Abstract
Background
Retinal detachment (RD) with proliferative vitreoretinopathy (PVR) often requires surgery. During surgery, a tamponade agent is needed to reduce the rate of recurrent retinal detachment.
Objectives
The objective of this review was to evaluate the benefits and adverse outcomes of surgery with various tamponade agents.
Search methods
We searched the Cochrane Controlled Register (CENTRAL), MEDLINE, EMBASE, Latin America and Carribbean Health Sciences (LILACS) and the UK Clinical Trials Gateway (UKCTG). There were no language or date restrictions in the search for trials. The electronic databases were last searched on 9 July 2009.
Selection criteria
We included randomized clinical trials comparing patients treated with various tamponade agents.
Data collection and analysis
Two individuals screened the search results independently. One study with two trials was eligible for inclusion in the review.
Main results
One study with two trials was included in the review. The first trial randomized 151 eyes to receive either silicone oil or sulfur hexafluoride (SF6) gas tamponades; the second trial randomized 271 eyes to receive either silicone oil or perfluropropane (C3F8 ) gas tamponades. In patients with RD associated with PVR, pars plana vitrectomy and infusion of either silicone oil or perfluropropane gas appear comparable for a broad variety of cases. Sulfur hexafluoride gas was associated with worse anatomic and visual outcomes than either silicone oil or perfluropropane gas.
Authors’ conclusions
The use of either C3F8 or silicone oil appears reasonable for most patients with RD associated with PVR. Because there do not appear to be any major differences in outcomes between the two agents, the choice of a tamponade agent should be individualized for each patient.
Plain language summary
Tamponade in surgery for retinal detachment associated with proliferative vitreoretinopathy
Retinal detachment (RD) remains a significant cause of vision loss. Most recurrent RDs are associated with varying degrees of proliferative vitreoretinopathy (PVR), or the growth of fibrous membranes (similar to scar tissue) along the surface of the retina. The only proven therapy for RD with PVR is surgery. Injection of a tamponade agent is performed at the time of surgery to reduce the rate of fluid flow through open retinal tears, which would cause recurrent RD. The major tamponade agents available today are various gases and silicone oils. One study consisting of two independently randomized clinical trials was included in this review. The Silicone Study compared the use of silicone oil tamponades to either sulfur hexafluoride (SF6) gas or perfluropropane (C3F8) gas tamponades in patients undergoing surgery to treat RD associated with PVR. When silicone oil was compared to SF6 gas, eyes randomized to receive silicone oil were more likely to achieve a final visual acuity of 5/200 or better at one year, and more likely to achieve macular attachment at one year; both of these differences were statistically significant. When silicone oil was compared with C3F8 gas, there were no statistically significant differences between the groups with respect to visual acuity or macular attachment at one year. The use of either C3F8 gas or silicone oil appears to offer similar benefits, in terms of their ability to reattach the retina and to preserve or improve visual function.
Background
Description of the condition
Introduction
Retinal detachment (RD) remains a significant cause of vision loss. A variety of surgical techniques are available to treat RD. In general, these procedures have a very high rate of successful anatomic retinal reattachment for primary RD (overall above 90%) (Schwartz 2004). The recent Scleral Buckling versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment (SPR) study, which excluded many relatively straightforward cases, reported single operation success rates between 60% to 80%, depending on the subgroup, and 73% overall (Heimann 2007). Most recurrent RDs are associated with varying degrees of proliferative vitreoretinopathy (PVR), or the growth of fibrous membranes (similar to scar tissue) along the surface of the retina, which leads to traction on the retina (TRSTC 1983).
Epidemiology
Recurrent RD with PVR occurs in about 5% to 10% of patients (Charteris 2002). Major risk factors include RD in the inferior (lower) portion of the eye (Singh 1986), severe ocular trauma (Kruger 2002), and giant retinal tear (Scott 2002). Other reported risk factors for recurrent RD with PVR include: the inability to identify a retinal break, the use of pars plana vitrectomy in the initial repair, preoperative PVR, preoperative choroidal detachment, and relatively greater use of cryopexy ( Cowley 1989). Recurrent RD with PVR may require multiple additional surgeries and is associated with poorer visual outcomes. These additional surgeries are associated with significantly increased costs (Patel 2004).
Presentation and diagnosis
Proliferative vitreoretinopathy is usually diagnosed within the first few months after RD surgery. Symptoms include decreased vision in the affected eye. The diagnosis is made by dilated fundus examination in the office or outpatient clinic.
Description of the intervention
Vitreoretinal surgery is standard treatment for recurrent RD with PVR. Pars plana vitrectomy (PPV), removal of the epiretinal membranes, treatment of the retinal breaks, and injection of a tamponade agent are performed. In some cases, removal of the lens (either the crystalline lens or a previously placed intraocular lens) is performed. Tamponade is necessary to reduce the rate of fluid flow through open retinal tears, which would cause recurrent RD. The major tamponade agents available today are various gases and silicone oils. Currently available gases include air, sulfur hexafluoride (SF6), hexafluroethane (C2F6 ) and perfluropropane (C3F8). The major advantage of gas tamponade is that the gas spontaneously dissipates, usually over several weeks. Currently available silicone oils come in 1000-centistoke and 5000-centistoke viscosities. Silicone oil is permanent and may eventually require surgical removal.
There are several investigational tamponade agents, including heavy silicone oil (polydimethylsiloxane (PDMS) 1000) ( Tognetto 2005), perfluorohexylethan (O62) (Hoerauf 2005), perfluoro-n-octane (PFO) (Rofail 2005), a mixture of perfluorohexyloctane (F6H8) in silicone oil (Stappler 2008), and a mixture of perfluorohexyloctane (F6H8) in PDMS 1000 (Tognetto 2008; Heimann 2008). Various tamponade agents with a specific gravity greater than that of water have shown evidence of toxicity in animal models, in rat retinal cell cultures in vitro, and in clinical reports (Eckardt 1990; Matteucci 2007; Singh 2001).
Tamponade agents are useful in four broad categories of patients with RD:
Patients with primary RD, treated with PPV as a first-line procedure. These patients are generally treated with gas tamponade, rather than silicone oil.
Patients with complex or recurrent RD associated with PVR. These patients are the focus of this review. These patients are generally treated with either gas or silicone oil.
Patients with RD associated with giant retinal tear. These patients are generally treated with either gas or silicone oil.
Patients with inferior RD, treated with PPV as a first-line procedure. Some surgeons are using heavy liquids, such as PFO or heavy silicone oil, as investigational agents in these patients.
How the intervention might work
Tamponade agents are believed to work by reducing or eliminating fluid vectors through open retinal breaks until the applied retinopexy (typically photocoagulation or cryopexy) creates a permanent seal. Gases, such as SF6 and C3F8, spontaneously dissipate, while silicone oil is permanent and may eventually require removal.
Why it is important to do this review
The various tamponade agents offer different advantages and disadvantages in terms of safety and effectiveness (Krzystolik 2000; Young 2005). A systematic review may assist surgeons in selection of a tamponade agent.
Objectives
The objective of this review was to assess the relative safety and effectiveness of various tamponade agents used with PPV for RD complicated by PVR. The specific comparisons depended on the trials we identified in the search. We intended to compare:
the various gas tamponade agents with each other;
the two silicone oil preparations to each other;
the various gas agents versus the various silicone oils;
the established agents (gases, silicone oil) versus the investigational agents.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials. There were no limitations on the various treatment arms compared.
Types of participants
We included trials in which participants underwent surgical repair of RD associated with PVR. There were no limitations with respect to age or cause of RD.
Types of interventions
We included trials which studied agents used as tamponade in the treatment of RD associated with PVR, such as air, sulfur hexafluoride (SF6), hexafluroethane (C2F6), perfluropropane (C3F8) and silicone oil, as well as investigational agents such as heavy silicone oil (polydimethylsiloxane 1000), perfluorohexylethan (O62), and perfluoro-n-octane (PFO).
Types of outcome measures
Primary outcomes
The primary outcome for this review was visual acuity at one year. We analyzed outcomes at additional times of follow up as reported in included trials. We intended to compare visual acuity as a dichotomous outcome (the proportion of participants who lost 3 or more lines of logMAR visual acuity; patients who lost 1 or 2 lines of logMAR visual acuity were considered stabilized) and also as a continuous outcome (mean logMAR scores). We considered other dichotomous and continuous visual acuity outcomes as reported in included trials.
Secondary outcomes
The secondary outcome for this review was macular attachment at one year.
Adverse effects (severe, minor)
Severe:
Retina detached at one year;
Visual acuity worse than 20/200 (regardless of anatomic outcome).
Minor:
Intraocular pressure (IOP) greater than 21 mmHg;
Visually significant cataract.
Quality of life measures
We intended to examine patient satisfaction, subjective visual improvement and other quality of life measures evaluated using a validated scale.
Economic data
We intended to summarize direct and indirect costs of surgery and rehabilitation and any other economic data in the included studies.
Follow up
We restricted studies to those with at least one year of follow-up. We believe that shorter follow-up periods are less clinically relevant.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library, Issue 3, 2009), MEDLINE (January 1950 to July 2009), EMBASE (January 1980 to July 2009), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to July 2009) and the UK Clinical Trials Gateway (UKCTG). There were no language or date restrictions in the search for trials. The electronic databases were last searched on 9 July 2009.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4) and the UKCTG (Appendix 5).
Searching other resources
We searched the reference lists of the studies included in the review for other potential inclusions. We did not search conference proceedings for the purpose of this review. Although we did not initially intend to contact individuals or organizations specifically for this review, because we did not believe that doing so would add significantly to the data obtainable through published trials, we contacted the investigators of included studies for clarification of methods and other data reported in published manuscripts.
Data collection and analysis
Selection of studies
At least two authors, working independently, reviewed the titles and abstracts resulting from the searches. Each author reviewed the full text manuscripts of all possibly or definitely relevant studies to determine eligibility for inclusion. We resolved any discrepancies through discussion. We did not mask trial details in this process.
Data extraction and management
Extraction of study characteristics
We extracted the following information for each trial.
Methods: method of allocation, masking (blinding), exclusions after randomization, losses to follow up and compliance, unusual study design.
Participants: country where participants enrolled, number randomized, age, sex, inclusion/exclusion criteria.
Interventions: test intervention, comparison intervention (control), duration of intervention.
Outcomes: visual acuity, retinal reattachment rate, complication rates, adverse effects, quality of life and economic outcomes.
Notes: additional details (such as funding sources).
Data extraction and entry
Two authors, working independently, extracted the outcome data. One review author entered the data into RevMan 5 and a second author verified the data entry. Main outcome measures were visual acuity, retinal reattachment rate and various complication rates. This included dichotomous data (such as retinal reattachment, percentage of participants who lost 3 or more lines of logMAR visual acuity) as well as continuous data (such as logMAR visual acuity).
Assessment of risk of bias in included studies
We reviewed the risk of bias of included studies as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). At least two authors assessed the risk of bias for each included study according to the following criteria:
Selection bias (randomized sequence generation and allocation concealment).
Performance bias (masking of participants and researchers).
Attrition bias (incomplete outcome data adequately addressed).
Detection bias (masking of outcome assessors).
Reporting bias (free of selective outcome reporting).
Each area of potential bias was judged as ‘Yes’ for low risk of bias, ‘No’ for high risk of bias, or ‘Unclear’ risk of bias. We considered methods such as centralized randomization, and use of sequential opaque envelopes as evidence of adequate allocation concealment. We evaluated any exclusions after randomization, losses to follow up and differential reasons for losses to follow up in the treatment groups. Any discrepancies were resolved through discussion.
We recognized that masking of patients and surgeons (performance bias) and masking of persons assessing outcome (detection bias) may not be possible in studies comparing gas to silicone oil. However, if we identified studies that had successfully masked outcome data (such as studies in which visual acuity is obtained by an examiner masked to the tamponade agent), these studies were emphasized.
Measures of treatment effect
We reported unpooled risk ratios with 95% confidence intervals for dichotomous outcomes visual acuity and macular attachment for Silicone Study 1992a and Silicone Study 1992b. If continuous outcomes are included in future updates of the review, we will calculate mean differences or standardized mean differences.
We intended to focus on ‘all gases versus silicone oil’, but the outcomes as reported in the included studies were not appropriately compared in this manner. This will be considered for future updates of the review.
Unit of analysis issues
The unit of analysis for outcomes was eyes of individuals.
Dealing with missing data
Primary authors of included studies were contacted to provide 12 month visual acuity and macula status outcome data. Data were not imputed using available information, but will be considered for future updates of the review. The assumptions made during imputation will be indicated.
Assessment of heterogeneity
We intended to test for heterogeneity using the Chi2 test and the I2 value, but since no pooled estimates were included, tests of heterogeneity were not applicable. If data synthesis is considered at the time of an update to this review, we will follow the following guidelines. If the I2 value is greater than 50% we will consider it to indicate substantial heterogeneity. In such a situation we will not report a pooled estimate. Instead, we will report a narrative or tabulated summary of the included studies. We will use a random-effects model to incorporate the heterogeneity if the I2 value is less than 50% unless there are fewer than three studies. If we detect no statistical or clinical heterogeneity (from details listed in the table of included studies), we will use a fixed-effect model.
Assessment of reporting biases
Assessment of reporting biases will be explored, as appropriate, in future updates of the review according to the guidelines in Chapter 10 of the Cochrane Handbook for Systematic Review of Interventions (Sterne 2008).
Data synthesis
No pooled estimates of included studies are reported. If pooled estimates are considered for future updates of the review, we will follow the guidelines in Chapter 9 of the Cochrane Handbook for Systematic Review of Interventions (Deeks 2008).
Subgroup analysis and investigation of heterogeneity
We will consider subgroup analyses as appropriate in future updates of this review and will consult the guidelines for investigating heterogeneity in Chapter 9 of the Cochrane Handbook for Systematic Review of Interventions (Deeks 2008). One possible strategy is to divide patients by surgical history, such as patients with chronic RD with PVR and no previous surgery, patients with recurrent RD following scleral buckling only, and patients with recurrent RD following PPV and previous intravitreal tamponade (gas or oil). Another possible strategy is to divide patients with certain high-risk clinical features, such as patients with giant retinal tear, patients with open-globe trauma, and pediatric patients.
Sensitivity analysis
We planned to examine the impact of the exclusion of unpublished and industry-funded studies in sensitivity analyses, but this is not applicable to the current systematic review.
Results
Description of studies
Results of the search
Our literature search yielded a total of 348 titles and abstracts. After independent review of the references by two review authors we identified 19 studies which appeared to be relevant.
Included studies
We identified one study that consisted of two trials that met our inclusion criteria. Enrollment for the first trial comparing silicone oil to SF6 gas occurred between September 1985 to September 1987 (Silicone Study 1992a). For the second part of the study period, SF6 gas was replaced with the longer-lasting C3F8 gas. Enrollment for the second trial comparing silicone oil to C3F8 occurred between September 1987 to October 1990 (Silicone Study 1992b). Patients aged 18 years or older, and with RD associated with PVR, were offered randomization. One eye per patient was randomized and grouped as (Group 1) eyes that had not undergone prior vitrectomy or (Group 2) eyes that had undergone vitrectomy but without silicone oil injection. The first trial included 113 eyes in Group 1 and 38 eyes in Group 2; the second trial included 132 eyes in Group 1 and 139 eyes in Group 2. The exclusion criteria were uncontrolled concomitant eye disease, a history of blunt trauma within three months of entry into the study, a history of penetrating trauma, a giant retinal tear of 90 degrees or greater, proliferative diabetic retinopathy, and any medical condition that could preclude participation in a three-year study.
There was one reference for an ongoing study (HSO Study) that has yet to publish results, described in the ‘Characteristics of ongoing studies’ table, and one reference for an American Academy of Ophthalmology (AAO) abstract (Oncel 2006) that may be relevant to this review, currently listed in the ‘Characteristics of studies awaiting classification’ table until further details are reported.
Characteristics of ongoing studies
| HSO Study | |
| Study name | The Heavy Silicone Oil versus Standard Silicone Oil Study (HSO) |
| Methods | Randomized controlled trial to determine safety and efficacy of vitrectomy with two types of endotamponades |
| Participants | Multicenter study enrolling patients with inferior and posterior PVR grade C-A6, P12 according to Machemer or inferior detachment with giant retinal tear in the inferior hemisphere |
| Interventions | Two-arm parallel group design:
|
| Outcomes | Main endpoints: Complete retinal attachment at 12 months and change of visual acuity at 12 months postoperatively |
| Secondary endpoints: Complete retinal attachment before endotamponade removal, quality of life analysis, and the evaluation of the number of retina affecting re-operation within 1 year of follow-up | |
| Other endpoints: Emulsification of the compound, cataract formation, number of eyes with constantly raised intraocular pressure from baseline, and the number of eyes with glaucoma | |
| Starting date | January 2004 |
| Contact information | Antonia M Joussen, Department of Ophthalmology, University of Düsseldorf, Moorenstrasse 5, Düsseldorf, Germany; |
| Notes | The present article describes the methods and design. The investigators have not published results. |
Characteristics of studies awaiting classification
| Oncel 2006 | |
| Methods | Randomized controlled trial of 45 participants with complicated retinal detachments |
| Participants | Patients with complicated retinal detachments |
| Interventions | Heavy silicone oil (viscosity of 1400cSt, density = 1.06 gr/cm3) |
| Standard silicone oil | |
| Outcomes | Retinal reattachment (time of follow-up is unknown) |
| Notes | Conference abstract from the American Academy of Ophthalmology Meeting (2006) |
Excluded studies
We excluded the remaining 16 studies, listed in the ‘Characteristics of excluded studies’ table with reasons for exclusion.
Characteristics of excluded studies
| Avci 2001 | |
| Reason for exclusion | Patients were not randomized |
| Brazitikos 2005 | |
| Reason for exclusion | Investigated tamponade agents after pars plana vitrectomy surgery |
| Gao 1993 | |
| Reason for exclusion | Case series |
| Hammer 1997 | |
| Reason for exclusion | Study reported only 180 days of follow-up |
| Hutchins 2003 | |
| Reason for exclusion | Case series |
| Krasnik 1999 | |
| Reason for exclusion | Not relevant to the review |
| Latecka-Krajewska 1998 | |
| Reason for exclusion | Not randomized |
| Li 1995 | |
| Reason for exclusion | Case series |
| Lu 2002 | |
| Reason for exclusion | Retrospective study |
| Pastor 1998 | |
| Reason for exclusion | Retrospective study |
| Pertile 1999 | |
| Reason for exclusion | Not relevant to the review |
| Peyman 1987 | |
| Reason for exclusion | Average follow-up 8.4 months |
| Soheilian 2006 | |
| Reason for exclusion | Retrospective study |
| Stefer 1991 | |
| Reason for exclusion | Case series |
| Tufail 1997 | |
| Reason for exclusion | Not relevant to the review |
| van Effenterre 1987 | |
| Reason for exclusion | Case series |
Risk of bias in included studies
Two trials, reported as the Silicone Study, met the inclusion criteria for this review (Silicone Study 1992a; Silicone Study 1992b). Since the two included trials were part of the same study protocol, they follow the same design, methods, and analyses (Azen 1991 in Silicone Study 1992a). This study was of good methodological quality and at low risk of bias (Figure 1).
Figure 1.
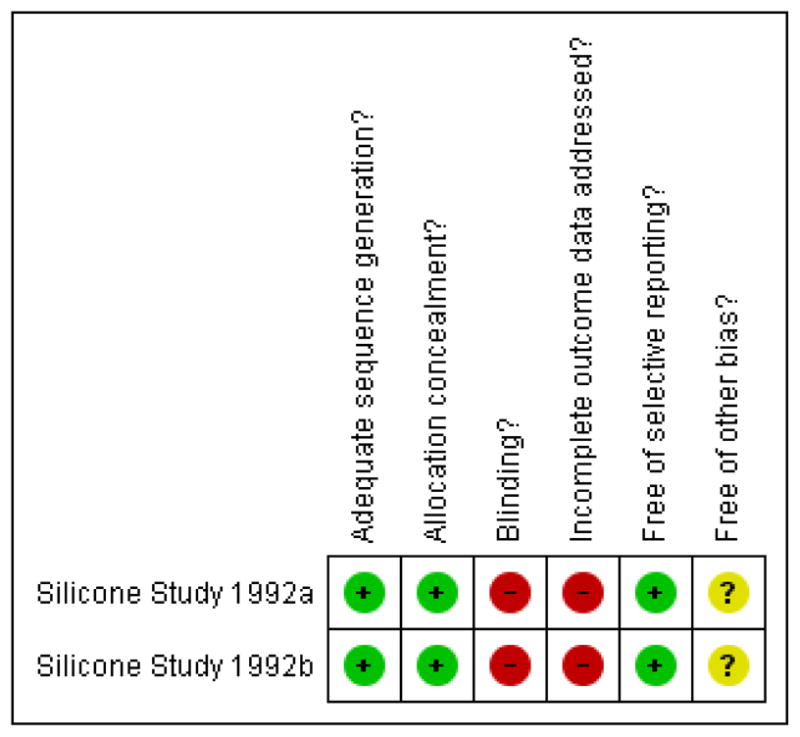
Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Allocation
The randomization scheme was centralized through the Data Coordinating Center and employed stratification and blocking methods to ensure equal treatment assignments within each clinical center. The treatment allocation was adequately concealed for the randomization process with sequential opaque envelopes delivered to each study site and opened at the time of tamponade injection.
Blinding
The study outcome assessors and surgeons were not masked.
Incomplete outcome data
Last observation carried forward method was used for missing data. Data were inferred for patients who missed intermediate examinations, but attended prior and subsequent examinations only when findings were deemed consistent. In the event that a retinal detachment reoccurred during the study period and required surgery, patients were analyzed using the original randomized treatment allocation. Randomized participants from a study center that ceased recruitment during the study period were excluded from the analysis (12 participants from the first trial and six from the second trial).
Selective reporting
This study appeared to be free of selective reporting since primary and secondary outcomes were published a priori in a methods paper (Azen 1991 in Silicone Study 1992a).
Other potential sources of bias
Fourteen baseline characteristics were compared between treatment arms (age, sex, study eye, prior scleral buckle, other ocular surgery, mean duration of RD, Retina Society classification, visual acuity, refractive status, IOP, corneal status, aqueous flare, aqueous cell, and neovascularization). The Silicone Study investigators reported one statistically significant difference in baseline characteristics between eyes of patients assigned to receive SF6 gas and those assigned to receive silicone oil (Silicone Study 1992a). The estimated duration of RD was greater in Group 2 eyes (eyes of patients with prior vitrectomy but without silicone oil injection) randomized to SF6 compared to Group 2 eyes randomized to silicone oil.
Effects of interventions
The Silicone Study conducted two trials, one comparing silicone oil (1000 centistokes) with SF6, and one comparing silicone oil (1000 centistokes) with C3F8. When silicone oil (1000 centistokes) was compared with SF6, the study investigators reported that Group 1 eyes randomized to receive silicone oil were more likely to achieve a visual acuity of 5/200 or better at one year, and more likely to achieve macular attachment at one year; both of these differences were statistically significant. There was no statistically significant difference between the groups with respect to IOP greater than or equal to 30 mmHg. At baseline, about 60% of the eyes were pseudophakic or aphakic, and the crystalline lens was subsequently removed in 81% of the phakic eyes (Silicone Study 1992a). At 24 months, there was no difference in the proportion of eyes of achieving a visual acuity of 5/200 among Group 1 eyes randomized to silicone oil or SF6 (risk ratio (RR): 1.57; 95% confidence interval (CI): 0.93, 2.66; Analysis 1.1, Figure 2), but Group 1 eyes randomized to silicone oil were more likely than eyes randomized to SF to achieve macular attachment at 24 months (RR: 1.37; 95% CI: 1.01, 1.86; Analysis 1.2, Figure 3).
Figure 2.
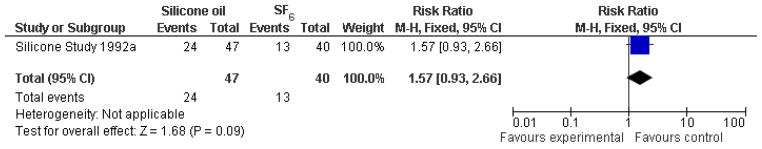
(Analysis 1.1). Forest plot of comparison: 1 Silicone oil versus SF6, outcome: 1.1 Visual acuity ≥ 5/200 at 24 months.
Figure 3.
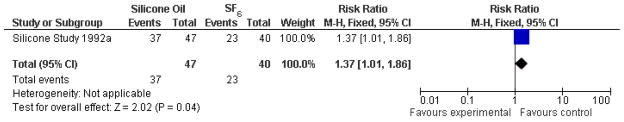
(Analysis 1.2). Forest plot of comparison: 1 Silicone oil versus SF6, outcome: 1.2 Macular attachment at 24 months.
When silicone oil (1000 centistokes) was compared with C3F8, there were no statistically significant differences between the groups with respect to visual acuity of 5/200 or better at one year or last follow-up examination (Analysis 2.1, Figure 4), macular attachment at one year or last follow-up examination (Analysis 2.2, Figure 5), or IOP greater than or equal to 30 mmHg. In the subgroup of eyes which had not undergone previous PPV (Group 1), eyes randomized to receive C3F8 were associated with an increased likelihood of achieving macular attachment beyond one year following surgery, which was of borderline statistical significance. Overall, eyes randomized to receive either C3F8 or silicone oil achieved better anatomic and visual outcomes than eyes randomized to receive SF6 (Silicone Study 1992b).
Figure 4.
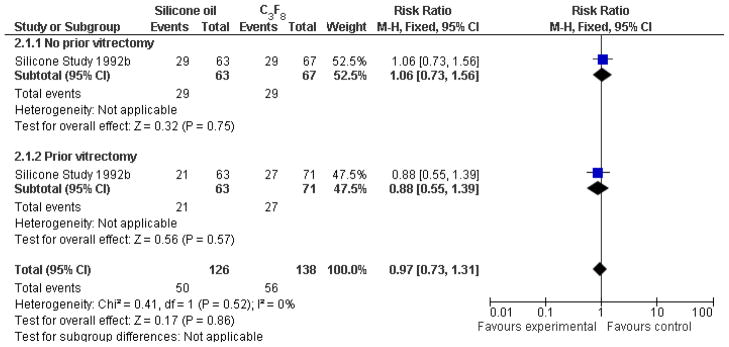
(Analysis 2.1). Forest plot of comparison: 2 Silicone oil versus perfluropropane (C3F8), outcome: 2.1 Visual acuity ≥ 5/200 at last follow-up examination.
Figure 5.
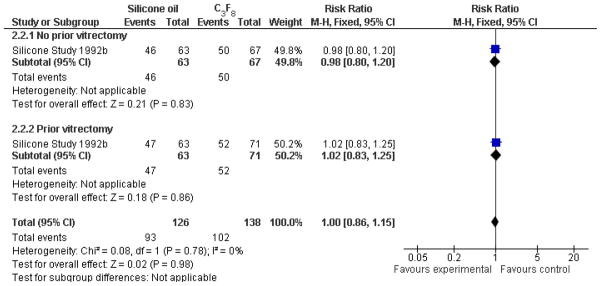
(Analysis 2.2). Forest plot of comparison: 2 Silicone oil versus perfluropropane (C3F8), outcome: 2.2 Macular attachment at last follow-up examination.
SF6, C3F8, and silicone oil can worsen cataracts. However, it seems unlikely that cataract progression played a major role in the visual outcomes, because most eyes were pseudophakic or aphakic at one year. In the silicone oil versus SF6 study, about 40% of the eyes were phakic at baseline, and the lens was subsequently removed in 81% of these eyes (Silicone Study 1992a). In the silicone oil versus C3F8 study, 48% of eyes were phakic at baseline, and the lens was subsequently removed in about 90% of these eyes (Silicone Study 1992b).
The Silicone Study recorded visual acuity using Diabetic Retinopathy Vitrectomy Study protocol and charts. The Silicone Study did not specifically address quality of life measurements and economic analysis.
Discussion
Summary of main results
The Silicone Study was a series of two well-designed prospective, multicenter, randomized clinical trials of patients with RD associated with PVR. The first trial, comparing silicone oil to SF6, was conducted between 1985 to 1987. The second trial, comparing silicone oil to C3F8, was conducted between 1987 to 1990. Pars plana vitrectomy and infusion of either silicone oil or C3F8 gas appeared to show comparable results for final visual acuities of 5/200 or better at one year and macular attachments at one year. SF6 gas was associated with worse anatomic and visual outcomes than either silicone oil or perfluropropane gas, although some of these differences diminished after two years.
Overall completeness and applicability of evidence
In the intervening two decades since the study began, there have been many advances in vitrectomy instrumentation, intraoperative viewing systems, and surgical techniques. The silicone oil used in the Silicone Study was not approved by the U.S. Food and Drug Administration (FDA) and differed in many respects from the higher quality, more purified oils used today.
In addition, although SF6 and C3F8 are still used today, many surgeons now prefer 5000-centistoke silicone oil to the 1000-centistoke oil used in the Silicone Study, although anatomic and visual outcomes are generally similar with either oil viscosity (Scott 2005).
Perfluoro-n-octane (PFO) became available in 1988 as an intraoperative tool to achieve retinal reattachment. Perfluoro-n-octane was not available for any of the patients enrolled in the first trial (oil versus SF6), which completed enrolment in 1987. Perfluoro-n-octane was available for some, but not all, patients enrolled in the second trial (oil versus C3F8). In addition, the investigational use of PFO and other heavy liquids as intermediate-term tamponade agents was not described until more recently.
The Silicone Study also excluded participants with a history of penetrating trauma, giant retinal tears greater than 90 degrees, and proliferative diabetic retinopathy. For these reasons, the results reported by the Silicone Study may not be applicable to patients undergoing contemporary surgical procedures.
Authors’ conclusions
Implications for practice
Based on results from the Silicone Study, patients with recurrent RD associated with PVR appear to have good results with PPV with either C3F8 gas or silicone oil tamponades. There is a suggestion that C3F8 may have certain advantages with respect to long-term anatomic outcomes in some patients, although the visual results appear similar between the tamponade agents. The choice of tamponade agent is usually made on an individual, patient-by-patient basis. Factors to be considered include the configuration of the detachment, the location of the retinal breaks, the lens status, the visual status of the fellow eye, the patient’s ability to comply with postoperative positioning requirements, the patient’s need to travel by air in the early postoperative period, and individual physician and patient preferences.
As tamponade agents, C3F8 and silicone oil appear to have visual and anatomic advantages over SF6, especially within the first year after surgery, but SF6 may be a reasonable choice in certain clinical situations.
Implications for research
The Silicone Study delineated various relative advantages and disadvantages of 1000-centistoke silicone oil, SF6, and C3F8 as tamponade agents. Formal evaluation of 5000-centistoke silicone oil in a prospective clinical trial appears warranted, but to our knowledge there are no plans to do so at this time. Future research may develop alternative tamponade agents. Properties of an ideal tamponade agent include: optical clarity, lack of toxicity, no effect on the eye’s refractive state, no effect on IOP or cataract formation, inhibition of cellular migration, inhibition of gliosis or glial proliferation, and density greater than water, which would reduce the postoperative positioning requirements for many patients.
Characteristics of included studies
| Silicone Study 1992a | ||
|
| ||
| Methods | Unmasked, multicenter randomized clinical trial | |
| Eyes of patients were stratified as follows (only one eye per patient randomized): | ||
| Eyes that had not undergone prior vitrectomy (Group 1) | ||
| Eyes that had undergone vitrectomy but without silicone oil injection (Group 2) | ||
| Number of eyes randomized: 113 (Group 1) and 38 (Group 2) | ||
|
| ||
| Participants | Inclusion criteria: Patients with proliferative vitreoretinopathy with C-3 grade, at least age 18, visual acuity better than light perception, and sufficient contracture so intraocular dissection was required | |
| Exclusion criteria: Patients with uncontrolled concomitant eye disease, occurrence of blunt trauma to the eye within 3 months of randomization, history of penetrating trauma to the eye, presence of a giant tear ≥90°, presence of proliferative diabetic retinopathy, and the existence of any condition that would prevent 3-year follow-up | ||
|
| ||
| Interventions | Type of tamponade | |
| Sulfur hexafluoride gas (SF6): 49 eyes (Group 1), 15 eyes (Group 2) | ||
| Silicone oil: 52 eyes (Group 1), 23 eyes (Group 2) | ||
|
| ||
| Outcomes | Visual acuity, retinal reattachment, refraction; development or change in ocular complications affecting the cornea, iris, or lens; and measurements of intraocular pressure at 10 days, and 1, 3, 6, 12, 18, 24, and 36 months following randomization | |
| Number of eyes included in the 24 month analysis: 40 of 49 eyes (Group 1) randomized to sulfur hexafluoride; 47 of 52 eyes (Group 1) randomized to silicone oil | ||
|
| ||
| Notes | Trial dates: September 1, 1985 to June 30, 1991; one center terminated follow-up in 1988 and patient data were excluded (n = 12 from Group 1). | |
| Twelve month visual acuity and macula status outcomes were displayed in graphs; investigators contacted for 12 month outcomes. | ||
|
| ||
| Risk of bias table | ||
|
| ||
| Item | Authors’ judgement | Support for judgement |
|
| ||
| Adequate sequence generation? | Yes | Randomization scheme generated by the Data Coordinating Center; Stratification and blocking methods employed to ensure equal treatment assignments within each clinical center. |
|
| ||
| Allocation concealment? | Yes | Investigators used sealed envelopes supplied in limited numbers by the Data Coordinating Center. |
|
| ||
| Blinding? | No | “A surgeon cannot be masked to the treatment during the operative procedure. During follow-up examinations, silicone fluid produces a characteristic appearance in the eye unlike that of a long-acting gas, making it impossible to mask study technicians.” |
|
| ||
| Incomplete outcome data addressed? | No | Last observation carried forward method used for missing data, but data inferred only if “consistent” findings for prior and subsequent examinations. Randomized participants from a center that ceased recruitment (n = 12) and randomized participants with a history of prior vitrectomy (n = 38) were excluded from the analysis. |
|
| ||
| Free of selective reporting? | Yes | This study appeared to be free of selective reporting. Primary and secondary outcomes were reported in a prior manuscript describing trial design and participant baseline characteristics. |
|
| ||
| Free of other bias? | Unclear | The baseline estimated duration of retinal detachment was greater in Group 2 eyes (eyes of patients with prior vitrectomy but without silicone oil injection) randomized to SF6 compared to Group 2 eyes randomized to silicone oil. Trial sponsored by the National Eye Institute. Silicone oil provided by the Dow Corning Corporation. |
|
| ||
| Silicone Study 1992b | ||
|
| ||
| Methods | Unmasked, multicenter randomized clinical trial | |
| Eyes of patients were stratified as follows (only one eye per patient randomized): | ||
| Eyes that had not undergone prior vitrectomy (Group 1) | ||
| Eyes that had undergone vitrectomy but without silicone oil injection (Group 2) | ||
| Number of eyes randomized: 132 (Group 1) and 139 (Group 2) | ||
|
| ||
| Participants | Inclusion criteria: Patients with proliferative vitreoretinopathy (C-3 grade), at least age 18, visual acuity better than light perception, and sufficient contracture so intraocular dissection was required | |
| Exclusion criteria: Patients with uncontrolled concomitant eye disease, occurrence of blunt trauma to the eye within 3 months of randomization, history of penetrating trauma to the eye, presence of a giant tear ≥90°, presence of proliferative diabetic retinopathy, and the existence of any condition that would prevent 3-year follow-up | ||
|
| ||
| Interventions | Type of tamponade | |
| Perfluropropane gas (C3F8): 67 eyes (Group 1), 71 eyes (Group 2) | ||
| Silicone oil: 64 eyes (Group 1), 63 eyes (Group 2) | ||
|
| ||
| Outcomes | Visual acuity, retinal reattachment, refraction; development or change in ocular complications affecting the cornea, iris, or lens; and measurements of intraocular pressure at 10 days, and 1, 3, 6, 12, 18, 24, and 36 months following randomization | |
| Number of eyes included in the last follow-up analysis: 67 of 67 eyes (Group 1) and 71 or 71 eyes (Group 2) randomized to C3F8 ; 63 of 64 eyes (Group 1) and 63 of 63 eyes (Group 2) randomized to silicone oil. One patient randomized to silicone oil in Group 1 died after the baseline visit. | ||
|
| ||
| Notes | Trial dates: September 1, 1987 to June 30, 1991; one center terminated follow-up in 1988 and patient data were excluded (n = 1 from Group 1; n = 5 from Group 2) | |
| Twelve month visual acuity and macula status outcomes were displayed in graphs; investigators contacted for 12 month outcomes. | ||
|
| ||
| Risk of bias table | ||
|
| ||
| Item | Authors’ judgement | Support for judgement |
|
| ||
| Adequate sequence generation? | Yes | Randomization scheme generated by the Data Coordinating Center; Stratification and blocking methods employed to ensure equal treatment assignments within each clinical center. |
|
| ||
| Allocation concealment? | Yes | Investigators used sealed envelopes supplied in limited numbers by the Data Coordinating Center. |
|
| ||
| Blinding? | No | “A surgeon cannot be masked to the treatment during the operative procedure. During follow-up examinations, silicone fluid produces a characteristic appearance in the eye unlike that of a long-acting gas, making it impossible to mask study technicians.” |
|
| ||
| Incomplete outcome data addressed? | No | Last observation carried forward method used for missing data, but data inferred only if “consistent” findings for prior and subsequent examinations. Randomized participants (n = 6) from center that ceased recruitment were excluded from the analysis. |
|
| ||
| Free of selective reporting? | Yes | This study appeared to be free of selective reporting. Primary and secondary outcomes were reported in a prior manuscript describing trial design and participant baseline characteristics. |
|
| ||
| Free of other bias? | Unclear | No differences in baseline characteristics; Trial sponsored by the National Eye Institute. Silicone oil provided by the Dow Corning Corporation. |
Acknowledgments
We acknowledge Iris Gordon, the Trials Search Co-ordinators for Cochrane Eyes and Vision Group (CEVG), for designing and conducting the electronic searches. We acknowledge support provided by the CEVG, US Project, which is funded by Contract N01-EY-2-1003, National Eye Institute, National Institutes of Health. We also acknowledge Peter Gehlbach, Barbara Hawkins, Kate Henshaw and Tianjing Li for their comments on the protocol version of this review.
Sources of support
Internal sources
Bascom Palmer Eye Institute, USA
External sources
Partially supported by NIH center grant P30-EY014801, USA
Unrestricted grant to the University of Miami from Research to Prevent Blindness, USA
Contract N01-EY2-1003, National Eye Institute, National Institutes of Health, USA
Appendices
1 CENTRAL search strategy
-
#1
MeSH descriptor Retinal Detachment
-
#2
MeSH descriptor Retinal Perforations
-
#3
MeSH descriptor Vitreous Detachment
-
#4
retina* near/2 break*
-
#5
retina* near/2 tear*
-
#6
retina* near/2 detach*
-
#7
retina* near/2 perforat*
-
#8
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
-
#9
MeSH descriptor Silicone Oils
-
#10
silicone oil*
-
#11
tamponade*
-
#12
MeSH descriptor Sulfur Hexafluoride
-
#13
sulfur hexafluoride*
-
#14
hexafluoroethane*
-
#15
MeSH descriptor Fluorocarbons
-
#16
MeSH descriptor Dimethylpolysiloxanes
-
#17
perfluoropropane*
-
#18
polydimethylsiloxane*
-
#19
perfluorohexylethan*
-
#20
perfluoro-n-octane
-
#21
(#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20)
-
#22
(#8 AND #21)
2 MEDLINE search strategy
randomized controlled trial.pt.
(randomized or randomised).ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1–7
exp animals/
exp humans/
9 not (9 and 10)
8 not 11
exp retinal detachment/
exp retinal perforation/
exp vitreous detachment/
(retina$ adj2 break$).tw.
(retina$ adj2 tear$).tw.
(retina$ adj2 detach$).tw.
(retina$ adj2 perforat$).tw.
or/13–19
exp silicone oils/
silicone oil$.tw.
tamponade$.tw.
exp sulfur hexafluoride/
sulfur hexafluoride$.tw.
hexafluoroethane$.tw.
exp fluorocarbons/
exp dimethylpolysiloxanes/
perfluoropropane$.tw.
polydimethylsiloxane$.tw.
perfluorohexylethan$.tw.
perfluoro-n-octane.tw.
or/21–32
20 and 33
12 and 34
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
3 EMBASE search strategy
exp randomized controlled trial/
exp randomization/
exp double blind procedure/
exp single blind procedure/
random$.tw.
or/1–5
(animal or animal experiment).sh.
human.sh.
7 and 8
7 not 9
6 not 10
exp clinical trial/
(clin$ adj3 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
exp placebo/
placebo$.tw.
random$.tw.
exp experimental design/
exp crossover procedure/
exp control group/
exp latin square design/
or/12–21
22 not 10
23 not 11
exp comparative study/
exp evaluation/
exp prospective study/
(control$ or prospectiv$ or volunteer$).tw.
or/25–28
29 not 10
30 not (11 or 23)
11 or 24 or 31
retina detachment/
retina tear/
vitreous body detachment/
(retina$ adj2 break$).tw.
(retina$ adj2 tear$).tw.
(retina$ adj2 detach$).tw.
(retina$ adj2 perforat$).tw.
or/33–39
silicone oil/
silicone oil$.tw.
tamponade$.tw.
sulfur hexafluoride/
sulfur hexafluoride$.tw.
hexafluoroethane$.tw.
fluorocarbon/
dimeticone/
perfluoropropane$.tw.
polydimethylsiloxane$.tw.
perfluorohexylethan$.tw.
perfluoro-n-octane.tw.
or/41–52
40 and 53
32 and 54
4 LILACS search strategy
retina$ and detach$ or perforat$ or break$ or tear and silicone or sulfur hexafluoride$ or hexafluoroethane$ or fluorocarbon$ or dimethylpolysiloxane$ or perfluoropropane$ or polydimethylsiloxane$ or perfluorohexylethan$
5 UK Clinical Trials Gateway search strategy
(tamponade or oil) and retina*
Data and analyses
1 Silicone oil versus sulfur hexafluoride (SF6)
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 1.1 Visual acuity ≥ 5/200 at 24 months | 1 | 87 | Risk Ratio(M-H, Fixed, 95% CI) | 1.57[0.93, 2.66] |
| 1.2 Macular attachment at 24 months | 1 | 87 | Risk Ratio(M-H, Fixed, 95% CI) | 1.37[1.01, 1.86] |
2 Silicone oil versus perfluropropane (C3F8)
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 2.1 Visual acuity ≥ 5/200 at last follow-up examination | 1 | 264 | Risk Ratio(M-H, Fixed, 95% CI) | 0.97[0.73, 1.31] |
| 2.1.1 No prior vitrectomy | 1 | 130 | Risk Ratio(M-H, Fixed, 95% CI) | 1.06[0.73, 1.56] |
| 2.1.2 Prior vitrectomy | 1 | 134 | Risk Ratio(M-H, Fixed, 95% CI) | 0.88[0.55, 1.39] |
| 2.2 Macular attachment at last follow-up examination | 1 | 264 | Risk Ratio(M-H, Fixed, 95% CI) | 1.00[0.86, 1.15] |
| 2.2.1 No prior vitrectomy | 1 | 130 | Risk Ratio(M-H, Fixed, 95% CI) | 0.98[0.80, 1.20] |
| 2.2.2 Prior vitrectomy | 1 | 134 | Risk Ratio(M-H, Fixed, 95% CI) | 1.02[0.83, 1.25] |
Footnotes
Declarations of interest
Harry W. Flynn, Jr., MD is a co-author on several of the studies that were eligible for inclusion in this review.
Contributions of authors
Conceiving the review: SGS
Designing the review: SGS
Coordinating the review: SGS
Undertaking manual searches: SGS, ES
Screening search results: SGS, ES
Organizing retrieval of papers: SGS, ES
Screening retrieved papers against inclusion criteria: SGS, ES
Appraising quality of papers: SGS, ES
Abstracting data from papers: SGS, ES, AE
Writing to authors of papers for additional information: SGS
Obtaining and screening data on unpublished studies: SGS
Data management for the review: SGS, ES, AE
Entering data into RevMan: SGS, ES, AE
Analysis of data: SGS, HWF, ES, AE
Interpretation of data: SGS, HWF
Writing the review: SGS, WHL, HWF, ES, AE
Securing funding for the review: SGS
Guarantor for the review: SGS
References to studies
Included studies
- Silicone Study 1992a.Abrams GW, Azen SP, Barr CC, Lai MY, Hutton WL, Trese MT, et al. The incidence of corneal abnormalities in the Silicone Study. Silicone Study Report 7. Archives of Ophthalmology. 1995;113(6):764–9. doi: 10.1001/archopht.1995.01100060090039. [DOI] [PubMed] [Google Scholar]; Abrams GW, Azen SP, McCuen BW, 2nd, Flynn HW, Jr, Lai MY, Ryan SJ. Vitrectomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy: results of additional and long-term follow-up. Silicone Study report 11. Archives of Ophthalmology. 1997;115(3):335–43. doi: 10.1001/archopht.1997.01100150337005. [DOI] [PubMed] [Google Scholar]; * Anonymous. Vitrectomy with silicone oil or sulfur hexafluoride gas in eyes with severe proliferative vitreoretinopathy: results of a randomized clinical trial. Silicone Study Report 1. Archives of Ophthalmology. 1992;110(6):770–9. doi: 10.1001/archopht.1992.01080180042027. [DOI] [PubMed] [Google Scholar]; Azen SP, Boone DC, Barlow W, McCuen BW, Walonker AF, Anderson MM, et al. Methods, statistical features, and baseline results of a standardized, multicentered ophthalmologic surgical trial: the Silicone Study. Controlled Clinical Trials. 1991;12(3):438–55. doi: 10.1016/0197-2456(91)90022-e. [DOI] [PubMed] [Google Scholar]; Barr CC, Lai MY, Lean JS, Linton KL, Trese M, Abrams G, et al. Postoperative intraocular pressure abnormalities in the Silicone Study. Silicone Study Report 4. Ophthalmology. 1993;100(11):1629–35. doi: 10.1016/s0161-6420(93)31425-9. [DOI] [PubMed] [Google Scholar]; Blumenkranz MS, Azen SP, Aaberg T, Boone DC, Lewis H, Radtke N, et al. Relaxing retinotomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy. Silicone Study Report 5. The Silicone Study Group. American Journal of Ophthalmology. 1993;116(5):557–64. doi: 10.1016/s0002-9394(14)73196-4. [DOI] [PubMed] [Google Scholar]; Brown GC, Brown MM, Sharma S, Busbee B, Landy J. A cost-utility analysis of interventions for severe proliferative vitreoretinopathy. American Journal of Ophthalmology. 2002;133(3):365–71. doi: 10.1016/s0002-9394(01)01423-4. [DOI] [PubMed] [Google Scholar]; Cox MS, Azen SP, Barr CC, Linton KL, Diddie KR, Lai MY, et al. Macular pucker after successful surgery for proliferative vitreoretinopathy. Silicone Study Report 8. Ophthalmology. 1995;102(12):1884–91. doi: 10.1016/s0161-6420(95)30779-8. [DOI] [PubMed] [Google Scholar]; Diddie KR, Azen SP, Freeman HM, Boone DC, Aaberg TM, Lewis H. Anterior proliferative vitreoretinopathy in the silicone study. Silicone Study Report Number 10. Ophthalmology. 1996;103(7):1092–9. doi: 10.1016/s0161-6420(96)30562-9. [DOI] [PubMed] [Google Scholar]; Hutton WL, Azen SP, Blumenkranz MS, Lai MY, McCuen BW, Han DP, et al. The effects of silicone oil removal. Silicone Study Report 6. Archives of Ophthalmology. 1994 Jun;112(6):778–85. doi: 10.1001/archopht.1994.01090180076038. [DOI] [PubMed] [Google Scholar]; Lean J, Azen SP, Lopez PF, Qian D, Lai MY, McCuen B. The prognostic utility of the Silicone Study Classification System. Silicone Study Report 9. Silicone Study Group. Archives of Ophthalmology. 1996;114(3):286–92. doi: 10.1001/archopht.1996.01100130282009. [DOI] [PubMed] [Google Scholar]; McCuen BW, 2nd, Azen SP, Stern W, Lai MY, Lean JS, Linton KL, et al. Vitrectomy with silicone oil or perfluoropropane gas in eyes with severe proliferative vitreoretinopathy. Silicone Study Report 3. Retina. 1993;13(4):279–84. doi: 10.1097/00006982-199313040-00002. [ClinicalTrials.gov: NCT00000140] [DOI] [PubMed] [Google Scholar]
- Silicone Study 1992b.Anonymous. Vitrectomy with silicone oil or perfluoropropane gas in eyes with severe proliferative vitreoretinopathy: results of a randomized clinical trial. Silicone Study Report 2. Archives of Ophthalmology. 1992;110(6):780–92. doi: 10.1001/archopht.1992.01080180052028. [ ClinicalTrials.gov: NCT00000140] [DOI] [PubMed] [Google Scholar]
Excluded studies
- Avci 2001.Avci R. Cataract surgery and transpupillary silicone oil removal through a single scleral tunnel incision under topical anesthesia; sutureless surgery. International Ophthalmology. 2001;24(6):337–41. doi: 10.1023/b:inte.0000006833.64874.55. [DOI] [PubMed] [Google Scholar]
- Brazitikos 2005.Brazitikos PD, Androudi S, Christen WG, Stangos NT. Primary pars plana vitrectomy versus scleral buckle surgery for the treatment of pseudophakic retinal detachment: a randomized clinical trial. Retina. 2005;25(8):957–64. doi: 10.1097/00006982-200512000-00001. [DOI] [PubMed] [Google Scholar]
- Gao 1993.Gao R, Lu L, Zhang S, Tang S, Liu Q, Hu Z. Study on application of the silicone oil in the reattachment of complicated retinal detachment. Yan Ke Xue Bao [Eye Science] 1993;9(3):146–8. [PubMed] [Google Scholar]
- Hammer 1997.Hammer M, Margo CE, Grizzard WS. Complex retinal detachment treated with silicone oil or sulfur hexafluoride gas: a randomized clinical trial. Ophthalmic Surgery & Lasers. 1997;28(11):926–31. [PubMed] [Google Scholar]
- Hutchins 2003.Hutchins RK, Kaufman AH, Augsburger JJ. Perfluoro-octane internal tamponade when using a temporary keratoprosthesis during retinal detachment repair. Retina. 2003;23(1):106–10. doi: 10.1097/00006982-200302000-00020. [DOI] [PubMed] [Google Scholar]
- Krasnik 1999.Krásnik V, Strmen P, Furdová A, Hasa J. Ultrasonic diagnosis of retinal detachment after internal tamponade with silicone oil [Ultrazvukova diagnostika amocie sietnice po vnutornej tamponade silikonovym olejom] Ceska a Slovenska Oftalmologie. 1998;54(5):305–9. [PubMed] [Google Scholar]
- Latecka-Krajewska 1998.Latecka-Krajewska B, Nawrocki J, Bogorodzki B. Usefulness of different silicone oils for intraocular tamponade [Ocena przydatnosci roznych rodzajow oleju sylikonowego do tamponady wewnatrzgalkowej] Klinka Oczna. 1998;100(5):295–300. [PubMed] [Google Scholar]
- Li 1995.Li X, Jiang Y, Zkang X. A comparison of attachment rates between SF6 and silicone oil tamponades following vitrectomy for treatment of complicated retinal detachment. Zhonghua Yan Ke Za Zhi [Chinese Journal of Ophthalmology] 1995;31(4):250–4. [PubMed] [Google Scholar]
- Lu 2002.Lu L, Li Y, Cai S, Yang J. Vitreous surgery in highly myopic retinal detachment resulting from a macular hole. Clinical & Experimental Ophthalmology. 2002;30(4):261–5. doi: 10.1046/j.1442-9071.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- Pastor 1998.Pastor JC, Zarco JM, Del Nozal MJ, Pampliega A, Marinero P. Clinical consequences of the use of highly purified silicone oil: Comparative study of highly and less purified silicone oil. European Journal of Ophthalmology. 1998;8(3):179–83. doi: 10.1177/112067219800800311. [DOI] [PubMed] [Google Scholar]
- Pertile 1999.Pertile G, Claes C. Silicone oil vs. gas for the treatment of full-thickness macular hole. Bulletin de la Société Belge d’Ophtalmologie. 1999;274:31–6. [PubMed] [Google Scholar]
- Peyman 1987.Peyman GA, Kao GW, de Corral LR. Randomized clinical trial of intraocular silicone vs. gas in the management of complicated retinal detachment and vitreous hemorrhage. International Ophthalmology. 1987;10(4):221–34. doi: 10.1007/BF00155629. [DOI] [PubMed] [Google Scholar]
- Soheilian 2006.Soheilian M, Mazareei M, Mohammadpour M, Rahmani B. Comparison of silicon oil removal with various viscosities after complex retinal detachment surgery. BMC Ophthalmology. 2006;6:21. doi: 10.1186/1471-2415-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefer 1991.Stefer U, Wiedemann P, Weber J, Heimann K. Functional results after vitrectomy with silicone oil injection. European Journal of Ophthalmology. 1991;1(2):89–95. doi: 10.1177/112067219100100207. [DOI] [PubMed] [Google Scholar]
- Tufail 1997.Tufail A, Schwartz SD, Gregor ZJ. Prophylactic argon laser retinopexy prior to removal of silicone oil: a pilot study. Eye. 1997;11(Pt 3):328–30. doi: 10.1038/eye.1997.69. [DOI] [PubMed] [Google Scholar]
- van Effenterre 1987.van Effenterre G, Haut J, Larricart P, Abi-Rached J, Vachet JM. Gas tamponade as a single technique in the treatment of retinal detachment: is vitrectomy needed? A comparative study of 120 cases. Graefes Archive for Clinical & Experimental Ophthalmology. 1987;225(4):254–8. doi: 10.1007/BF02150143. [DOI] [PubMed] [Google Scholar]
Studies awaiting classification
- Oncel 2006.Oncel M, Acikalin B. Heavy silicone oil vs. standard silicone oil for the management of complicated retinal detachment: A prospective randomized study. American Academy of Ophthalmology. 2006:301. [Google Scholar]
Ongoing studies
- HSO Study.Joussen AM, Kirchhof B, Schrage N, Ocklenburg C, Hilgers RD. Heavy silicone oil versus standard silicone oil as vitreous tamponade in inferior PVR (HSO Study): design issues and implications. Acta Ophthalmologica Scandinavica. 2007;85(6):623–30. doi: 10.1111/j.1600-0420.2007.00898.x. [ISRCTN Register: ISRCTN47399029 ; Study Name: The Heavy Silicone Oil versus Standard Silicone Oil Study] [DOI] [PubMed] [Google Scholar]
Other references
Additional references
- Charteris 2002.Charteris DG, Sethi CS, Lewis GP, Fisher SK. Proliferative vitreoretinopathy - developments in adjunctive treatment and retinal pathology. Eye. 2002;16(4):369–74. doi: 10.1038/sj.eye.6700194. [DOI] [PubMed] [Google Scholar]
- Cowley 1989.Cowley M, Conway BP, Campochiaro PA, Kaiser D, Gaskin H. Clinical risk factors for proliferative vitreoretinopathy. Archives of Ophthalmology. 1989;107(8):1147–51. doi: 10.1001/archopht.1989.01070020213027. [DOI] [PubMed] [Google Scholar]
- Deeks 2008.Deeks JJ, Higgins JPT, Altman DG, Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2008. Chapter 9: Analysing data and undertaking meta-analyses. Version 5.0.1 [updated September 2008] Available from www.cochrane-handbook.org. [Google Scholar]
- Eckardt 1990.Eckardt C, Schmidt D, Czank M. Intraocular tolerance to silicone oils of different specific gravities: an experimental study. Ophthalmologica. 1990;201(3):133–9. doi: 10.1159/000310141. [DOI] [PubMed] [Google Scholar]
- Glanville 2006.Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association. 2006;94(2):130–6. [PMC free article] [PubMed] [Google Scholar]
- Heimann 2007.Heimann H, Bartz-Schmidt KU, Bornfeld N, Weiss C, Hilgers RD, Foerster MH. Scleral Buckling versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment Study Group. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology. 2007;114(12):2142–54. doi: 10.1016/j.ophtha.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Heimann 2008.Heimann H, Stappler T, Wong D. Heavy tamponade 1: a review of indications, use, and complications. Eye (London, England) 2008;22(10):1342–59. doi: 10.1038/eye.2008.61. [DOI] [PubMed] [Google Scholar]
- Higgins 2008.Higgins JPT, Altman DG, Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2008. Chapter 8: Assessing risk of bias in included studies. Version 5.0.1 [updated September 2008] Available from www.cochrane-handbook.org. [Google Scholar]
- Hoerauf 2005.Hoerauf H, Roider J, Kobuch K, Laqua H. Perfluorohexylethan (O62) as ocular endotamponade in complex vitreoretinal surgery. Retina. 2005;25(4):479–88. doi: 10.1097/00006982-200506000-00014. [DOI] [PubMed] [Google Scholar]
- Kruger 2002.Kruger EF, Nguyen QD, Ramos-Lopez M, Lashkari K. Proliferative vitreoretinopathy after trauma. International Ophthalmology Clinics. 2002;42(3):129–43. doi: 10.1097/00004397-200207000-00015. [DOI] [PubMed] [Google Scholar]
- Krzystolik 2000.Krzystolik MG, D’Amico DJ. Complications of intraocular tamponade: silicone oil versus intraocular gas. International Ophthalmology Clinics. 2000;40(1):187–200. doi: 10.1097/00004397-200040010-00018. [DOI] [PubMed] [Google Scholar]
- Matteucci 2007.Matteucci A, Formisano G, Paradisi S, Carnovale-Scalzo G, Scorcia G, Caiazza S, et al. Biocompatibility assessment of liquid artificial vitreous replacements: relevance of in vitro studies. Survey of Ophthalmology. 2007;52(3):289–99. doi: 10.1016/j.survophthal.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Patel 2004.Patel NN, Bunce C, Asaria RH, Charteris DG. Resources involved in managing retinal detachment complicated by proliferative vitreoretinopathy. Retina. 2004;24(6):883–7. doi: 10.1097/00006982-200412000-00007. [DOI] [PubMed] [Google Scholar]
- Rofail 2005.Rofail M, Lee LR. Perfluoro-n-octane as a postoperative vitreoretinal tamponade in the management of giant retinal tears. Retina. 2005;25(7):897–901. doi: 10.1097/00006982-200510000-00013. [DOI] [PubMed] [Google Scholar]
- Schwartz 2004.Schwartz SG, Mieler WF. Management of primary rhegmatogenous retinal detachment. Comprehensive Ophthalmology Update. 2004;5:285–94. [Google Scholar]
- Scott 2002.Scott IU, Murray TG, Flynn HW, Jr, Feuer WJ, Schiffman JC Perfluoron Study Group. Outcomes and complications associated with giant retinal tear management using perfluoro-n-octane. Ophthalmology. 2002;109(10):1828–33. doi: 10.1016/s0161-6420(02)01184-3. [DOI] [PubMed] [Google Scholar]
- Scott 2005.Scott IU, Flynn HW, Jr, Murray TG, Smiddy WE, Davis JL, Feuer WJ. Outcomes of complex retinal detachment repair using 1000- vs 5000-centistoke silicone oil. Archives of Ophthalmology. 2005;123(4):473–8. doi: 10.1001/archopht.123.4.473. [DOI] [PubMed] [Google Scholar]
- Singh 1986.Singh AK, Glaser BM, Lemor M, Michels RG. Gravity-dependent distribution of retinal pigment epithelial cells dispersed into the vitreous cavity. Retina. 1986;6(2):77–80. doi: 10.1097/00006982-198600620-00002. [DOI] [PubMed] [Google Scholar]
- Singh 2001.Singh J, Ramaesh K, Wharton SB, Cormack G, Chawla HB. Perfluorodecalin-induced intravitreal inflammation. Retina. 2001;21(3):247–51. doi: 10.1097/00006982-200106000-00009. [DOI] [PubMed] [Google Scholar]
- Stappler 2008.Stappler T, Heimann H, Wong D, Gibran SK, Groenewald C, Pearce IA. Heavy tamponade 2 Densiron 68 in routine clinical practice: anatomical and functional outcomes of a consecutive case series. Eye (London, England) 2008;22(10):1360–5. doi: 10.1038/eye.2008.62. [DOI] [PubMed] [Google Scholar]
- Sterne 2008.Sterne JAC, Egger M, Moher D, Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2008. Chapter 10: Addressing reporting biases. Version 5.0.1 [updated September 2008] Available from www.cochrane-handbook.org. [Google Scholar]
- Tognetto 2005.Tognetto D, Minutola D, Sanguinetti G, Ravalico G. Anatomical and functional outcomes after heavy silicone oil tamponade in vitreoretinal surgery for complicated retinal detachment. A pilot study. Ophthalmology. 2005;112(9):1574–8. doi: 10.1016/j.ophtha.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Tognetto 2008.Tognetto D, Lepori L, Lapasin R, Minutola D, Sanguinetti G, Michelone L, et al. A new heavy internal tamponade in vitreoretinal surgery: an in vitro study. Eye. 2008;22(8):1082–8. doi: 10.1038/eye.2008.144. [DOI] [PubMed] [Google Scholar]
- TRSTC 1983.Anonymous. The Retina Society Terminology Committee. The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology. 1983;90(2):121–5. doi: 10.1016/s0161-6420(83)34588-7. [DOI] [PubMed] [Google Scholar]
- Young 2005.Young TA, D’Amico DJ. Controversies in proliferative vitreoretinopathy tamponade and pharmacologic agents. International Ophthalmology Clinics. 2005;45(5):163–71. doi: 10.1097/01.iio.0000176368.93887.2c. [DOI] [PubMed] [Google Scholar]


