Abstract
Purpose.
To determine the effect of the nitric oxide donor, sodium nitroprusside (SNP), and the nitric oxide synthase (NOS) inhibitor, L-nitro-arginine-methylester (L-NAME), on IOP, mean arterial pressure (MAP), pupil diameter (PD), refraction (Rfx), aqueous humor formation (AHF), and outflow facility (OF) in monkeys.
Methods.
Monkeys were treated with single or multiple topical treatments of 500 μg SNP or L-NAME to one eye. IOP was determined by Goldmann applanation tonometry, PD with vernier calipers in room light, Rfx by Hartinger coincidence refractometry, AHF by fluorophotometry, and MAP with a blood pressure monitor. OF was determined by two-level constant pressure perfusion following anterior chamber exchange.
Results.
Following four topical treatments with 500 μg SNP, 30 minutes apart, IOP was significantly decreased from 2 to 6 hours compared with the contralateral control with the maximum IOP reduction of 20% at 3 hours (P < 0.001). PD, Rfx, and AHF were unchanged. Effects on MAP were variable. OF after SNP exchange was significantly increased by 77% (P < 0.05) at 10−3 M. Topical L-NAME had no effect on IOP, PD, Rfx, or MAP.
Conclusions.
Enhancement of nitric oxide concentration at targeted tissues in the anterior segment may be a useful approach for IOP reduction for glaucoma therapy. Additional studies are warranted before conclusions can be made regarding the effect of NOS inhibition on ocular physiology in nonhuman primates.
Keywords: nitric oxide, intraocular pressure, outflow facility
Topical sodium nitroprusside lowered IOP in cynomolgus monkeys without affecting aqueous humor formation. Intracameral sodium nitroprusside increased outflow facility.
Introduction
Nitric oxide is synthesized in the eye from L-arginine by nitric oxide synthase (NOS), and activates cyclic guanosine monophosphate (cGMP) that results in mediation of relaxation responses in the trabecular meshwork and ciliary muscle1,2 as well as in vascular smooth muscle cells in the aqueous drainage system.3 NOS has been reported in ocular structures in the anterior segment of the primate eye as well as in layers of the retina and its circulation. Nitric oxide has the potential to be involved with both protective and damaging responses related to glaucoma. Therefore, selected inhibition or enhancement of nitric oxide synthesis in various parts of the eye could potentially be used for glaucoma therapy.4
In human glaucoma eyes there are dramatic reductions in staining indicative of NOS activity in ciliary muscle, trabecular meshwork, and Schlemm's canal compared with control eyes5,6 that are unrelated to the use of multiple glaucoma therapies, or the severity of the disease.5
NOS activity, equated with nicotinamide adenine dinucleotide phosphate (NADPH)-diaphorase staining, localizes strongly to the longitudinal as compared with the circular regions of the ciliary muscle in healthy human eyes.6,7 Regionalized staining is less apparent in monkey ciliary muscle. Only minor staining is detected in trabecular meshwork. Ciliary processes show staining associated with stroma and nonpigmented epithelial cells.7
In vivo, there have been conflicting reports of the effect of the nitric oxide donors on IOP.8–14 One study in rabbits reports that due to the role of nitric oxide in episcleral vasoregulation, topical treatment with a nitric oxide donor, such as sodium nitroprusside (SNP), should elicit vasodilation and increased episcleral venous pressure, and therefore would increase IOP so long as the other components of aqueous fluid dynamics are unchanged.9 Indeed, in anesthetized rabbits, topical administration of the nitric oxide donor SNP increased IOP measured via direct cannulation without changing aqueous flow as measured by fluorophotometry.9 This increase in IOP is consistent with what was found in other rabbit studies with or without anesthesia.8,11 However, a reduction in IOP as measured by pneumatonometry was demonstrated in conscious rabbits following topical treatment with SNP.9
Similar conflicting results have been reported in monkeys12,13 and humans.14 In cynomolgus monkeys, one study reports that nitrovasodilators (nitroglycerine or hydralazine) decrease IOP when compared with baseline measured on a different day. Outflow facility (OF) was also increased at specific concentrations.12 Conversely, no reduction in IOP was found after topical nitroglycerin administration to healthy and glaucomatous monkeys when IOP was compared with baseline measured on the same day.13 In humans, small increases in IOP were observed at two different doses of topical hydralazine.8
Recently, a study has described a marginal decrease in IOP in transgenic mice that overexpress endothelial NOS in vivo. An increase in OF was reported in enucleated transgenic mouse eyes. L-nitro-arginine-methylester (L-NAME) administration effectively inhibited this effect such that there was no difference in the pressure–flow relationship from wild-type mice.15 This study suggests that nitric oxide plays a role in mediating conventional, pressure-dependent OF via trabecular meshwork mechanosensitivity to maintain normal OF and IOP.
The goal of the current study was to conduct a more systematic study of the effects of nitric oxide elevation and suppression on ocular physiology in living nonhuman primates.
Methods
Animals and Anesthesia
Twenty-one adult cynomolgus (Macaca fascicularis) monkeys of either sex, weighing 3.0 to 7.4 kg, and ranging in age from 4 to 14 years were studied. Monkeys were determined to be free of ocular abnormalities by slit-lamp biomicroscopic examination prior to any experimental protocol. Monkeys were anesthetized with intramuscular (i.m.) ketamine (10–20 mg/kg initial; 1–10 mg/kg supplemental) for topical drop administration, IOP, and fluorophotometry experiments. Monkeys were anesthetized with i.m. ketamine (10–20 mg/kg initial) followed by intravenous pentobarbital anesthesia (15 mg/kg initial; 5–10 mg/kg supplemental) for OF studies. Anesthesia was maintained continuously for the duration of the experimental protocols. For all measurements and examinations, monkeys were in a prone position with the eyes pointing straight ahead and maintained approximately 4 to 8 cm above the heart. For all eye drop administration, monkeys were in a supine position with the eyelid held open for drop administration and for 30 seconds after each drop. Drops were administered to the central cornea 1 minute apart for multiple drops at a given time point. All experiments were conducted in accordance with the University of Wisconsin Institutional Animal Care and Use Committee and National Institutes of Health Guidelines, and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
IOP, Pupil Diameter (PD), and Refraction (Rfx)
Baseline IOP was determined by ‘minified' Goldmann applanation tonometry using a Haag Streit slit lamp (Haag-Streit AG, Koeniz, Switzerland), PD was measured in room light using vernier calipers (Fisher Science Education Traceable Digital Carbon Fiber Calipers; Fisher Scientific, Waltham, MA), and Rfx was determined using a Hartinger coincidence refractometer (Zeiss, Jena, Germany).16,17
All agents were freshly prepared in PBS immediately prior to the first administration. Test agents and vehicles were kept refrigerated between dosings on a given day.
In one set of experiments (n = 8), the nitric oxide donor, SNP (T1/2 ≤ 10 minutes at 37°C; Sigma-Aldrich, St. Louis, MO) was administered to one eye; PBS vehicle to the contralateral eye. SNP was given as a single topical treatment at baseline (50 μg in 2×5 μL drops: total dose = 50 μg), or as multiple topical treatments (500 μg in 5×5 μL drops administered at 0, 1, 2, and 3 hours or at 0, 0.5, 1, 1.5 hours: total dose = 2 mg).
In another experiment (n = 4), the purported longer acting nitric oxide donor,10 S-nitroso-n-acetyl-DL-penicillamine (SNAP; T1/2 = 5 hours in aqueous at 37°C, Sigma-Aldrich) was given as a single topical treatment (500 μg in 5×5 μL drops: total dose = 500 μg) to one eye; PBS vehicle to the contralateral eye.
In a separate set of experiments (n = 8), the NOS inhibitor, L-NAME (Sigma-Aldrich) was administered to one eye; PBS vehicle to the contralateral eye. L-NAME was given as multiple topical treatments (500 μg in 2×5 μL drops administered at 0 and 0.5 hours: total dose = 1 mg).
IOP was measured hourly (every 0.5 hours on some occasions, to determine the time frame of the drug effect) for up to 6 hours. Slit-lamp biomicroscopy (to determine the presence of biomicroscopic cells or flare) was performed at baseline and 3 and 6 hours post treatment.
Mean Arterial Blood Pressure (MAP) and Heart Rate (HR)
MAP values were recorded via a cuff attached to a Cardell Veterinary Monitor Model 9402 (Sharn Veterinary, Inc., Tampa, FL). Values for each time point represent the average of two to four measurements taken with the cuff applied to the arm and/or leg after IOP was measured. MAP and HR were taken at baseline, 1, 2, 3, 4, 5, and 6 hours.
Aqueous Humor Formation (AHF)
AHF was determined by ocular scanning fluorophotometry (Fluorotron Master; OcuMetrics, Inc., Mountain View, CA) as previously described.18 The afternoon preceding fluorophotometry, five 2 μL drops of a 5% sodium fluorescein solution were administered 30 seconds apart to the supine animal, beginning 5 minutes after administration of 1 to 2 × 30 μL drop(s) of topical 0.5% proparacaine HCl. This regimen maintained corneal fluorescein concentrations of greater than or equal to 200 ng/mL throughout the measurement period. Baseline fluorophotometry was done at least 1 week prior to treatment with SNP or vehicle. Measurements were done every 30 minutes for 3 hours, beginning 30 minutes after treatment. IOP was measured prior to treatment and again after the last scan at 3 hours post treatment. Baseline fluorophotometry was repeated at 1 to 11 weeks post treatment. Biomicroscopy was performed and IOP was measured at baseline and after the last scan.
Outflow Facility
Total OF was determined by two-level constant pressure perfusion of the anterior chamber.19 The anterior chambers of both eyes were cannulated with one branched (superiorly) and one nonbranched (inferiorly) 26-gauge needle. One end of the branched needle was attached to an elevated reservoir containing Bárány's perfusand, and the other to a physiologic pressure transducer (Gould & Statham P23 Db Series; Gould, Inc., Oxnard, CA) via polyethylene tubing. The nonbranched needle was attached to clamped polyethylene tubing. Baseline OF was measured for approximately 45 minutes. The tubing from the nonbranched needle was then attached to a variable-speed infusion pump (Harvard Apparatus Model #944; Harvard Apparatus, Millis, MA; or Model #210; KD Scientific, Inc., Hollistan, MA). The anterior chamber contents of one eye were then exchanged over approximately 10 minutes with 2 mL (200 μL/min) of test compound in Bárány's perfusand; the opposite eye with 2 mL Bárány's perfusand only. Reservoirs were closed for 15 minutes and filled with the corresponding solution. Reservoirs were then reopened and OF measured for an additional 60 minutes. In some cases, the anterior chamber contents of one eye (same eye as treated previously) was then exchanged as above with a higher dose; the opposite eye with Bárány's perfusand and OF measured for another 60 minutes.
Data Analysis
Data are mean ± SEM. IOP, MAP, HR, and pupil data were evaluated by repeated measures ANOVA with post-test Tukey-Kramer Multiple Comparisons (Instat GraphPad v.3.06; GraphPad Software, Inc., La Jolla, CA). Significance for all data was also determined by the two-tailed paired t-test for differences compared with 0.0 or for ratios compared with 1.0 unless otherwise noted. For ratio analysis of AHF and OF measurements, post treatment values were first compared with their respective baseline values and then the ratio of these ratios was compared between contralateral eyes.
Sample size calculation for paired organs20 conducted based on the mean and standard error of the current data verifies that at n equals 8 to 10, we have sufficient power to detect a physiologic response greater than or equal to 25% of baseline for a two-sided test and 5% significance.
Results
IOP, PD, Rfx, HR, and MAP
SNP.
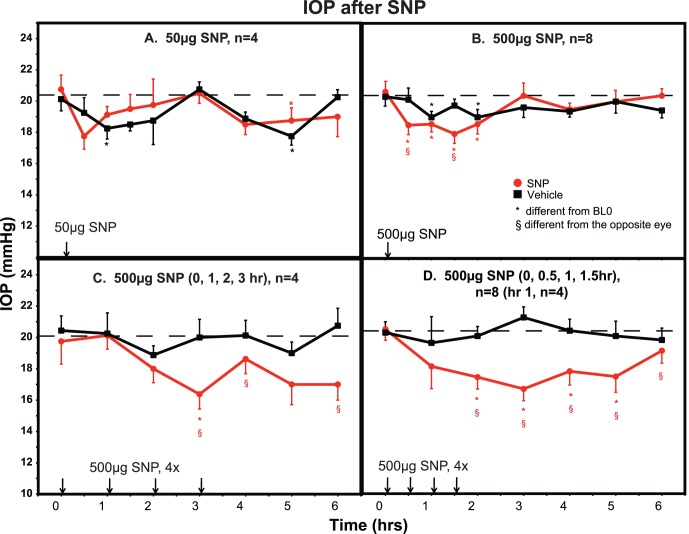
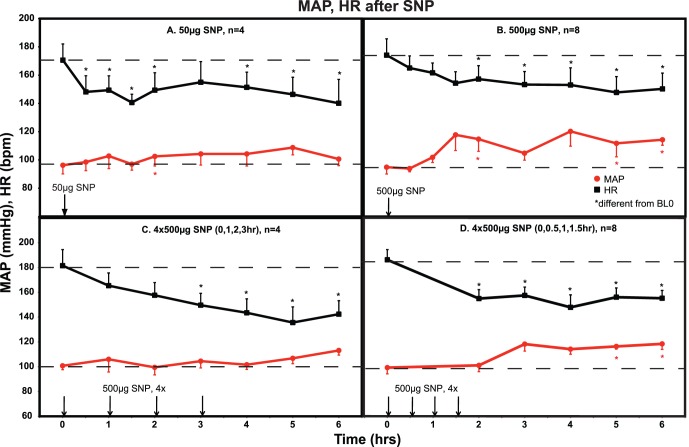
An IOP dose-response relationship was found following topical treatment with the nitric oxide donor SNP. Sustained IOP lowering was only achieved when the 500 ug dose was administered at 30 minute intervals (Fig. 1D). Using this regimen, IOP was significantly lowered by 2.5 to 5 mm Hg (10–20%, two-tailed paired t-test, P < 0.01; ANOVA, P < 0.0001) during the interval 2 to 5 hours post treatment compared with the contralateral control eye. SNP did not significantly affect PD or Rfx during any treatment regimen. MAP for all protocols was unaffected or was variably elevated over the 6-hour measurement period. Heart rate trended downward over the 6-hour measurement period for all protocols (Fig. 2).
Figure 1.
IOP after topical SNP. Following a single treatment with 50 μg (A) or 500 μg (B) of SNP, IOP was decreased by 10% to 15% at various time points compared with vehicle-treated eyes. Four hourly treatments with 500 μg SNP (C) prolonged the IOP reduction, but this was not significantly different from the vehicle-treated eye except at the 3-hour time point. Treatment with 500 μg SNP at four 30-minute intervals (D) produced a significant 15% to 20% reduction in IOP that was sustained for several hours. *Significantly different from baseline prior to the first treatment (BL0) or §compared with the opposite eye, P < 0.05. ANOVA analysis showed a significant decrease in IOP in treated compared with control eyes following a single dose of 50 μg SNP ([A], P = 0.049), 500 μg SNP ([B], P = 0.035), and after treatment with 500 μg SNP at 30-minute intervals (four doses, [D], P < 0.0001). Results for 500 μg SNP at 1 hour intervals (four doses, [C]) approached significance (P = 0.054).
Figure 2.
MAP and HR after topical SNP. HR was significantly decreased with all treatment regimens. MAP was essentially unchanged or variably increased. *Significantly different from baseline prior to the first treatment (BL0), P < 0.05. ANOVA analysis over the 6-hour interval showed a significant decrease in HR at all doses ([A], P = 0.0002; [B], P = 0.035; [C], P < 0.0001; [D], P = 0.0005). MAP was significantly increased for (B) (P = 0.005) and (D) (P = 0.006).
SNAP.
A single treatment with 500 μg SNAP (5×5 μL) had no effect on IOP compared with the vehicle control (not shown); therefore, no further testing was carried out with this compound.
L-NAME.
The NOS inhibitor L-NAME did not significantly affect IOP compared with the contralateral control eye, although IOP was slightly, but significantly, reduced by 1 to 2 mm Hg at different time points in both eyes compared with their respective pretreatment baselines (Fig. 3). The IOP in untreated monkey eyes can decrease gradually over a 6-hour period under ketamine anesthesia. This is a variable response on a given day even in the same group of monkeys. We have reported this gradual decline on several other occasions.21,22 HR and MAP were unaffected. Rfx and PD were no different between treated and control eyes at any time point.
Figure 3.
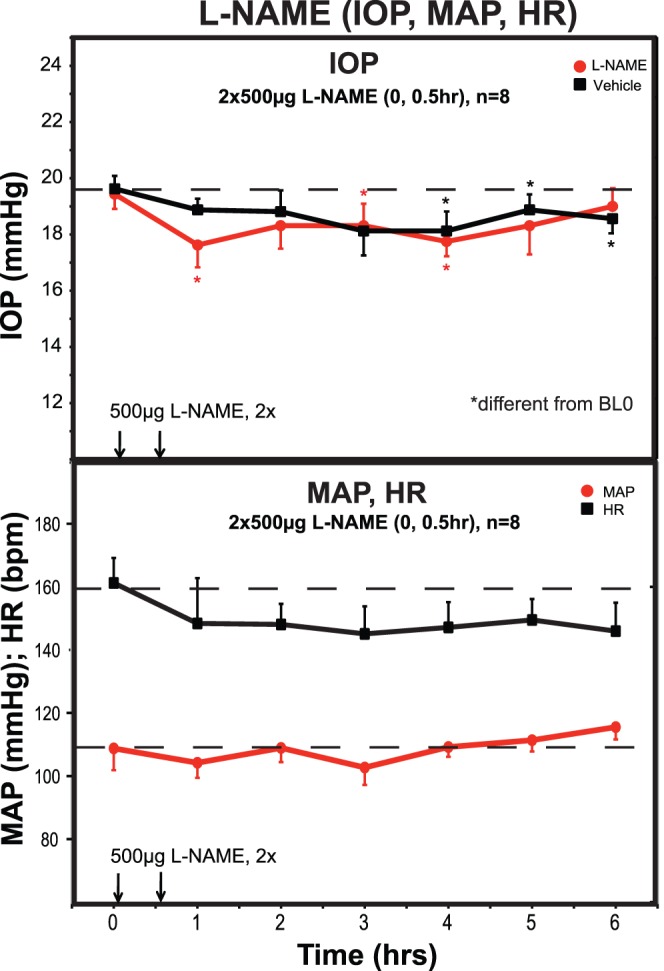
L-NAME effects on IOP, MAP, HR. Topical treatment with the NOS inhibitor L-NAME had no effect on IOP in treated compared with vehicle control eyes, although IOP in both eyes were significantly less than baseline at various time points. MAP and HR were also unaffected, although there was a tendency for HR to decrease with time as was seen after SNP in Figure 2. *Significantly different from baseline prior to the first treatment (BL0), P < 0.05. ANOVA analysis showed no effect of L-NAME on IOP, MAP, or HR.
Aqueous Humor Formation
Topical treatment with 500 μg SNP at 30-minute intervals for four treatments had no effect on AHF during the interval 0.5 to 3 hours post treatment when comparing treated with control eyes after correcting for their respective baselines taken on a separate day (Table 1). IOP, measured after the last fluorophotometric scan at 3 hours, was significantly reduced in SNP-treated eyes (P < 0.001) compared with contralateral controls. In contralateral control eyes, IOP was unchanged compared with same day baseline.
Table 1.
Aqueous Humor Formation and IOP After Topical SNP
|
Aqueous Humor Formation, μL/min/mm Hg |
||||||
|
Treated |
Control |
Trt/Cont |
Trt/BLavg |
Cont/BLavg |
(Trt/BLavg)/(Cont/BLavg) |
|
| BL1 | 1.58 ± 0.15 | 1.51 ± 0.15 | 1.06 ± 0.04 | |||
| BL2 | 1.51 ± 0.16 | 1.76 ± 0.25 | 0.90 ± 0.06 | |||
| BLavg | 1.54 ± 0.12 | 1.63 ± 0.17 | 0.98 ± 0.04 | |||
| SNP | 1.57 ± 0.16 | 1.48 ± 0.20 | 1.10 ± 0.07 | 1.01 ± 0.06 | 0.90 ± 0.07 | 1.12 ± 0.07 |
|
IOP, mmHg |
Trt-BL |
Cont-BL |
Trt-BL – Cont-BL |
|||
| BL | 20.38 ± 0.23 | 19.50 ± 0.35 | ||||
| SNP | 16.50 ± 0.38 | 19.38 ± 0.26 | −3.88 ± 0.53* | −0.13 ± 0.38 | −3.75 ± 0.54* | |
= 8; SNP = 500 μg at 30-minute intervals × 4; IOP at 3 hours post initial treatment. BL, baseline; Trt, treated; Cont, control.
Significant by the two-tailed paired t-test for differences compared with 0.0: P < 0.001.
Outflow Facility
SNP.
Baseline OF during the 10−5M and 10−4M SNP studies was significantly different between treated and control eyes, but was within the normal range for cynomolgus monkeys.23 OF was not altered following treatment with 10−5M or 10−4M SNP (n = 9) when comparing treated and contralateral control eyes after correction for baseline. OF was significantly increased by 77% (P < 0.05) in eyes treated with 10−3 M SNP (n = 8) compared with contralateral control eyes before and after correcting for baseline measurements. OF in control eyes did not differ significantly when compared with ipsilateral baseline measurements. All eight of the monkeys used in the 10−3 M SNP studies were common to the 10−5 M and 10−4 M SNP studies (Table 2).
Table 2. .
Outflow Facility After Anterior Chamber Exchange With SNP
|
Outflow Facility, μL/min/mm Hg |
T/C |
T/BL |
C/BL |
(T/BL)/(C/BL) |
||
|
Treated |
Control |
|||||
| BL1 | 0.35 ± 0.03 | 0.28 ± 0.03 | 1.32 ± 0.12* | |||
| 10−5 M SNP | 0.49 ± 0.06 | 0.37 ± 0.05 | 1.42 ± 0.17* | 1.41 ± 0.15* | 1.30 ± 0.10* | 1.13 ± 0.15 |
| 10−4 M SNP | 0.57 ± 0.12 | 0.40 ± 0.05 | 1.40 ± 0.21 | 1.57 ± 0.31 | 1.50 ± 0.18* | 1.09 ± 0.18 |
| BL2 | 0.35 ± 0.06 | 0.32 ± 0.05 | 1.07 ± 0.06 | |||
| 10−3 M SNP | 0.55 ± 0.06 | 0.32 ± 0.04 | 1.88 ± 0.27* | 1.86 ± 0.29* | 1.04 ± 0.05 | 1.77 ± 0.27* |
= 9 for BL1, 10−5 M, and 10−4 M SNP; N = 8 for BL2 and 10−3 M SNP studies, which were conducted on a separate occasion. All of the monkeys in the 10−3 M SNP studies were common to the 10−4 M and 10−5 M SNP studies. T, treated; C, control.
Significant by the two-tailed paired t-test for ratios compared with 1.0: P < 0.05.
At the conclusion of the OF measurements, after needle withdrawal, blood was seen in the anterior chambers of both eyes of three of nine monkeys which received the 10−4 M dose, and in both eyes of all eight monkeys that received the 10−3 M dose. Subjectively, this is a much higher frequency of occurrence than is typically seen following needle withdrawal at the conclusion of OF measurements with other compounds.
Discussion
One role for nitric oxide in the anterior segment may be to modulate outflow resistance either directly at the level of the trabecular meshwork, Schlemm's canal, and collecting channels, or indirectly through alteration in the tone of the longitudinal ciliary muscle. Nitrovasodilators were shown to relax bovine trabecular meshwork1 as well as bovine and monkey ciliary muscle1,2,24 strips precontracted with carbachol in vitro. Small increases in OF are produced by modulation of the nitric oxide system in organ-cultured human anterior segments.25 Nitric oxide decreases cell volume in the trabecular meshwork and increases OF in porcine eye anterior segments, suggesting that the nitric oxide–induced alterations in cell volume may regulate outflow resistance.26 IOP and AHF are decreased in isolated pig eyes perfused with nitrovasodilators,27 suggesting mechanisms independent of ocular vasculature. Systemic administration of L-NAME to rabbits resulted in an increase in MAP, but a dramatic reduction in IOP, possibly due to a reduction in ciliary blood flow sufficient to impair aqueous production.28,29
Early studies reported conflicting results of the effects of nitrovasodilators on aqueous humor dynamics in the living primate eye. Nitroglycerin and hydralazine increased OF in monkey eyes by 92% and 28%, respectively, but only after intracameral bolus injection of 10−3 M.12 Nitroglycerin may or may not decrease IOP when applied topically to monkey eyes.12,13 Topical administration of hydralazine to ocular normotensive humans resulted in an increase in IOP accompanied by conjunctival hyperemia.14 Intravenous administration of L-arginine lowered IOP in healthy human volunteers and increased nitrite levels in the aqueous humor of rabbits compared with controls, presumably as a result of conversion to nitric oxide by NOS.30 In a different study, no effect of intravenous L-arginine on IOP in humans was detected.31 Study ethnicities, design, and analysis may account for some of the differences in findings between these two studies.
Caution is advised in comparing results from different nitric oxide donor compounds delivered via different routes to different species since there may be confounding effects of these compounds other than those resulting from nitric oxide production alone. Some additional mechanisms need to be verified in ocular tissues.
Hydralazine-induced vasodilatation is associated with powerful stimulation of the sympathetic nervous system, which results in increased heart rate and contractility, increased plasma renin activity, and fluid retention; all of these effects counteract the antihypertensive effect of hydralazine. Although most of the sympathetic activity is due to a baroreceptor-mediated reflex, hydralazine may stimulate the release of norepinephrine from sympathetic nerve terminals and augment myocardial contractility directly.32
Nitroglycerin is extensively metabolized, yielding dinitrates, mononitrates, nitric oxide, and inorganic nitric oxide. A number of enzymes/proteins have been reported to metabolize nitroglycerin, including glutathione S-transferase, cytochrome P-450, and an uncharacterized vascular microsomal enzyme. Nonproteinous thiols can also react with nitroglycerin to generate nitric oxide or S-nitrosothiol. Nitroglycerin administration can lead to increased vascular oxidative stress resulting in multiple changes in signal transduction and gene regulation. Studies have shown that other nitric oxide donors such as SNP, S-nitrosothiols, and organic nitrites, either do not induce or are less effective in inducing vascular tolerance when compared with organic nitrates.33
SNP is not without its confounding issues as well. SNP was originally thought to spontaneously release nitric oxide. However, a membrane-bound nicotine adenine dinucleotide oxidoreductase appears to contribute to the release of nitric oxide from nitroprusside, but not nitroglycerin, in calf pulmonary artery.34 In addition, SNP is reported to degrade to cyanide in vivo and with light exposure. Maximal degradation of SNP under our subdued room light conditions is calculated to be 10% for topical IOP experiments for the protocol giving the maximum IOP response (500 μg at 30-minute intervals for 2 hours). Maximal degradation during OF measurements would be 10% for a 2 hour experiment (1 dose of SNP) or 20% for a 2-dose, 3 hour experiment. As stated in Arnold et al,35 this degradation would not be expected to alter the extent of nitric oxide available for producing the physiologic response. Cyanide production under these circumstances would be expected to be less than 0.01% of the SNP dose. It is possible that cyanide toxicity may have contributed to the increased incidence of hemorrhage into the anterior chamber upon removing the needles from eyes perfused with the highest dose of SNP (10−3 M).
In the current studies, the nitric oxide donor SNP was effective in lowering IOP following repeated topical administration of a high dose at short intervals. Thus far, our data suggest that the mechanism for this IOP lowering response is, at least partly, due to an increase in OF, when a very high dose of 10−3 M SNP was utilized intracamerally. This intracameral concentration is comparable to what would be expected following topical delivery of 2 mg SNP (4×500 μg) assuming 1% corneal penetration36 and no loss over the 1.5 hours of delivery (30-minute intervals). This high concentration requirement may be due to a low rate of penetration into the target tissues, or decreased concentration of the drug at the target sites due to the diverse biologic roles of nitric oxide and its short half-life (T1/2 ≤ 10 minutes at 37°C) in living tissues.37,38 As a reference point, in humans with congestive heart failure and life-threatening high blood pressure, the maximum recommended dose for intravenous administration of SNP (see package insert for Nitropress; Hospira, Inc., Lake Forest, IL) is 100 μg/kg delivered over a 10-minute period (for a 70-kg human, this would amount to 7 mg).
Additional support for an effect of nitric oxide on outflow was recently reported. Transgenic mice over expressing endothelial NOS39 were found to have lower IOP15 compared with wild-type mice. However, both mean aortic pressure and pulmonary artery pressure are also reduced by 30% in the transgenic mice.39 In situ, transgenic mice eyes (n = 4) demonstrated increased pressure-dependent drainage compared with wild-type mice.15 This effect on pressure-dependent drainage was reversed with the NOS inhibitor L-NAME.
Thus, the nitric oxide system may be a signal/transduction arm that mediates response to stressors such as mechanical deformation of the trabecular meshwork caused by stress–strain, shear–stress, and other pressure-related phenomena, or biochemical, hormonal, or metabolic alterations in the surrounding milieu.40
Enhancement of uveoscleral outflow, may be another component involved in the IOP lowering mechanism, as suggested by our previous studies on ciliary muscle relaxation in vitro,2 we did not measure the effects of SNP on uveoscleral outflow in living animals, nor did we measure episcleral venous pressure in the current study. It could be beneficial to study the effect of SNP on those parameters in nonhuman primates in the future.
Results from the current study reporting a decrease in IOP in monkeys in response to the nitrovasodilator SNP are in contrast to reports of the opposite effect on IOP in rabbits.8,9,11 These conflicting results may be due to species differences. They also illustrate that the nitric oxide story is complicated and that its actions should be described and interpreted cautiously. Another possible species-related response difference in our study was the lack of an IOP response to topical SNAP in our monkeys (n = 4) as compared with the purported longer acting and greater IOP-lowering response obtained in rabbit studies.10 Additional studies in monkeys were not carried out with SNAP since the superior IOP-lowering response to SNP was chosen for mechanistic studies.
The NOS inhibitor L-NAME did not have an effect on IOP in the current study in contrast to the reduction in IOP produced by L-NAME in rabbit studies.9,28,29 However, effects of L-NAME may be limited by the amount of NOS in the eye at the time of drug administration, unintended reactions in the eye, and/or the ability of the compound to localize to the regions of the eye with the highest NOS.2,6,9
L-NAME has been shown to be a muscarinic antagonist, which could result in a reduction of ciliary muscle tension on the trabecular meshwork leading to a decrease in OF.41 In the current studies, we did not detect any changes in PD or Rfx in response to L-NAME treatment that would suggest a muscarinic antagonist effect. Additional studies are warranted before conclusions can be made regarding the effect of NOS inhibition on ocular physiology and the responses to other classes of pharmaceuticals in nonhuman primates.
The nitric oxide system could potentially be targeted to enhance aqueous outflow and lower IOP in glaucoma. Since NOS levels appear to be diminished in glaucomatous eyes,5,6 pharmacotherapy would have to bypass this part of the nitric oxide pathway. A nitric oxide–releasing prostaglandin analog was shown to produce a larger IOP reduction compared with latanoprost alone in ocular hypertensive rabbits, dogs, and monkeys.42 Gene therapy in targeted sites where pharmacologic delivery of nitric oxide donors may not reach sufficient concentrations for therapeutic purposes may be a viable option as well.43 Endothelial NOS would be a likely candidate.39 However, caution is advised since manipulating nitric oxide homeostasis could be fraught with potential problems (e.g., elimination of vascular autoregulation).
Acknowledgments
The authors thank Beth Hennes, Jessica McDonald, Matthew Szaniawski, Alex Katz, Caitlin Kuehn, and Jeremy Kemmerling, who assisted with the experiments.
Supported by grants from the National Institutes of Health/National Eye Institute (R01 EY018567, P30 EY016665, P51 RR000167); Research to Prevent Blindness, Inc., New York, NY, unrestricted departmental and Physician-Scientist awards; Ocular Physiology Research and Education Foundation; and Walter Helmerich Chair from the Retina Research Foundation.
Disclosure: G.W. Heyne, None; J.A. Kiland, None; P.L. Kaufman, None; B.T. Gabelt, None
References
- 1. Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994; 35: 2515–2520 [PubMed] [Google Scholar]
- 2. Gabelt BT, Kaufman PL, Rasmussen CA. Effect of nitric oxide compounds on monkey ciliary muscle in vitro. Exp Eye Res. 2011; 93: 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Selbach JM, Rohen JW, Steuhl KP, Lütjen-Drecoll E. Angioarchitecture and innervation of the primate anterior episclera. Curr Eye Res. 2005; 30: 337–344 [DOI] [PubMed] [Google Scholar]
- 4. Becquet F, Courtois Y, Goureau O. Nitric oxide in the eye: multifaceted roles and diverse outcomes. Surv Ophthalmol. 1997; 42: 71–82 [DOI] [PubMed] [Google Scholar]
- 5. Nathanson JA, McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995; 36: 1774–1784 [PubMed] [Google Scholar]
- 6. Chen Z, Gu Q, Kaufman PL, Cynader MS. Histochemical mapping of NADPH-diaphorase in monkey and human eyes. Curr Eye Res. 1998; 17: 370–379 [DOI] [PubMed] [Google Scholar]
- 7. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci. 1995; 36: 1765–1773 [PubMed] [Google Scholar]
- 8. Krupin T, Weiss A, Becker B, Holmberg N, Fritz C. Increased intraocular pressure following topical azide or nitroprusside. Invest Ophthalmol Vis Sci. 1977; 16: 1002–1007 [PubMed] [Google Scholar]
- 9. Zamora DO, Keil JW. Episcleral venous pressure responses to topical nitroprusside and n-nitro-l-arginine methyl ester. Invest Ophthalmol Vis Sci. 2010; 51: 1614–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carreiro S, Anderson S, Gukasyan HJ, Krauss A, Prasanna G. Correlation of in vitro and in vivo kinetics of nitric oxide donors in ocular tissues. J Ocul Pharmacol Ther. 2009; 25: 105–112 [DOI] [PubMed] [Google Scholar]
- 11. Funk RH, Gehr J, Rohen JW. Short-term hemodynamic changes in episcleral arteriovenous anastomoses correlate with venous pressure and IOP changes in the albino rabbit. Curr Eye Res. 1996; 15: 87–93 [DOI] [PubMed] [Google Scholar]
- 12. Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and outflow facility in monkeys. Exp Eye Res. 1994; 58: 99–105 [DOI] [PubMed] [Google Scholar]
- 13. Wang R-F, Podos SM. Effect of the topical application of nitroglycerin on intraocular pressure in normal and glaucomatous monkeys. Exp Eye Res. 1995; 60: 337–339 [DOI] [PubMed] [Google Scholar]
- 14. Larsson L-I, Maus TL, Brubaker RF, Nathanson JA. Topically applied hydralazine: effects on systemic cardiovascular parameters, blood–aqueous barrier, and aqueous humor dynamics in normotensive humans. J Ocul Pharmacol. 1995; 11: 145–156 [DOI] [PubMed] [Google Scholar]
- 15. Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011; 52: 9438–9444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaufman PL, Davis GE. “Minified” Goldmann applanating prism for tonometry in monkeys and humans. Arch Ophthalmol. 1980; 98: 542–546 [DOI] [PubMed] [Google Scholar]
- 17. Croft MA, Kiland J, Gange SJ, Aref A, Pelzek CD, Kaufman PL. Comparison of Goldmann tonometry measurements using creamer vs fluorescein in cynomolgus monkeys. In: Lakshminarayanan V. ed Basic and Clinical Applications of Vision Science. The Professor Jay M. Enoch Festschrift Volume, Documenta Ophthalmologica Proceedings Series, Vol 60. Dorderecht, The Netherlands: Kluwer; 1997: 213–216 [Google Scholar]
- 18. Rasmussen CA, Gabelt BT, Kaufman PL. Aqueous humor dynamics in monkeys in response to the kappa opioid agonist bremazocine. Trans Am Ophthalmol Soc. 2007; 105: 225–239 [PMC free article] [PubMed] [Google Scholar]
- 19. Bárány EH. Simultaneous measurement of changing intraocular pressure and outflow facility in the vervet monkey by constant pressure infusion. Invest Ophthalmol Vis Sci. 1964; 3: 135–143 [PubMed] [Google Scholar]
- 20. Friedman LM, Furberg CD, DeMets DL. Sample size. In: Fundamentals of Clinical Trials. New York, NY: Springer; 2010: 133–167 [Google Scholar]
- 21. Gabelt BT, Okka M, Dean RT, Kaufman PL. Aqueous humor dynamics in monkeys after topical R-DOI. Invest Ophthalmol Vis Sci. 2005; 46: 4691–4694 [DOI] [PubMed] [Google Scholar]
- 22. Gabelt BT, Robinson JC, Hubbard WC, et al. Apraclonidine and brimonidine effects of anterior ocular and cardiovascular physiology in normal and sympathectomized monkeys. Exp Eye Res. 1994; 59: 633–644 [DOI] [PubMed] [Google Scholar]
- 23. Kaufman P, Erickson K, Bárány E. Effect of repeated anterior chamber perfusion on the intraocular pressure and total outflow facility in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1983; 24: 159–164 [PubMed] [Google Scholar]
- 24. Kamikawatoko S, Tokoro T, Ishida A, et al. Nitric oxide relaxes bovine ciliary muscle contracted by carbachol through elevation of cyclic GMP. Exp Eye Res. 1998; 66: 1–7 [DOI] [PubMed] [Google Scholar]
- 25. Schneemann A, Dijkstra BG, van den Berg TJ, Kamphuis W, Hoyng PFJ. Nitric oxide/guanylate cyclase pathways and flow in anterior segment perfusion. Graefes Arch Clin Exp Ophthalmol. 2002; 240: 936–941 [DOI] [PubMed] [Google Scholar]
- 26. Dismuke WM, Mbadugha CC, Ellis DZ. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am J Physiol Cell Physiol. 2008; 294: C1378–C1386 [DOI] [PubMed] [Google Scholar]
- 27. Shahidullah M, Yap M, To CH., Cyclic GMP. sodium nitroprusside and sodium azide reduce aqueous humour formation in the arterially perfused pig eye. Br J Pharmacol. 2005; 145: 84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kiel JW. Modulation of choroidal autoregulation in rabbits. Exp Eye Res. 1999; 69: 413–429 [DOI] [PubMed] [Google Scholar]
- 29. Kiel JW, Reitsamer HA, Walker JS, Kiel FW. Effects of nitric oxide synthase inhibition on ciliary blood flow, aqueous production and intraocular pressure. Exp Eye Res. 2001; 73: 355–364 [DOI] [PubMed] [Google Scholar]
- 30. Chuman H, Chuman T, Nao-i N, Sawada A. The effect of L-arginine on intraocular pressure in the human eye. Curr Eye Res. 2000; 20: 511–516 [PubMed] [Google Scholar]
- 31. Garhöfer G, Resch H, Lung S, Weigert G, Schmetterer L. Intravenous administration of L-arginine increases retinal and choroidal blood flow. Am J Ophthalmol. 2005; 140: 69–76 [DOI] [PubMed] [Google Scholar]
- 32. Oates JA, Brown NJ. Antihypertensive agents and the drug therapy of hypertension. In: Hardman JG, Limbird LE. eds Goodman and Gilman's The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill; 2001: 871–900 [Google Scholar]
- 33. Fung H-L. Biochemical mechanism of nitroglycerin action and tolerance: is this old mystery solved? Ann Rev Pharmacol Toxicol. 2004; 44: 67–85 [DOI] [PubMed] [Google Scholar]
- 34. Mohazzab-H KM, Kaminski PM, Agarwal R, Wolin MS. Potential role of a membrane-bound NADH oxidoreductase in nitric oxide release and arterial relaxation to nitroprusside. Circ Res. 1999; 84: 220–228 [DOI] [PubMed] [Google Scholar]
- 35. Arnold WP, Longnecker DE, Epstein RM. Photodegradation of sodium nitroprusside: biological activity and cyanide release. Anesthesiology. 1984; 61: 254–260 [DOI] [PubMed] [Google Scholar]
- 36. Burstein NL, Anderson JA. Corneal penetration and ocular bioavailability of drugs. J Ocul Pharmacol. 1985; 1: 309–326 [DOI] [PubMed] [Google Scholar]
- 37. Bredt D, Snyder S. Nitric oxide: a physiologic messenger molecule. Ann Rev Biochem. 1994; 63: 175–195 [DOI] [PubMed] [Google Scholar]
- 38. Beckman J, Koppenol W. Nitric oxide, superoxide, and peroxynitrite: the good, the bad and the ugly. Am J Physiol. 1996; 271: C1424–C1437 [DOI] [PubMed] [Google Scholar]
- 39. Van Haperen R, Cheng C, Mees BME, et al. Functional expression of endothelial nitric oxide synthase fused to green fluorescent protein in transgenic mice. Am J Pathol. 2003; 163: 1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaufman PL. Highlighted/invited editor's selection commentary on Stamer W, Lei Y: eNOS, a pressure-dependent regulator of intraocular pressure ( Invest Ophthalmol Vis Sci. 2011; 52: 9438–9444). Int Glauc Rev. 2012; 13-4: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buxton IL, Cheek DJ, Eckman D, Westfall DP, Sanders KM, Keef KD. NO-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993; 72: 387–395 [DOI] [PubMed] [Google Scholar]
- 42. Borghi V, Bastia E, Guzzetta M, et al. A novel nitric oxide releasing prostaglandin analog, NCX 125, reduces intraocular pressure in rabbit, dog, and primate models of glaucoma. J Ocul Pharmacol Ther. 2010; 26: 125–131 [DOI] [PubMed] [Google Scholar]
- 43. Kaufman PL, Tian B, Gabelt BT, Liu X. Outflow enhancing drugs and gene therapy in glaucoma. In: Weinreb R, Krieglstein G, Kitazawa Y. eds Glaucoma in the 21st Century. London, UK: Harcourt-Mosby; 2000: 117–128 [Google Scholar]