Abstract
Purpose.
The zebrafish lens is well suited for studies of physiology and development due to its rapid formation in the embryo and genetic accessibility. Aquaporin 0 (AQP0), a lens-specific membrane protein, is required for lens clarity. Zebrafish have two copies of AQP0 (Aqp0a and b), whereas mammals have a single, multifunctional protein. Here we demonstrate a reliable knockdown/rescue system in zebrafish and use it to provide evidence for subfunctionalization of Aqp0a and b, as well as to show that calcium-mediated regulation of Aqp0a in zebrafish lenses is necessary for transparency.
Methods.
Coinjection of antisense oligonucleotides and DNA rescue constructs into zebrafish embryos, followed by evaluation of the developing fish for cataracts, was used to analyze the functions of Aqp0a and b. The water permeability and regulation characteristics of each rescue protein were tested in a Xenopus oocyte swelling assay.
Results.
Both copies of AQP0 are necessary for lens clarity in the zebrafish, and neither is sufficient. Water permeability is necessary but also insufficient. Phosphorylation and regulation of Aqp0a are required for its function.
Conclusions.
In the zebrafish lens, the two closely related AQP0s have acquired distinct functions that are both necessary for lens development and clarity. Regulation of AQP0 water permeability, a well-studied phenomenon in vitro, may be physiologically relevant in the living lens.
Keywords: cataract, AQP0, zebrafish, subfunctionalization
A novel knockdown/rescue assay in zebrafish demonstrates that regulation of AQP0 water permeability is required for lens transparency and shows subfunctionalization of the two copies of zebrafish AQP0.
Introduction
Defects in aquaporin 0 (AQP0) result in the formation of both congenital and age-related cataract. The AQP0 protein forms a water channel that is regulated by pH, calcium, and phosphorylation.1 Calmodulin (CaM) mediates the calcium regulation of AQP0 water permeability depending on the phosphorylation state of serine residues contained in the CaM-binding site in the C-terminus of AQP0.2,3 In addition, AQP0 can function as a cell adhesion/junction–forming protein.4–8 However, despite extensive studies of AQP0 permeability regulation, its functions in lens development and physiology remain unclear.
The zebrafish (Danio rerio) lens closely resembles the mammalian lens in its development and physiology, and disruption of AQP0 can cause cataract.9–11 Although at initial stages of lens development the zebrafish lens delaminates from the surface ectoderm as a solid mass of cells rather than a lens vesicle, the adult morphologies of the mammalian and zebrafish lens fiber cells are indistinguishable,12 and proteomic studies comparing zebrafish and mammalian lenses have indicated overwhelming similarities.10,13 Zebrafish have two AQP0 genes, products of a genome duplication event that occurred approximately 350 million years ago.14 Following genome duplications, a duplicated gene can be nonfunctionalized (evolved to serve no function or keep its original function and remain as a duplicate), neofunctionalized (evolved to produce a new function), or subfunctionalized (evolved to maintain some subset of the original gene's function).15 We have previously shown that knockdown of either zebrafish AQP0 causes cataracts at larval stages.11
Our new evidence suggests that the two zebrafish AQP0 genes have undergone subfunctionalization, which facilitates a genetic analysis of AQP0 function. Furthermore, our new data show that MIPfun, an AQP0 protein from killifish (Fundulus heteroclitus), characterized by Virkki et al.,16 is capable of rescuing knockdown of either Aqp0a or Aqp0b. It is unknown if MIPfun is the only AQP0 in killifish due to the lack of a full genome sequence. For the purposes of this study, however, MIPfun can be considered similar to mammalian AQP0, as it contains all functions necessary for lens clarity in the zebrafish. Because it can rescue the knockdown of either zebrafish AQP0, we used MIPfun as a tool to help us dissect the various functions of AQP0.
The conservation of lens development and morphology, combined with the subfunctionalization of the duplicated AQP0 gene in zebrafish, makes the model system a powerful tool for the exploration of the role of AQP0 function and regulation in lens development. With the implementation of our knockdown/rescue system, and the knowledge that MIPfun can rescue either copy of AQP0 in zebrafish, we have a full set of tools with which to explore the multiple functions of AQP0. Here we show that both copies of AQP0 are necessary for clarity of the zebrafish lens, Aqp0a requires water-channel function and Aqp0b does not, Aqp0a requires the ability to phosphorylate and dephosphorylate residue 231 in the C-terminal CaM binding domain, and calcium-mediated regulation of Aqp0a water permeability is required for lens clarity in zebrafish. Although AQP0 permeability regulation by phosphorylation has been well studied in vitro,3 this last finding demonstrates the necessity of AQP0 permeability regulation in vivo and is confirmed by experiments using gating-deficient MIPfun.
Materials and Methods
Oocyte Swelling Assay
Relative permeabilities of various water channels were determined as described previously.1–3,11,17–19 DNA encoding the AQP of choice was cloned into a transcription vector (pXβG) containing a BglII cloning site flanked by the 5′ and 3′ untranslated regions (UTRs) of the Xenopus laevis β-Globin gene and driven by the T3 transcription promotor. RNA was generated using the mMessage mMachine T3 kit (Life Technologies, Foster City, CA) and injected (10 ng/oocyte) into X. laevis oocytes. After 2 days, permeability was assessed by moving oocytes from 100% ND96 to 30% or 50% ND96 (adjusted to 1.8 mM calcium) and monitoring swelling rates using National Institutes of Health ImageJ software (Bethesda, MD).
Knockdown/Rescue Assay
The animal protocols used in this study adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Zebrafish (AB strain) were raised and maintained under standard laboratory conditions.20 Morpholinos (MOs) against Aqp0a (Aqp0a-MO) and Aqp0b (Aqp0b-MO) were used as previously described.11 Injection of 2 ng per embryo of Aqp0a-MO or 4 ng of Aqp0b-MO achieved significant levels of cataract with minimal general toxicity.11 Rescue constructs were generated by cloning the coding sequences of Aqp0a, Aqp0b, AQP1, AQP0, and MIPfun into the pEGFP vector (Life Technologies) downstream of a 200-bp fragment of the human βB1-crystallin promoter.21 Mutant constructs were generated by a standard PCR mutagenesis protocol using KOD polymerase (EMD Millipore, Billerica, MA) and DpnI (Promega, Madison, WI). Table 1 contains information about each of the injected constructs used in this study. Of the resultant DNA constructs, 50 pg per embryo were coinjected with MOs in the following way: each clutch of zebrafish embryos was split into four groups, one uninjected control, one MO-only control, one MO + self-rescue control, and one MO + experimental rescue. After 3 days, fish were evaluated for the presence of focal nuclear cataract by standard light microscopy. We anesthetized the fish and counted how many had focal cataracts, as described previously.11 Statistical significance was determined using the Test of Independence (G-statistic test) as implemented in R.22 Two comparisons were made per experiment: MO + self-rescue control versus MO-only control, and MO + experimental rescue versus MO-only control. A Bonferroni correction was used to correct for multiple comparisons, adjusting the P value cutoff from the desired familial error rate of α = 5% (0.05) to the per-comparison error rate of 2.5% (0.025). As a result of this experimental design, there is variation between experiments in the extent of rescue for the same constructs. In other words, the results of all experiments were not pooled because doing so would artificially reduce the resultant P values. We do not speculate as to the source of intraexperimental variation, as it does not affect the statistical significance or interpretation of any of our findings.
Table 1.
Nucleic Acid Constructs
| Construct |
Mechanism |
Purpose |
Attributes |
Source |
| Aqp0a-MO | Morpholino injection | Knockdown | Synthetic (GeneTools) | |
| Aqp0b-MO | Morpholino injection | Knockdown | Synthetic (GeneTools) | |
| Aqp0a | Transgenic expression | Self/cross-rescue | Zebrafish (D. rerio) | |
| Aqp0aN8Q | Transgenic expression | Rescue experiment | No permeability | Zebrafish (D. rerio) |
| Aqp0b | Transgenic expression | Self/cross-rescue | Zebrafish (D. rerio) | |
| MIPfun | Transgenic expression | Rescue experiment | Killifish (F. heteroclitus) | |
| MIPfunN68Q | Transgenic expression | Rescue experiment | No permeability | Killifish (F. heteroclitus) |
| MIPfunS231A | Transgenic expression | Rescue experiment | Unphosphorylatable | Killifish (F. heteroclitus) |
| MIPfunS231D | Transgenic expression | Rescue experiment | Phosphomimetic | Killifish (F. heteroclitus) |
| MIPfunY75G | Transgenic expression | Rescue experiment | No calcium regulation | Killifish (F. heteroclitus) |
| AQP1 | Transgenic expression | Rescue experiment | Human (Homo sapiens sapiens) | |
| AQP0 | Transgenic expression | Rescue experiment | Bovine (Bos taurus) |
Results
Establishment of the Knockdown/Rescue System
We previously demonstrated that knockdown of Aqp0a, Aqp0b, or both, using antisense MOs targeted to their 5′ UTRs, results in cataract.11 Attempts at mRNA injection to rescue knockdown of Aqp0a caused early embryonic lethality, possibly due to expression of an inappropriate water permeability in cells outside of the lens where AQP0 is exclusively expressed (RNA rescue was effective for Aqp0b). To circumvent this problem, DNA rescue constructs were generated containing a 200-bp fragment of the human βB1-crystallin promoter, which drives expression from early lens placode stages through 72 hours post fertilization (hpf).21 Sequences of variant proteins whose rescue capacity we wished to test were cloned into this DNA construct. Each experiment used four test conditions: uninjected controls, MO-based knockdown (negative controls), “self-rescue” controls injected with the MO and the corresponding DNA rescue construct (positive controls), and embryos injected with the MO and the experimental DNA rescue construct. Each group was assessed for the presence of cataract at 72 hpf, and a Test of Independence (G-statistic test) was used to determine statistical significance. In the rare case (<1%) that uninjected controls were observed to have naturally occurring cataracts, the entire batch was excluded from the study.
Both Aqp0a and Aqp0b Are Required for Lens Clarity
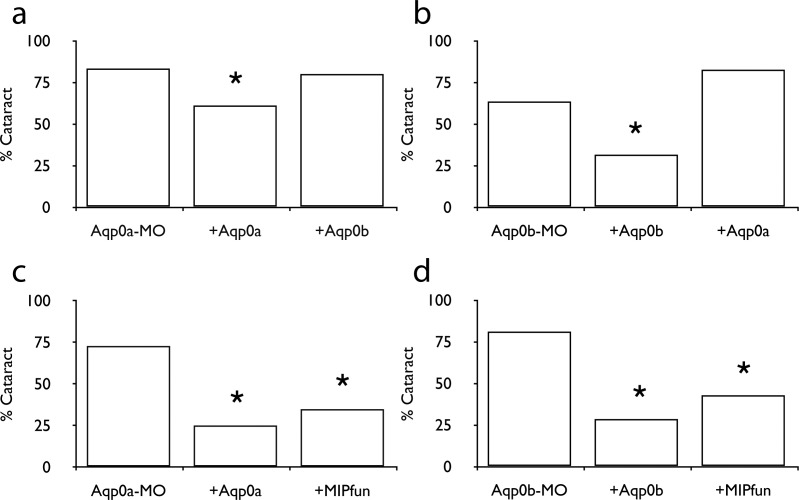
Knockdown of either Aqp0a, Aqp0b, or both led to cataracts at 72 hpf.11 To test if their functions were redundant, we injected DNA encoding one copy into embryos deficient for the other. In both cases, self-rescue resulted in a statistically significant decrease in the incidence of a cataract phenotype, while both cross-rescues failed to alter the percentage of fish with cataracts (Figs. 1a, 1b; Tables 2, 3). Although overexpression of bovine AQP0 failed to rescue knockdown of either zebrafish Aqp0 (data not shown), overexpression of MIPfun rescued knockdown of either Aqp0a or Aqp0b (Figs. 1c, 1d; Tables 4, 5).
Figure 1.
Both Aqp0a and Aqp0b are necessary for lens clarity. (a) The percentage of fish with cataract caused by MO against Aqp0a is reduced by injection of exogenous Aqp0a DNA, but not by injection of Aqp0b. (b) Cataract caused by MO against Aqp0b is reduced by injection of exogenous Aqp0b DNA, but not by injection of Aqp0a. (c, d) Injection of exogenous MIPfun DNA can rescue cataract caused by MO against either Aqp0a or Aqp0b. Asterisks indicate P is less than 0.025 between indicated group and MO-alone group.
Table 2.
Knockdown and Self-Rescue of Aqp0a
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0a 2 ng | 70 | 14 | 84 | 83.3 |
| +drAqp0a 0.05 ng | 22 | 14 | 36 | 61.1 |
| +drAqp0b 0.05 ng | 24 | 6 | 30 | 80.0 |
| Total | 116 | 24 | 150 |
Table 3.
Knockdown and Self-Rescue of Aqp0b
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0b 4 ng | 61 | 35 | 96 | 63.5 |
| +drAqp0b 0.05 ng | 6 | 13 | 19 | 31.6 |
| +drAqp0a 0.05 ng | 19 | 4 | 23 | 82.6 |
| Total | 86 | 52 | 138 |
Table 4.
Knockdown and Rescue of Aqp0a by MIPfun
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0a 2ng | 24 | 9 | 33 | 72.2 |
| +drAqp0a 0.05ng | 7 | 21 | 28 | 25.0 |
| +MIPfun 0.05ng | 8 | 15 | 23 | 34.7 |
| Total | 39 | 45 | 84 |
Table 5.
Knockdown and Rescue of Aqp0b by MIPfun
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0b 2 ng | 30 | 7 | 37 | 81.1 |
| +drAqp0b 0.05 ng | 6 | 15 | 21 | 28.6 |
| +MIPfun 0.05 ng | 3 | 4 | 7 | 42.9 |
| Total | 39 | 26 | 65 |
Water Channel Function Is Necessary for Aqp0a But Not Aqp0b
To test if Aqp0b is functionally equivalent to Aqp0a, we designed two fish AQP0s (Aqp0aN68Q and MIPfunN68Q) lacking water permeability. These AQP0s contain an asparagine-to-glutamine substitution in one of the two proton-blocking NPA domains, which are conserved among all canonical AQPs.23,24 Both Aqp0aN68Q and MIPfunN68Q lacked water permeability in the oocyte swelling assay despite normal expression (Figs. 2a, 2b). When tested in the zebrafish knockdown/rescue system, both Aqp0aN68Q and MIPfunN68Q failed to rescue Aqp0a knockdown while MIPfunN68Q rescued knockdown of Aqp0b (Figs. 2c–e; Tables 6–8). These experiments show that Aqp0b is not merely an Aqp0a lacking water permeability and further support the hypothesis that Aqp0a and Aqp0b have nonoverlapping functions. Even more importantly, they show that Aqp0b, in spite of its high-sequence conservation with Aqp0a, is not a cryptic water channel turned on at certain stages of development by an unknown mechanism (at least through 3 days postfertilization).
Figure 2. .
Water channel function is necessary (but insufficient) for Aqp0a but not Aqp0b. Water channel dead (N68Q) Aqp0a (a) or MIPfun (b) do not have water permeability in the Xenopus oocyte swelling assay, and neither can rescue cataract caused by Aqp0a-MO ([c, d], respectively). (e) MIPfunN68Q can rescue cataracts caused by Aqp0b-MO. (f) hAQP1, an unregulated and high permeability AQP, cannot rescue cataract caused by Aqp0a-MO. Asterisks indicate P is less than 0.025 between indicated group and MO-alone group.
Table 6.
Knockdown and Failure of Rescue of Aqp0b by Aqp0aN68Q
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0a 2 ng | 20 | 4 | 37 | 81.1 |
| +drAqp0a 0.05 ng | 6 | 16 | 21 | 28.6 |
| +drAqp0aN68Q 0.05 ng | 13 | 9 | 7 | 42.9 |
| Total | 39 | 26 | 65 |
Table 8.
Knockdown and Rescue of Aqp0b by Aqp0aN68Q
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0b 4 ng | 21 | 3 | 24 | 87.5 |
| +drAqp0b 0.05 ng | 7 | 21 | 28 | 25.0 |
| +MIPfunN68Q 0.05 ng | 6 | 17 | 23 | 26.1 |
| Total | 34 | 41 | 75 |
Table 7.
Knockdown and Failure of Rescue of Aqp0b by Aqp0aN68Q
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0a 2 ng | 27 | 5 | 32 | 84.4 |
| +drAqp0a 0.05 ng | 4 | 17 | 21 | 19.0 |
| +MIPfunN68Q 0.05 ng | 12 | 5 | 17 | 70.6 |
| Total | 43 | 27 | 70 |
To test if water permeability is the only function of Aqp0a, we attempted to rescue Aqp0a knockdown with a construct encoding AQP1, the human AQP expressed in red blood cells, kidney podocytes, and other cell types with high osmotic demands.25 AQP1 has a similar permeability to Aqp0a but is not regulated by calcium or pH.25,26 In the zebrafish knockdown/rescue system, hAQP1 failed to rescue Aqp0a knockdown (Fig. 2f; Table 9) suggesting that Aqp0a is not simply an open water channel; it requires regulated permeability or may interact with fish-specific accessory proteins that AQP1 cannot.
Table 9.
Knockdown and Rescue of Aqp0b by Aqp0aN68Q
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0a 2 ng | 41 | 8 | 49 | 83.7 |
| +drAqp0a 0.05 ng | 3 | 8 | 11 | 27.3 |
| +hAQP1 0.05 ng | 10 | 3 | 13 | 76.9 |
| Total | 54 | 19 | 73 |
Phosphorylation and Permeability Regulation of Aqp0a Are Required for Lens Clarity
The regulation of AQP0 water permeability has been well studied in vitro but has not been definitively shown to be physiologically relevant. Although there are indications that regulation is required in vivo,27 other reports show the ability of AQP1 (an unregulated water channel) to partially rescue mice deficient for AQP0.28 We created phosphorylation-deficient and pseudophosphorylated versions of MIPfun to test whether or not AQP regulation by phosphorylation is required for lens clarity. Serine 231 (S231) is conserved among all AQP0s and is the only target of phosphorylation within the putative CaM binding site in MIPfun. Two mutants of MIPfun, S231A and S231D, were tested for regulation in the oocyte swelling assay and were not hyperactivated in zero calcium (Fig. 3a). Previous studies have indicated that the role of phosphorylation in calcium-mediated AQP0 regulation is complex.3,29 Additionally, some of the previously studied phosphorylation sites in bovine AQP0 are absent in the fish AQP0s. S231, however, is conserved among all AQP0s studied to date. Combined with our demonstration that both the S231A and S231D mutations lead to MIPfun water channels lacking calcium-mediated regulation, these regulation-deficient mutants were used purely to examine the role of phosphoregulation of AQP0 in vivo.
Figure 3. .
Regulation of Aqp0a by phosphorylation is required for lens clarity. (a) MIPfunS231A and D do not respond to calcium in the Xenopus oocyte swelling assay. Neither can rescue cataract caused by Aqp0a-MO ([b, c], respectively), while both can rescue cataract caused by Aqp0b-MO ([d, e], respectively). Asterisks indicate P is less than 0.025 between indicated group and MO-alone group.
We tested MIPfun S231A and S231D for their ability to rescue knockdown of zebrafish Aqp0a and b. Both significantly reduced the number of embryos with cataracts when coinjected with Aqp0b-MO, confirming that they are efficiently expressed in the embryonic lens and suggesting that Aqp0b does not require S231 to be differentially phosphorylated (capable of being both phosphorylated and dephosphorylated) (Figs. 3c–f; Tables 10–13).
Table 10.
Knockdown and Failure of Rescue of Aqp0a by MIPfunS231A
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0a 2 ng | 14 | 4 | 18 | 77.8 |
| +drAqp0a 0.05 ng | 5 | 12 | 17 | 29.4 |
| +MIPfunS231A 0.05 ng | 12 | 3 | 15 | 80.0 |
| Total | 31 | 19 | 50 |
Table 13.
Knockdown and Rescue of Aqp0b by MIPfunS231D
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0b 4 ng | 12 | 3 | 17 | 70.6 |
| +drAqp0b 0.05 ng | 3 | 10 | 9 | 33.3 |
| +MIPfunS231D 0.05 ng | 4 | 10 | 16 | 25.0 |
| Total | 29 | 13 | 42 |
Table 11.
Knockdown and Rescue of Aqp0b by MIPfunS231A
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0b 4 ng | 14 | 3 | 17 | 82.3 |
| +drAqp0b 0.05 ng | 5 | 12 | 17 | 29.44 |
| +MIPfunS231A 0.05 ng | 10 | 12 | 22 | 45.5 |
| Total | 29 | 27 | 56 |
Table 12.
Knockdown and Failure of Rescue of Aqp0a by MIPfunS231D
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0a 2 ng | 13 | 4 | 17 | 76.5 |
| +drAqp0a 0.05 ng | 2 | 7 | 9 | 22.2 |
| +MIPfunS231D 0.05 ng | 14 | 2 | 16 | 87.5 |
| Total | 29 | 13 | 42 |
In contrast, neither construct reduced the incidence of cataract when coinjected with Aqp0a-MO, suggesting that S231 must be differentially phosphorylated in Aqp0a for lens clarity in vivo. In other words, it is required that S231 be sequentially phosphorylated and dephosphorylated (or the reverse), or for subpopulations of Aqp0a to be phosphorylated while others are not, for lens clarity. This result is compatible with mass spectrometry experiments showing a variety of phosphorylation states of AQP0 in human and bovine lenses.29 Additionally, these experiments provide indirect evidence for a requirement of AQP0 to be regulated in vivo because neither MIPfunS231A nor MIPfunS231D, both of which lack calcium-mediated regulation, are able to rescue Aqp0a knockdown.
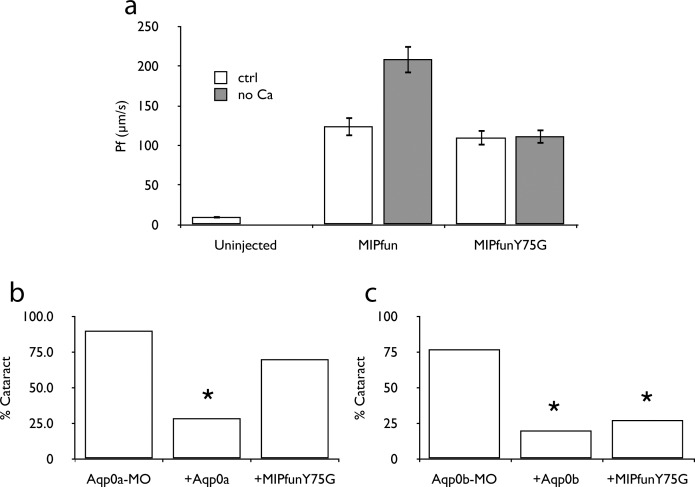
To show conclusively that regulated permeability of AQP0 is required for lens clarity, we attempted to rescue Aqp0a and Aqp0b knockdown with a regulation deficient mutant of MIPfun. Residues at positions 75 and 149 make up a constriction in the pore of AQP0, and previous studies by our group have shown that a Y149G mutation in bovine AQP0 abrogates regulation by calcium.30 Positions 149 and 75 appear to be swapped in the gating constriction of MIPfun, and we have found that a Y75G mutation similarly abrogated calcium regulation of MIPfun in our oocyte swelling assay (Fig. 4a). When introduced to the zebrafish knockdown/rescue assay, the MIPfunY75G construct rescued knockdown of Aqp0b but not Aqp0a (Figs. 4b, 4c; Tables 14, 15). These results, combined with the results from the phosphorylation-deficient and pseudophosphorylated mutation rescues, indicate regulated permeability of AQP0 regulation is physiologically relevant.
Figure 4. .
Gating of Aqp0a is required for lens clarity. (a) MIPfunY75G does not respond to calcium in the Xenopus oocyte swelling assay. The Y75G variant cannot rescue cataract caused by Aqp0a-MO (b), but can rescue cataract caused by Aqp0b-MO (c). Asterisks indicate P is less than 0.025 between indicated group and MO-alone group.
Table 14.
Knockdown and Failure of Rescue of Aqp0a by MIPfunY75G
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0a 2 ng | 9 | 1 | 10 | 90.0 |
| +drAqp0a 0.05 ng | 4 | 10 | 14 | 28.6 |
| +MIPfunY75G 0.05 ng | 7 | 3 | 10 | 70.0 |
| Total | 20 | 14 | 34 |
Table 15.
Knockdown and Rescue of Aqp0b by MIPfunS231D
| Constructs Injected |
Cataract |
Normal |
Total |
% |
| MO drAqp0b 4 ng | 10 | 3 | 13 | 76.9 |
| +drAqp0b 0.05 ng | 2 | 8 | 10 | 20.0 |
| +MIPfunS231D 0.05 ng | 3 | 8 | 11 | 27.3 |
| Total | 15 | 19 | 34 |
Discussion
Studies of AQP0 in mammalian model organisms and expression systems in vivo have been limited in their ability to distinguish between its multiple functions in lens cells. Here we show that subfunctionalization of two Aqp0s in zebrafish has separated its water permeability from other functions. Knockdown/rescue experiments reveal that both Aqp0a and Aqp0b are necessary for lens clarity, and neither can substitute for the other, providing clear evidence for this subfunctionalization event. Attempts to prevent cataracts in Aqp0a- and Aqp0b-deficient fish with various mutant forms of MIPfun also confirm predictions from molecular models that hypothesize a conserved mechanism for regulating water permeability in AQP0s involving AQP0-CaM interaction and the action of a pore constriction/gate composed of residues 75 and 149.30–32
Our results suggest that zebrafish Aqp0a is a water channel and functions as such in vivo, whereas Aqp0b is not (Fig. 2). However, elevated water permeability alone fails to rescue loss of Aqp0a, implying that water permeability may be tightly regulated for lens clarity. The failure of human AQP1 to rescue knockdown of Aqp0a may indicate that, even though water permeability is essential for the function of Aqp0a, it is not sufficient. Human AQP1 lacks the regulation that we have shown is essential for Aqp0a. It is possible, however, that other differences between human AQP1 and Aqp0a are responsible for the inability of human AQP1 to rescue Aqp0a knockdown. Our results further suggest that regulation requires residues capable of being both phosphorylated and dephosphorylated and possibly participating in gating of the pore of AQP0 to prevent cataract formation in the knockdown/rescue system. The failure of regulation-competent bovine AQP0 to rescue knockdown of either zebrafish Aqp0s (data not shown) suggests that the pattern of regulation in the fish differs somewhat from that in mammals. The interaction between AQP0 and its known companion proteins, such as BFSP, connexin 50, AKAP2,33,34 and possibly other unknown binding partners, could be sufficiently different in the fish to prevent AQP0 and AQP1 from participating in the same modes of water permeability regulation available to MIPFun and Aqp0a.
This is among the first in vivo studies to validate the extensive literature on AQP0 regulation, providing support for the lens circulation model as described by Mathias et al.35 The lens circulation is proposed to be driven by the sodium-potassium ATPase in lens epithelial cells on the surface of the lens.35–37 Sodium is allowed to enter lens fiber cells through an unidentified channel that is absent in lens epithelial cells.35 The action of these transporters and channels produces a sodium gradient that, according to the model, drives osmotic flow of water from the exterior to the interior of the lens through the interstitial spaces.35 The proposed role of AQP0 in the lens circulation model is to allow water into lens fiber cells from the interstitial spaces.34,35,37 Once inside fiber cells, water and other solutes are transported back to the surface of the lens through gap junctions driven by hydrostatic pressure.37,38 Thus AQP0 must support osmotically driven water flow into lens fiber cells against a hydrostatic pressure gradient. To accomplish this task, without allowing all of the water to enter the superficial layers of lens fiber cells in the lens cortex, the rate of water flow through AQP0 channels in the cortex may be different than that of channels in the lens nucleus. Previous studies have shown that bovine AQP0 water permeability can be regulated in several ways, including by intracellular calcium concentration through the action of CaM, which in turn is regulated by the phosphorylation state of serine residues in the C-terminus of bovine AQP0.1–3,39 This study provides evidence that these regulatory mechanisms, regulation by calcium (Fig. 4) and by phosphorylation/dephosphorylation (Fig. 3), may be relevant in vivo.
Although the distinct functions of Aqp0b in lens development and clarity in the zebrafish are unclear, previous studies suggest AQP0 plays a role in lens cell adhesion.4–8 Both Aqp0a and Aqp0b may have adhesive functionalities, which can be tested in the zebrafish system. Our laboratory is currently developing reliable methods for assaying the adhesive properties of aquaporins to determine whether Aqp0a, Aqp0b, or both can act as adhesive proteins. Future work is planned to use these methods to design adhesion-deficient mutational variants of Aqp0a and Aqp0b that can be tested in the zebrafish knockdown/rescue assay. In addition to self-adhesion, bovine AQP0 binds AKAP2, BFSP1, and other regulatory and cytoskeletal proteins.33,34 As these interactions are better characterized, experiments can be designed to test the in vivo relevance of these interactions in the knockdown/rescue assay. More broadly, the knockdown/rescue assay in zebrafish can be used to study a multitude of lens-specific proteins in vivo, and can potentially be extended as an in vivo method to study any protein for which tissue-specific expression and the availability of tissue-specific promotors exist.
Acknowledgments
The authors thank Bei-En Chang, National Taiwan University, for the generous gift of the human crystallin promotor.
Supported by National Institutes of Health, National Eye Institute Grant EY5661 (JEH); National Institutes of Health, National Institute of Dental and Craniofacial Research Grant DE13828 (TFS); and the National Library of Medicine Biomedical Informatics Research Training Program Award LM007443 (DMC).
Disclosure: D.M. Clemens, None; K.L. Németh-Cahalan, None; L. Trinh, None; T. Zhang, None; T.F. Schilling, None; J.E. Hall, None
References
- 1. Németh-Cahalan KL. Hall JE. pH and calcium regulate the water permeability of aquaporin 0. J Biol Chem. 2000; 275: 6777–6782 [DOI] [PubMed] [Google Scholar]
- 2. Németh-Cahalan KL, Kalman K, Hall JE. Molecular basis of pH and Ca2+ regulation of aquaporin water permeability. J Gen Physiol. 2004; 123: 573–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalman K, Németh-Cahalan KL, Froger A, Hall JE. Phosphorylation determines the calmodulin-mediated Ca2+ response and water permeability of AQP0. J Biol Chem. 2008; 283: 21278–21283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zampighi GA, Hall JE, Ehring GR, Simon SA. The structural organization and protein composition of lens fiber junctions. J Cell Biol. 1989; 108: 2255–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michea LF, de la Fuente M, Lagos N. Lens major intrinsic protein (MIP) promotes adhesion when reconstituted into large unilamellar liposomes. Biochemistry. 1994; 33: 7663–7669 [DOI] [PubMed] [Google Scholar]
- 6. Michea L, Andrinolo D, Ceppi H, Lagos N. Biochemical evidence for adhesion-promoting role of major intrinsic protein isolated from both normal and cataractous human lenses. Exp Eye Res. 1995; 61: 293–301 [DOI] [PubMed] [Google Scholar]
- 7. Gonen T, Cheng Y, Kistler J, Walz T. Aquaporin-0 membrane junctions form upon proteolytic cleavage. J Mol Biol. 2004; 342: 1337–1345 [DOI] [PubMed] [Google Scholar]
- 8. Kumari SS, Varadaraj K. Intact AQP0 performs cell-to-cell adhesion. Biochem Biophys Res Commun. 2009; 390: 1034–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greiling TMS, Clark JI. Early lens development in the zebrafish: a three-dimensional time-lapse analysis. Dev Dyn. 2009; 238: 2254–2265 [DOI] [PubMed] [Google Scholar]
- 10. Greiling TMS, Houck SA, Clark JI. The zebrafish lens proteome during development and aging. Mol Vis. 2009; 15: 2313–2325 [PMC free article] [PubMed] [Google Scholar]
- 11. Froger A, Clemens DM, Kalman K, Németh-Cahalan KL, Schilling TF, Hall JE. Two distinct aquaporin 0s required for development and transparency of the zebrafish lens. Invest Ophthalmol Vis Sci. 2010; 51: 6582–6592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dahm R, Schonthaler HB, Soehn AS, van Marle J, Vrensen GFJM. Development and adult morphology of the eye lens in the zebrafish. Exp Eye Res. 2007; 85: 74–89 [DOI] [PubMed] [Google Scholar]
- 13. Posner M, Hawke M, Lacava C, Prince CJ, Bellanco NR, Corbin RW. A proteome map of the zebrafish (Danio rerio) lens reveals similarities between zebrafish and mammalian crystallin expression. Mol Vis. 2008; 14: 806–814 [PMC free article] [PubMed] [Google Scholar]
- 14. Postlethwait J, Amores A, Cresko W, Singer A, Yan YL. Subfunction partitioning, the teleost radiation and the annotation of the human genome. Trends Genet. 2004; 20: 481–490 [DOI] [PubMed] [Google Scholar]
- 15. Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999; 151: 1531–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Virkki LV, Cooper GJ, Boron WF. Cloning and functional expression of an MIP (AQP0) homolog from killifish (Fundulus heteroclitus) lens. Am J Physiol Regul Integr Comp Physiol. 2001; 281: R1994–R2003 [DOI] [PubMed] [Google Scholar]
- 17. Ehring GR, Zampighi G, Horwitz J, Bok D, Hall JE. Properties of channels reconstituted from the major intrinsic protein of lens fiber membranes. J Gen Physiol. 1990; 96: 631–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chandy G, Zampighi GA, Kreman M, Hall JE. Comparison of the water transporting properties of MIP and AQP1. J Membr Biol. 1997; 159: 29–39 [DOI] [PubMed] [Google Scholar]
- 19. Kalman K, Németh-Cahalan KL, Froger A, Hall JE. AQP0-LTR of the Cat Fr mouse alters water permeability and calcium regulation of wild type AQP0. Biochim Biophys Acta. 2006; 1758: 1094–1099 [DOI] [PubMed] [Google Scholar]
- 20. Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). 4th ed. Eugene, OR: University of Oregon Press; 2000. [Google Scholar]
- 21. Hou HH, Kuo MYP, Luo YW, Chang BE. Recapitulation of human βB1-crystallin promoter activity in transgenic zebrafish. Dev Dyn. 2006; 235: 435–443 [DOI] [PubMed] [Google Scholar]
- 22. Hurd P. G test code. 2001. Available at: http://www.psych.ualberta.ca/∼phurd/cruft/g.test.r. Accessed December 12, 2011. [Google Scholar]
- 23. Wree D, Wu B, Zeuthen T, Beitz E. Requirement for asparagine in the aquaporin NPA sequence signature motifs for cation exclusion. FEBS J. 2011; 278: 740–748 [DOI] [PubMed] [Google Scholar]
- 24. Benga G. Water channel proteins (later called aquaporins) and relatives: past, present, and future. IUBMB Life. 2009; 61: 112–133 [DOI] [PubMed] [Google Scholar]
- 25. Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992; 256: 385–387 [DOI] [PubMed] [Google Scholar]
- 26. Saparov SM, Kozono D, Rothe U, Agre P, Pohl P. Water and ion permeation of aquaporin-1 in planar lipid bilayers. Major differences in structural determinants and stoichiometry. J Biol Chem. 2001; 276: 31515–31520 [DOI] [PubMed] [Google Scholar]
- 27. Varadaraj K, Kumari S, Shiels A, Mathias RT. Regulation of aquaporin water permeability in the lens. Invest Ophthalmol Vis Sci. 2005; 46: 1393–1402 [DOI] [PubMed] [Google Scholar]
- 28. Varadaraj K, Kumari S, Mathias R. Transgenic expression of AQP1 in the fiber cells of AQP0 knockout mouse: effects on lens transparency. Exp Eye Res. 2010; 91: 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schey KL, Fowler JG, Schwartz JC, Busman M, Dillon J, Crouch RK. Complete map and identification of the phosphorylation site of bovine lens major intrinsic protein. Invest Ophthalmol Vis Sci. 1997; 38: 2508–2515 [PubMed] [Google Scholar]
- 30. Reichow SL, Clemens DM, Freites JA, et al. Allosteric mechanism of water-channel gating by Ca2+-calmodulin. Nat Struc Mol Bio. 2013. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jensen MO, Dror RO, Xu H, et al. Dynamic control of slow water transport by aquaporin 0: implications for hydration and junction stability in the eye lens. Proc Natl Acad Sci U S A. 2008; 105: 14430–14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xin L, Su H, Nielsen CH, Tang C, Torres J, Mu Y. Water permeation dynamics of AqpZ: a tale of two states. Biochim Biophys Acta. 2011; 1808: 1581–1586 [DOI] [PubMed] [Google Scholar]
- 33. Gold MG, Reichow SL, O'Neill SE, et al. AKAP2 anchors PKA with aquaporin-0 to support ocular lens transparency. EMBO Mol Med. 2012; 4: 15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hall JE. Through a glass darkly. EMBO Mol Med. 2012; 4: 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mathias RT, Kistler J, Donaldson P. The lens circulation. J Membr Biol. 2007; 216: 1–16 [DOI] [PubMed] [Google Scholar]
- 36. Candia OA, Zamudio AC. Regional distribution of the Na+ and K+ currents around the crystalline lens of rabbit. Am J Physiol Cell Physiol. 2002; 282: C252–C262 [DOI] [PubMed] [Google Scholar]
- 37. Gao J, Sun X, Moore LC, White TW, Brink PR, Mathias RT. Lens intracellular hydrostatic pressure is generated by the circulation of sodium and modulated by gap junction coupling. J Gen Physiol. 2011; 137: 507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gong X, Cheng C, Xia C. Connexins in lens development and cataractogenesis. J Membr Biol. 2007; 218: 9–12 [DOI] [PubMed] [Google Scholar]
- 39. Reichow SL, Gonen T. Noncanonical binding of calmodulin to aquaporin-0: implications for channel regulation. Structure. 2008; 16: 1389–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]