | 7.6 (±1.6) | 89 (±17) | 5.1 (±1.2) | 12.7 (±3.6) |
| 54R | 750 (±200) | 186 (±29) | 2590 (±390) | 217 (±57) | 440 (±41) |
| 54S | 24.4 (±0.82) | 4.16 (±0.67) | 15.8 (±4.4) | 0.49 (±0.13) | 8.3 (±1.6) |
We started by preparing and characterizing two amide B-region surrogates of the parent thiourea 2. Whereas acetamide 4 showed a reduction in binding affinity and antagonism by an order of magnitude, the propanamide (α-methyl acetamide) 54 exhibited higher affinity (Ki = 46.2 nM) and more potent antagonism (Ki(ant) = 7.6 nM) compared to 2. This result prompted us to investigate in detail the structure activity relationships of the A-region 4-methylsulfonylaminophenyl group in the series while the B- and C-regions were fixed as the propanamide and the 4-t-butylbenzyl group, respectively.
The unsubstituted analogue 53 showed a two-fold reduction in the binding affinity and antagonism compared to 54, indicating that the fluoro atom is crucial for activity. The replacement of fluoro in 54 with bulkier halogens, such as chloro (55), bromo (56) and iodo (57), led to an increase in binding affinity, but with a 4-fold reduction in their potencies as antagonists. The 3,5-difluoro substituted analogue 58 exhibited a 2-fold enhancement in binding affinity and similar antagonism compared to 54. On the other hand, the 2-halogen analogues, 2-fluoro (59) and 2-chloro (60), exhibited much reduced activities compared to the corresponding 3-halogen analogues 54 and 55, probably due to a weaker electron-withdrawing effect toward the 4-sulfonamide in 59 and steric hinderance with the α–methyl in 60.
As electron-withdrawing and hydrophilic groups, the cyano and carboxylate groups were introduced to the 3-position to provide 61–65. Unfortunately, all of these compounds were found to be very weak or inactive antagonists.
As electron-donating groups, cyclic amines, methyl and methoxy groups were introduced to the 3-position. Whereas the 3-piperidinyl (66), morpholinyl (67), N-Boc piperazinyl (68) and piperazinyl analogues (69) showed marked loss of activity, the 3-methyl analogue (70) retained high potency in binding affinity and antagonism comparable with 54. The 3-methoxy analogue (71) was intermediate, showing 10 to 30-fold reduced activity in binding affinity and antagonism.
The analysis of SAR indicated that electron withdrawing and lipophilic substituents at the 3-position appeared to be favorable for high binding and potent antagonism and the 3-fluoro substitution (54) was validated as the best for receptor antagonism.
For further exploration of the activity revealed by the racemate 54, we prepared the two optically pure enantiomers of 54 and assessed their receptor binding and functional activities in rat and human (Table 2). Previously, our SAR investigation of the α-alkyl amide 6 B-region in a series of simplified RTX analogues had indicated that the stereocenter at the α-position was crucial for determining the potencies, binding affinities, and antagonism, with the S-configuration of the α-alkyl demonstrating high receptor potency.18 As anticipated, the receptor potencies revealed that the interaction of 54 was stereospecific. The (S)-isomer (54S) proved to be the active isomer with a Ki(binding) = 24.4 nM and a Ki(ant) = 4.16 nM for rTRPV1, values which were ca. two-fold more potent than the respective values for 54. The activity of 54R, in contrast, was 30- to 40-fold weaker.
The high potency of 54 was confirmed for human TRPV1. In hTRPV1, 54 showed similar potencies in binding affinity and antagonism compared to those in rTRPV1 with Ki = 89 nM, Ki(CAP) = 5.1 nM and Ki(pH) = 12.7 nM. The stereospecific activity of the active isomer (54S) in binding affinity and functional antagonism was likewise observed with the hTRPV1, showing Ki = 15.8 nM, Ki(CAP) = 0.49 nM and Ki(pH) = 8.3 nM while 54R again showed low potencies.
The further in vivo evaluation of 54 and 54S will be presented elsewhere.
3.2. Molecular Modeling
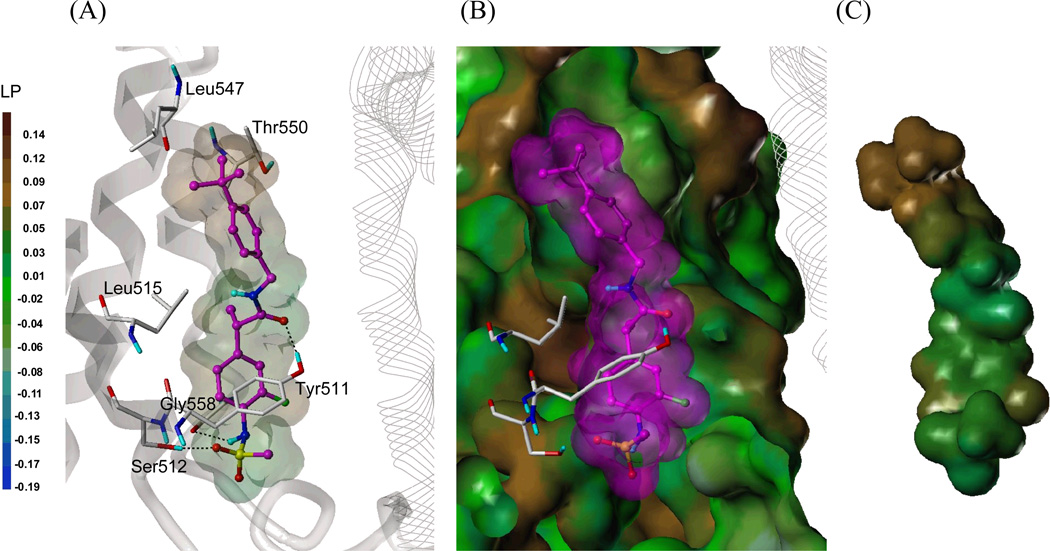
We have built the tetramer homology model of rat TRPV1 (rTRPV1) and performed the docking studies of the representative TRPV1 ligands.20 The rat and human TRPV1 have more than 85% sequence identity, and only five residues in the ligand binding site are different. Using the rTRPV1 model, the human TRPV1 (hTRPV1) model was constructed and refined by energy minimization.
As shown in Figure 2, the binding site of hTRPV1 has a deep bottom hole, surrounded by Tyr511 and Ser512, and an upper hydrophobic region with Leu547. The docking study showed that the sulfonylaminobenzyl group (A-region) of 54S occupied the deep bottom hole. An oxygen atom of the sulfonamide group appeared to form a hydrogen bond with Ser512 and its –NH could make a hydrogen bond with Gly558. In addition, the carbonyl group in the B-region participated in hydrogen bonding with Tyr511. Moreover, the highly hydrophobic 4-t-butylbenzyl group (C-region) extended nicely toward the upper hydrophobic region of the binding site and made a hydrophobic interaction with Leu547.
Figure 2. Predicted binding mode of 54S in hTRPV1 homology model with surface representations.
(A) Docked mode of 54S. The key interacting residues are marked and displayed as a capped-stick representation with carbon atoms in white. The helices are colored in gray and the helices of the neighboring monomer are displayed in a line ribbon representation. The ligand is depicted as a ball-and-stick with carbon atoms in magenta and its van der Waals surface is presented with lipophilic potential property (LP) which ranges from brown (highest lipophilic area) to blue (highest hydrophilic area). Hydrogen bonds are drawn in black dashed lines, and non-polar hydrogens are undisplayed for clarity. (B) Surface representations of the docked 54S and hTRPV1. The Fast Connolly surface of hTRPV1 was generated by MOLCAD and colored by the lipophilic potential. For clarity, the surface of hTRPV1 is Z-clipped and that of the ligand is in its carbon color. (C) Van der Waals surface of 54S colored by the lipophilic potential.
It appeared that the S-stereochemistry of α-methyl in the B-region contributes to proper positioning of the B- and C-regions toward the upper region in the binding site. Consequently, 54S fully occupied the binding site with these important interactions and it could explain the high potency of 54S. This result is consistent with our previous structure-activity relationship (SAR) studies of the B-region in a series of simplified RTX analogues in which the (S)-propanamide proved to be the active isomer.18
4. Conclusion
The structure activity relationships of the A-region in a series of N-4-t-butylbenzyl 2-(4-methylsulfonylaminophenyl) propanamides as TRPV1 antagonists have been investigated. The analysis indicates that the 3-fluoro analogue 54 is validated as the best with high binding affinity and potent antagonism for both rTRPV1 and hTRPV1 in CHO cells. The two chiral isomers of 54 were synthesized and its (S)-configuration (54S) was found to be the active one, showing approximately a 2-fold enhancement in receptor activity compared to 54, whereas the (R)-configuration showed marked loss of activity.
A docking study of 54S with our hTRPV1 homology model indicates that the hydrophobic 4-t-butylbenzyl group (C-region) extended nicely toward the upper hydrophobic region of the binding site, making hydrophobic interactions with Leu547, while the 2-(3-fluoro-4-methylsulfonylaminophenyl) propanamide (A/B-regions) occupied the deep bottom hole, forming critical hydrogen bonds with Tyr511, Ser512 and Gly558 for high potency.
5. Experimental
5.1. Chemistry
5.1.1. General
All chemical reagents were commercially available. Melting points were determined on a melting point Buchi B-540 apparatus and are uncorrected. Silica gel column chromatography was performed on silica gel 60, 230–400 mesh, Merck. Proton NMR spectra were recorded on a JEOL JNM-LA 300 at 300 MHz and Bruker Analytik, DE/AVANCE Digital 400 at 400 MHz. Chemical shifts are reported in ppm units with Me4Si as a reference standard. Mass spectra were recorded on a VG Trio-2 GC-MS. Combustion analyses were performed on an EA 1110 Automatic Elemental Analyzer, CE Instruments.
5.1.2. General Procedure for Alkylation
To a stirred solution of potassium t-butoxide (20 mmol) in DMF (20 mL) was added a mixture of nitrobenzene 4 (10 mmol) and ethyl 2-chloropropionate (10 mmol) at 0 °C dropwise. After being stirred for 10 min at 0 °C, the mixture was quenched with 1 N HCl solution, diluted with water and extracted with diethyl ether several times. The combined organic layers were washed with water and brine, dried over MgSO4, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using EtOAc:hexanes (1:10) as eluant.
5.1.2.1. Ethyl 2-(3-fluoro-4-nitrophenyl)propionate (5)
68% yield, yellow oil
1H NMR (CDCl3) δ 8.02 (dd, 1 H, J = 7.8, 8.0 Hz, H-5), 7.2–7.3 (m, 2 H, H–2,6), 4.14 (m, 2 H, CO2CH2CH3), 3.78 (q, 1 H, J = 7.1 Hz, CHCH3), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3), 1.22 (t, 3 H, J = 7.08 Hz, CO2CH2CH3)
5.1.2.2. Ethyl 2-(3-chloro-4-nitrophenyl)propionate (6)
64% yield, yellow oil
1H NMR (CDCl3) δ 7.87 (d, 1 H, J = 8.4 Hz, H-5), 7.51 (d, 1 H, J = 1.8 Hz, H-2), 7.36 (dd, 1 H, J = 8.4, 1.8 Hz, H-6), 4.15 (m, 2 H, CO2CH2CH3), 3.77 (q, 1 H, J = 7.2 Hz, CHCH3), 1.53 (d, 3 H, J = 7.2 Hz, CHCH3), 1.24 (t, 3 H, J = 7.08 Hz, CO2CH2CH3)
5.1.2.3. Ethyl 2-(3-bromo-4-nitrophenyl)propionate (7)
52% yield, yellow oil
1H NMR (CDCl3) δ 7.83 (d, 1 H, J = 8.4 Hz, H-5), 7.69 (d, 1 H, J = 2 Hz, H-2), 7.40 (dd, 1 H, J = 8.4, 2 Hz, H-6), 4.15 (m, 2 H, CO2CH2CH3), 3.75 (q, 1 H, J = 7.1 Hz, CHCH3), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3), 1.24 (t, 3 H, J = 7.08 Hz, CO2CH2CH3)
5.1.2.4. Ethyl 2-(3-iodo-4-nitrophenyl)propionate (8)
32% yield, yellow oil
1H NMR (CDCl3) δ 7.97 (d, 1 H, J = 2 Hz, H-2), 7.84 (d, 1 H, J = 8.4 Hz, H-5), 7.43 (dd, 1 H, J = 8.4, 2 Hz, H-6), 4.15 (m, 2 H, CO2CH2CH3), 3.72 (q, 1 H, J = 7.1 Hz, CHCH3), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3), 1.24 (t, 3 H, J = 7.08 Hz, CO2CH2CH3)
5.1.2.5. Ethyl 2-(3,5-difluoro-4-nitrophenyl)propionate (9)
56% yield, yellow oil
1H NMR (CDCl3) δ 7.08 (bd, 2 H), 4.16 (m, 2 H, CO2CH2CH3), 3.74 (q, 1 H, J = 7.1 Hz, CHCH3), 1.53 (d, 3 H, J = 7.1 Hz, CHCH3), 1.25 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.2.6. Ethyl 2-(2-fluoro-4-nitrophenyl)propionate (10)
36% yield, yellow oil
1H NMR (CDCl3) δ 8.03 (dd, 1 H, J = 8.6, 2.2 Hz, H-3), 7.93 (dd, 1 H, J = 9.5, 2.2 Hz, H-5), 7.52 (t, 1 H, J = 8.3 Hz, H-6), 4.17 (q, 2 H, J = 7.1 Hz, CO2CH2CH3), 4.08 (q, 1 H, J = 7.1 Hz, CHCH3), 1.55 (d, 3 H, J = 7.1 Hz, CHCH3), 1.23 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.2.7. Ethyl 2-(2-chloro-4-nitrophenyl)propionate (11)
28% yield, yellow oil
1H NMR (CDCl3) δ 8.26 (d, 1 H, J = 2.4 Hz, H-3), 8.11 (dd, 1 H, J = 8.6, 2.4 Hz, H-5), 7.55 (d, 1 H, J = 8.6 Hz, H-6), 4.27 (q, 1 H, J = 7.1 Hz, CHCH3), 4.18 (m, 2 H, CO2CH2CH3), 1.55 (d, 3 H, J = 7.1 Hz, CHCH3), 1.23 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.2.8. Ethyl 2-(3-cyano-4-nitrophenyl)propionate (12)
20% yield, yellow oil
1H NMR (CDCl3) δ 8.31 (d, 1 H, J = 8.4 Hz, H-5), 7.87 (d, 1 H, J = 2 Hz, H-2), 7.77 (dd, 1 H, J = 8.4, 2 Hz, H-6), 4.17 (m, 2 H, CO2CH2CH3), 3.88 (q, 1 H, J = 7.2 Hz, CHCH3), 1.58 (d, 3 H, J = 7.2 Hz, CHCH3), 1.25 (t, 3 H, J = 7.2 Hz, CO2CH2CH3)
IR (neat) 2236 (CN), 1731 (CO) cm−1
5.1.2.9. Ethyl 2-(3-t-butoxycarbonyl-4-nitrophenyl)propionate (13)
44% yield, yellow oil
1H NMR (CDCl3) δ 7.79 (d, 1 H, J = 8.3 Hz, H-5), 7.58 (d, 1 H, J = 1.8 Hz, H-2), 7.49 (dd, 1 H, J = 8.3, 1.8 Hz, H-6), 4.11 (m, 2 H, CO2CH2CH3), 3.78 (q, 1 H, J = 7.2 Hz, CHCH3), 1.53 (s, 9 H, C(CH3)3), 1.50 (d, 3 H, J = 7.2 Hz, CHCH3), 1.19 (t, 3 H, J = 7.08 Hz, CO2CH2CH3)
5.1.2.10. Ethyl 2-(3-piperidino-4-nitrophenyl)propionate (14)
prepared from 5b by the condensation with piperidine.
93% yield, yellow oil
1H NMR (CDCl3) δ 7.73 (d, 1 H, J = 8.4 Hz, H-5), 7.02 (d, 1 H, J = 1.8 Hz, H-2), 6.88 (dd, 1 H, J = 8.4, 1.8 Hz, H-6), 4.14 (m, 2 H, CO2CH2CH3), 3.69 (q, 1 H, J = 7.1 Hz, CHCH3), 3.02 (m, 4 H, CH2NCH2), 1.65–1.75 (m, 4 H), 1.60 (m, 2 H), 1.49 (d, 3 H, J = 7.1 Hz, CHCH3), 1.23 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.2.11. Ethyl 2-(3-morpholino-4-nitrophenyl)propionate (15)
prepared from 5b by the condensation with morpholine.
58% yield, yellow oil
1H NMR (CDCl3) δ 7.77 (d, 1 H, J = 8.4 Hz, H-5), 7.05 (d, 1 H, J = 1.8 Hz, H-2), 7.00 (dd, 1 H, J = 8.4, 1.8 Hz, H-6), 4.14 (m, 2 H, CO2CH2CH3), 3.85 (m, 4 H, CH2OCH2), 3.72 (q, 1 H, J = 7.1 Hz, CHCH3), 3.07 (m, 4 H, CH2NCH2), 1.51 (d, 3 H, J = 7.1 Hz, CHCH3), 1.23 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.2.12. Ethyl 2-(3-(tert-butoxycarbonyl)piperazino-4-nitrophenyl)propionate (16)
prepared from 5b by the condensation with N-(tert-butoxycarbonyl)piperazine.
48% yield, yellow oil
1H NMR (CDCl3) δ 7.77 (d, 1 H, J = 8.4 Hz, H-5), 7.06 (d, 1 H, J = 1.8 Hz, H-2), 7.00 (dd, 1 H, J = 8.4, 1.8 Hz, H-6), 4.14 (m, 2 H, CO2CH2CH3), 3.72 (q, 1 H, J = 7.1 Hz, CHCH3), 3.12 (m, 8 H, 2×CH2NCH2), 1.50 (d, 3 H, J = 7.1 Hz, CHCH3), 1.49 (s, 9 H, C(CH3)3), 1.23 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.3. General Procedure for Nitro Reduction
Method A
A suspension of nitro compound 5 (5 mmol) and 10% Pd-C (500 mg) in EtOH (30 mL) was hydrogenated under a balloon of hydrogen for 1 h and filtered through Celite. The filtrate was concentrated in vacuo and the residue was purified by flash column chromatography on silica gel using EtOAc:hexanes (1:4) as eluant.
Method B
A suspension of nitro compound 5 (5 mmol) and activated iron (0.28 g, 5 mmol) in acetic acid (30 mL) was heated at 90 °C for 1 min. After being cooled at room temperature, the mixture was diluted with EtOH, filtered and the filtrate was concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using EtOAc:hexanes (1:4) as eluant.
5.1.3.1. Ethyl 2-(4-amino-3-fluorophenyl)propionate (17)
Method A, 94% yield, a colorless oil
1H NMR (CDCl3) δ 6.96 (dd, 1 H, J = 1.7, 11.9 Hz, H-2), 6.87 (dd, 1 H, J = 1.7, 8.3 Hz, H-6), 6.71 (dd, 1 H, J = 8.3, 11.9 Hz, H-5), 4.11 (m, 2 H, CO2CH2CH3), 3.58 (q, 1 H, J = 7.1 Hz, CHCH3), 3.45 (bs, 2 H, NH2), 1.43 (d, 3 H, J = 7.1 Hz, CHCH3), 1.20 (t, 3 H, J = 7.05 Hz, CO2CH2CH3)
5.1.3.2. Ethyl 2-(4-amino-3-chlorophenyl)propionate (18)
Method B, 88% yield, yellow oil
1H NMR (CDCl3) δ 7.20 (d, 1 H, J = 2 Hz, H-2), 7.00 (dd, 1 H, J = 2, 8.1 Hz, H-6), 6.71 (d, 1 H, J = 8.1 Hz, H-5), 4.11 (m, 2 H, CO2CH2CH3), 4.00 (bs, 2 H, NH2), 3.56 (q, 1 H, J = 7.1 Hz, CHCH3), 1.44 (d, 3 H, J = 7.1 Hz, CHCH3), 1.21 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.3.3. Ethyl 2-(4-amino-3-bromophenyl)propionate (19)
Method B, 45% yield, yellow oil
1H NMR (CDCl3) δ 7.36 (d, 1 H, J = 2 Hz, H-2), 7.05 (dd, 1 H, J = 2, 8.2 Hz, H-6), 6.71 (d, 1 H, J = 8.2 Hz, H-5), 4.11 (m, 2 H, CO2CH2CH3), 4.03 (bs, 2 H, NH2), 3.56 (q, 1 H, J = 7.1 Hz, CHCH3), 1.43 (d, 3 H, J = 7.1 Hz, CHCH3), 1.21 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.3.4. Ethyl 2-(4-amino-3-iodophenyl)propionate (20)
Method B, 34% yield, yellow oil
1H NMR (CDCl3) δ 7.57 (d, 1 H, J = 2 Hz, H-2), 7.08 (dd, 1 H, J = 2, 8.3 Hz, H-6), 6.69 (d, 1 H, J = 8.3 Hz, H-5), 4.12 (m, 2 H, CO2CH2CH3), 4.05 (bs, 2 H, NH2), 3.54 (q, 1 H, J = 7.1 Hz, CHCH3), 1.43 (d, 3 H, J = 7.1 Hz, CHCH3), 1.21 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.3.5. Ethyl 2-(4-amino-3,5-difluorophenyl)propionate (21)
Method A, 99% yield, yellow oil
1H NMR (CDCl3) δ 6.79 (ddd, 2 H), 4.12 (m, 2 H, CO2CH2CH3), 3.68 (bs, 2 H, NH2), 3.56 (q, 1 H, J = 7.1 Hz, CHCH3), 1.43 (d, 3 H, J = 7.1 Hz, CHCH3), 1.22 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.3.6. Ethyl 2-(4-amino-2-fluorophenyl)propionate (22)
Method A, 96% yield, colorless oil
1H NMR (CDCl3) δ 7.04 (t, 1 H, J = 8.3 Hz, H-6), 6.41 (dd, 1 H, J = 8.2, 2.2 Hz, H-5), 6.35 (dd, 1 H, J = 11.9, 2.2 Hz, H-3), 4.12 (m, 2 H, CO2CH2CH3), 3.87 (q, 1 H, J = 7.1 Hz, CHCH3), 3.64 (bs, 2 H, NH2), 1.43 (d, 3 H, J = 7.1 Hz, CHCH3), 1.20 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.3.7. Ethyl 2-(4-amino-2-chlorophenyl)propionate (23)
Method A, 97% yield, yellow oil
1H NMR (CDCl3) δ 7.08 (d, 1 H, J = 8.4 Hz, H-6), 6.69 (d, 1 H, J = 2.4 Hz, H-3), 6.55 (dd, 1 H, J = 8.6, 2.4 Hz, H-5), 4.13 (m, 2 H, CO2CH2CH3), 4.07 (q, 1 H, J = 7.1 Hz, CHCH3), 3.69 (bs, 1 H, NH2), 1.43 (d, 3 H, J = 7.1 Hz, CHCH3), 1.21 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.3.8. Ethyl 2-(4-amino-3-cyanophenyl)propionate (24)
Method A, 42% yield, yellow oil
1H NMR (CDCl3) δ 7.25–7.35 (m, 2 H, H-2 & H-6), 6.70 (d, 1 H, J = 8.4 Hz, H-5), 4.36 (bs, 2 H, NH2), 4.12 (m, 2 H, CO2CH2CH3), 3.58 (q, 1 H, J = 7.1 Hz, CHCH3), 1.44 (d, 3 H, J = 7.1 Hz, CHCH3), 1.22 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.3.9. Ethyl 2-[4-amino-3-(t-butoxycarbonyl)phenyl]propionate (25)
Method A, 89% yield, colorless oil
1H NMR (CDCl3) δ 7.74 (d, 1 H, J = 2.2 Hz, H-2), 7.25 (dd, 1 H, J = 2.2, 8.6 Hz, H-6), 6.65 (d, 1 H, J = 8.6 Hz, H-5), 4.16 (m, 2 H, CO2CH2CH3), 5.70 (bs, 2 H, NH2), 3.63 (q, 1 H, J = 7.1 Hz, CHCH3), 1.62 (s, 9 H, C(CH3)3), 1.48 (d, 3 H, J = 7.1 Hz, CHCH3), 1.26 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.3.10. Ethyl 2-(4-amino-3-piperidinophenyl)propionate (26)
Method A, 72% yield, yellow oil
1H NMR (CDCl3) δ 6.92 (d, 1 H, J = 2 Hz, H-2), 6.84 (dd, 1 H, J = 2, 8.1 Hz, H-6), 6.66 (d, 1 H, J = 8.1 Hz, H-5), 4.10 (m, 2 H, CO2CH2CH3), 3.92 (bs, 2 H, NH2), 3.58 (q, 1 H, J = 7.1 Hz, CHCH3), 2.83 (m, 4 H, CH2NCH2), 1.65–1.75 (m, 4 H), 1.57 (m, 2 H), 1.44 (d, 3 H, J = 7.1 Hz, CHCH3), 1.20 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.3.11. Ethyl 2-(4-amino-3-morpholinophenyl)propionate (27)
Method A, 90% yield, yellow oil
1H NMR (CDCl3) δ 6.93 (d, 1 H, J = 2 Hz, H-2), 6.89 (dd, 1 H, J = 2, 8.3 Hz, H-6), 6.69 (d, 1 H, J = 8.3 Hz, H-5), 4.10 (m, 2 H, CO2CH2CH3), 3.93 (bs, 2 H, NH2), 3.84 (m, 4 H, CH2OCH2), 3.59 (q, 1 H, J = 7.1 Hz, CHCH3), 2.92 (m, 4 H, CH2NCH2), 1.45 (d, 3 H, J = 7.1 Hz, CHCH3), 1.21 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.3.12. Ethyl 2-[4-amino-3-N-(tert-butoxycarbonyl)piperazinophenyl]propionate (28)
Method A, 94% yield, yellow oil
1H NMR (CDCl3) δ 6.90 (d, 1 H, J = 2 Hz, H-2), 6.88 (dd, 1 H, J = 2, 8 Hz, H-6), 6.69 (d, 1 H, J = 8 Hz, H-5), 4.10 (m, 2 H, CO2CH2CH3), 3.93 (bs, 2 H, NH2), 3.5–3.6 (m, 5 H, CHCH3 and CH2N(Boc)CH2), 2.86 (m, 4 H, CH2NCH2), 1.49 (s, 9 H, C(CH3)3), 1.44 (d, 3 H, J = 7.1 Hz, CHCH3), 1.21 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.4. General Procedure for Mesylation
A mixture of amine 6 (4 mmol) and methanesulfonyl chloride (6 mmol) in pyridine (10 mL) was stirred at 0 °C for 10 min. After aqueous workup, the residue was purified by flash column chromatography on silica gel using EtOAc:hexanes (1:2) as eluant.
5.1.4.1. Ethyl 2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionate (29)
91% yield, white solid mp = 81 °C
1H NMR (CDCl3) δ 7.50 (t, 1 H, J = 8.3 Hz, H-5), 7.0–7.1 (m, 2 H, H-2,6), 6.55 (bs, 1 H, NHSO2), 4.12 (m, 2 H, CO2CH2CH3), 3.68 (q, 1 H, J = 7.1 Hz, CHCH3), 3.02 (s, 3 H, SO2CH3), 1.48 (d, 3 H, J = 7.1 Hz, CHCH3), 1.22 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.4.2. Ethyl 2-[3-chloro-4-(methylsulfonylamino)phenyl]propionate (30)
90% yield, colorless oil
1H NMR (CDCl3) δ 7.60 (d, 1 H, J = 8.4 Hz, H-5), 7.40 (d, 1 H, J = 2 Hz, H-2), 7.25 (dd, 1 H, J = 8.4, 2 Hz, H-6), 6.78 (bs, 1 H, NHSO2), 4.14 (m, 2 H, CO2CH2CH3), 3.67 (q, 1 H, J = 7.1 Hz, CHCH3), 3.02 (s, 3 H, SO2CH3), 1.49 (d, 3 H, J = 7.1 Hz, CHCH3), 1.23 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.4.3. Ethyl 2-[3-bromo-4-(methylsulfonylamino)phenyl]propionate (31)
96% yield, yellow oil
1H NMR (CDCl3) δ 7.60 (d, 1 H, J = 8.4 Hz, H-5), 7.55 (d, 1 H, J = 2 Hz, H-2), 7.28 (dd, 1 H, J = 8.4, 2 Hz, H-6), 6.76 (bs, 1 H, NHSO2), 4.13 (m, 2 H, CO2CH2CH3), 3.67 (q, 1 H, J = 7.1 Hz, CHCH3), 3.01 (s, 3 H, SO2CH3), 1.49 (d, 3 H, J = 7.1 Hz, CHCH3), 1.23 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.4.4. Ethyl 2-[3-iodo-4-(methylsulfonylamino)phenyl]propionate (32)
95% yield, yellow oil
1H NMR (CDCl3) δ 7.77 (d, 1 H, J = 2 Hz, H-2), 7.58 (d, 1 H, J = 8.4 Hz, H-2), 7.32 (dd, 1 H, J = 8.4, 2 Hz, H-6), 4.14 (m, 2 H, CO2CH2CH3), 3.65 (q, 1 H, J = 7.1 Hz, CHCH3), 3.01 (s, 3 H, SO2CH3), 1.48 (d, 3 H, J = 7.1 Hz, CHCH3), 1.23 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.4.5. Ethyl 2-[3,5-difluoro-4-(methylsulfonylamino)phenyl]propionate (33)
95% yield, yellow oil
1H NMR (CDCl3) δ 7.05 (d, 2 H, J = 8.4 Hz), 4.14 (m, 2 H, CO2CH2CH3), 3.71 (q, 1 H, J = 7.1 Hz, CHCH3), 3.47 (s, 3 H, SO2CH3), 1.50 (d, 3 H, J = 7.1 Hz, CHCH3), 1.25 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.4.6. Ethyl 2-[2-fluoro-4-(methylsulfonylamino)phenyl]propionate (34)
92% yield, colorless oil
1H NMR (CDCl3) δ 7.27 (t, 1 H, J = 8.1 Hz, H-6), 7.02 (dd, 1 H, J = 11, 2.2 Hz, H-3), 6.94 (dd, 1 H, J = 8.4, 2.2 Hz, H-5), 4.16 (m, 2 H, CO2CH2CH3), 3.96 (q, 1 H, J = 7.1 Hz, CHCH3), 3.04 (s, 3 H, SO2CH3), 1.49 (d, 3 H, J = 7.1 Hz, CHCH3), 1.23 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.4.7. Ethyl 2-[2-chloro-4-(methylsulfonylamino)phenyl]propionate (35)
89% yield, colorless oil
1H NMR (CDCl3) δ 7.32 (d, 1 H, J = 2.4 Hz, H-3), 7.28 (d, 1 H, J = 8.4 Hz, H-6), 7.16 (dd, 1 H, J = 8.6, 2.4 Hz, H-5), 4.1–4.2 (m, 3 H, CO2CH2CH3 and CHCH3), 3.02 (s, 3 H, SO2CH3), 1.48 (d, 3 H, J = 7.1 Hz, CHCH3), 1.24 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.4.8. Ethyl 2-[3-cyano-4-(methylsulfonylamino)phenyl]propionate (36)
98% yield, yellow oil
1H NMR (CDCl3) δ 7.55–7.7 (m, 3 H), 4.15 (m, 2 H, CO2CH2CH3), 3.72 (q, 1 H, J = 7.1 Hz, CHCH3), 3.12 (s, 3 H, SO2CH3), 1.51 (d, 3 H, J = 7.1 Hz, CHCH3), 1.24 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.4.9. Ethyl 2-[3-(t-butoxycarbonyl)-4-(methylsulfonylamino)phenyl]propionate (37)
99% yield, colorless oil
1H NMR (CDCl3) δ 7.84 (d, 1 H, J = 2.2 Hz, H-2), 7.61 (d, 1 H, J = 8.6 Hz, H-5), 7.43 (dd, 1 H, J = 8.6, 2.2 Hz, H-6), 4.08 (m, 2 H, CO2CH2CH3), 3.66 (q, 1 H, J = 7.1 Hz, CHCH3), 3.00 (s, 3 H, SO2CH3), 1.56 (s, 9 H, C(CH3)3), 1.44 (d, 3 H, J = 7.1 Hz, CHCH3), 1.19 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.4.10. Ethyl 2-[3-piperidino-4-(methylsulfonylamino)phenyl]propionate (38)
96% yield, yellow oil
1H NMR (CDCl3) δ 7.79 (bs, 1 H, NHSO2), 7.45 (d, 1 H, J = 8.4 Hz, H-5), 7.15 (d, 1 H, J = 2 Hz, H-2), 7.07 (dd, 1 H, J = 8.4, 2 Hz, H-6), 4.14 (m, 2 H, CO2CH2CH3), 3.64 (q, 1 H, J = 7.1 Hz, CHCH3), 3.04 (s, 3 H, SO2CH3), 2.77 (m, 4 H, CH2NCH2), 1.65–1.75 (m, 4 H), 1.59 (m, 2 H), 1.47 (d, 3 H, J = 7.1 Hz, CHCH3), 1.22 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.4.11. Ethyl 2-[3-morpholino-4-(methylsulfonylamino)phenyl]propionate (39)
92% yield, yellow oil
1H NMR (CDCl3) δ 7.72 (bs, 1 H, NHSO2), 7.46 (d, 1 H, J = 8.4 Hz, H-5), 7.18 (d, 1 H, J = 2 Hz, H-2), 7.13 (dd, 1 H, J = 8.4, 2 Hz, H-6), 4.13 (m, 2 H, CO2CH2CH3), 3.86 (m, 4 H, CH2OCH2), 3.66 (q, 1 H, J = 7.1 Hz, CHCH3), 3.08 (s, 3 H, SO2CH3), 2.87 (m, 4 H, CH2NCH2), 1.48 (d, 3 H, J = 7.1 Hz, CHCH3), 1.23 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.4.12. Ethyl 2-[3-N-(tert-butoxycarbonyl)piperazino-4-(methylsulfonylamino)phenyl] propionate (40)
99% yield, yellow oil
1H NMR (CDCl3) δ 7.75 (bs, 1 H, NHSO2), 7.45 (d, 1 H, J = 8.4 Hz, H-5), 7.15 (d, 1 H, J = 2 Hz, H-2), 7.13 (dd, 1 H, J = 8.4, 2 Hz, H-6), 4.13 (m, 2 H, CO2CH2CH3), 3.66 (q, 1 H, J = 7.1 Hz, CHCH3), 3.61 (m, 4 H, CH2N(Boc)CH2), 3.09 (s, 3 H, SO2CH3), 2.83 (m, 4 H, CH2NCH2), 1.49 (s, 9 H, C(CH3)3), 1.47 (d, 3 H, J = 7.1 Hz, CHCH3), 1.22 (t, 3 H, J = 7.1 Hz, CO2CH2CH3)
5.1.5. General Procedure for Hydrolysis
A solution of ester 7 (2 mmol) in H2O and THF (1:2, 30 mL) was treated with lithium hydroxide (6 mmol) and stirred for 4 h at room temperature. The mixture was diluted with H2O and CH2Cl2, acidified with 1 N HCl solution and extracted with CH2Cl2 several times. The combined organic layers were washed with water and brine, dried over MgSO4 and concentrated in vacuo. The residue was crystallized by diethyl ether and n-hexane.
5.1.5.1. 2-[3-Fluoro-4-(methylsulfonylamino)phenyl]propionic acid (41)
97% yield, white solid, mp = 120 °C
1H NMR (CDCl3) δ 7.52 (t, 1 H, J = 8.04 Hz, H-5), 7.1–7.15 (m, 2 H, H-2,6), 6.60 (bs, 1 H, NHSO2), 3.73 (q, 1 H, J = 7.1 Hz, CHCH3), 3.03 (s, 3 H, SO2CH3), 1.51 (d, 3 H, J = 7.1 Hz, CHCH3)
5.1.5.2. 2-[3-Chloro-4-(methylsulfonylamino)phenyl]propionic acid (42)
92% yield, pink solid, mp = 133–135 °C
1H NMR (CDCl3) δ 10.19 (bs, 1 H, CO2H), 7.60 (d, 1 H, J = 8.4 Hz, H-5), 7.41 (d, 1 H, J = 1.8 Hz, H-2), 7.26 (dd, 1 H, J = 8.4, 1.8 Hz, H-6), 6.91 (bs, 1 H, NHSO2), 3.72 (q, 1 H, J = 7.1 Hz, CHCH3), 3.02 (s, 3 H, SO2CH3), 1.51 (d, 3 H, J = 7.1 Hz, CHCH3)
5.1.5.3. 2-[3-Bromo-4-(methylsulfonylamino)phenyl]propionic acid (43)
99% yield, white solid, mp = 117–118 °C
1H NMR (CDCl3) δ 7.62 (d, 1 H, J = 8.4 Hz, H-5), 7.56 (d, 1 H, J = 2 Hz, H-2), 7.30 (dd, 1 H, J = 8.4, 2 Hz, H-6), 6.76 (bs, 1 H, NHSO2), 3.72 (q, 1 H, J = 7.1 Hz, CHCH3), 3.02 (s, 3 H, SO2CH3), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3)
5.1.5.4. 2-[3-Iodo-4-(methylsulfonylamino)phenyl]propionic acid (44)
99% yield, white solid, mp = 102–103 °C
1H NMR (CD3OD) δ 7.88 (d, 1 H, J = 1.8 Hz, H-2), 7.3–7.4 (m, 2 H, H-5 and H-6), 3.52 (q, 1 H, J = 7.1 Hz, CHCH3), 2.97 (s, 3 H, SO2CH3), 1.39 (d, 3 H, J = 7.1 Hz, CHCH3)
5.1.5.5. 2-[3,5-Difluoro-4-(methylsulfonylamino)phenyl]propionic acid (45)
92% yield, white solid, mp = 78–79 °C
1H NMR (CDCl3) δ 7.00 (d, 2 H, J = 8.4 Hz), 6.03 (bs, 1 H, NHSO2), 3.73 (q, 1 H, J = 7.1 Hz, CHCH3), 3.22 (s, 3 H, SO2CH3), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3)
5.1.5.6. 2-[2-Fluoro-4-(methylsulfonylamino)phenyl]propionic acid (46)
86% yield, pink solid, mp = 134–136 °C
1H NMR (CDCl3) δ 7.29 (t, 1 H, J = 8.1 Hz, H-6), 7.02 (dd, 1 H, J = 11, 2.2 Hz, H-3), 6.94 (dd, 1 H, J = 8.4, 2.2 Hz, H-5), 6.82 (bs, 1 H, NHSO2), 4.02 (q, 1 H, J = 7.1 Hz, CHCH3), 3.05 (s, 3 H, SO2CH3), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3)
5.1.5.7. 2-[2-Chloro-4-(methylsulfonylamino)phenyl]propionic acid (47)
84% yield, pink solid, mp = 188–190°C
1H NMR (CD3OD) δ 7.24 (d, 1 H, J = 8.4 Hz, H-6), 7.21 (d, 1 H, J = 2.4 Hz, H-3), 7.07 (dd, 1 H, J = 8.6, 2.4 Hz, H-5), 4.02 (q, 1 H, J = 7.1 Hz, CHCH3), 2.88 (s, 3 H, SO2CH3), 1.35 (d, 3 H, J = 7.1 Hz, CHCH3)
5.1.5.8. 2-[3-Cyano-4-(methylsulfonylamino)phenyl]propionic acid (48)
38% yield, white solid, mp = 108–111 °C
1H NMR (CD3OD) δ 8.67 (d, 1 H, J = 2.2 Hz, H-2), 8.60 (dd, 1 H, J = 8.4, 2.2 Hz, H-6), 8.43 (d, 1 H, J = 8.4 Hz, H-5), 5.98 (bs, 2 H, NH2), 4.64 (q, 1 H, J = 7.1 Hz, CHCH3), 4.05 (s, 3 H, SO2CH3), 2.18 (d, 3 H, J = 7.1 Hz, CHCH3)
5.1.5.9. 2-[3-(t-Butoxycarbonyl)-4-(methylsulfonylamino)phenyl]propionic acid (49)
87% yield, yeollw solid, mp = 79–81 °C
1H NMR (CDCl3) δ 10.53 (s, 1 H, CO2H), 7.87 (d, 1 H, J = 2.2 Hz, H-2), 7.64 (d, 1 H, J = 8.6 Hz, H-5), 7.46 (dd, 1 H, J = 8.6, 2.2 Hz, H-6), 3.71 (q, 1 H, J = 7.1 Hz, CHCH3), 3.02 (s, 3 H, SO2CH3), 1.57 (s, 9 H, C(CH3)3), 1.48 (d, 3 H, J = 7.1 Hz, CHCH3)
5.1.5.10. 2-[3-Piperidino-4-(methylsulfonylamino)phenyl]propionic acid (50)
83% yield, white solid, mp = 152 °C
1H NMR (CDCl3) δ 7.46 (d, 1 H, J = 8.4 Hz, H-5), 7.05–7.2 (m, 2 H, H-2 and H-6), 3.69 (q, 1 H, J = 7.1 Hz, CHCH3), 3.06 (s, 3 H, SO2CH3), 2.79 (m, 4 H, CH2NCH2), 1.68–1.8 (m, 4 H), 1.60 (m, 2 H), 1.50 (d, 3 H, J = 7.1 Hz, CHCH3)
5.1.5.11. 2-[3-Morpholino-4-(methylsulfonylamino)phenyl]propionic acid (51)
93% yield, white solid, mp = 180 °C
1H NMR (CDCl3) δ 7.47 (d, 1 H, J = 8.4 Hz, H-5), 7.18 (d, 1 H, J = 1.8 Hz, H-2), 7.15 (dd, 1 H, J = 8.4, 1.8 Hz, H-6), 6.91 (bs, 1 H, NHSO2), 3.86 (m, 4 H, CH2OCH2), 3.70 (q, 1 H, J = 7.1 Hz, CHCH3), 3.08 (s, 3 H, SO2CH3), 2.86 (m, 4 H, CH2NCH2), 1.51 (d, 3 H, J = 7.1 Hz, CHCH3)
5.1.5.12. 2-[3-(N-tert-Butoxycarbonyl)piperazino-4-(methylsulfonylamino)phenyl]propionic acid (52)
93% yield, white solid, mp = 139–142 °C
1H NMR (CDCl3) δ 7.47 (d, 1 H, J = 8.4 Hz, H-5), 7.12–7.16 (m, 2 H, H-2 and H-6), 3.70 (q, 1 H, J = 7.1 Hz, CHCH3), 3.59 (m, 4 H, CH2N(Boc)CH2), 3.08 (s, 3 H, SO2CH3), 2.80 (m, 4 H, CH2NCH2), 1.50 (d, 3 H, J = 7.1 Hz, CHCH3), 1.49 (s, 9 H, C(CH3)3)
5.1.6. Generel Procedure for Coupling
A mixture of acid 8 (10 mmol), 4-t-butylbenzylamine (12 mmol) and 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride (12 mmol) in CH2Cl2 (20 mL) was stirred for 12 h at room temperature. The reaction mixture was filtered off and the filtrate was concentrated. The residue was purified by flash column chromatography on silica gel using EtOAc:hexanes as eluant.
5.1.6.1. N-(4-tert-Butylbenzyl)-2-[4-(methylsulfonylamino)phenyl]propionamide (53)
prepared from the carboxylic acid previously reported by the general coupling procedure.
93% yield, white solid, mp = 77–79 °C
1H NMR (CDCl3) δ 7.32 (dt, 2 H), 7.27 (dt, 2 H), 7.18 (dt, 2 H), 7.11 (dt, 2 H), 6.96 (bs, 1 H, NHSO2), 5.73 (bt, 1 H, NH), 4.38 (ddd, 2 H, ArCH2NH), 3.55 (q, 1 H, J = 7.1 Hz, CHCH3), 2.98 (s, 3 H, SO2CH3), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3), 1.29 (s, 9 H, C(CH3)3)
IR (KBr) 3277, 2963, 1649, 1512, 1464, 1333, 1228, 1153 cm−1
MS (EI) m/z 388 (M+)
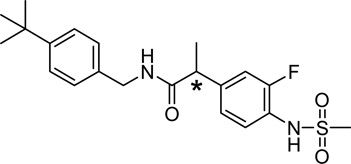
5.1.6.2. N-(4-tert-Butylbenzyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (54)
78% yield, white solid, mp = 52–54 °C
1H NMR (CDCl3) δ 7.48 (t, 1 H, J = 8.3 Hz, H-5), 7.32 (bd, 2 H, Ar), 7.1–7.2 (m, 4 H, Ar), 6.73 (bs, 1 H, NHSO2), 5.83 (bt, 1 H, NHCO), 4.36 (ddd of AB, 2 H, ArCH2NH), 3.52 (q, 1 H, J = 7.1 Hz, CHCH3), 3.00 (s, 3 H, SO2CH3), 1.50 (d, 3 H, J = 7.1 Hz, CHCH3), 1.29 (s, 9 H, C(CH3)3)
IR (KBr) 3286, 2964, 1650, 1511, 1331, 1157, 1116 cm−1
MS (FAB) m/z 407 (MH+)
5.1.6.3. N-(4-tert-Butylbenzyl)-2-[3-chloro-4-(methylsulfonylamino)phenyl]propionamide (55)
68% yield, white solid, mp = 126–129 °C
1H NMR (CDCl3) δ 7.60 (d, 1 H, J = 8.2 Hz, H-5), 7.43 (d, 1 H, J = 2 Hz, H-2), 7.34 (bd, 2 H, Ar), 7.24 (dd, 1 H, J = 8.2, 2 Hz, H-6), 7.14 (bd, 2 H, Ar), 6.75 (bs, 1 H, NHSO2), 5.68 (bt, 1 H, NHCO), 4.38 (ddd of AB, 2 H, ArCH2NH), 3.50 (q, 1 H, J = 7.1 Hz, CHCH3), 3.01 (s, 3 H, SO2CH3), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3), 1.30 (s, 9 H, C(CH3)3)
IR (KBr) 3287, 2963, 1648, 1497, 1331, 1236, 1157 cm−1
MS (FAB) m/z 423 (MH+)
5.1.6.4.N-(4-tert-Butylbenzyl)-2-[3-bromo-4-(methylsulfonylamino)phenyl]propionamide (56)
76% yield, white solid, mp = 66–67 °C
1H NMR (CDCl3) δ 7.55–7.6 (m, 2 H, H-2 and H-5), 7.33 (d, 2 H, J = 8.1 Hz, Ar), 7.27 (dd, 1 H, J = 1.8, 8.6 Hz, H-6), 7.12 (d, 2 H, J = 8.1 Hz, Ar), 6.80 (bs, 1 H, NHSO2), 5.91 (bt, 1 H, NHCO), 4.36 (ddd of AB, 2 H, ArCH2NH), 3.50 (q, 1 H, J = 7.1 Hz, CHCH3), 2.98 (s, 3 H, SO2CH3), 1.50 (d, 3 H, J = 7.1 Hz, CHCH3), 1.29 (s, 9 H, C(CH3)3)
IR (KBr) 3292, 2963, 1649, 1493, 1387, 1330, 1233, 1157, 1044 cm−1
MS (FAB) m/z 467 (MH+)
5.1.6.5. N-(4-tert-Butylbenzyl)-2-[3-iodo-4-(methylsulfonylamino)phenyl]propionamide (57)
75% yield, white solid, mp = 71 °C
1H NMR (CDCl3) δ 7.80 (d, 1 H, J = 2 Hz, H-2), 7.59 (d, 1 H, J = 8.3 Hz, H-5), 7.3–7.37 (m, 3 H, Ar), 7.13 (d, 2 H, J = 8.1 Hz, Ar), 6.60 (bs, 1 H, NHSO2), 5.67 (bt, 1 H, NHCO), 4.39 (ddd of AB, 2 H, ArCH2NH), 3.48 (q, 1 H, J = 7.1 Hz, CHCH3), 3.01 (s, 3 H, SO2CH3), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3), 1.30 (s, 9 H, C(CH3)3)
IR (KBr) 3299, 2963, 1649, 1539, 1486, 1385, 1329, 1231, 1115, 1036 cm−1
MS (FAB) m/z 515 (MH+)
5.1.6.6. N-(4-tert-Butylbenzyl)-2-[3,5-difluoro-4-(methylsulfonylamino)phenyl]propionamide (58)
70% yield, white solid, mp = 80–81 °C
1H NMR (CDCl3) δ 7.35 (dt, 2 H), 7.15 (bd, 2 H, Ar), 6.99 (dt, 2 H, Ar), 6.16 (bs, 1 H, NHSO2), 5.76 (bt, 1 H, NHCO), 4.38 (ddd of AB, 2 H, J = 5.7, 14.5, 33.7 Hz, ArCH2NH), 4.12 (q, 1 H, J = 7.1 Hz, CHCH3), 3.02 (s, 3 H, SO2CH3), 1.50 (d, 3 H, J = 7.1 Hz, CHCH3), 1.30 (s, 9 H, C(CH3)3)
IR (KBr) 3376, 2962, 1653, 1511, 1454, 1331, 1231, 1155, 1023, 1155, 1023 cm−1
MS (FAB) m/z 425 (MH+)
5.1.6.7. N-(4-tert-Butylbenzyl)-2-[2-fluoro-4-(methylsulfonylamino)phenyl]propionamide (59)
63% yield, white solid, mp = 111–113 °C
1H NMR (CDCl3) δ 7.3–7.38 (m, 3 H, H-6 and Ar), 7.28 (bs, 1 H, NHSO2), 7.15 (bd, 2 H, Ar), 7.02 (dd, 1 H, J = 11.4, 2.2 Hz, H-3), 6.87 (dd, 1 H, J = 8.4, 2.2 Hz, H-5), 5.88 (bt, 1 H, NHCO), 4.41 (ddd of AB, 2 H, ArCH2NH), 3.84 (q, 1 H, J = 7.1 Hz, CHCH3), 3.00 (s, 3 H, SO2CH3), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3), 1.30 (s, 9 H, C(CH3)3)
IR (KBr) 3277, 2963, 1652, 1509, 1396, 1328, 1266, 1151, 1117 cm−1
MS (FAB) m/z 407 (MH+)
5.1.6.8. N-(4-tert-Butylbenzyl)-2-[2-chloro-4-(methylsulfonylamino)phenyl]propionamide (60)
46% yield, white solid, mp = 134–136 °C
1H NMR (CDCl3) δ 7.44 (d, 1 H, J = 8.4 Hz, H-6), 7.34 (bd, 2 H, Ar), 7.29 (d, 1 H, J = 2.2 Hz, H-3), 7.15 (bd, 2 H, Ar), 7.07 (dd, 1 H, J = 8.4, 2.2 Hz, H-5), 5.88 (bt, 1 H, NHCO), 4.40 (ddd of AB, 2 H, ArCH2NH), 3.84 (q, 1 H, J = 7.1 Hz, CHCH3), 3.00 (s, 3 H, SO2CH3), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3), 1.30 (s, 9 H, C(CH3)3)
IR (KBr) 3286, 2963, 1650, 1608, 1494, 1376, 1324, 1154 cm−1
MS (FAB) m/z 423 (MH+)
5.1.6.9. N-(4-tert-Butylbenzyl)-2-[3-cyano-4-(methylsulfonylamino)phenyl]propionamide (61)
30% yield, white solid, mp = 102–105 °C
1H NMR (CDCl3) δ 7.67 (d, 1 H, J = 8.4 Hz, H-5), 7.63 (d, 1 H, J = 1.8 Hz, H-2), 7.58 (dd, 1 H, J = 8.4, 1.8 Hz, H-6), 7.35 (bd, 2 H, Ar), 7.15 (bd, 2 H, Ar), 5.73 (bt, 1 H, NHCO), 4.38 (ddd of AB, 2 H, ArCH2NH), 3.51 (q, 1 H, J = 7.1 Hz, CHCH3), 3.11 (s, 3 H, SO2CH3), 1.53 (d, 3 H, J = 7.1 Hz, CHCH3), 1.31 (s, 9 H, C(CH3)3)
IR (KBr) 3285, 2963, 2231, 1649, 1501, 1404, 1334, 1157, 1114 cm−1
MS (FAB) m/z 414 (MH+)
5.1.6.10. N-(4-tert-Butylbenzyl)-2-[3-(t-butoxycarbonyl)-4-(methylsulfonylamino)phenyl]propionamide (62)
53% yield, white solid, mp = 75–77 °C
1H NMR (CDCl3) δ 7.90 (d, 1 H, J = 2.2 Hz, H-2), 7.67 (d, 1 H, J = 8.6 Hz, H-5), 7.50 (dd, 1 H, J = 8.6, 2.2 Hz, H-6), 7.33 (bd, 2 H, Ar), 7.13 (bd, 2 H, Ar), 5.74 (bt, 1 H, NHCO), 4.38 (ddd of AB, 2 H, ArCH2NH), 3.55 (q, 1 H, J = 7.1 Hz, CHCH3), 3.04 (s, 3 H, SO2CH3), 1.60 (s, 9 H, CO2C(CH3)3), 1.53 (d, 3 H, J = 7.1 Hz, CHCH3), 1.30 (s, 9 H, C(CH3)3)
IR (KBr) 2966, 1678, 1500, 1395, 1330, 1253, 1153, 1089 cm−1
MS (FAB) m/z 489 (MH+)
5.1.6.11. N-(4-tert-Butylbenzyl)-2-[3-carboxyl-4-(methylsulfonylamino)phenyl]propionamide (63)
prepared from 62 by acid hydrolysis.
74% yield, white solid, mp = 180–183 °C
1H NMR (CD3OD) δ 8.45 (bt, 1 H, NH), 8.12 (d, 1 H, J = 2.2 Hz, H-2), 7.64 (d, 1 H, J = 8.6 Hz, H-5), 7.56 (dd, 1 H, J = 8.6, 2.2 Hz, H-6), 7.30 (bd, 2 H, Ar), 7.11 (bd, 2 H, Ar), 4.29 (bs, 2 H, ArCH2NH), 3.69 (q, 1 H, J = 7.1 Hz, CHCH3), 3.04 (s, 3 H, SO2CH3), 1.46 (d, 3 H, J = 7.1 Hz, CHCH3), 1.27 (s, 9 H, C(CH3)3)
IR (KBr) 3429, 2964, 1678, 1626, 1503, 1338, 1206, 1153 cm−1
MS (EI) m/z 432 (M+)
5.1.6.12. N-(4-tert-Butylbenzyl)-2-[3-(methoxycarbonyl)-4-(methylsulfonylamino)phenyl]propionamide (64)
prepared from 63 by the diazomethane reaction.
79% yield, white solid, mp = 142–144 °C
1H NMR (CDCl3) δ 10.38 (s, 1 H, NHSO2), 8.03 (d, 1 H, J = 2.2 Hz, H-2), 7.70 (d, 1 H, J = 8.6 Hz, H-5), 7.51 (dd, 1 H, J = 8.6, 2.2 Hz, H-6), 7.33 (bd, 2 H, Ar), 7.13 (bd, 2 H, Ar), 5.69 (bt, 1 H, NHCO), 4.38 (ddd of AB, 2 H, ArCH2NH), 3.94 (s, 3 H, CO2CH3), 3.53 (q, 1 H, J = 7.1 Hz, CHCH3), 3.05 (s, 3 H, SO2CH3), 1.54 (d, 3 H, J = 7.1 Hz, CHCH3), 1.30 (s, 9 H, C(CH3)3)
IR (KBr) 3296, 2961, 1688, 1608, 1503, 1398, 1332, 1258, 1156, 1088 cm−1
MS (FAB) m/z 447 (MH+)
5.1.6.13. N-(4-tert-Butylbenzyl)-2-[3-(benzylamino)carbonyl-4-(methylsulfonylamino)phenyl]propionamide (65)
prepared from 63 by coupling with benzyl amine.
88% yield, white solid, mp = 79–81 °C
1H NMR (CDCl3) δ 7.65 (d, 1 H, J = 8.6 Hz, H-5), 7.61 (d, 1 H, J = 2.2 Hz, H-2), 7.3–7.38 (m, 8 H), 7.11 (bd, 2 H, Ar), 5.84 (bt, 1 H, NHCO), 4.60 (d, 2 H, J = 5.88 Hz, NHCH2Ph), 4.35 (ddd of AB, 2 H, ArCH2NH), 3.48 (q, 1 H, J = 7.1 Hz, CHCH3), 2.97 (s, 3 H, SO2CH3), 1.50 (d, 3 H, J = 7.1 Hz, CHCH3), 1.29 (s, 9 H, C(CH3)3)
IR (KBr) 3303, 2963, 1644, 1537, 1333, 1267, 1152 cm−1
MS (FAB) m/z 522 (MH+)
5.1.6.14. N-(4-tert-Butylbenzyl)-2-[3-piperidino-4-(methylsulfonylamino)phenyl]propionamide (66)
86% yield, white solid, mp = 125 °C
1H NMR (CDCl3) δ 7.78 (bs, 1 H, NHSO2), 7.45 (d, 1 H, J = 8.4 Hz, H-5), 7.31 (bd, 2 H, Ar), 7.15 (d, 1 H, J = 2 Hz, H-2), 7.10 (bd, 2 H, Ar), 7.05 (m, 1 H, J = 8.4, 2 Hz, H-6), 5.59 (bt, 1 H, NHCO), 4.38 (d of AB, 2 H, J = 5.7 Hz, ArCH2NH), 3.52 (q, 1 H, J = 7.1 Hz, CHCH3), 3.04 (s, 3 H, SO2CH3), 2.75 (m, 4 H, CH2NCH2), 1.65–1.75 (m, 4 H), 1.6 (m, 2 H), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3), 1.29 (s, 9 H, C(CH3)3)
IR (KBr) 3290, 2937, 1648, 1501, 1335, 1242, 1157 cm−1
MS (FAB) m/z 472 (MH+)
5.1.6.15. N-(4-tert-Butylbenzyl)-2-[3-morpholino-4-(methylsulfonylamino)phenyl]propionamide (67)
84% yield, white solid, mp = 78 °C
1H NMR (CDCl3) δ 7.69 (bs, 1 H, NHSO2), 7.46 (d, 1 H, J = 8.2 Hz, H-5), 7.32 (bd, 2 H, Ar), 7.18 (d, 1 H, J = 1.8 Hz, H-2), 7.08–7.15 (m, 3 H, H-6 and Ar), 5.63 (bt, 1 H, NHCO), 4.38 (d of AB, 2 H, J = 5.5 Hz, ArCH2NH), 3.85 (m, 4 H, CH2OCH2), 3.52 (q, 1 H, J = 7.1 Hz, CHCH3), 3.08 (s, 3 H, SO2CH3), 2.84 (m, 4 H, CH2NCH2), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3), 1.29 (s, 9 H, C(CH3)3)
IR (KBr) 3293, 2962, 1650, 1505, 1455, 1333, 1156, 1115 cm−1
MS (FAB) m/z 474 (MH+)
5.1.6.16. N-(4-tert-Butylbenzyl)-2-[3-(N-tert-butoxycarbonyl)piperazino-4-(methylsulfonylamino)phenyl]propionamide (68)
88% yield, white solid, mp = 103 °C
1H NMR (CDCl3) δ 7.66 (bs, 1 H, NHSO2), 7.46 (d, 1 H, J = 8.2 Hz, H-5), 7.32 (bd, 2 H, Ar), 7.15 (d, 1 H, J = 1.8 Hz, H-2), 7.08–7.13 (m, 3 H, H-6 and Ar), 5.60 (bt, 1 H, NHCO), 4.38 (ddd of AB, 2 H, ArCH2NH), 3.58 (m, 4 H, CH2N(Boc)CH2), 3.49 (q, 1 H, J = 7.1 Hz, CHCH3), 3.08 (s, 3 H, SO2CH3), 2.79 (m, 4 H, CH2NCH2), 1.55 (d, 3 H, J = 7.1 Hz, CHCH3), 1.49 (s, 9 H, C(CH3)3), 1.30 (s, 9 H, C(CH3)3)
IR (KBr) 3294, 2965, 1690, 1502, 1366, 1333, 1245, 1160 cm−1
MS (FAB) m/z 573 (MH+)
5.1.6.17. N-(4-tert-Butylbenzyl)-2-[3-piperazino-4-(methylsulfonylamino)phenyl]propionamide (69)
prepared from 9o by acid hydrolysis.
96% yield, white solid, mp = 92 °C
1H NMR (CDCl3) δ 7.46 (d, 1 H, J = 8.3 Hz, H-5), 7.32 (bd, 2 H, Ar), 7.18 (d, 1 H, J = 1.8 Hz, H-2), 7.08–7.13 (m, 3 H, H-6 and Ar), 5.60 (bt, 1 H, NHCO), 4.38 (d of AB, 2 H, J = 5 Hz, ArCH2NH), 3.52 (q, 1 H, J = 7.1 Hz, CHCH3), 3.06 (s, 3 H, SO2CH3), 3.03 (m, 4 H, CH2NCH2), 2.80 (m, 4 H, CH2NCH2), 1.52 (d, 3 H, J = 7.1 Hz, CHCH3), 1.29 (s, 9 H, C(CH3)3)
IR (KBr) 3293, 2960, 1651, 1506, 1456, 1333, 1155 cm−1
MS (FAB) m/z 473 (MH+)
5.1.6.18. N-(4-tert-Butylbenzyl)-2-[3-methyl-4-(methylsulfonylamino)phenyl]propionamide (70)
To a solution of 56 (110 mg, 0.27 mmol) and tetramethyl tin (72 mg, 0.4 mmol) in toluene (3 mL) was added tetrakis(triphenylphosphine)palladium (31 mg, 0.027 mmol, 0.1 eq). The mixture was refluxed for 6 h under a dry N2 atmosphere. The reaction mixture was cooled to ambient temperature, filtered through Celite and concentrated in vacuo. The residue was extracted with EtOAc several times. The combined organic layers were washed with saturated aqueous NaCl and dried over MgSO4. Concentration in vacuo and subsequent purification by column chromatography (using EaOAc-hexane = 1 : 1) gave 9q in 47 % yield.
white solid, mp = 72–74 °C
1H NMR (CDCl3) 7.41~7.10 (m, 7 H, Ar-H), 6.29 (bs, 1 H, NHSO2), 5.67 (bs, 1 H, NHCO), 4.56–4.29 (m, 2 H, ArCH2NH), 3.52 (q, 1 H, J = 6.39 Hz, CHCH3), 3.01 (s, 3 H, SO2CH3), 2.30 (s, 3 H, ArCH3), 1.52 (d, 3 H, J = 7.14 Hz, CHCH3), 1.30 (s. 9 H, C(CH3)3)
IR(KBr) 3285, 2953, 1650, 1501, 1488, 1321, 1156, 1024 cm−1
MS (FAB) m/z 403 (MH+)
5.1.6.19. N-(4-tert-Butylbenzyl)-2-[3-methoxy-4-(methylsulfonylamino)phenyl]propionamide (71)
prepared from the carboxylic acid previously reported by the general coupling procedure.
83% yield, white solid, mp = 74–76 °C
1H NMR (CDCl3) δ 7.1–7.5 (m, 5 H), 6.85–6.9 (m, 2 H), 6.75 (bs, 1 H, NHSO2), 5.75 (bt, 1 H, NHCO), 4.39 (ddd of AB, 2 H, ArCH2NH), 3.85 (s, 3 H, OCH3), 3.54 (q, 1 H, J = 7.1 Hz, CHCH3), 2.94 (s, 3 H, SO2CH3), 1.53 (d, 3 H, J = 7.1 Hz, CHCH3), 1.31 (s, 9 H, C(CH3)3)
IR (KBr) 3286, 2961, 1652, 1548, 1338, 1271, 1156, 1026 cm−1
MS (FAB) m/z 419 (MH+)
5.1.6.20. N-(4-tert-Butylbenzyl)-(2S)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (54S)
prepared from the chiral (S)-carboxylic acid previously reported by the general coupling procedure.
98% yield, [α] = −15.5 (c 0.5, CHCl3)
The spectra are identical to those of racemate 54.
5.1.6.21. N-(4-tert-Butylbenzyl)-(2R)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (54R)
The compound was prepared by following the procedure described for the synthesis of 1–51.
96% yield, [α] = +18.4 (c 0.5, CHCl3)
The spectra are identical to those of racemate 54.
5.2. Molecular Modeling
Based on our rat TRPV1 tetramer homology model **[ref#1], the human TRPV1 (hTRPV1) model was constructed as a tetramer. In the binding site, five residues are different between rat and human, and they were mutated as Ile514Met, Val518Leu, Val525Ala, Ser526Thr, and Met547Leu. Then, the side chains and backbone within 6 Å of the mutated residues were energy minimized using the Protein Composition Tool in SYBYL 8.1.1 (Tripos Int., St. Louis, MO, USA). The ligand structure was generated with Concord and energy minimized using the MMFF94s force field and MMFF94 charge until the rms of the Powell gradient was 0.05 kcal mol−1A−1 in SYBYL. The flexible docking study on the hTRPV1 model was performed using GOLD v.5.0.1 (Cambridge Crystallographic Data Centre, Cambridge, UK). It uses a genetic algorithm (GA) and allows for full ligand flexibility and partial protein flexibility. The binding site was defined as 8 Å around capsaicin which was extracted from the docking result in the rTRPV1 model and merged into the aligned hTRPV1 model. The side chains of the important six residues for ligand binding (i.e., Tyr511, Ser512, Leu515, Leu547, Thr550, and Asn551) were set to be flexible with ‘crystal mode’. The default parameters were used with the GoldScore scoring function, and 30 docking runs per ligand were performed. All computation calculations were undertaken on an Intel® XeonTM Quad-core 2.5 GHz workstation with Linux Cent OS release 5.5.
Acknowledgement
This research was supported by Grants R11-2007-107-02001-0 from the National Research Foundation of Korea (NRF), the National Core Research Center (NCRC) program (No. 2011-0006244) of MEST and NRF through the Center for Cell Signaling & Drug Discovery Research at Ewha Womans University, and the intramural program of the National Institutes of Health, Center for Cancer Research, National Cancer Institute (project Z1A BC 005270).
References and Notes
- 1.Szallasi A, Blumberg PM. Pharmacol. Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- 2.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 4.Zygmunt PM, Petersson J, Andersson DA, Chuang H-H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 5.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Proc. Natl. Acad. Sci. USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walpole CSJ, Wrigglesworth R. Capsaicin in the Study of Pain. San Diego, CA: Academic Press; 1993. p. 63. [Google Scholar]
- 7.Appendino G, Szallasi A. Life Sci. 1997;60:681–696. doi: 10.1016/s0024-3205(96)00567-x. [DOI] [PubMed] [Google Scholar]
- 8.Szallasi A, Cruz F, Geppetti P. Trends in Mol. Med. 2006;12:545–554. doi: 10.1016/j.molmed.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Voight EA, Kort ME. Exp. Opin. Ther. Pat. 2010;20:1–16. doi: 10.1517/13543776.2010.497756. [DOI] [PubMed] [Google Scholar]
- 10.Lazar J, Gharat L, Khairathkar-Joshi N, Blumberg PM, Szallasi A. Exp. Opin. Drug Discov. 2009;4:159–180. doi: 10.1517/17460440802681300. [DOI] [PubMed] [Google Scholar]
- 11.Gunthorpe MJ, Chizh BA. Drug Disc. Today. 2009;14:56–67. doi: 10.1016/j.drudis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Wong GY, Gavva NR. Brain Res. Rev. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Kym PR, Kort ME, Hutchins CW. Biochem. Pharmacol. 2009;78:211–216. doi: 10.1016/j.bcp.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Szabo T, Welter JD, Toth A, Tran R, Lee J, Kang SU, Lee Y-S, Min KH, Suh Y-G, Park M-K, Park H-G, Park Y-H, Kim H-D, Oh U, Blumberg PM, Lee J. Mol. Pharm. 2002;62:947–956. doi: 10.1124/mol.62.4.947. [published erratum appears in Mol. Pharmacol. 2003, 63, 958] [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Toth A, Tran R, Szabo T, Welter JD, Blumberg PM, Lee J, Kang S-U, Lim J-O, Lee J. Mol. Pharm. 2003;64:325–333. doi: 10.1124/mol.64.2.325. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Lee J, Kang M, Shin M-Y, Kim J-M, Kang S-U, Lim J-O, Choi H-K, Suh Y-G, Park H-G, Oh U, Kim H-D, Park Y-H, Ha H-J, Kim Y-H, Toth A, Wang Y, Tran R, Pearce LV, Lundberg DJ, Blumberg PM. J. Med. Chem. 2003;46:3116–3126. doi: 10.1021/jm030089u. [DOI] [PubMed] [Google Scholar]
- 17.Suh Y-G, Lee Y-S, Min K-H, Park O-H, Kim J-K, Seung H-S, Seo S-Y, Lee B-Y, Nam YH, Lee K-O, Kim H-D, Park H-G, Lee J, Oh U, Lim J-O, Kang S-U, Kil M-J, Koo J-Y, Shin SS, Joo Y-H, Kim JK, Jeong Y-S, Kim S-Y, Park Y-H. J. Med. Chem. 2005;48:5823–5836. doi: 10.1021/jm0502790. [DOI] [PubMed] [Google Scholar]
- 18.Ryu H, Jin M-K, Kang S-U, Kim SY, Kang DW, Lee J, Pearce LV, Pavlyukovets VA, Morgan MA, Tran R, Toth A, Lundberg DJ, Blumberg PM. J. Med. Chem. 2008;51:57–67. doi: 10.1021/jm701049p. [DOI] [PubMed] [Google Scholar]
- 19.Stahly GP, Stahly BC, Lilje KC. J. Org. Chem. 1984;49:579–580. [Google Scholar]
- 20.Lee JH, Lee Y, Ryu H, Kang DW, Lee J, Lazar J, Pearce LV, Pavlyukovets VA, Blumberg PM, Choi S. J. Comput. Aided Mol. Des. 2011 doi: 10.1007/s10822-011-9421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]