Abstract
Background
Associations of epicardial fat volume (EFV) measured on non-contrast cardiac computed tomography (NCT) include coronary plaque, myocardial ischemia and adverse cardiac events.
Objectives
This study aimed to define the relationship of EFV to coronary plaque type, severe coronary stenosis, and to the presence of high-risk plaque features (HRPFs).
Methods
We retrospectively evaluated 402 consecutive patients, with no prior history of coronary artery disease, who underwent same day non-contrast cardiac computed tomography (NCT) and coronary CT angiography (CTA). EFV was measured on NCT using validated, semi-automated, software. The coronary arteries were evaluated for coronary plaque type [calcified (CP), non-calcified (NCP) or partially-calcified (MP)] and coronary stenosis severity ≥70% using coronary CTA. For patients with NCP and PCP, 2 high risk plaque features were evaluated: Low-attenuation plaque and positive remodeling.
Results
There were 402 patients with a median age of 66 years (range 23–92) of whom 226 (56%) were male. The EFV was larger in patients with CP (112 ± 55 cm3 vs. 89 ± 39 cm3), PCP (110 ± 57 cm3 vs. 98 ± 45 cm3) and NCP (115 ± 44 cm3 vs. EFV 100 ± 52 cm3. In the 192 patients with PCP or NCP, on multivariable analysis, after adjusting for conventional cardiovascular risk factors, EFV was an independent predictor of ≥70% coronary artery stenosis (OR 3.0, 95% CI 1.3–6.6, p=0.008), any high risk plaque features (OR 1.7, 95% CI 0.9–3.4, p=0.04) and low attention plaque (OR 2.4, 95% CI 1.1–5.1, p=0.02), but not of positive remodeling.
Conclusions
Epicardial fat volume is larger in patients with CP, PCP and NCP. In patients with NCP and PCP, EFV is significantly associated with severe coronary stenosis, high risk plaque features and low attenuation plaque.
Keywords: Epicardial fat volume, coronary computed tomography angiography, coronary artery stenosis, high-risk plaque features
Epicardial fat volume (EFV), measured on non-contrast enhanced computed tomography (NCT), has emerged as an important parameter in understanding the pathophysiology of coronary atherosclerosis. Recent studies have demonstrated a relationship between EFV and the presence and severity of coronary plaque as assessed by coronary artery calcification 1,2. Furthermore, there is now emerging data that suggests that this parameter may be related to myocardial ischemia 3, acute coronary syndrome 4 and also prognosis 4,5. However, despite these observations, there remains uncertainty as to how epicardial fat may exert these detrimental effects. One potential hypothesis that has emerged is that epicardial fat volume may exert a local paracrine effect upon adjacent coronary artery segments and lead to local inflammation and changes in plaque architecture 6. Supporting this hypothesis are data showing that specific morphological plaque characteristics identified on coronary computed tomography angiography (CTA) are associated with culprit coronary lesions and an increased risk of future cardiac events 7–10.
Although EFV has been shown to be related to coronary plaque 11,12 and coronary stenosis 13–15, whether or not EFV is related to high risk plaque features (HRPFs) is a subject of ongoing investigation. Oka et al showed an independent association between EFV and HRPFs in patients with non-calcified coronary plaques 16, and Schlett et al. have shown EFV to be associated to HRPFs in 13 patients with a high risk coronary lesion presenting with chest pain 17. The relationship between EFV and thin-capped fibroatheroma has also been recently confirmed on invasive coronary angiography and optical coherence tomography 18. One area of current uncertainty is the association of EFV to severe coronary stenosis, plaque type and HRPFs in patients with stable symptoms being referred for coronary CTA. The principal aims of the current study were to investigate the relationship of EFV to coronary plaque type, severe coronary stenoses and HRPFs in stable patients with either non-calcified or partially calcified coronary plaques.
Methods
Patients
We retrospectively studied 402 consecutive patients who underwent same-day coronary CTA and NCT at Cedars-Sinai Medical Center from January 2009 through December 2009. Patients were excluded if they had a prior history of coronary artery disease (myocardial infarction, coronary stenting and prior bypass surgery), if their body mass index (BMI) was beyond limits set by mean BMI ± 2 standard deviations, and if their image quality was not considered good or excellent by an expert reader. Cardiovascular risk factors were determined by preset criteria. Hypertension was defined as a systolic blood pressure of >140 mmHg, a diastolic blood pressure of >90 mmHg, or antihypertensive drug use. Smoking was defined as a current smoker or past heavy smoker (>20 package-years). Diabetes mellitus was defined as a previously established diagnosis, insulin or oral hypoglycemic therapy, fasting glucose of >126 mg/dL, or non-fasting glucose of >200 mg/dL. Family history of coronary artery disease was defined as myocardial infarction, coronary revascularization, or sudden cardiac death in a first-degree male relative <55 years old or female relative <65 years old. Epicardial fat volume of all patients was measured on NCT using semi-automated quantitative software. Detailed assessment of coronary plaque severity, type, and morphological characteristics was performed on coronary CTA images.
Non-contrast enhanced CT scan
All subjects underwent NCT on a on a dual-source CT scanner (SOMATOM Definition, Siemens Medical Solutions, Forchheim, Germany). The scan extended from the aortic arch to the diaphragm and was obtained in a single breath hold. Heart-rate dependent ECG triggering was performed, typically at 45% to 60% of the RR interval. The field of view was 35 cm. Matrix size was 512 × 512. Tube voltage was 120 kVp with multislice scanning. The slice thickness was 3 mm for electron-beam CT and 2.5 mm for multislice CT. Each set of NCT images were evaluated for coronary calcium score (CCS) by an experienced reader blinded to results of epicardial fat measurements, using semi-automatic, commercially available software (ScImage, Los Altos, CA, USA). CCS was calculated using the Agatston method; total CCS was the sum of calcified plaque scores of all coronary arteries 19.
Epicardial fat quantification
Epicardial fat quantification was performed on the NCT scan using validated software (QFAT) developed at the Cedars-Sinai Medical Center 20. Scans were presented to blinded experienced readers in random order. Readers identified the superior epicardial fat boundary at the take-off of the right pulmonary artery, and the inferior boundary at the first slice where the posterior descending artery was first visualized. Five to 10 contour points were then traced on the pericardium at each slice from the upper slice limit to the lower slice in the axial view (total number of slices ranged 20–40 for typical subjects). From these control points, piecewise cubic Catmull-Rom spline functions were automatically generated to form a smooth, closed pericardial contour (Figure 1). The EFV was then automatically calculated (reported in cm3), using contiguous 3-dimensional voxels with Hounsfield Units between −190 and −30 as the range of attenuation values defining fat 21–23. The reproducibility of QFAT software measurement of epicardial fat volume has previously been shown to be high for both intrascanner (MDCT-MDCT) and interscanner (EBCT-MDCT) data (correlation coefficient ≥0.98). The reproducibility coefficient values are lowest (4.3% for EFV) for intrascanner same-observer measurement. For intrascanner cross-observer measurement, reproducibility values are 10.7% for EFV 24.
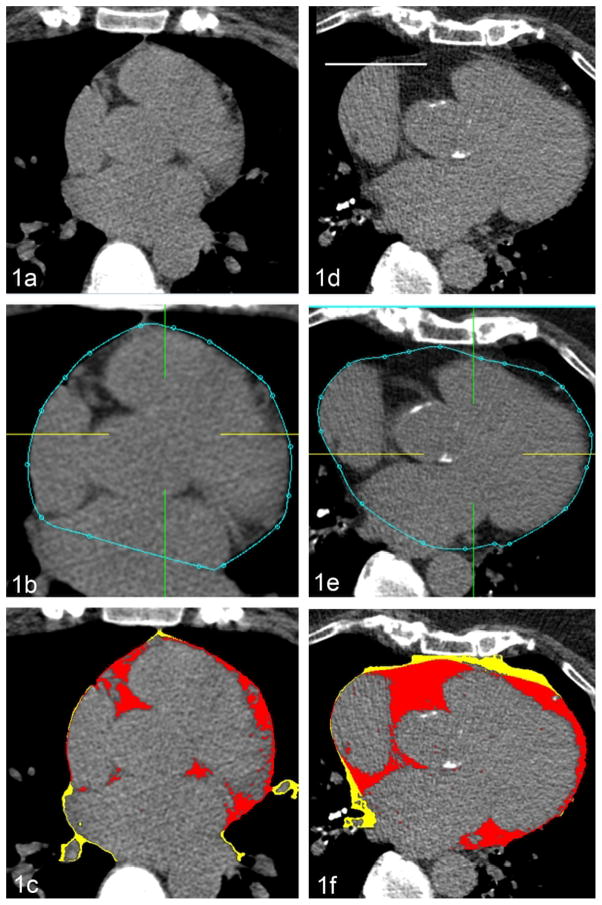
Figure 1.
The measurement of EFV in a patient with low (Figs. 1a–c) and high (Figs. 1d–f) EFV. The user identifies the pericardium and places approximately 10 contour points manually on the pericardium at each slice from the upper slice limit to the lower slice in the axial view. From these control points, the software (Q-FAT) automatically quantifies all fat voxels within the pericardial contour (blue line) and generates the EFV (red).
EFV – epicardial fat volume.
Coronary CTA image acquisition and reconstruction
Coronary CTA was performed on a dual-source CT scanner (SOMATOM Definition, Siemens Medical Solutions, Forchheim, Germany). The imaging protocol has been previously described in detail 25,26. Beta-blockade with metoprolol was used to achieve a heart rate of <70 beats-per-minute, and 0.4 mg nitroglycerin spray (Sciele Pharma, Alpharetta, GA, USA) was administered 3–5 minutes prior to the scan. Eighty mls of intravenous contrast (Omnipaque, GE Healthcare, Princeton, NJ, USA) followed by 50–80 ml of saline at a rate of 5 ml/s were power-injected into the antecubital vein. Ascending aorta contrast-triggered (100 Hounsfield Units), ECG-gated helical scanning was then performed in a single breath-hold. Scanning parameters included heart-rate dependent pitch (0.2–0.45), 330 ms gantry rotation-time, 100 or 120 kVp tube-voltage depending on patient body-mass index 26, and 330–350 mAs reference tube current. The acquired coronary CTA data was reconstructed in mid-diastole and at end-systole using 0.6 mm slice-thickness (0.75 mm if BMI was >35 kg/m2), 0.3 mm slice increment, 250 mm field-of-view, 512 × 512 matrix, and B26f “medium-smooth” kernel. If reconstruction from standard phases of the cardiac cycle resulted in uninterpretable segments, additional phases were reconstructed and analyzed.
Coronary CTA Analysis
For the purposes of this research, plaque assessment on coronary CTA was independently performed by 2 experienced readers using axial images, oblique multiplanar reformations, and oblique maximum intensity projections 27. Plaque type was classified into calcified plaque (CP - plaque consisting of only calcium), non-calcified plaque (NCP - plaque that was calcium free) and partially calcified plaque (PCP - plaque that had calcified and non-calcified components). Stenosis severity was manually quantified using the luminal diameter ratio between the sites of maximal stenosis and proximal healthy reference, as previously reported 28. Severe coronary stenosis was defined as ≥70% reduction in luminal diameter. For each non-calcified and partially calcified plaque, readers determined the presence of 2 HRPFs, shown to predict subsequent adverse cardiovascular events (Fig. 2): LAP, defined as visually distinct intra-plaque hypo-attenuation containing Hounsfield Units ≤30, and PR, defined as maximal outer arterial wall diameter along the plaque exceeding proximal reference by ≥5% (see Figure 2). Patients were classified as having none, one, or two HRPFs. All readers were blinded to the results of the EFV measurements. Consensus was used to resolve discrepancies. Study quality was graded on a five-point qualitative scale ranging from uninterpretable, to excellent. Only studies graded as being good (some minor artifact present but all coronary segments evaluable) and excellent (no artifact present, all coronary segments evaluable) were included in the study.
Figure 2.
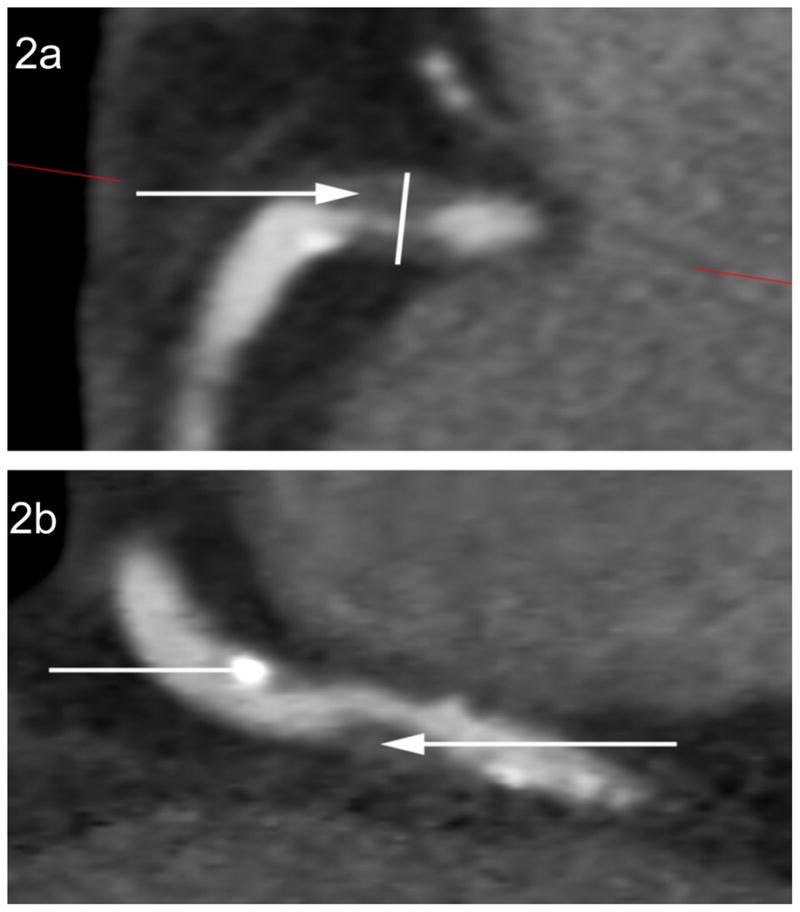
The assessment of HRPFs. Fig. 1a demonstrates the presence of three high-risk plaque features within the proximal right coronary artery: a >70% stenosis, LAP (closed arrow) and PR at the site of non-calcified plaque (open arrow). Fig. 2b demonstrates the presence of a proximal partially calcified plaque within the right coronary artery (open arrow) and a more distal non-calcified plaque with a LAP component (closed arrow).
HRPFs – high-risk plaque features; LAP – low attenuation plaque; PR – positive remodelling.
Statistical analysis
All continuous variables included in the analysis are presented as mean ± SD. Variables with non-normal distributions are presented as median with range. Univariate analyses were performed on continuous variables using the two-sample t-test for normally distributed variables and the Mann-Whitney U test for non-normally distributed data. The distribution of EFV was positively skewed, therefore natural-log transformed values were used throughout, unless otherwise specified. Spearman’s correlation coefficient was used to assess the relationship between continuous variables. Multivariable logistic regression was used to determine the predictors of severe coronary stenosis and HRPFs using age, gender, BMI, hypertension, hypercholesterolemia, diabetes mellitus, active smoking, a family history of premature coronary disease and EFV as covariates. Statistical significance for all analyses was set at the 5% level. All data were collected and analyzed using SPSS for MAC (Version 17, IBM, Somers, NY, USA).
Results
There were 402 patients with a median age of 66 (23–92) years of whom 226 were men (56%). Coronary CTA was predominantly performed for symptoms of chest pain in 227 (56.4%), shortness of breath 107 (26.6%), as a pre-operative assessment in 15 (4%) and for an abnormal resting ECG in 15 (4%) patients. Other indications included equivocal stress test results and prior positive coronary calcium scores. Table 1 shows the baseline demographics of the population. Epicardial fat volume was weakly correlated to increasing age (r=0.29, p<0.001), BMI (r=0.42, <0.001) and also body surface area (r=0.39, p<0.001). On univariate analysis, EFV was also larger in men [102 cm3 (16–476) vs. 85 cm3 (19–310), p<0.001], in patients with hypertension [107 cm3 (16–310) vs. 81 (19–476), p<0.001], hypercholesterolemia [101 cm3 (30–310) vs. 84 cm3 (16–476), p=0.001] and a prior smoking history [127 cm3 (42–266) vs. 83 cm3 (16–476), p=0.007], but not in patients with diabetes mellitus or a significant family history of coronary artery disease.
Table 1.
Baseline demographics
| Total | |
|---|---|
| n | 402 |
| Age | 66 (23–92) |
| Gender M:F (%) | 56:44 |
| BMI kg/m2 | 26.7 ± 4.4 |
| Hypercholesterolemia n (%) | 253 (63%) |
| Smoking n (%) | 41 (10%) |
| Diabetes mellitus n (%) | 55 (13.7%) |
| Family History n(%) | 148 (37%) |
| Hypertension n (%) | 215 (54%) |
| EFV (cm3) | 103 ± 51 |
| Log EFV (cm3) | 4.52 ± 0.49 |
| CCS (AU) | 57 (0 – 5272) |
Abbreviations
AU – Agatston Units; BMI – body mass index; CCS – coronary calcium score; EFV – epicardial fat volume.
Epicardial fat volume and type of coronary artery plaque
Coronary CTA detected coronary plaques in 294 (73%) patients and no plaques in 108 (27%) patients. Calcified plaques were present in 237 (59%) patients with a median plaque number of 3 (range 1–12). Partially calcified coronary plaques were present in 166 (41%) patients with a median plaque number of 2 (range 1–8). Non-calcified plaques were present in 76 (19%) of patients with a median plaque number of 1 (range 1–6). EFV was larger in patients with coronary plaques than patients without (EFV with plaque: 108 ± 53 cm3 vs. EFV without plaque: 89 ± 41 cm3, p <0.001). EFV was larger in patients with CP (EFV with CP 112 ± 55 cm3 vs. n = 165, EFV without CP 89 ± 39 cm3, p <0.001), PCP (EFV with PCP 110 ± 57 cm3 vs. n = 236, EFV without PCP 98 ± 45 cm3, p = 0.02), and in patients with NCP (EFV with NCP 115 ± 44 cm3 vs n = 326, EFV without NCP 100 ± 52 cm3, p = 0.03).
Epicardial fat volume and number of coronary artery plaques
EFV was weakly correlated to the total number of plaques (p<0.001, r=0.28), the number of calcified plaques (p<0.001, r=0.28), the number of partially calcified plaques (p=0.009, r=0.13), the number of non-calcified plaques (p=0.003, r=0.15), and the coronary calcium score (p<0.001, r=0.27).
Epicardial fat volume and presence of severe coronary stenosis
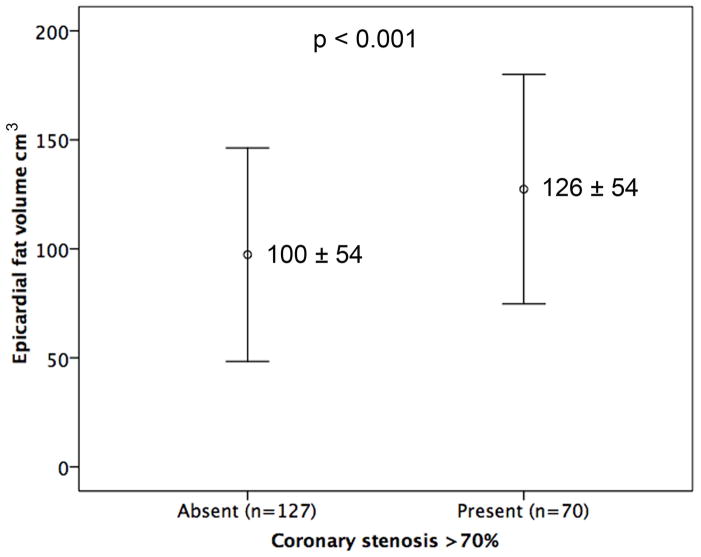
There were 70 (36%) patients with a coronary stenosis ≥70%. In these patients EFV was larger (EFV with ≥70% stenosis 126 ± 54 cm3 vs. 100 ± 54 cm3 without, p<0.001) (Fig. 3a) as was CCS [CCS with ≥70% stenosis 28 AU (0–3681) vs. 544 AU (6–4537) without, p<0.001]. On multivariable logistic regression analysis for the determinants of coronary stenosis ≥70%, age (OR 1.0, 95% CI 1.0–1.1, p=0.009), diabetes mellitus (OR 2.1, 95% CI 1.3–3.4, p=0.004, smoking (OR 3.1, 95% CI 1.4–6.9), p=0.005 and EFV (OR 3.0, 95% CI 1.3–6.6, p=0.008) were significant independent determinants (Table 2).
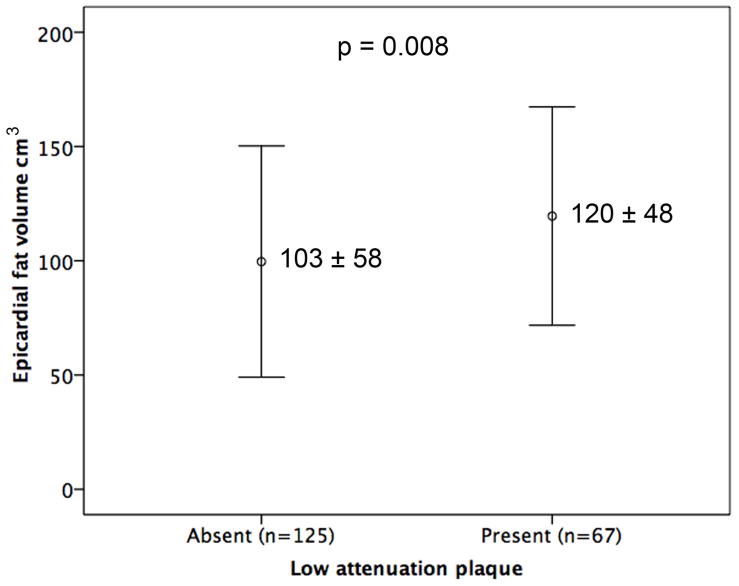
Figure 3.
Comparison of EFV (mean ± SD) in patients with and without ≥ 70% stenosis (Fig. 3a), LAP (Fig. 3b) and PR (Fig. 3c).
EFV – epicardial fat volume; LAP – low attenuation plaque; PR – positive remodelling.
Table 2.
Multivariable logistic regression analysis for the prediction of severe coronary stenosis (≥70%)
| Odds Ratio | 95% Confidence Intervals | P | |
|---|---|---|---|
| Age | 1.0 | 1.0 – 1.1 | 0.009 |
| BMI | 1.0 | 0.9 – 1.0 | 0.3 |
| Diabetes mellitus | 2.1 | 1.3 – 3.4 | 0.004 |
| Hypercholesterolemia | 1.9 | 0.9 – 3.6 | 0.1 |
| Smoking | 3.1 | 1.4 – 6.9 | 0.005 |
| Family history | 1.0 | 0.6 – 1.9 | 0.9 |
| Hypertension | 1.3 | 0.7 – 2.4 | 0.4 |
| Log EFV | 3.0 | 1.3 – 6.6 | 0.008 |
Abbreviations
BMI – body mass index; CCS – coronary calcium score; EFV – epicardial fat volume.
Epicardial fat volume and high-risk plaque features
At least one high-risk plaque feature was present in 113 (59%) of the 192 patients. Table 3 outlines the demographics of the patients with and without HRPFs. Coronary artery calcium score category was higher in patients with HRPFs than those without (p<0.001). At least one plaque exhibited LAP in 67 (35%) patients and PR in 93 (48%) patients. EFV was larger in patients with at least 1 plaque exhibiting LAP (EFV with LAP 120 ± 48 cm3 vs. 103 ± 58 cm3 without, p=0.008) (Fig. 3b), PR (EFV with PR 115 ± 54 cm3 vs. 104 ± 56 cm3 without, p=0.07) (Fig. 3c), in patients who had either LAP or PR (EFV with HRPFs 116 ± 53 cm3 vs. 99 ± 57 cm3 without, p=0.009) and in patients with both LAP and PR (EFV with LAP and PR 119 ± 47 vs. 101 ±51 cm3, p=0.006).
Table 3.
Comparison of risk factors in patients with and without high-risk plaque features (HRPFs)
| HRPFs (−) | HRPFs (+) | p | |
|---|---|---|---|
| n | 289 | 113 | |
| Age | 63 (23 – 92) | 66 (41 – 87) | 0.01 |
| BMI kg/m2 | 26.5 ± 4.5 | 26.9 ± 4.1 | 0.3 |
| Hypercholesterolemia n (%) | 169 (59%) | 84 (74.3%) | 0.003 |
| Smoking n (%) | 25 (8.7%) | 16 (14.2%) | 0.1 |
| Diabetes mellitus n (%) | 31 (11.1%) | 23 (20.3%) | 0.01 |
| Family History n (%) | 108 (37.4%) | 40 (35.4%) | 0.7 |
| Hypertension n (%) | 148 (51.2%) | 67 (59.3%) | 0.1 |
| EFV (cm3) | 98 ± 50 | 116 ± 53 | <0.001 |
| Log EFV (cm3) | 4.5 ± 0.5 | 4.7 ±0.4 | <0.001 |
| CCS (AU) | 18 (0 – 5272) | 268 (0 – 2600) | <0.001 |
|
| |||
| CCS Category (AU) | |||
| 0 n (%) | 110 (38%) | 4 (4%) | |
| 1–99 n (%) | 84 (29 %) | 29 (26%) | <0.001 |
| 100–399 n (%) | 53 (18%) | 35 (31%) | |
| >400 n (%) | 42 (15%) | 45 (30%) | |
Abbreviations
AU – Agatston Units; BMI – body mass index; CCS – coronary calcium score; EFV – epicardial fat volume.
On multivariable logistic regression analysis for the determinants of having at least 1 HRPF, diabetes mellitus, hypercholesterolemia and EFV (OR 1.7, 95% CI 0.9–3.4, p=0.038) were significantly associated with having an HRPF. For LAP, diabetes mellitus and EFV (OR 2.4, 95% CI 1.1–5.1, p=0.02) were independently associated to LAP. For PR, EFV was not associated to the presence of PR. For both LAP and PR, diabetes mellitus, smoking, hypertension and EFV (OR 2.6, 95% CI 1.1–6.2, p=0.03) were significantly associated with having both HRPFs. (Table 4).
Table 4.
Multivariable logistic regression analysis for the prediction of any high-risk plaque features (HRPFs) low attenuation plaque (LAP), positive remodelling (PR) and concomitant LAP and PR
| Any HRPFs | LAP | PR | LAP + PR | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
|
| ||||||||
| Age | 1.0 (1.0 – 1.0) | 0.1 | 1.0 (1.0 – 1.1) | 0.03 | 1.0 (1.0 – 1.0) | 0.5 | 1.0 (1.0 – 1.0) | 0.3 |
| BMI | 1.0 (0.9 – 1.1) | 0.6 | 1.0 (0.9 – 1.0) | 0.5 | 1.0 (0.9 – 1.1) | 0.7 | 1.0 (0.9 – 1.1) | 0.5 |
| Diabetes mellitus | 1.6 (0.9 – 2.5) | 0.04 | 1.8 (1.1 – 2.8) | 0.02 | 1.8 (1.1 – 2.7) | 0.01 | 2.2 (1.3 – 3.7) | 0.002 |
| Hypercholesterolemia | 1.5 (0.8 – 2.6) | 0.047 | 1.5 (0.8 – 2.8) | 0.2 | 1.8 (1.0 – 3.1) | 0.04 | 1.7 (0.8 – 3.6) | 0.2 |
| Smoking | 1.8 (0.8 – 4.2) | 0.2 | 2.1 (1.0 – 4.8) | 0.06 | 1.6 (0.8 – 3.4) | 0.2 | 2.5 (1.0 – 6.1) | 0.040 |
| Family history | 0.9 (0.6 – 1.6) | 0.8 | 1.0 (0.6 – 1.8) | 0.9 | 1.1 (0.7 – 1.8) | 0.8 | 1.3 (0.7 – 2.6) | 0.4 |
| Hypertension | 0.6 (0.4 – 1.1) | 0.7 | 0.7 (0.4 – 1.3) | 0.3 | 0.8 (0.4 – 1.3) | 0.3 | 0.5 (0.2 – 0.9) | 0.03 |
| Log EFV | 1.7(0.9 – 3.4) | 0.04 | 2.4 (1.1 – 5.1) | 0.02 | 1.8 (1.0 – 3.4) | 0.07 | 2.6 (1.1 – 6.2) | 0.03 |
Abbreviations
BMI – body mass index; EFV – epicardial fat volume.
Discussion
In the current study we show that EFV is larger in patients with CP, PCP and also NCP. Furthermore we show that EFV is associated with severe coronary stenosis (≥70%) and also to HRPFs in patients with NCP or PCP when clinical characteristics and BMI are accounted for.
Association of epicardial fat volume to coronary disease
We found sizeable differences in epicardial fat volume in patients who showed any type of coronary plaque and also in patients who had a significant coronary stenosis ≥70%. These findings are consistent with prior studies that have suggested that EFV is associated with significant coronary disease. Iwasaki et al. 14 measured epicardial fat volume in 197 patients who underwent 64 slice coronary CTA and observed on univariate analysis higher EFV in patients with significant coronary stenosis (≥50%) than those patients without. Wang et al. 15 showed that epicardial fat thickness within the left atrioventricular groove was independently related to the extent and severity of significant coronary artery disease (≥50%), and Ueno et al. 29 have shown that EFV is related independently to chronic total occlusions. This finding supports the hypothesis that EFV is associated to not only the presence of coronary plaque disease but also its severity.
Association of epicardial fat volume to high-risk plaque features
There is now emerging evidence that epicardial fat volume, as well as being associated with atherosclerosis and plaque composition 30, is associated with adverse outcome. Studies have shown that EFV is associated with acute coronary syndrome4, myocardial ischemia 3,13 and also cardiac events 5,14. Despite this there remains uncertainty as to how exactly EFV exerts its detrimental effect. A potential explanation is that EFV causes a local inflammatory response on adjacent coronary arterial segments that may in turn mediate atherosclerosis. In the current study we therefore examined whether EFV was related to the plaque characteristics of positive remodelling and low attenuation plaque. These two features on coronary CTA are considered to be high-risk plaque features by virtue of their association with acute and future coronary events 7–10. After adjusting for conventional cardiovascular risk factors and BMI, we found that EFV was related to having at least one HRPF, 2 HRPFs and also LAP. Our findings are consistent with prior published work. Schlett et al. 17 evaluated the relationship of EFV to high-risk coronary lesions in 358 patients presenting with acute chest pain. The authors defined a high risk coronary lesion as one having a ≥50% luminal narrowing and at least two high risk plaque features (from PR, LAP and spotty calcification). In the 13 patients with a high-risk coronary lesion the authors showed an independent association to EFV after adjusting for conventional cardiovascular risk factors and BMI, but not for CCS. In another study, Oka et al. additionally studied 357 patients undergoing coronary CTA and examined the relationship of EFV to non-calcified plaque characteristics 16. The authors showed that EFV was related to PR and LAP after adjusting for conventional cardiovascular risk factors and coronary artery calcium score. In contrast to the current study, the authors did not examine partially calcified plaques as well as non-calcified plaques. Recently Ito et al. have also shown that epicardial fat volume measured on cardiac CT is associated with thin-capped fibroatheroma detected by invasive optical coherence tomography and also to acute coronary syndrome patients 18. The findings from these studies suggest a possible relationship between EFV and high-risk plaque morphology, which in turn may explain the observations that EFV is elevated in patients experiencing ACS 4, adverse cardiac events 5 and also ischemia on single photon emission computed tomography (SPECT) 3.
Limitations
In the current study we used a tube voltage of 100 or 120 kVp to minimize the radiation dose. In previous studies evaluating HRPFs, higher tube voltages were used including 135 kVp by Motoyama et al7 and in the study by Kitigawa et al, all patients were studied using 120 kVp 8. It is possible that the use of lower tube voltage may have reduced our detection rate of certain HRPFs. Only patients with good or excellent coronary CTA studies were included. Limitations in the spatial resolution of coronary CTA may reduce its capability in accurately describing LAP and PR. However, two recent studies have confirmed that the assessment of these adverse plaque characteristics by coronary CTA correlates well with that measured by intra-vascular ultrasound (IVUS). Marwan et al. 31 compared 40 predominantly lipid rich plaques using IVUS and dual source CT and showed that plaques with a cut-off of 5.5% pixels with an attenuation <30HU had a sensitivity of 95%, a specificity of 80% and a positive predictive value of 93% for the detection of lipid rich plaques by IVUS. Similarly, Gauss et al. 32 in a comparative study between IVUS and coronary CTA demonstrated a sensitivity of 83 and specificity of 78% for the detection of PR using a remodelling index of 1.1. Since the current study was retrospective in design, no measurements of visceral fat or inflammatory markers were available. Although the mean EFV values in the current study were lower than in previous studies this most likely reflects varied methodologies of EFV quantification and differing patient cohorts. The findings of the current study additionally need to be confirmed in similar cohorts of patients, and other cohorts with varying demographic and risk factor profiles. There was no long term follow up data for major adverse cardiac events in the current study and further studies are indicated to further evaluate the prognostic potential of EFV in patients undergoing NCT.
Conclusions
Epicardial fat volume is elevated in patients with calcified, partially calcified and non-calcified plaque and is associated with severe coronary stenosis. In patients with non-calcified plaque and partially calcified plaque, EFV is independently associated with high-risk plaque features. Our findings suggest that EFV may be a useful additional measurement in risk stratifying patients who undergo routine CCS and may indirectly provide prognostic information above and beyond the assessment of stenosis severity on coronary CTA.
Acknowledgments
This work was partly supported by grants from the Eisner, Glazer, and Lincy Foundations (to Dr. Berman), and from the National Institute of Biomedical Imaging and Bioengineering (R21EB006829 to Dr. Dey).
Footnotes
Conflict of interests
None to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gorter PM, de Vos AM, van der Graaf Y, Stella PR, Doevendans PA, Meijs MF, Prokop M, Visseren FL. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol. 2008;102:380–5. doi: 10.1016/j.amjcard.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Dey D, Wong ND, Tamarappoo B, Nakazato R, Gransar H, Cheng VY, Ramesh A, Kakadiaris I, Germano G, Slomka PJ, Berman DS. Computer-aided non-contrast CT-based quantification of pericardial and thoracic fat and their associations with coronary calcium and Metabolic Syndrome. Atherosclerosis. 2010;209:136–41. doi: 10.1016/j.atherosclerosis.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamarappoo B, Dey D, Shmilovich H, Nakazato R, Gransar H, Cheng VY, Friedman JD, Hayes SW, Thomson LE, Slomka PJ, Rozanski A, Berman DS. Increased pericardial fat volume measured from noncontrast CT predicts myocardial ischemia by SPECT. JACC Cardiovasc Imaging. 2010;3:1104–12. doi: 10.1016/j.jcmg.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harada K, Amano T, Uetani T, Tokuda Y, Kitagawa K, Shimbo Y, Kunimura A, Kumagai S, Yoshida T, Kato B, Kato M, Marui N, Ishii H, Matsubara T, Murohara T. Cardiac 64-Multislice Computed Tomography Reveals Increased Epicardial Fat Volume in Patients With Acute Coronary Syndrome. Am J Cardiol. 2011;108:1119–23. doi: 10.1016/j.amjcard.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda-Peats R, Ramesh A, Wong ND, Shaw LJ, Slomka PJ, Berman DS. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352–60. doi: 10.1016/j.jcmg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 7.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–26. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Kitagawa T, Yamamoto H, Horiguchi J, Ohhashi N, Tadehara F, Shokawa T, Dohi Y, Kunita E, Utsunomiya H, Kohno N, Kihara Y. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging. 2009;2:153–60. doi: 10.1016/j.jcmg.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Pflederer T, Marwan M, Schepis T, Ropers D, Seltmann M, Muschiol G, Daniel WG, Achenbach S. Characterization of culprit lesions in acute coronary syndromes using coronary dual-source CT angiography. Atherosclerosis. 2010;211:437–44. doi: 10.1016/j.atherosclerosis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND, Virmani R, Kondo T, Ozaki Y, Narula J. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 11.Ding J, Kritchevsky SB, Harris TB, Burke GL, Detrano RC, Szklo M, Jeffrey Carr J. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16:1914–9. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kalsch H, Seibel RM, Erbel R, Mohlenkamp S. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211:195–9. doi: 10.1016/j.atherosclerosis.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Nakazato R, Dey D, Cheng VY, Gransar H, Slomka PJ, Hayes SW, Thomson LE, Friedman JD, Min JK, Berman DS. Epicardial fat volume and concurrent presence of both myocardial ischemia and obstructive coronary artery disease. Atherosclerosis. 2012;221:422–6. doi: 10.1016/j.atherosclerosis.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki K, Matsumoto T, Aono H, Furukawa H, Samukawa M. Relationship between epicardial fat measured by 64-multidetector computed tomography and coronary artery disease. Clin Cardiol. 2011;34:166–71. doi: 10.1002/clc.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TD, Lee WJ, Shih FY, Huang CH, Chen WJ, Lee YT, Shih TT, Chen MF. Association of epicardial adipose tissue with coronary atherosclerosis is region-specific and independent of conventional risk factors and intra-abdominal adiposity. Atherosclerosis. 2010;213:279–87. doi: 10.1016/j.atherosclerosis.2010.07.055. [DOI] [PubMed] [Google Scholar]
- 16.Oka T, Yamamoto H, Ohashi N, Kitagawa T, Kunita E, Utsunomiya H, Yamazato R, Urabe Y, Horiguchi J, Awai K, Kihara Y. Association between epicardial adipose tissue volume and characteristics of non-calcified plaques assessed by coronary computed tomographic angiography. Int J Cardiol. 2012;161:45–9. doi: 10.1016/j.ijcard.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Schlett CL, Ferencik M, Kriegel MF, Bamberg F, Ghoshhajra BB, Joshi SB, Nagurney JT, Fox CS, Truong QA, Hoffmann U. Association of pericardial fat and coronary high-risk lesions as determined by cardiac CT. Atherosclerosis. 2012;222:129–34. doi: 10.1016/j.atherosclerosis.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito T, Nasu K, Terashima M, Ehara M, Kinoshita Y, Kimura M, Tanaka N, Habara M, Tsuchikane E, Suzuki T. The impact of epicardial fat volume on coronary plaque vulnerability: insight from optical coherence tomography analysis. Eur Heart J Cardiovasc Imaging. 2012;13:408–15. doi: 10.1093/ehjci/jes022. [DOI] [PubMed] [Google Scholar]
- 19.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 20.Dey D, Suzuki Y, Suzuki S, Ohba M, Slomka PJ, Polk D, Shaw LJ, Berman DS. Automated quantitation of pericardiac fat from noncontrast CT. Invest Radiol. 2008;43:145–53. doi: 10.1097/RLI.0b013e31815a054a. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, Arai T, Kotani K, Funahashi T, Yamashita S, Matsuzawa Y. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–6. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 22.Kvist H, Chowdhury B, Grangard U, Tylen U, Sjostrom L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351–61. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 23.Sjostrom L, Kvist H, Cederblad A, Tylen U. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am J Physiol. 1986;250:E736–45. doi: 10.1152/ajpendo.1986.250.6.E736. [DOI] [PubMed] [Google Scholar]
- 24.Nakazato R, Shmilovich H, Tamarappoo BK, Cheng VY, Slomka PJ, Berman DS, Dey D. Interscan reproducibility of computer-aided epicardial and thoracic fat measurement from noncontrast cardiac CT. J Cardiovasc Comput Tomogr. 2011;5:172–9. doi: 10.1016/j.jcct.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dey D, Lee CJ, Ohba M, Gutstein A, Slomka PJ, Cheng V, Suzuki Y, Suzuki S, Wolak A, Le Meunier L, Thomson LE, Cohen I, Friedman JD, Germano G, Berman DS. Image quality and artifacts in coronary CT angiography with dual-source CT: initial clinical experience. J Cardiovasc Comput Tomogr. 2008;2:105–14. doi: 10.1016/j.jcct.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Gutstein A, Dey D, Cheng V, Wolak A, Gransar H, Suzuki Y, Friedman J, Thomson LE, Hayes S, Pimentel R, Paz W, Slomka P, Le Meunier L, Germano G, Berman DS. Algorithm for radiation dose reduction with helical dual source coronary computed tomography angiography in clinical practice. J Cardiovasc Comput Tomogr. 2008;2:311–22. doi: 10.1016/j.jcct.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Ferencik M, Ropers D, Abbara S, Cury RC, Hoffmann U, Nieman K, Brady TJ, Moselewski F, Daniel WG, Achenbach S. Diagnostic accuracy of image postprocessing methods for the detection of coronary artery stenoses by using multidetector CT. Radiology. 2007;243:696–702. doi: 10.1148/radiol.2433060080. [DOI] [PubMed] [Google Scholar]
- 28.Cheng V, Gutstein A, Wolak A, Suzuki Y, Dey D, Gransar H, Thomson L, Hayes S, Friedman J, Berman D. Moving beyond binary grading of coronary arterial stenoses on coronary computed tomographic angiography: insights for the imager and referring clinician. JACC Cardiovasc Imaging. 2008;1:460–71. doi: 10.1016/j.jcmg.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Ueno K, Anzai T, Jinzaki M, Yamada M, Jo Y, Maekawa Y, Kawamura A, Yoshikawa T, Tanami Y, Sato K, Kuribayashi S, Ogawa S. Increased epicardial fat volume quantified by 64-multidetector computed tomography is associated with coronary atherosclerosis and totally occlusive lesions. Circ J. 2009;73:1927–33. doi: 10.1253/circj.cj-09-0266. [DOI] [PubMed] [Google Scholar]
- 30.Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010;210:150–4. doi: 10.1016/j.atherosclerosis.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Marwan M, Taher MA, El Meniawy K, Awadallah H, Pflederer T, Schuhback A, Ropers D, Daniel WG, Achenbach S. In vivo CT detection of lipid-rich coronary artery atherosclerotic plaques using quantitative histogram analysis: a head to head comparison with IVUS. Atherosclerosis. 2011;215:110–5. doi: 10.1016/j.atherosclerosis.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Gauss S, Achenbach S, Pflederer T, Schuhback A, Daniel WG, Marwan M. Assessment of coronary artery remodelling by dual-source CT: a head-to-head comparison with intravascular ultrasound. Heart. 2011;97:991–7. doi: 10.1136/hrt.2011.223024. [DOI] [PubMed] [Google Scholar]