Abstract
Adolescent alcohol use is associated with myriad adverse consequences and contributes to the leading causes of mortality among youth. Despite the magnitude of this public health problem, evidenced-based treatment initiatives for alcohol use disorders in youth remain inadequate. Identifying promising pharmacological approaches may improve treatment options. Naltrexone is an opiate receptor antagonist that is efficacious for reducing drinking in adults by attenuating craving and the rewarding effects of alcohol. Implications of these findings for adolescents are unclear, however, given that randomized trials of naltrexone with youth are nonexistent. We conducted a randomized, double-blinded, placebo-controlled crossover study, comparing naltrexone (50 mg/daily) and placebo in 22 adolescent problem drinkers aged 15 – 19 years (M = 18.36, SD = 0.95; 12 females). The primary outcome measures were alcohol use, subjective responses to alcohol consumption, and alcohol-cue-elicited craving assessed in the natural environment using ecological momentary assessment methods, and craving and physiological reactivity assessed using standard alcohol cue reactivity procedures. Results showed that naltrexone reduced the likelihood of drinking and heavy drinking (p’s ≤ .03), blunted craving in the laboratory and in the natural environment (p’s ≤ .04), and altered subjective responses to alcohol consumption (p’s ≤ .01). Naltrexone was generally well tolerated by participants. This study provides the first experimentally controlled evidence that naltrexone reduces drinking and craving, and alters subjective responses to alcohol in a sample of adolescent problem drinkers, and suggests larger clinical trials with long-term follow ups are warranted.
Keywords: adolescents, alcohol sensitivity, craving, cue reactivity, naltrexone
Introduction
Adolescence is a key period in the development of alcohol use disorders, with nearly 15% of youth meeting diagnostic criteria for alcohol abuse or dependence by 18 years of age (Merikangas and McClair, 2012; Swendsen et al., 2012). Yet less than one-third of treated youth experience sustained benefit from existing psychosocial interventions (Chung and Maisto, 2006). Inadequate treatment for this age group is an important public health concern given that alcohol misuse during adolescence predicts future alcohol dependence in adulthood (Buu et al., 2011). Although pharmacotherapy research has expanded treatment options for adults with drinking problems, medication development for adolescents has not progressed. Randomized controlled pharmacotherapy trials for alcohol problems in youth are few and published reports bear substantial limitations that preclude inferences about the efficacy of the medication studied. This gap in knowledge impedes treatment practices, as the safety and efficacy of medications for adolescents cannot be inferred from adult data (Bridge et al., 2007).
Naltrexone is an opiate receptor antagonist that is efficacious for treating alcohol dependence in adults. In most clinical trials, naltrexone lowered the risk of relapse and reduced the frequency of drinking and heavy drinking days, with a modest effect size (g = 0.20; see Maisel et al., in press). Considering its promise, researchers have attempted to elucidate the behavioral mechanisms by which naltrexone exerts beneficial effects. Retrospective patient reports in the initial clinical trials suggested that naltrexone reduced day-to-day craving and subjective high following alcohol consumption (Anton et al., 1999; O’Malley et al., 1992). These observations spurred researchers to more carefully investigate naltrexone’s effects in controlled laboratory settings. Studies found naltrexone blunted craving in response to alcohol and alcohol cues (Anton et al., 2004; Davidson et al., 1999; Drobes et al., 2004; O’Malley et al., 2002) and dampened the reinforcing effects of alcohol (Drobes et al., 2004; McCaul et al., 2000; Na and Lee, 2002; O’Malley et al., 2002; Ray and Hutchison, 2007; Setiawan et al., 2011). Studies also tested whether naltrexone intensifies alcohol-induced sedation, but generally found little effect (de Wit et al., 1999; Drobes et al., 2004, Ray et al., 2008). On the whole, these data suggest that naltrexone reduces drinking primarily by dampening craving and alcohol’s reinforcing effects.
Despite beneficial effects of naltrexone on adult drinking, implications of these findings for adolescents are unclear. Youth exhibit clinical characteristics that differ from adults, and adolescence is associated with substantial neuronal remodeling in brain regions that govern alcohol sensitivity (for review, see Spear, 2011). Inasmuch as naltrexone affects drinking by altering the subjective responses to alcohol, adolescents’ unique patterns of alcohol sensitivity may influence how naltrexone affects youth. Published reports of its effects on adolescent drinking, however, are limited to case studies and open-label trials (Deas et al., 2005). Although most reports claim naltrexone reduces drinking and craving, causal inferences cannot be drawn from these studies.
In this randomized, double-blinded, placebo-controlled crossover study, we examined the effects of naltrexone on adolescents’ drinking, reactivity to alcohol cues, and subjective responses to alcohol consumption in real time in their natural environments using ecological momentary assessment (EMA) methods. We also tested the effects of naltrexone on adolescents’ reactivity to alcohol cues in a controlled laboratory setting. Empirical study of adolescents’ responses to alcohol has relied almost exclusively on animal models due to restrictions on administering alcohol to underage drinkers. A primary goal of this study was to surmount this challenge by using an EMA approach to test naltrexone’s effects on teenagers’ momentary subjective responses to alcohol in their natural environments. Momentary assessments are particularly important when the phenomena of interest are subject to rapid change, such as craving and acute responses to alcohol. Other advantages of EMA include the large number of repeated observations (i.e., boosting statistical power), the ability to track compliance and eliminate the possibility of ‘faking’ compliance by recording the time and date of each entry, the low incidence of missing data because questions cannot be skipped, and the ecological validity of findings.
Informed by adult research, we tested four hypotheses. First, we tested the hypothesis that naltrexone, as compared to placebo, reduces alcohol use in adolescent problem drinkers. Second, we tested the hypothesis that naltrexone dampens the subjective reinforcing effects of alcohol consumption (i.e., stimulation). Third, we tested the hypothesis that naltrexone blunts alcohol cue-elicited craving in the natural environment. Lastly, we tested the hypothesis that naltrexone dampens alcohol cue-induced craving and physiological reactivity in a laboratory setting. Given that this is the first controlled pharmacotherapy study on adolescent drinking, we also explored the effects of naltrexone on ratings of sedation and alcohol high during drinking episodes.
Materials and Methods
Participant Selection
Adolescents were recruited from the community to participate in a study of how a medication affects teenagers’ reactions to alcohol. Inclusion criteria were 15 to 19 years old, consumed alcohol ≥ 2 times weekly in the past 30 days, able to read simple English, and postpubescent. Exclusion criteria were history of alcohol treatment or treatment seeking, opiate use in the past 30 days, current or lifetime opiate use disorder based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; American Psychiatric Association, 2000), positive urine toxicology screen for narcotics, amphetamines, sedative hypnotics, or opiates; alcohol withdrawal (> 10 on the Clinical Institute Withdrawal Assessment for Alcohol; Sullivan et al., 1989), suicidal or psychotic, and medical conditions or medications that contraindicated taking naltrexone (medications stabilized for ≥4 weeks were permissible, medications known to affect drinking were exclusionary). Females were ineligible if they were pregnant, nursing, or unwilling to use birth control.
Procedures
Study design
This double blind crossover trial compared naltrexone (up to 50mg daily) and placebo. Participants were randomized to each condition for 8 to 10 days (M = 9.93, SD = 0.34) in counterbalanced order with a 4- to 11-day washout period (M = 4.52, SD = 1.72) to allow for clearance of naltrexone (Gonzalez and Brogden, 1988). At the end of each condition participants underwent a laboratory-based alcohol cue reactivity assessment (CRA); variability in the duration of each arm permitted flexibility in scheduling CRA sessions. Conditions typically began on Thursdays, included two weekends, and avoided events that might impact drinking (e.g., holiday breaks). We contacted participants daily to assess side effects. Procedures were identical across conditions, except for the medication administered. No instructions were given to reduce or otherwise alter drinking habits. The Brown University Institutional Review Board approved this study.
Schedule of assessments
Volunteers completed a telephone screening (N = 461). Potentially eligible youths underwent additional in-person screening. The study was fully described to participants and, if < 18 years, their parents. Consent was obtained from 18 and 19 year olds and from the parents of minors; minors provided assent. Adolescents completed baseline assessments and learned our EMA protocol, which was designed for this study and implemented on handheld devices (Samsung Electronics, Ridgefield Park, NJ). EMA response options included: visual analog bars (converted to discrete point scales); multiple checkboxes when more than one option was appropriate; and categorical checkboxes when only one response was warranted. Other features made it user-friendly, such as an alarm-clock feature to avoid assessments while sleeping.
Participants learned to discern standard alcoholic drink volumes using a graphic manual that depicted standard drinks by beverage type. To simplify the instructional set, participants were instructed to initiate begin- and end- drink reports on their handheld device directly before and after each standard drink, respectively. However, the EMA battery was delivered only before the first drink and after the first three drinks of a drinking episode. We selected three drinks based on evidence that the minimum blood alcohol concentration (BAC) at which reinforcing effects are reported is 0.04 g/dl (Davidson et al., 1997). Participants also responded to device-initiated auditory prompts (random assessments), which occurred once randomly within 3hr blocks and did not overlap with drinking, to assess craving outside drinking episodes. The program recorded if participants failed to respond within 2min. Youths could ‘suspend’ random assessments for up to 7hrs when necessary (e.g., school, driving). Finally, each morning participants recorded the number and type of standard drinks consumed the previous day.
Alcohol cue reactivity
CRA sessions mirrored published protocols (Miranda et al., 2010). All participants tested negative for breath alcohol using an Alco-Sensor IV breathalyzer (Intoximeters Inc., Saint Louis, MO) before the session. Cigarette users smoked their last cigarette 1hr prior to cue exposure. Experimental manipulations occurred in a sound-attenuated room equipped with a one-way mirror. Participants were fitted with a Scholar II 507EP blood pressure cuff (Criticare Systems Inc., Waukesha, WI) and underwent a 3min period to habituate to the inflation cycle (approximately every 40s) and setting. Participants were then presented with a glass of water accompanied by its commercially labeled bottle. Audio recordings instructed participants to sniff the glass when high tones signaled and stop sniffing when low tones signaled; thirteen 5s olfactory exposures occurred in variable intervals during each trial. Following the water trial, participants underwent a 3min relaxation period followed by two alcohol cue exposure trials that were identical to the water trial except the glass contained their most commonly consumed alcoholic beverage and was accompanied by its commercially labeled bottle. At the end of each trial, participants rated their craving (see Measures). Trials were presented in the same order for all participants because of known carryover effects (Monti et al., 1987).
Medication administration and compliance
Naltrexone was compounded into 25mg capsules. Placebo capsules contained inert filler and were identical to naltrexone capsules except for content. Participants were prescribed one capsule the first two days of each condition and two capsules daily thereafter. Compliance was assessed using the medication event monitoring system (MEMS; Aardex Group Ltd., Geneva, Switzerland), an electronic bottle cap that records the date/time the bottle was opened. At CRA appointments youths ingested the medication at our laboratory 1hr prior to procedures.
Measures
Alcohol use
Baseline drinking was assessed using the 90-day timeline follow-back interview (TLFB; Sobell and Sobell, 1992). Drinking during the trial was assessed using the EMA program and TLFB. EMA data were our primary outcome measure, with missing data culled from the TLFB (Carney et al., 1998).
Momentary subjective responses
Two items from the stimulation (energized, excited) and sedation (sedated, sluggish) subscales of the Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993) were administered to reduce burden. Items were selected based on an unpublished principal components analysis of data from college-age heavy drinkers. Youths rated items on visual analog scales from 0 (not at all) to 10 (extremely); items were combined into a mean score for each dimension. Urge to drink (i.e., craving) and high were measured using single items rated from 0 (no urge and not at all, respectively) to 10 (strongest ever and extremely, respectively). This craving assessment is widely used in alcohol administration and EMA studies (Tidey et al., 2008). The measure of high originated from the Subjective High Assessment Scale (SHAS; Schuckit, 1984) and was selected because it strongly correlates with the total SHAS score across BAC levels (Ray et al., 2009). Participants also rated their craving during random assessments using the same scale.
Estimated blood alcohol concentration
Subjective effects of alcohol are dose-dependent (Anton et al., 2004; Drobes et al., 2004; Ray and Hutchison, 2007), therefore we estimated BAC (eBAC) levels at each drink report using a standard algorithm (see Piasecki et al., in press; Ray et al., 2010a).
Person-level variables
Demographic and clinical information was collected at baseline. Psychiatric diagnoses, including alcohol use disorders, were derived using the Kiddie Schedule for Affective Disorders for School-Age Children (KSADS; Kaufman et al., 1997). Diagnostic decisions were based on adolescents’ reports, made by case consensus, and used for descriptive purposes. To further describe the sample, adolescents also completed the Rutgers Alcohol Problem Index (RAPI; White and Labouvie, 1989), a continuous measure of alcohol-related problems, and the Kaufman Brief Intelligence Test (Kaufman and Kaufman, 1990).
Event-level variables
In the natural environment, participants recorded whether alcohol was directly visible (e.g., bottle, glass, etc.) at random assessments. Other event-level data were collected to include as covariates in models examining cue-elicited craving in the natural environment. EMA software date and time stamped each entry. We classified entries based on whether they occurred on a weekend (6 p.m. on Friday through 6 p.m. on Sunday). Additionally, time of day was represented by four exclusive 6hr blocks, with 6 p.m. to midnight serving as the reference category. Participants recorded their location from a list of options (home, friend’s house, other’s house, school, work, public place, vehicle, other location); home served as the reference category. Participants also reported others present by selecting all applicable options (mother, father, brother, sister, child, other relative, boy/girlfriend, friend, teacher, other, no one). Each entry was coded for the presence (1) or absence (0) of peers. Finally, entries were categorized as occurring on drinking (1) or nondrinking (0) days.
Laboratory measures
Urge to drink was measured during CRA procedures using the same item delivered during EMA. The Alcohol Urge Questionnaire (AUQ; Bohen, Krahn, & Staehler, 1995), an 8-item measure of craving, was also administered in the laboratory. Craving was assessed immediately after each cue exposure. Measures of physiological arousal during cue exposures included mean arterial pressure (MAP) and heart rate measured in beats per minute.
Statistical Methods
Analyses were performed using the SPSS statistical package, version 19.0 (IBM, Armonk, NY). Comparisons between participants and youths screened but not enrolled were evaluated using independent sample t tests and chi-squared analyses. We tested for differences in paired proportions of side effects between conditions using the McNemar statistic, with side effect categories coded as present (1) or not present (0). To garner full benefit of the extensive collection of repeated observations from each participant, we used generalized estimating equation (GEE) models to analyze our primary outcomes (Zegar, Liang, & Albert, 1988). GEE models are essentially regression equations that allow for inclusion of participants with some missing data and varying numbers of observations while controlling for autocorrelation. Several covariance structures were compared using the quasi-likelihood under the independence model criterion to select the optimum working correlation matrix (Pan, 2001). An autoregressive structure provided the best fit for all data except subjective responses to alcohol; an unstructured matrix provided the best fit for subjective response data. Models assumed a normal link function when the dependent measure was continuous and a logit link function when the outcome of interest was binary. Additionally, in all models condition was coded with an orthogonal contrast (-0.5 for placebo vs. 0.5 for naltrexone) and treatment order was included as a between-subjects covariate to control for possible order effects.
We first examined the effect of condition on drinking outcomes. The primary units of analysis were repeated daily assessments of drinking variables. This variable followed a count distribution (i.e., number of standard drinks) with overdispersion due to positive skewness. We therefore used a binomial distribution (nondrinking day = 0, drinking day = 1) to analyze this outcome. We also used GEE analyses to predict heavy drinking days using a binomial distribution (nonheavy or nondrinking day = 0, heavy drinking day = 1); heavy drinking days were defined as ≥ 5 standard drinks for males and ≥ 4 standard drinks for females.
Our next set of analyses examined the effects of condition on adolescents’ subjective responses to alcohol consumption. Adult studies indicate subjective responses to alcohol are heavily influenced by the biphasic nature of intoxication. We evaluated whether drink reports were recorded during the ascending or descending limb of the blood alcohol curve by computing successive differences in eBAC across reports within each drinking episode. Results identified a small number of reports recorded during the descending limb (n = 3, 1.4%). To facilitate interpretation of our data, we restricted analyses to data collected in the ascending limb. Separate models tested the main and interactive effects of condition and eBAC on each dependent variable. Only subjective high required transformation (logarithmic) to correct for positive skewness. To disentangle within-person drink-to-drink variation in eBAC and subjective intoxication from the effects of between-person variability in typical eBAC and subjective intoxication, we entered both momentary eBAC after each of the first three drinks each day and each participant’s average eBAC level across the trial into all models (Palta, 2003). The momentary variable reflects the within-person effect, whereas the average variable reflects the between-person effect of typical intoxication. All variables were standardized to ease interpretation of results; the model coefficients represent differences in standard deviation units associated with the predictors (effect size d).
We also tested whether naltrexone dampened craving in the natural environment outside of drinking episodes after accounting for event- and person-level covariates. Data were culled from random assessments. Assessments recorded during or after drinking episodes each day (n = 59, 3.8%) were excluded from analyses to curtail confounding effects with alcohol intoxication. The single-item measure of craving was square-root-transformed due to positive skewness and standardized.
Laboratory CRA data were also analyzed using GEE. Separate models tested the main and interactive effects of condition and cue type (water vs. alcohol) on craving and physiological outcomes (i.e., heart rate, MAP). Craving measures were square-root-transformed due to positive skewness, and craving and physiological measures were standardized.
Results
Sample Characteristics
Twenty-eight adolescents entered the study and were randomized. Participants did not differ from youths screened but not enrolled on demographic (age, sex, race, and ethnicity; ps > .10) or baseline drinking characteristics (percent drinking and heavy drinking days; ps > .08). Figure 1 illustrates the flow of participants through the study. Six participants did not complete either arm of the study and were excluded from analyses (see Figure 1). One participant completed all measurements during the naltrexone arm but did not complete the placebo arm due to time constraints. This participant was included in the analyses. Table 1 depicts the characteristics of the final sample. The final sample (N = 22) was 15 to 19 years of age and more than two-thirds met criteria for an AUD; 27.3% met criteria for abuse (Mage of onset = 16.93, SD = 2.17) and 50.0% met criteria for dependence (Mage of onset = 17.20, SD =1.81).
Figure 1.
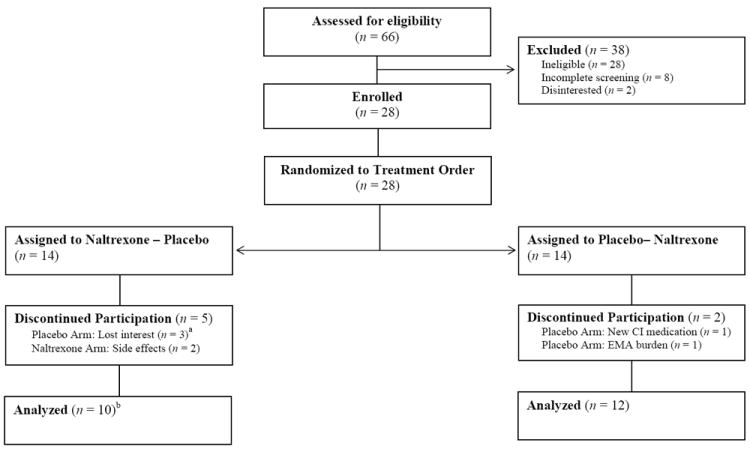
Participant flow through the double-blind crossover study; CI = contraindicated; a Two participants did not complete all measures in the naltrexone arm, proceeded to the placebo arm, and then discontinued participation. b Analyses included one participant who completed all measurements in the naltrexone arm but discontinued during the placebo arm.
Table 1.
Summary of Participant Characteristics at Baseline by Sex
| Characteristic | Males (n = 10)
|
Females (n = 12)
|
Full Sample
|
|---|---|---|---|
| N (%) or M ± SD | N (%) or M ± SD | N (%) or M ± SD | |
| Age | 18.00 ± 1.25 | 18.67 ± 0.49 | 18.36 ± 0.95 |
| Race | |||
| White | 7 (70.0) | 9 (75.0) | 16 (72.7) |
| African-American | 0 (0.0) | 1 (8.3) | 1 (4.5) |
| American Indian | 1 (10.0) | 0 (0.0) | 1 (4.5) |
| Asian/Pacific Islander | 2 (20.0) | 2 (16.7) | 4 (18.1) |
| Ethnicity (Hispanic)a | 3 (30.0) | 0 (0.0) | 3 (13.6) |
| Full scale IQ score | 100.70 ± 16.55 | 106.92 ± 17.98 | 104.09 ± 16.74 |
| Disruptive behavior disorderb | 3 (30.0) | 1 (8.3) | 4 (18.2) |
| Mood disorderb | 1 (10.0) | 0 (0.0) | 1 (4.5) |
| Anxiety disorderb | 0 (0.0) | 1 (8.3) | 1 (4.5) |
| Cigarette smokerb | 5 (50.0) | 1 (8.3) | 6 (27.3) |
| Cannabis use disorderb | 5 (50.0) | 2 (16.7) | 7 (31.8) |
| Alcohol abuseb | 3 (30.0) | 3 (25.0) | 6 (27.3) |
| Alcohol dependentb | 4 (40.0) | 7 (58.3) | 11 (50.0) |
| AUD symptom countb | 3.4 ± 2.88 | 4.33 ± 2.27 | 3.91 ± 2.54 |
| RAPI | 6.00 ± 5.42 | 9.75 ± 8.80 | 8.05 ± 7.54 |
| Drinking daysc | 26.44 ± 11.06 | 28.70 ± 8.14 | 27.68 ± 9.40 |
| Drinks per drinking dayc | 4.82 ± 1.76 | 3.74 ± 1.35 | 4.23 ± 1.61 |
| Heavy drinking daysc | 12.56 ± 8.73 | 15.37 ± 8.70 | 14.09 ± 8.62 |
Note.
Ethnicity and race were not mutually exclusive;
Diagnoses were identified in accordance with the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) using the Kiddie Schedule for Affective Disorders for School-Age Children;
Derived from the 90-day Timeline Follow-Back interview conducted at baseline;
AUD = Alcohol Use Disorder; RAPI = Rutgers Alcohol Problem Index
Medication Compliance and Tolerability
Participants completed a similar number of days in each condition, t(20) = 1.37, p = .19 (naltrexone: M = 9.86, SD = 0.48; placebo: M = 10.00, SD = 0.00). Participants were highly compliant with the medication regimen, with an average compliance rate of 95.0% in the placebo arm (range = 80% to 100%) and 97.2% in the naltrexone arm (range = 80% to 100%), and condition was not associated with daily compliance, OR = 1.85, p = .33, 95% CI [0.53, 6.44]. We also tested whether participants were less likely to take medication on drinking days compared to nondrinking days and found no association, OR = 2.24, p = .24, 95% CI [0.59, 8.50]. Regarding side effects, two participants withdrew during the naltrexone arm due to gastrointestinal symptoms (i.e., nausea, loss of appetite, vomiting). We tested for differences in paired proportions of side effects between conditions among those who completed both arms of the study using the McNemar statistic. Completers were marginally more likely to report nausea while taking naltrexone (p = .06). Otherwise, there were no differences between conditions in terms of the paired proportions of side effects reported (ps > .13). Table 2 summarizes side effects reported by 10% or more of the sample.
Table 2.
Summary of Adverse Events Reported by ≥ 10% of Participants in Either Arm of the Study
| Adverse Event | Naltrexone
|
Placebo
|
McNemar’s Test p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Neurocognitive | |||||
| Difficulty sleeping | 4 | 19.0 | 1 | 4.8 | 0.38 |
| Drowsiness | 4 | 19.0 | 4 | 19.0 | 1.00 |
| Excessive tiredness | 4 | 19.0 | 0 | 0.0 | 0.13 |
| Fatigue or lack of energy | 8 | 38.1 | 8 | 38.1 | 1.00 |
| Headache | 5 | 23.8 | 4 | 19.0 | 1.00 |
| Gastrointestinal | |||||
| Abdominal pain | 1 | 4.8 | 3 | 14.3 | 0.50 |
| Decreased appetite | 3 | 14.3 | 0 | 0.0 | 0.25 |
| Nausea | 7 | 33.3 | 2 | 9.5 | 0.06 |
| Otolaryngolic | |||||
| Nasal symptoms | 1 | 4.8 | 5 | 23.8 | 0.22 |
| Sore throat | 2 | 9.5 | 4 | 19.0 | 0.63 |
| Sneezing | 3 | 14.3 | 3 | 14.3 | 1.00 |
| Cough/dry mouth | 2 | 9.5 | 7 | 33.3 | 0.13 |
Note. McNemar’s test was used to test paired proportions of side effects between medication conditions. Consequently, analyses included participants who completed both arms of the study (n = 21). This approach excluded two participants who withdrew during the naltrexone arm due to gastrointestinal symptoms and one participant who completed the naltrexone arm only. This participant endorsed the following adverse events: fatigue or lack of energy, difficulty sleeping, and drowsiness.
Findings in the Natural Environment
EMA Compliance
Participants completed 1,551 random assessments during the trial, of which 1,493 (96%) occurred prior to the onset of drinking each day. We evaluated participants’ compliance with random assessments by calculating the percentage of assessments completed by each participant in each study arm and averaged rates across participants. Participants completed 86.1% (SD = 7.1) of random assessments in the placebo arm and 86.9% (SD = 9.38) in the naltrexone arm, with no significant difference between conditions, t(20) = −.43, p = .68.
Drinking outcomes
EMA and TLFB drinking data were highly correlated in both conditions in terms of percent drinking (rs = 0.85, 0.90) and heavy drinking (rs = 0.72, 0.78) days (ps < .001), supporting our decision to use EMA data in analyses. On average, participants consumed alcohol on 3.1 days (SD = 2.0; 30.7%) in the placebo arm and on 2.4 days (SD = 1.4; 24.6%) in the naltrexone arm. With respect to heavy drinking days, participants drank heavily on an average of 1.6 days (SD = 1.8; 15.3%) while assigned to placebo compared to 1.1 days (SD = 1.0; 9.3%) while assigned to naltrexone. Naltrexone reduced the likelihood of drinking on a study day, OR = 0.69, 95% CI [0.50, 0.97], p = .03, effect size d = 0.17). Participants were also less likely to drink heavily while taking naltrexone compared to placebo, OR = 0.54, 95% CI [0.35, 0.81], p = .003, effect size d = 0.20. The frequency distribution of individual responses shows that 48% of participants had fewer drinking days in the naltrexone arm compared to placebo, and 48% had fewer heavy drinking days (see Figure 2).
Figure 2.
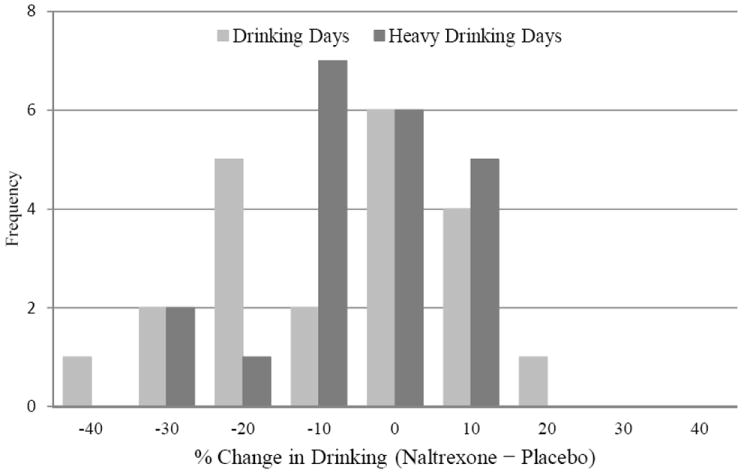
Frequency of percent change in drinking days and heavy drinking days from the naltrexone to the placebo arm (i.e., naltrexone – placebo) of the study among participants who completed both arms of the study (n = 21).
Subjective responses to alcohol
Participants recorded data for 213 alcoholic drinks during the study, with fewer drinks recorded in the naltrexone arm (n = 87) compared to placebo (n = 126). As shown in Table 3 and Figure 3, condition produced a main effect on alcohol-induced stimulation, sedation, and high. Specifically, participants reported lower stimulation and greater sedation while in the naltrexone arm compared to placebo. Naltrexone also potentiated subjective high, however, the Condition × Average eBAC interaction in this model was marginally significant (p = .06), suggesting that individuals with higher average eBAC levels experienced greater high while drinking in the naltrexone arm compared to placebo. Additionally, the Condition × Momentary eBAC interaction was a significant predictor of craving, such that naltrexone blunted alcohol-induced urge to drink more strongly at higher eBAC levels.
Table 3.
Summary of GEE Models Predicting Momentary Subjective Responses from Medication Condition Fitting Between- and Within-Individual Effects for eBAC
| Model and predictor variables | β | SE | 95% CI
|
p | |
|---|---|---|---|---|---|
| LL | UL | ||||
| Craving | |||||
| Average eBAC | −0.06 | 0.13 | −0.32 | 0.19 | .628 |
| Momentary eBAC | 0.05 | 0.05 | −0.05 | 0.15 | .353 |
| Medication condition | −0.11 | 0.07 | −0.24 | 0.03 | .109 |
| Medication condition × average eBAC | 0.24 | 0.14 | −0.03 | 0.51 | .083 |
| Medication condition × momentary eBAC | −0.43 | 0.17 | −0.76 | −0.11 | .010 |
| Stimulation | |||||
| Average eBAC | −0.28 | 0.14 | −0.55 | −0.02 | .035 |
| Momentary eBAC | 0.16 | 0.03 | 0.10 | 0.22 | < .001 |
| Medication condition | 0.35 | 0.08 | 0.20 | 0.50 | < .001 |
| Medication condition × average eBAC | −0.13 | 0.11 | −0.33 | 0.08 | .239 |
| Medication condition × momentary eBAC | −0.16 | 0.10 | −0.36 | 0.04 | .112 |
| Sedation | |||||
| Average eBAC | 0.09 | 0.14 | −0.17 | 0.36 | .490 |
| Momentary eBAC | 0.00 | 0.04 | −0.08 | 0.08 | .960 |
| Medication condition | 0.66 | 0.07 | 0.53 | 0.79 | < .001 |
| Medication condition × average eBAC | −0.08 | 0.18 | −0.43 | 0.27 | .651 |
| Medication condition × momentary eBAC | 0.13 | 0.12 | −0.11 | 0.36 | .285 |
| High | |||||
| Average eBAC | −0.39 | 0.13 | −0.63 | −0.14 | .002 |
| Momentary eBAC | 0.23 | 0.04 | 0.16 | 0.30 | < .001 |
| Medication condition | 0.39 | 0.05 | 0.29 | 0.50 | < .001 |
| Medication condition × average eBAC | 0.28 | 0.15 | −0.01 | 0.57 | .057 |
| Medication condition × momentary eBAC | 0.03 | 0.08 | −0.12 | 0.18 | .714 |
Note. GEE = generalized estimating equations; eBAC = estimated blood alcohol concentration; CI = confidence interval; LL = lower limit; UL = upper limit; Average eBAC = average eBAC across all momentary drink reports during the monitoring period and reflects the between-person effect; Momentary eBAC = person-centered eBAC and reflects the within-person effect. Subjective responses are continuous and standardized variables. In all models, medication condition was coded with an orthogonal contrast (-0.5 for placebo vs. 0.5 for naltrexone) and treatment order was included as a between-subjects covariate to control for possible order effects. The reported coefficients represent the standardized effects (effect size d).
Figure 3.
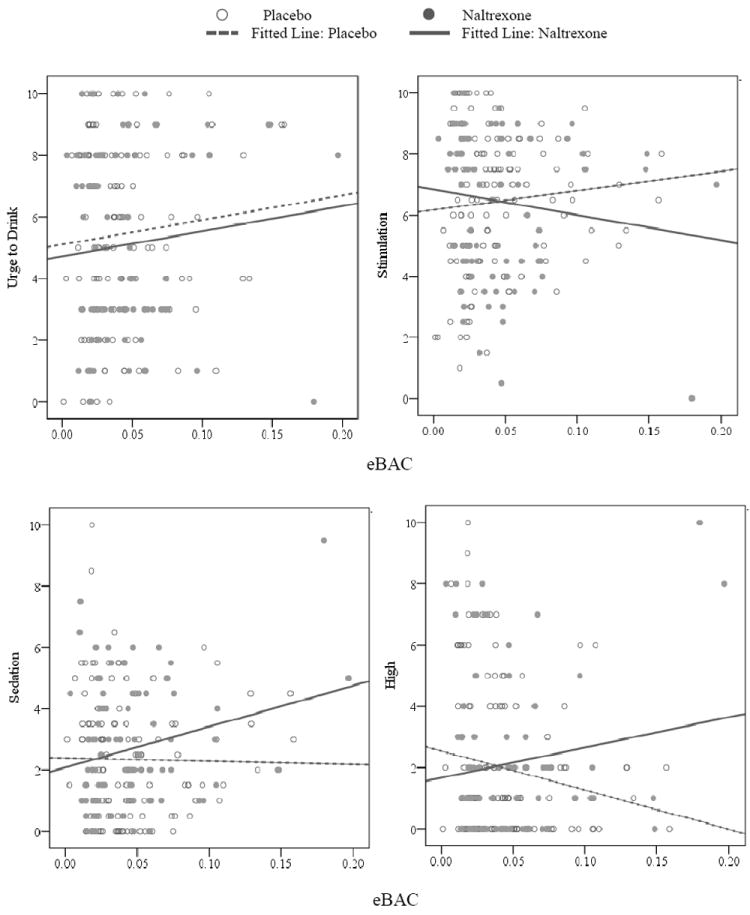
Predicted raw values for subjective alcohol response from momentary estimated blood alcohol concentrations (eBAC) as a function of medication condition. Best fitting lines for the naltrexone and placebo arms are illustrated in each panel.
Urge to drink (random assessments)
Youths reported that alcohol was directly visible in 218 (14.6%) of the 1,492 random assessments completed. When alcohol was present, participants were typically at home or a friend’s house (73.4%). We modeled the main and interactive effects of alcohol cues and condition on urge to drink in the natural environment while controlling for event- and person-level covariates. As summarized in Table 4, some event-level covariates were associated with heightened levels of craving (i.e., presence of peers, drinking day, friend’s house), while others were associated with lower levels of craving (i.e., time of day). As hypothesized, there was a Condition × Alcohol Cue interaction (p = .02), such that the presence of alcohol cues potentiated craving in the placebo condition but significantly less so in the naltrexone condition (see Figure 4), indicating that adolescents experience increased craving when exposed to alcohol cues in the natural environment and that naltrexone blunts this effect.
Table 4.
Summary of GEE Models Predicting Momentary Craving in the Natural Environment from Alcohol Cues, Medication Condition, and Occasion- and Person-Level Covariates
| Predictor | Craving
|
|||
|---|---|---|---|---|
| β | 95% CI
|
p | ||
| LL | UL | |||
| Alcohol cues | 0.13 | 0.00 | 0.25 | .048 |
| Medication condition | −0.09 | −0.26 | 0.08 | .302 |
| Alcohol cues × medication condition | −0.42 | −0.77 | −0.08 | .016 |
| Treatment order | 0.38 | −0.17 | 0.93 | .172 |
| Occasion-level covariates | ||||
| Time of day | ||||
| 12 a.m. – 5:59 a.m. | −0.14 | −0.27 | −0.01 | .042 |
| 6 a.m. – 11:59 a.m. | −0.36 | −0.58 | −0.14 | .001 |
| 12 p.m. – 5:59 p.m. | −0.21 | −0.28 | −0.13 | < .001 |
| 6 p.m. – 11:59 a.m. (reference) | —— | —— | —— | —— |
| Drinking day | 0.22 | 0.07 | 0.37 | .004 |
| Weekend | 0.07 | −0.02 | 0.16 | .136 |
| Peers present | 0.09 | 0.00 | 0.17 | .046 |
| Location | ||||
| Friend’s house | 0.26 | 0.02 | 0.49 | .033 |
| Other’s house | 0.03 | −0.10 | 0.16 | .686 |
| School | −0.09 | −0.18 | 0.00 | .052 |
| Work | 0.29 | −0.30 | 0.88 | .339 |
| Public location | 0.02 | −0.07 | 0.11 | .620 |
| Vehicle | 0.06 | −0.07 | 0.18 | .389 |
| Other location | −0.03 | −0.14 | 0.09 | .673 |
| Home (reference) | —— | —— | —— | —— |
| Person-level covariates | ||||
| Age (centered predictor) | −0.09 | −0.37 | 0.20 | .548 |
| Female | −0.14 | −0.85 | 0.57 | .697 |
Note. GEE = generalized estimating equations; CI = confidence interval; LL = lower limit; UL = upper limit.
The coefficients reported for alcohol cues and medication condition represent standardized effects (effect size d).
Figure 4.
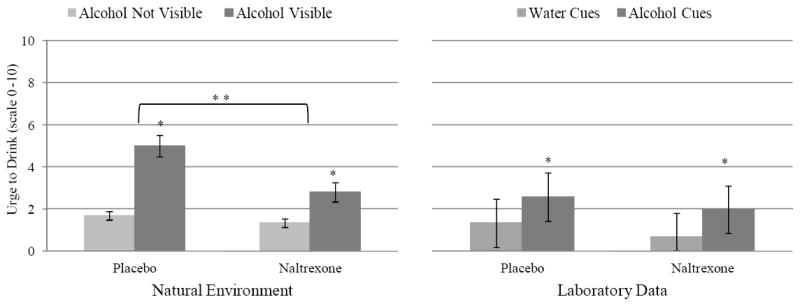
Marginal means for raw scores depicting momentary rating of craving during alcohol cue reactivity by medication condition across the laboratory and natural environments. Error bars represent 95% confidence intervals for the estimated means. Single asterisks denote significant main effect of cue type (p’s ≤ .05). Double asterisks denote a significant Cue Type × Medication Condition interaction (p = .02), such that cues elicited greater craving in the placebo arm than the naltrexone arm.
Laboratory Findings
Urge to drink
As illustrated in Figure 4, analysis of the single-item measure of craving showed a main effect of cue type in the CRA, β = 0.55, 95% CI [0.26, 0.84], p < .001, such that alcohol cues increased urge to drink relative to water cues. Neither the main effect of condition, β = −0.25, 95% CI [−0.58, 0.08], p = .14, nor the Cue Type × Condition interaction, β = 0.01, 95% CI [−0.29, 0.32], p = .93, was significant. Analysis of the AUQ indicated a similar main effect of cue type, β = 0.46, 95% CI [0.16, 0.76], p = .003, as well as a significant main effect of condition, β = −0.29, 95% CI [−0.56, −0.02], p = .04; participants reported less craving on the AUQ while taking naltrexone compared to placebo (see Figure 5). The Cue Type × Condition interaction was not significant, β = −0.07, 95% CI [−0.26, 0.12], p = .46, suggesting that naltrexone attenuated tonic levels of craving measured by the AUQ across cue exposures.
Figure 5.
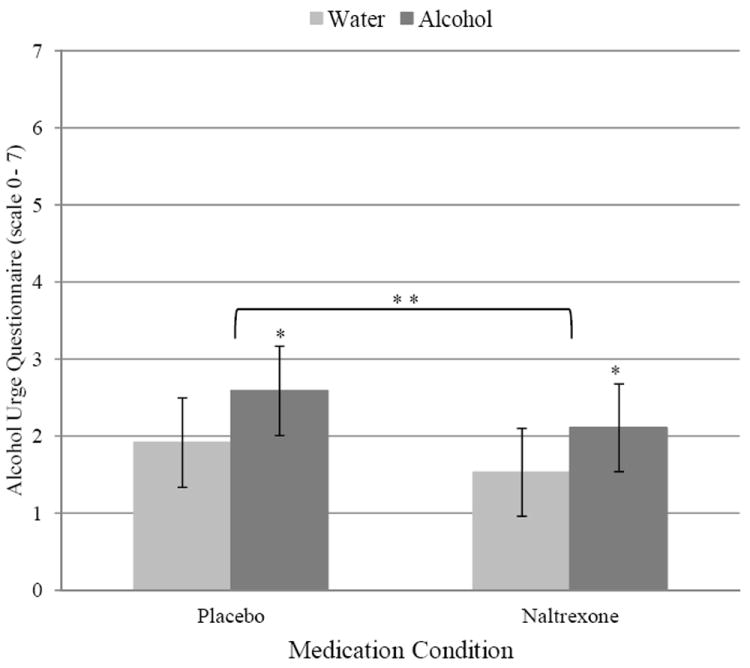
Marginal means for raw scores depicting the main effects of Beverage Cue Type and Medication Condition on craving in the laboratory, as measured using the Alcohol Urge Questionnaire. Error bars represent 95% confidence intervals for the estimated means. Single asterisks denote significant main effect of cue type (p = .003). Double asterisks denote significant main effect of medication condition (p = .04).
Physiological reactivity
Cue type produced a main effect on MAP, β = 0.29, 95% CI [0.10, 0.47], p = .002, such that participants had greater MAP while exposed to alcohol cues (M = 82.89, SD = 7.58) compared to water cues (M = 80.66, SD = 8.13). Neither the main effect of condition, β = −0.22, 95% CI [−0.60, 0.16], p = .25, nor the Cue Type × Condition interaction, β = 0.06, 95% CI [0.32, 0.45], p = .75, was significant. In terms of heart rate, the effects cue type, β = 0.04, 95% CI [−0.09, 0.18], p = .52, condition, β = −0.22, 95% CI [−0.48, 0.04], p = .09, and the Cue Type × Condition interaction, β = −0.13, 95% CI [−0.42, 0.16], p = .38, were nonsignificant.
Discussion
Adolescent problem drinkers were randomized to placebo and naltrexone using a crossover design. Naltrexone reduced the likelihood of drinking and heavy drinking. Additionally, naltrexone blunted craving across methods and contexts. EMA data from drinking episodes revealed that alcohol potentiated craving in a dose-dependent fashion, such that adolescents reported greater craving as their eBAC levels increased. Importantly, naltrexone blunted this effect. Results also showed that naltrexone blunted alcohol-induced stimulation and increased sedation. Naltrexone also potentiated high while drinking, especially among participants with greater average eBAC levels. EMA data from random assessments demonstrated that alcohol cues elicited craving outside drinking episodes and that naltrexone also dampened this response. Finally, laboratory data showed that alcohol cues elicited craving and physiological reactivity compared to water cues, and that naltrexone attenuated subjective craving assessed by the AUQ across both cue types. It is noteworthy, however, that alcohol cues elicited greater craving in the natural environment than in the laboratory. This relatively weak effect of alcohol cues on craving in the laboratory may have compromised our ability to detect alcohol-specific medication effects in a controlled environment.
Our finding that naltrexone reduced drinking is noteworthy given the brief medication period, the exclusion of treatment-seeking youths, and the fact that we did not include a behavioral intervention in order to isolate the pharmacological effects of naltrexone. Moreover, the magnitude of the estimated effects on drinking outcomes observed in this study, albeit modest, mirrors those found with adults (Maisel et al., in press). Our finding that naltrexone blunted craving is also significant. Although craving remains a cornerstone of research on alcoholism in adults, relatively few studies have examined this construct in adolescents. Our findings are consistent with initial research showing that craving is common among adolescent drinkers (Martin et al., 1995). In addition, this study offers further evidence that alcohol cues reliably elicit craving among adolescents under controlled conditions (Curtin et al., 2005; Thomas, Drobes, & Deas, 2005) and, perhaps more notably, extends previous work by demonstrating findings from the laboratory generalize to the natural environment. Effect size estimates indicated the effects of naltrexone on craving were in the medium range, which is similar to those observed with adults (Maisel et al., in press). Clinical data further underscore the potential relevance of these findings by showing that adolescents experience difficulty utilizing skills learned in treatment when faced with alcohol cues and that posttreatment relapses among teenagers are frequently associated with exposure to alcohol cues (Brown et al., 2000; Meyers, Brown, & Mott, 1993). Thus, our finding that naltrexone blunts craving across contexts may hold significant clinical utility. Moreover, recent data highlight the role of naltrexone in attenuating craving associated with goal directed (as opposed to habitual) drinking (Ray et al., 2010b). As such, naltrexone may be particularly effective for adolescents.
This study extends pharmacotherapy research on alcoholism in several meaningful ways. At the fundamental level, we demonstrated the utility of pairing laboratory paradigms with EMA methods to more fully capture the effects of medications on purported behavioral mechanisms of pharmacotherapy action. Our findings indicate that laboratory CRA methods, which are designed to simulate clinically-relevant phenomena under experimentally controlled conditions, may not adequately capture what happens in the real world. These novel findings highlight the utility of EMA methods for understanding addiction processes and testing treatment effects. This study also presents the first randomized controlled evidence that an opioid antagonist attenuates drinking and craving in adolescents, along with novel data on subjective responses to alcohol. Although the neuropharmacological mechanisms of naltrexone’s effects on drinking are not fully delineated, most animal studies suggest that competitive binding of opioid antagonists to opioid receptors attenuate the rewarding effects of alcohol by decreasing dopamine release in the mesolimbic pathway following alcohol ingestion (for review, see Ray et al., 2010b). Laboratory studies with adults generally support this notion by demonstrating that naltrexone and other opioid antagonists (e.g., nalmefene) blunt alcohol-induced stimulation (Anton et al., 2004). There is substantial remodeling of dopaminergic and other neurotransmitter systems during adolescence, however, including major changes in mesolimbic brain regions. Our findings showed that alcohol potentiates stimulation among adolescents and that naltrexone attenuates this effect. This suggests that alcohol exerts rewarding effects through similar mechanisms during adolescence as in adulthood.
Several limitations qualify our findings. First and foremost, drinking outcomes are inherently limited by the short duration of treatment. Although results support the promise of naltrexone for reducing adolescent drinking, this hypothesis must be tested in larger randomized clinical trials with long-term follow-up assessments. Second, we selected a sample of non-treatment-seeking teenagers, which may not represent the types of youth who engage in alcohol treatment. The majority of participants in this sample met diagnostic criteria for an alcohol use disorder, however, thereby increasing the applicability of our findings to clinical practice. Third, there were inherent limitations of our EMA approach to capturing drinking episodes, including the lack of a placebo control for alcohol consumption and the restriction of analyses to the ascending limb of the blood alcohol curve. It is possible that naltrexone also affected the descending limb in this sample (Ray et al., 2008). Finally, our sample size is small; an important goal for future research is to replicate these findings in a larger sample and to examine individual difference factors associated with patient responsiveness, such as sex or familial alcoholism.
On balance, this study provides the first experimental evidence that naltrexone reduces alcohol use and craving, and alters subjective responses to alcohol, in adolescent problem drinkers. This study supports our experimental paradigm, which combined human laboratory and EMA methods, as an innovative approach for testing medication effects. A major clinical implication of our findings is that naltrexone shows promise for treating alcohol misuse in this challenging population during an instrumental period in the development of alcohol use disorders.
Acknowledgments
The National Institute of Alcohol Abuse and Alcoholism at the National Institutes of Health supported this work (AA017273, AA019681). The authors thank Bethany Rallis, Justin Souliere, Jacqueline Lee, and Jason Frezza for their assistance with this project. Lara Ray is now at Department of Psychology, University of California, Los Angeles. Elizabeth K. Reynolds is now at Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine.
Footnotes
Authors Contribution
RM was responsible for the study concept. RM, LR, AB, PM, AJ, RS, JT, and CG were responsible for the study design. RM, AB, ER, AJ, TC, and JR were responsible for the acquisition of study data. TC and RS conducted medical exams on study applicants and provided medical coverage for this study. RM and AJ provided clinical coverage during alcohol cue reactivity assessments. RM, AB, ER, and CG were responsible for data analysis and all authors contributed to interpretation of findings. RM drafted the manuscript and all authors critically reviewed content and approved the final version for publication.
Disclosure/Conflict of Interest
CG is a consultant with ERT, which provides electronic diary solutions for use in clinical trials. LR is consulting with GlaxoSmithKline. RS is a consultant for D&A Pharma, CT San Remo and Transcept Pharmaceuticals. Otherwise, the authors have no conflicts to disclose.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: Temporal effects of drinking. Psychopharmacology. 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Waid R, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: Results of a placebo-controlled trial. Am J Psychiatry. 1999;156:1758–1764. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Bridge JA, Iyengar S, Salary CB, Barbe R, Birmaher B, Pincus HA, Ren L, Brent DA. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: A meta-analysis of randomized controlled trials. JAMA. 2007;297:1683–169. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Tate SR, Abrantes AM. The role of alcohol in adolescent relapse and outcome. J Psychoactive Drugs. 2000;32:107–115. doi: 10.1080/02791072.2000.10400216. [DOI] [PubMed] [Google Scholar]
- Buu A, Wang W, Schroder SA, Kalaida NL, Puttler LI, Zucker RA. Developmental emergence of alcohol use disorder symptoms and their potential as early indicators for progression to alcohol in dependence in a high risk sample: A longitudinal study from childhood to early adulthood. J Abnorm Psychol. doi: 10.1037/a0024926. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney MA, Tennen H, Afflect G, Del Boca FK, Kranzler HR. Levels and patterns of alcohol consumption using timeline follow-back, daily diaries and real-time “electronic interviews”. J Stud Alcohol. 1998;59:447–454. doi: 10.15288/jsa.1998.59.447. [DOI] [PubMed] [Google Scholar]
- Chung T, Maisto SA. Relapse to alcohol and other drug use in treated adolescents: Review and reconsideration of relapse as a change point in clinical course. ClinPsychol Rev. 2006;26:149–161. doi: 10.1016/j.cpr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Barnett NP, Colby SM, Rohsenow DJ, Monti PM. Cue reactivity in adolescents: Measurement of separate approach and avoidance reactions. J Stud Alcohol. 2005;66:332–343. doi: 10.15288/jsa.2005.66.332. [DOI] [PubMed] [Google Scholar]
- Davidson D, Camara P, Swift R. Behavioral effects and pharmacokinetics of low dose intravenous alcohol in humans. Alcohol Clin Exp Res. 1997;21:1294–1299. [PubMed] [Google Scholar]
- Davidson D, Palfai T, Bird C, Swift R. Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcohol Clin Exp Res. 1999;23:195–203. [PubMed] [Google Scholar]
- Deas D, May MP, Randall C, Johnson N, Anton R. Naltrexone treatment of adolescent alcoholics: An open-label pilot study. J Child Adolesc Psychopharmacol. 2005;15:723–728. doi: 10.1089/cap.2005.15.723. [DOI] [PubMed] [Google Scholar]
- de Wit H, Svenson J, York A. Nonspecific effect of naltrexone on ethanol consumption in social drinkers. Psychopharmacology. 1999;6:384–394. doi: 10.1007/s002130051085. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment seeking alcoholics and social drinkers. Alcohol Clin Exp Res. 2004;28:1362–1370. doi: 10.1097/01.alc.0000139704.88862.01. [DOI] [PubMed] [Google Scholar]
- Gonzalez JP, Brogden RN. Naltrexone: A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence. Drugs. 1988;35:192–213. doi: 10.2165/00003495-198835030-00002. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test manual. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present version and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful. Addiction. doi: 10.1111/j.1360-0443.2012.04054.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Kaczynski NA, Maisto SA, Bukstein OM, Moss HB. Patterns of DSM-IV alcohol misuse and dependence symptoms in adolescent drinkers. J Stud Alcohol. 1995;56:672–680. doi: 10.15288/jsa.1995.56.672. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology. 2000;22:480–492. doi: 10.1016/S0893-133X(99)00147-5. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, McClair VL. Epidemiology of substance use disorders. Hum Genet. 2012;131:779–789. doi: 10.1007/s00439-012-1168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers MG, Brown SA, Mott MA. Coping as a predictor of adolescent substance abuse treatment outcome. J Subst Abuse. 1993;5:15–29. doi: 10.1016/0899-3289(93)90120-z. [DOI] [PubMed] [Google Scholar]
- Miranda RM, MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, Swift R, Ray L, McGeary J. Effects of topiramate on urge to drink and the subjective effects of alcohol: A preliminary laboratory study. Alcohol Clin Exp Res. 2010;32:489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Zwick WR, Abrams DB, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Na C, Lee YS. Alcohol urge with plasma beta-endorphin change after alcohol challenge with naltrexone pretreatment in social drinkers. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:663–670. doi: 10.1016/s0278-5846(01)00315-3. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS. Naltrexone and coping skills therapy for alcohol dependence: A controlled study. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Palta M. Quantitative methods in population health: Extensions of ordinary regression. Hoboken, NJ: John Wiley & Sons, Inc; 2003. [Google Scholar]
- Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341X.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Wood PK, Shiffman S, Sher KJ, Heath AC. Responses to alcohol and cigarette use during ecologically assessed drinking episodes. Psychopharmacology. doi: 10.1007/s00213-012-2721-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A double-blind placebo-controlled study of the effects of naltrexone on alcohol sensitivity and genetic moderators of medication response. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: Clinical findings, mechanisms of action, and pharmacogenetics. CNS and Neurological Disorder – Drug Targets. 2010b;9:13–22. doi: 10.2174/187152710790966704. [DOI] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, Leventhal A, Hutchison KE. Catching the alcohol buzz: An examination of the latent factor structure of subjective intoxication. Alcohol Clin Exp Res. 2009;33:2154–2161. doi: 10.1111/j.1530-0277.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchinson KE, MacKillop J, Miranda RM, Audette A, Swift R, Monti PM. Effects of naltrexone during the descending limb of the blood alcohol curve. Am J Addict. 2008;17:257–264. doi: 10.1080/10550490802138400. [DOI] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Jr, Tidey J, McGeary JE, MacKillop J, Gwaltney C, Rohsenow DJ, Swift RM, Monti PM. Polymorphisms of the μ-opioid receptor and dopamine D4 receptor genes and subjective responses to alcohol in the natural environment. J Abnorm Psychol. 2010a;119:115–125. doi: 10.1037/a0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry. 1984;41:879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Cox SML, Gianoulakis C, Palmour RM, Benkelfat C, Leyton M. The effect of naltrexone on alcohol’s stimulant properties and self- administration behavior in social drinkers: Influence of gender and genotype. Alcohol Clin Exp Res. 2011;35:1134–1141. doi: 10.1111/j.1530-0277.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- Sobell LD, Sobell MD. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen K, editors. Measuring Alcohol Consumption. Human Press; Clifton, NJ: 1992. pp. 41–65. [Google Scholar]
- Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: Setting the stage for alcohol use disorders? Child Development Perspectives. 2011;5:231–238. doi: 10.1111/j.1750-8606.2011.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addiction. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Swendsen J, Burstein M, Case B, Conway KP, Dierker L, He J, Merikangas KR. Use and abuse of alcohol and illicit drugs in US adolescents: Results of the National Comorbidity Survey – Adolescent supplement. Arch Gen Psychiatry. 2012;69:390–398. doi: 10.1001/archgenpsychiatry.2011.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Drobes DJ, Deas D. Alcohol cue reactivity in alcohol-dependent adolescents. J Stud Alcohol. 2005;66:354–360. doi: 10.15288/jsa.2005.66.354. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranda R, Jr, McGeary JE, MacKillop J, Swift RM, Abrams DB, Shiffman S, Paty JA. Moderators of naltrexone’s effects on drinking, urge and alcohol effects in the natural environment. Alcohol Clin Exp Res. 2008;32:58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Labouvie EW. Towards the assessment of adolescent problem drinking. J Stud Alcohol. 1989;50:30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang K, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]


