Abstract
The transmembrane protein with epidermal growth factor (EGF) and two follistatin (FS) motifs 2 (TMEFF2) has a limited tissue distribution with strong expression only in brain and prostate. While TMEFF2 is overexpressed in prostate cancer indicating an oncogenic role, several studies indicate a tumor suppressor role for this protein. This dual mode of action is, at least in part, the result of metalloproteinase-dependent shedding that generates a soluble TMEFF2 ectodomain with a growth promoting function. While recent studies have shed some light on the biology of different forms of TMEFF2, little is known about the molecular mechanisms that influence its oncogenic/tumor suppressive function. In several non-prostate cell lines, it has been shown that a recombinant form of the TMEFF2 ectodomain can interact with platelet derived growth factor (PDGF)-AA to suppress PDGF receptor signaling and can promote ErbB4 and ERK1/2 phosphorylation. However, the role of the full length TMEFF2 in these pathways has not been examined. Using prostate cell lines, here we examine the role of TMEFF2 in ERK and Akt activation, two pathways implicated in prostate cancer progression and that have been shown to cross talk in several cancers. Our results show that different forms of TMEFF2 distinctly affect Akt and ERK activation and this may contribute to a different cellular response of either proliferation or tumor suppression.
Keywords: Prostate cancer, signaling pathways, Akt, ERK, phosphorylation, TMEFF2
Introduction
Prostate cancer (PCa) is the most commonly diagnosed non-cutaneous cancer and the second leading cause of cancer death in men [1]. Despite recent advances in treatment of localized PCa, effective therapies for the treatment of the advanced form of the disease are limited. The most common being disruption of androgen receptor (AR) signaling via hormone deprivation therapy, which although initially effective, ultimately leads to castration resistant prostate cancer (CRPC), a highly lethal form of the disease [2].
Essential to the development of new therapies for PCa is the understanding of the signaling pathways involved in the disease and the impact that these pathways have on each other during disease progression. The PTEN and MAPK pathways are often deregulated during PCa progression leading to aberrant activation of the Akt and ERK kinase activity as well as their downstream effectors [3,4]. Activation of the Akt signaling pathway promotes cell survival by inhibiting apoptosis while activation of ERK increases cell proliferation and both pathways may function together to promote tumorigenesis [5]. In fact, these pathways are known to regulate each other and co-regulate downstream functions [6,7]. Interestingly, although in some tumors phospho-ERK levels are very high [8-10], it has been reported that advanced PCa correlates with low phospho-ERK and high Akt levels [11], suggesting that the cross-talk between both pathways occurs during tumor progression.
TMEFF2 is a single pass type I transmembrane protein expressed in the embryo [12,13] and selectively in the adult brain and prostate [14-16]. TMEFF2 contains several potential biologically important features that suggest a role in signaling [13,16,17]. The extracellular (ecto) domain, which is cleaved from the membrane in an ADAM 17/gamma-secretase dependent fashion [18,19], consists of two follistatin (FS) modules and an epidermal growth factor-like (EGF) domain. The transmembrane domain and short cytoplasmic tail have features that resemble a potential G-protein coupled receptor [13,20].
TMEFF2 is up-regulated in a significant fraction of primary and metastatic prostate tumors suggesting a role in this disease [14-16,21]. The full length TMEFF2 protein functions as a tumor suppressor by inhibiting migration and invasion of prostate epithelial and prostate cancer cells [22,23] and by modulating apoptosis and growth of HEK293T cells [22], prostate cancer cells [15] and colorectal cancer cells as examined in an anchorage independent growth assay and a xenograft model [24]. In contrast, a recombinant form of the TMEFF2 ectodomain promotes increased cellular proliferation of HEK293 cells and some type of neurons [18,25]. In addition, pharmacological inhibition of TMEFF2 shedding from the membrane or TMEFF2 siRNA knockdown reduces cell proliferation of the LNCaP prostate cancer cell line [18]. In support of the proliferative role of the ectodomain, we have demonstrated that ectodomain-containing conditioned medium from cells expressing the TMEFF2 protein promotes growth of prostate and HEK293T cells [22].
At the molecular level, recombinant TMEFF2 ectodomain has been shown to modulate ERK activation by promoting phosphorylation of erbB4 and ERK1/2 and to interfere with platelet derived growth factor (PDGF) receptor signaling by binding and sequestering PDGF-AA from binding and signaling through this receptor [13,18,26]. The full length TMEFF2 protein also interacts with PDGF-AA [26], and with sarcosine-dehydrogenase (SARDH) the enzyme that catalyzes sarcosine conversion to glycine [22]. The TMEFF2-SARDH interaction modulates sarcosine levels and one carbon metabolism leading to changes in cellular invasion, possibly due to changes in the methylation potential of the cell [23]. In colon cancer cell lines, TMEFF2 overexpression leads to STAT1 upregulation and this appears to be required for the TMEFF2-mediated growth suppression effect [24].
The limited tissue distribution of TMEFF2, mainly expressed in brain and prostate [14-16], has drawn attention as a possible tool for conjugated antibody therapies. In addition, the occurrence of secreted forms of TMEFF2 (shed and spliced forms [27]) suggests a possible role as a biomarker. The potential therapeutic use of TMEFF2 stresses the need for understanding the molecular mechanism of action. Here we explore the effect of TMEFF2 full length and the ectodomain in the ERK and Akt signaling pathways. Our results indicate that these two different forms of the protein differentially regulate these pathways to either promote growth or to function as a tumor suppressor.
Materials and methods
Cell lines, plasmid constructs and materials
HEK293T and RWPE1 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA) and maintained in DMEM, or KSF media (Life Technologies, Grand Island, NY) at 37°C with 5% CO2. DMEM medium was supplemented with 10% v/v FBS, 100 units/mL penicillin, 100 μg/mL streptomycin, and Amphotericin B (Life Technologies, Grand Island, NY). The KSF medium was supplemented with human recombinant epidermal growth factor (EGF) and bovine pituitary extract (BPE) as recommended by the manufacturer in addition to 100 units/mL penicillin, 100 μg/mL streptomycin, and Amphotericin B. TMEFF2 full length or ectodomain expressing HEK293T cells or RWPE1 cells inducibly expressing full length TMEFF2 were previously described [22]. The TMEFF2-ΔGA mutant lacking 13 amino acids (343 to 355) from the cytoplasmic tail was generated using PCR and standard cloning strategies. The following primers were used to generate the deletion construct: a) TAGCTAGCAGTCATGGTGCTGTGGG; b) TACCATGGTGTGATGCAGAGGACC; c) TACCATGGCAAAATACAGGGCACTAC and d) TACTCGAGAGATTAACCTCGTGGACG.HEK293T and RWPE1 cells were transfected with the expression plasmids using Fugene HD transfection reagent (Promega, Madison, WI).Stable cell lines were generated by drug resistance and characterized for expression of the specific protein by western blot. Development of a system for inducible expression of the TMEFF2-ΔGA gene in RWPE1 cells was achieved using the Clontech’s Tet-On Advanced system as described before [22]. To inducibly express TMEFF2 in RWPE1 cells, cultures were grown in the presence of doxycycline (250 ng/ml; Clontech, Mountain View, CA). Epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) were obtained from Life Technologies, (Grand Island, NY) and R & D Systems, (Minneapolis, MN) and used at the concentrations specified in the text.
Cell culture and cell treatments
For experiments in which conditioned medium was added, RWPE-1 cells were growing in basal KSF medium without supplement for 30 min before growth medium was replaced with the specified conditioned medium. Cells were then incubated for 30 min, unless otherwise specified, before lysis. Conditioned medium was obtained from HEK293T cells stably transfected with a TMEFF2 ectodomain-expressing construct, a full length TMEFF2 expressing construct or the empty vector (EV) previously described [22]. Cells were grown at 70%-80% confluency, starved overnight and the conditioned medium collected and utilized to replace the growth medium. For PDGF-AA treatment, RWPE-1 cells were grown in basal KSF medium without supplements for 30 min before treatment with the indicated amount of PGDF-AA for 10 min, unless otherwise specified. For EGF treatment, RWPE-1 cells were grown in basal KSF medium without supplements for 3 h before treatment with 10 ng/ml EGF for 10 min.
Western blot analysis
For Western blot analysis, cells were lysed in buffer containing 20 mM sodium phosphate, pH 7.4; 150 mM sodium chloride, 1% Triton X- 100 and protease inhibitor cocktail (Sigma, St. Louis, MO). Protein concentrations were determined with BCA protein assay (Pierce, Rockford, IL) and proteins resolved by SDS-PAGE following transfer to Immobilon transfer membrane (BIORAD, Hercules, CA). Blots were blocked in 5% non-fat milk, and probed with the appropriate antibodies. Immunoreactive bands were visualized using ECL plus western blotting detection system (Pierce, Rockford, IL). To detect the presence of TMEFF2 in the conditioned medium, it was concentrated 20-fold in Amicon Ultra-4 Centrifugal Filter Devices (Millipore, Billerica, MA) and analyzed by western blot analysis using antibodies against TMEFF2 (Abcam, Cambridge, MA). Other antibodies were as follow: anti-TMEFF2 (Abcam, Cambridge, MA), anti-phospho-ERK1/2, anti-total-ERK, anti-phospho-Akt (Ser473), anti-total-Akt, anti-phospho-Smad2 (Cell Signaling Technology, Danvers, MA). Secondary antibodies: goat anti-mouse IgG1-HRP or goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit control IgG (Abcam, Cambridge, MA). Blocking antibodies added to the conditioned medium were all at 1 ug/ml working concentration.
Results
The full length TMEFF2 promotes ERK phosphorylation in response to epidermal growth factor
Expression of the full length, membrane-bound TMEFF2 inhibits growth, invasion and migration of HEK293T cells and several prostate epithelial and prostate cancer cell lines [15,22,23] indicating a tumor suppressor role for this protein. To further gain insight in the signaling pathways involved in TMEFF2 function, we analyzed the effect of the full length TMEFF2 on Ras/Raf/MEK/ERK, one of the main pathways involved in PCa progression. Of note, a recombinant form of the TMEFF2 ectodomain has been shown to promote activation of several components of the epidermal growth factor (EGF) receptor/MEK/ERK signaling pathway, including ERK1/2 phosphorylation [18]. Prostate epithelial RWPE1 cells that are induced to express TMEFF2 full length in response to doxycycline addition described in ref. [22] along with control cells transfected with the empty vector (EV) were used in these experiments. In addition, we created a deletion mutant, TMEFF2-ΔGA, lacking 13 consecutive basic-rich amino acids in the cytoplasmic domain of the protein, to examine its potential role in signaling. As shown in Figure 1A, expression of either the full length or the TMEFF2-ΔGA did not affect ERK phosphorylation when grown under normal conditions (compared to cells expressing the empty vector). However, stimulation with epidermal growth factor (EGF), a main EGFR/MEK/ERK activator, of cell cultures growing in KSF basal medium resulted in an increase in ERK phosphorylation in RWPE1 cells expressing full length TMEFF2 as compared to cells expressing the empty vector or cells left untreated (Figure 1B). Interestingly, deleting the 13 basic-rich amino acid region in the cytoplasmic domain (TMEFF2-ΔGA) prevents TMEFF2 from promoting ERK activation suggesting that losing that region of the protein affects its signaling ability. These results indicate that in RWPE1 prostate epithelial cells, the TMEFF2 full-length protein promotes ERK phosphorylation in response to EGF and that it requires a functional cytoplasmic domain for this effect.
Figure 1.
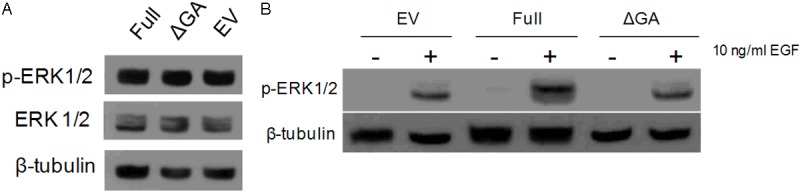
TMEFF2 promotes ERK1/2 phosphorylation in response to EGF. A: RWPE-1 cells inducibly expressing TMEFF2-Full, TMEFF2-ΔGA, or the EV control were grown in complete KSF medium, lysed and whole cell lysates were subjected to immunoblotting with anti-p-ERK1/2, anti-ERK1/2, and anti-β-tubulin antibodies. B: RWPE-1 cells induced to express TMEFF2-Full, TMEFF2-ΔGA, or the EV control were growing in basal KSF medium for 3 hours and then stimulated with 10 ng/ml of EGF for 10 minutes. Whole cell lysates were then subjected to immunoblotting with anti-p-ERK1/2 and anti-β-tubulin antibodies. Representative examples of at least two independent experiments showing similar results are shown.
PDGF-AA induces sustained phosphorylation of ERK in cells expressing TMEFF2
As stimulation of PDGF receptors activates the Ras/Raf/MEK/ERK and the Akt/PI3K pathways in numerous cells and PDGF-AA has been shown to interact with TMEFF2, we tested the effect of TMEFF2 on ERK and Akt activation using RWPE1 cells stimulated with PDGF. RWPE1 cells are known to express the PDGFα and β receptors [28]. First, we determined that ERK phosphorylation reaches a limit at a concentration of 25 ng/ml of PDGF-AA in the medium since increasing concentrations did not result in increased ERK phosphorylation (Figure 2A), and established a concentration of 50 ng/ml PDGF-AA in subsequent experiments. The addition of PDGF-AA to RWPE1 cells growing in basal KSF medium without supplements led to an early and transient phosphorylation of Akt which peaked within 10 minutes and returned to baseline within an hour (Figure 2B). Increased phosphorylation of ERK was not apparent until 1 hour after the stimulation and was highest at the last time point, obtained 4 hours after PDGF-AA addition (Figure 2B; of note, it is possible that it is further increased). Therefore, these two signaling pathways do not seem to overlap in cells treated with PDGF-AA. Similar to the results observed with EGF stimulation, overexpression of TMEFF2, while having no significant effect on Akt phosphorylation, promoted an early and robust induction of ERK phosphorylation that was apparent 10 minutes after the addition of PDGF-AA to the culture and increased progressively throughout the duration of the experiment (up to 4 hours; Figure 2C). In addition, overexpression of the TMEFF2-ΔGA mutant protein nearly completely reversed the effect on ERK phosphorylation to the level observed with the EV expressing RWPE1 cells suggesting that the presence of the cytoplasmic tail is required for the effect of TMEFF2 on ERK phosphorylation in response to PDGF-AA (Figure 2D). RWPE1 cells expressing the ectodomain protein, did not demonstrate an increase in ERK phosphorylation in response to PDGF-AA (Figure 2E). This result was expected since purified recombinant soluble TMEFF2 ectodomain has been shown to interact with PDGF-AA inhibiting its interaction with the PDGF receptor [26]. Since the TMEFF2 ectodomain expressed in our cells lacks the transmembrane domain and is secreted into the media, we hypothesized that it sequesters the PDGF-AA factor from its interaction with the receptor inhibiting its signaling.
Figure 2.
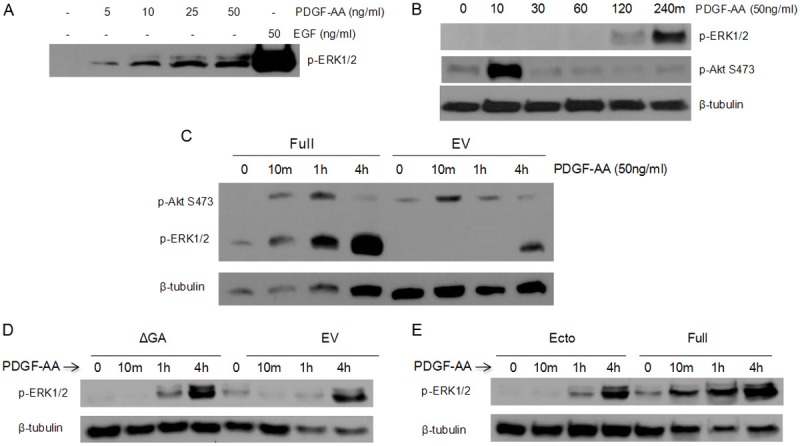
PDGF induces sustained phosphorylation of ERK in cells expressing TMEFF2. A: RWPE-1 cells were transferred to basal KSF medium for 30 minutes and then treated with various concentrations of PDGF-AA or 50 ng/ml of EGF. Whole cell lysates were then subjected to immunoblotting with anti-p-ERK1/2 antibody. B: RWPE-1 cells were transferred to basal KSF medium for 30 minutes and then treated with 50 ng/ml of PDGF-AA for indicated times. Whole cell lysates were then subjected to immunoblotting with anti-p-ERK1/2, anti-p-AKT S473, and anti-β-tubulin antibodies. C-E: RWPE-1 cells inducibly expressing TMEFF2-Ecto, TMEFF2-Full, TMEFF2-ΔGA, or the EV control were transferred to basal KSF medium for 30 minutes and then stimulated with 50 ng/ml of PDGF-AA for indicated times. Whole cell lysates were then subjected to immunoblotting with anti-p-ERK1/2, anti-p-AKT S473, and anti-β-tubulin antibodies. Representative examples of at least two independent experiments showing similar results are shown.
The ectodomain region of TMEFF2 inhibits ERK phosphorylation
The results presented above suggest that the TMEFF2 ectodomain may inhibit ERK phosphorylation in response to PDGF-AA. To further investigate the role of the TMEFF2 ectodomain in ERK phosphorylation, we determined whether ectodomain containing conditioned medium could directly modulate the activity of ERK in RWPE1 cells. Ectodomain containing conditioned medium was collected from exponentially growing HEK293T cell cultures overexpressing the TMEFF2 ectodomain. The presence of TMEFF2 ectodomain in the conditioned medium was analyzed by western blot using antibodies against the TMEFF2 ectodomain (Figure 3A). The collected conditioned medium was used to replace the growth medium of the RWPE1 cells, and the ERK1/2 phosphorylation was examined by western blot analysis using a rabbit polyclonal antibody. Addition of increasing amounts of conditioned medium from the TMEFF2 ectodomain overexpressing cultures resulted in a stepwise decrease in ERK phosphorylation in the RWPE1 cells (Figure 3B, left lanes). This effect was partially reversed by the addition of a monoclonal antibody to TMEFF2 (Figure 3B, central lanes) but not by a polyclonal antibody against this protein (Figure 3B, right lanes). IgG was used as control (Figure 3B, left lanes). The differential effect of the two antibodies is likely due to different binding specificities. These results indicate that inhibition of ERK phosphorylation is, at least in part, due to the presence of the TMEFF2 ectodomain in the conditioned medium. The presence of IgG did not affect the TMEFF2 ectodomain mediated inhibition of ERK phosphorylation, as a similar dose-dependent inhibition was observed with increasing amounts of ectodomain containing conditioned medium in the absence of IgGs (Figure 4A). Finally, using a fixed amount of conditioned medium, we compared the effect of ectodomain containing conditioned medium on ERK phosphorylation overtime. Conditioned medium collected from vector transfected HEK293T cultures was used as a control. The results (Figure 4B) indicated that although ERK phosphorylation occurs with similar kinetics in cells treated with TMEFF2 ectodomain containing or empty vector control conditioned medium, the extent of ERK phosphorylation was decreased when RWPE1 cells were treated with TMEFF2 ectodomain containing conditioned medium. These results indicate that in RWPE1 prostate epithelial cells, the effects of TMEFF2 ectodomain and the full-length protein on ERK activation are reversed and this could potentially explain the opposing functional roles of these two forms of the TMEFF2 protein.
Figure 3.
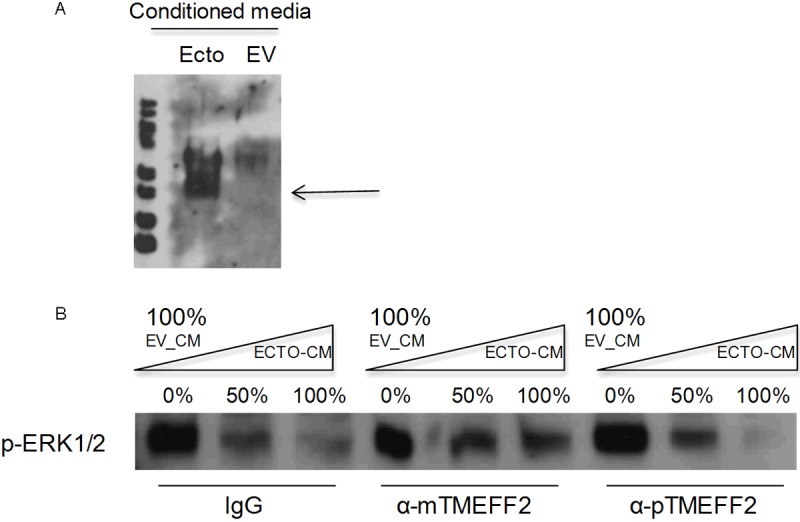
Conditioned medium from HEK293T cells expressing the ectodomain construct contains secreted TMEFF2 ectodomain and inhibits ERK phosphorylation. A: Exponentially growing HEK293T cells transfected with the TMEFF2-ectodomain construct or the empty vector (EV) as a control, were grown under serum starvation conditions for 24 hours. A sample of the conditioned medium was then collected, concentrated and subjected to immunoblotting with an anti-TMEFF2 antibody targeting the ectodomain region of the protein. The presence of TMEFF2 ectodomain in the conditioned medium is indicated by the arrow. B: RWPE-1 cells were transferred to basal KSF medium for 30 minutes before the medium was replaced with different amounts of ectodomain containing medium. Two different TMEFF2 antibodies were added to the conditioned medium to neutralize the TMEFF2 ectodomain. IgG was used as control. Whole cell lysates were prepared and subjected to immunoblotting with anti-p-ERK1/2 antibody. Representative examples of at least two independent experiments showing similar results are shown.
Figure 4.
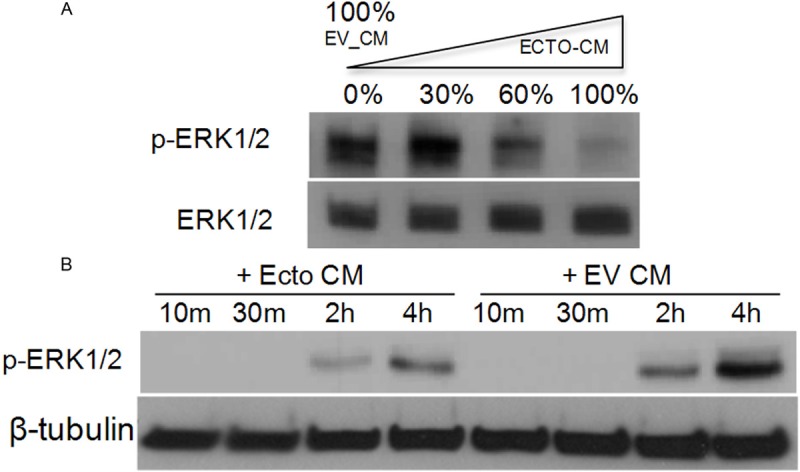
The ectodomain inhibits ERK1/2 phosphorylation in a dose dependent-manner but does not affect the activation kinetics. A: RWPE-1 cells were transferred to basal KSF medium for 30 minutes before the medium was replaced with different amounts of ectodomain containing conditioned medium. Whole cell lysates were then subjected to immunoblotting with anti-p-ERK1/2 and anti-ERK1/2 antibodies for normalization. B: RWPE-1 cells were transferred to basal KSF medium for 30 minutes before the medium was replaced with ectodomain containing conditioned medium for indicated times. Whole cell lysates were prepared and subjected to immunoblotting with anti-p-ERK1/2 and anti-β-tubulin antibodies for normalization. Representative examples of at least two independent experiments showing similar results are shown.
The TMEFF2 ectodomain promotes AKT activation
The results presented above were unexpected since previous reports indicate increased ERK phosphorylation in response to purified recombinant TMEFF2 ectodomain. Since the Ras-Raf-MEK-ERK and the PI3K-Akt pathways are known to demonstrate cross talk, impacting the outcome of the pathways [6], we analyzed whether treatment with ectodomain containing conditioned medium was able to induce changes in Akt activation that could ultimately affect ERK phosphorylation. Following the same protocol as described above, conditioned medium collected from HEK293T cells transfected with the TMEFF2 full length or the ectodomain constructs was used to replace the growth medium of cultures of RWPE1 cells, and the phosphorylation of ERK and AKT was analyzed 30 minutes after the medium replacement. As a control, conditioned medium collected from vector transfected HEK293T cultures was used (Figure 5). As described above, ectodomain containing conditioned medium-from the full length or ectodomain expressing cells-- decreased ERK phosphorylation. However, Akt phosphorylation was increased under these conditions, indicating an inverse correlation between ERK and Akt phosphorylation in response to the ectodomain in RWPE1 cells (Figure 5). These results are in agreement with data indicating that a proliferative stimulus can modulate the ERK pathway to prevent growth arrest by ERK-dependent up-regulation of cell cycle inhibitors [29,30] and with the fact that Akt can play a positive or negative role in the regulation of ERK depending on several variables, such as growth condition, stage of differentiation, etc [6]. Although TMEFF1, the only TMEFF2 homolog, has been shown to modulate Nodal signaling [31,32], we did not observe differences in Smad2 phosphorylation in response to TMEFF2 ectodomain conditioned medium (Figure 5). All together these results indicate that different forms of TMEFF2, full length or ectodomain, distinctly modulate the ERK and/or Akt pathways to exert different roles.
Figure 5.
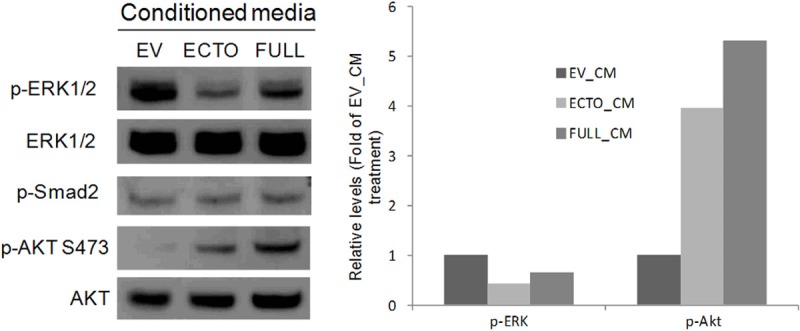
The TMEFF2 ectodomain promotes AKT phosphorylation and that inversely correlates with its effect on ERK phosphorylation. RWPE-1 cells transferred to basal KSF medium for 4 hours before the medium was replaced with ectodomain containing conditioned medium obtained from HEK293T cells that express the ectodomain (ECTO), the full length (FULL) or the empty vector (EV) constructs. Whole cell lysates were prepared and subjected to immunoblotting with anti-p-ERK1/2, anti-ERK1/2, anti-p-AKT S473, anti-AKT, and anti-p-smad2 antibodies (left). Quantitation of the results is shown (right). Representative examples of at least two independent experiments showing similar results are shown.
Discussion
In this study we report that TMEFF2, a protein with a role in prostate cancer, modulates the activity of the Ras/Raf/MEK/ERK and PI3K/Akt signaling pathways. Interestingly, while the full length TMEFF2 protein promotes a strong ERK activation in response to growth factors but had no effect in Akt activation, a shed form of the protein, the TMEFF2 ectodomain, inhibits ERK phosphorylation and promotes Akt activation. These opposing effects on ERK and Akt activation reflect a distinct role and effector site for each isoform; the full length functions as a tumor suppressor [15,22-24] from the membrane or from inside the cell after internalization [14,33,34], while the shed ectodomain promotes growth [18,22,25] and functions from outside the cell, potentially as a ligand.
The Ras/Raf/MEK/ERK and the PI3K/Akt signaling cascades play critical roles in control of cell survival, proliferation, differentiation, metabolism and cell motility and they are frequently activated during oncogenesis [3-5].Accumulating evidence indicates that in addition to their independent roles, these pathways regulate each other (cross-talk) during normal growth and oncogenesis. For example, activated Akt inhibits ERK activation by phosphorylating and inhibiting Raf, upstream of ERK [6,35], and by facilitating EGF-receptor (EGFR) degradation to inhibit signaling to its downstream pathways [36]. This cross inhibition by activated Akt is especially relevant to prostate cancer since deregulated expression and/or mutations of the phosphate and tensin (PTEN) homolog tumor suppressor gene, which lead to activation of AKT, occur with very high frequency in prostate cancer [37]. In fact, it has been reported that in advanced prostate cancer there are high levels of activated Akt, and this is inversely correlated with the level of ERK activation (low). In benign lesions, or low grade prostate cancer this relationship is reversed, demonstrating high phospho-ERK and low phospho-Akt levels [38-40]. These observations agree with a recently identified tumor suppressor role for ERK. High levels of ERK phosphorylation lead to an ERK-dependent protein degradation process and senescence [41].
Our results are consistent with these observations. The tumor suppressor full lengths TMEFF2 protein promotes strong ERK phosphorylation and requires the cytoplasmic tail for this effect, while the growth promoting ectodomain, activates Akt and inhibits ERK phosphorylation. TMEFF2 is cleaved from the membrane by ADAM17/Υ-secretase dependent cleavage that can be induced by inflammatory cytokines, i.e. TNFα [18,19,22], which are characteristic of the tumor microenvironment. Therefore, regulated TMEFF2 cleavage can modulate the function of TMEFF2, as it plays a role during tumor establishment and progression. Based on the results presented here, the functional switch from a tumor suppressor to a growth-promoting role could be mediated by the activation of the Akt pathway and subsequent silencing of the ERK-mediated tumor suppressor function. In addition, the distinct functions of the membrane-bound and soluble forms of TMEFF2 suggest that TMEFF2 may signal either as a ligand, a membrane bound receptor and/or as a co-receptor. Based on these results we propose a model (Figure 6) in which the different TMEFF2 forms distinctly modulate Akt and/or ERK signaling to exert different functions: A) full length TMEFF2 acting as a receptor or co-receptor promotes ERK phosphorylation; B) Shedding of TMEFF2 leads to ectodomain accumulation that can C) function as a ligand to an unknown receptor to promote Akt activation and subsequent Raf inhibition leading to low ERK phosphorylation and D) interact with growth factors (i.e. PDGF) to prevent interaction with their receptor and ERK activation.
Figure 6.
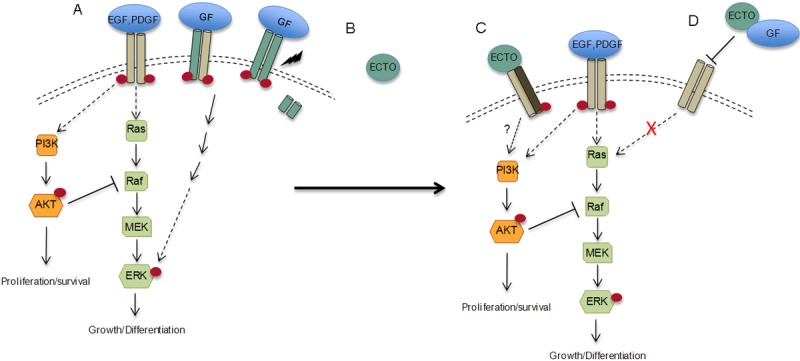
Model of the role of TMEFF2 in Akt and ERK activation. A: full length TMEFF2 acting as a receptor (green bars) or co-receptor (grey and green) promotes ERK phosphorylation; B: shedding of TMEFF2 leads to ectodomain accumulation (green circle) in the conditioned media that can C: function as a ligand to an unknown receptor to promote Akt activation and subsequent Raf inhibition leading to low ERK phosphorylation and D: interact with growth factors (i.e. PDGF) to prevent interaction with their promoter and ERK activation.
The data in Figure 4 indicates that the effect of the ectodomain-containing conditioned medium on ERK phosphorylation is partly reversed by a TMEFF2 blocking antibody suggesting that, at least in part, the effect on ERK phosphorylation is directly mediated by the TMEFF2 ectodomain. While the non-complete reversion of ERK phosphorylation could be due to failure of the antibody, a parallel explanation to the function of the TMEFF2 ectodomain, not depicted in our model (Figure 6), is that the cleaved TMEFF2 ectodomain may also have an indirect effect on ERK phosphorylation. In this role, expression/activity of the TMEFF2 ectodomain in HEK293T cells (utilized to collect the conditioned medium used in the experiments) would promote secretion of high levels of a factor (e.g. IGF) that can activate Akt in prostate epithelial RWPE1 cells. Whether direct or indirect, the ectodomain is functioning from the outside of the cell-potentially as a ligand- and therefore its effect may be cell specific depending on the repertoire of receptors present in the cell. In fact, several effects have been described for the ectodomain in different cell lines. Purified recombinant ectodomain, promotes growth of neurons [17] and non-transformed HEK293 cells [18] and phosphorylation of some of the components of the ERK signaling pathway-erbB4 in MNK28 gastric cancer cells and ERK1/2 in HEK293 cells [13,18] and corticotroph cells [42]. Interestingly, in corticotroph cells, ERK activation has been reported to occur as a consequence of Akt inhibition [42]. In contrast, ectodomain containing conditioned medium, although it promotes growth of HEK293T [22] and prostate epithelial RWPE1 cells [22], this effect, as demonstrated here, correlates with a decrease in ERK phosphorylation and an increase in Akt activation in prostate epithelial cells. Finally, using NR6 fibroblast, Lin et al. demonstrated that recombinant ectodomain binds to and competes with PDGF-AA from binding to its receptor, inhibiting PDGF-AA promoted growth [26].
The potential clinical relevance of the tumor suppressor role of the full length TMEFF2 has been documented in gliomas as an inverse correlation between TMEFF2 expression and both, the severity of the disease and the levels of PDGF-AA [26]. In prostate cancer cell lines, TMEFF2 interacts with SARDH [22] to modulate one carbon metabolism and the levels of sarcosine [22,23], an amino acid identified as a marker for prostate cancer progression [43]. TMEFF2-induced decrease in sarcosine levels partly correlates with its tumor suppressor role and, in fact, the TMEFF2 ectodomain, which promotes growth, does not modulate sarcosine levels since it does not interact with SARDH [22]. The role of TMEFF2 in one carbon metabolism may suggest an additional link to ERK activation. One carbon metabolism is comprised of several connected metabolic pathways that promote the folate-mediated transfer of one-carbon units necessary for DNA synthesis and repair. Folate is also essential in its 5-methyl-tetrahydrofolate (THF) form as a methyl donor in the remethylation of homocysteine to methionine, which is then converted to S-adenosylmethionine (SAM), the universal methyl donor [44]. By modulating one carbon metabolism, TMEFF2 has the potential to impact homocysteine levels, which have been reported to modulate the ERK and Akt signaling pathways [45].
Akt and the androgen receptor (AR) have been shown to cooperate in cancer progression [46] and it has been suggested that Akt may directly phosphorylate AR inhibiting AR transactivation, and blocking AR-induced apoptosis [47]. Based on our results the TMEFF2 ectodomain promotes Akt phosphorylation and thus negatively regulates AR activation, which in turn regulates TMEFF2 expression [15,48], providing an additional link between Akt and TMEFF2 mediated by the AR.
In conclusion, our results provide evidence of a role for TMEFF2 on Akt and ERK signaling pathways that may be relevant to prostate cancer tumorigenesis.
Acknowledgement
We greatly acknowledge Greg Tipton’s technical help and Tom Green, Dr. Lee and Dr. Asch for critical reading of the manuscript.
This work was supported in part by a grant from the National Cancer Institute (1R15CA155873). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013 doi: 10.1038/onc.2013.206. doi: 10.1038/onc.2013.206. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C, Abate-Shen C. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–64. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant S. Cotargeting survival signaling pathways in cancer. J Clin Invest. 2008;118:3003–6. doi: 10.1172/JCI36898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D’Assoro AB, Salisbury JL, Mazzarino MC, Stivala F, Libra M. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–79. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36:320–8. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt crosstalk. J Biol Chem. 2002;277:31099–106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 8.Bartholomeusz C, Gonzalez-Angulo AM, Liu P, Hayashi N, Lluch A, Ferrer-Lozano J, Hortobágyi GN. High ERK protein expression levels correlate with shorter survival in triple-negative breast cancer patients. Oncologist. 2012;17:766–74. doi: 10.1634/theoncologist.2011-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gee JM, Robertson JF, Ellis IO, Nicholson R. Phosphorylation of ERK1/2 mitogen-activated protein kinase is associated with poor response to anti-hormonal therapy and decreased patient survival in clinical breast cancer. Int J Cancer. 2001;95:247–54. doi: 10.1002/1097-0215(20010720)95:4<247::aid-ijc1042>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Kress TR, Raabe T, Feller SM. High Erk activity suppresses expression of the cell cycle inhibitor p27Kip1 in colorectal cancer cells. Cell Commun Signal. 2010;8:1. doi: 10.1186/1478-811X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, Kreisberg JI. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–71. [PubMed] [Google Scholar]
- 12.Heanue TA, Pachnis V. Expression profiling the developing mammalian enteric nervous system identifies marker and candidate Hirschsprung disease genes. Proc Natl Acad Sci USA. 2006;103:6919–24. doi: 10.1073/pnas.0602152103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchida T, Wada K, Akamatsu T, Yonezawa M, Noguchi H, Mizoguchi A, Kasuga M, Sakamoto C. A novel epidermal growth factor-like molecule containing two follistatin modules stimulates tyrosine phosphorylation of erbB-4 in MKN28 gastric cancer cells. Biochem Biophys Res Commun. 1999;266:593–602. doi: 10.1006/bbrc.1999.1873. [DOI] [PubMed] [Google Scholar]
- 14.Afar DE, Bhaskar V, Ibsen E, Breinberg D, Henshall SM, Kench JG, Drobnjak M, Powers R, Wong M, Evangelista F, O’Hara C, Powers D, DuBridge RB, Caras I, Winter R, Anderson T, Solvason N, Stricker PD, Cordon-Cardo C, Scher HI, Grygiel JJ, Sutherland RL, Murray R, Ramakrishnan V, Law DA. Preclinical validation of anti-TMEFF2-auristatin E-conjugated antibodies in the treatment of prostate cancer. Mol Cancer Ther. 2004;3:921–32. [PubMed] [Google Scholar]
- 15.Gery S, Sawyers CL, Agus DB, Said JW, Koeffler HP. TMEFF2 is an androgen-regulated gene exhibiting antiproliferative effects in prostate cancer cells. Oncogene. 2002;21:4739–46. doi: 10.1038/sj.onc.1205142. [DOI] [PubMed] [Google Scholar]
- 16.Glynne-Jones E, Harper ME, Seery LT, James R, Anglin I, Morgan HE, Taylor KM, Gee JM, Nicholson RI. TENB2, a proteoglycan identified in prostate cancer that is associated with disease progression and androgen independence. Int J Cancer. 2001;94:178–84. doi: 10.1002/ijc.1450. [DOI] [PubMed] [Google Scholar]
- 17.Horie M, Mitsumoto Y, Kyushiki H, Kanemoto N, Watanabe A, Taniguchi Y, Nishino N, Okamoto T, Kondo M, Mori T, Noguchi K, Nakamura Y, Takahashi E, Tanigami A. Identification and characterization of TMEFF2, a novel survival factor for hippocampal and mesencephalic neurons. Genomics. 2000;67:146–52. doi: 10.1006/geno.2000.6228. [DOI] [PubMed] [Google Scholar]
- 18.Ali N, Knauper V. Phorbol ester-induced shedding of the prostate cancer marker transmembrane protein with epidermal growth factor and two follistatin motifs 2 is mediated by the disintegrin and metalloproteinase-17. J Biol Chem. 2007;282:37378–88. doi: 10.1074/jbc.M702170200. [DOI] [PubMed] [Google Scholar]
- 19.Lin H, Wada K, Yonezawa M, Shinoki K, Akamatsu T, Tsukui T, Sakamoto C. Tomoregulin ectodomain shedding by proinflammatory cytokines. Life Sci. 2003;73:1617–27. doi: 10.1016/s0024-3205(03)00514-9. [DOI] [PubMed] [Google Scholar]
- 20.Eib DW, Martens GJ. A novel transmembrane protein with epidermal growth factor and follistatin domains expressed in the hypothalamo-hypophysial axis of Xenopus laevis. J Neurochem. 1996;67:1047–55. doi: 10.1046/j.1471-4159.1996.67031047.x. [DOI] [PubMed] [Google Scholar]
- 21.Mohler JL, Morris TL, Ford OH, Alvey RF, Sakamoto C, Gregory CW. Identification of differentially expressed genes associated with androgen-independent growth of prostate cancer. Prostate. 2002;51:247–55. doi: 10.1002/pros.10086. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Overcash R, Green T, Hoffman D, Asch AS, Ruiz-Echevarria MJ. The tumor suppressor activity of the transmembrane protein with epidermal growth factor and two follistatin motifs 2 (TMEFF2) correlates with its ability to modulate sarcosine levels. J Biol Chem. 2011;286:16091–100. doi: 10.1074/jbc.M110.193805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green T, Chen X, Ryan S, Asch A, Ruiz-Echevarria MJ. TMEFF2 and SARDH cooperate to modulate one carbon metabolism and invasion of prostate cancer cells. Prostate. 2013 doi: 10.1002/pros.22706. doi: 10.1002/pros.22706. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elahi A, Zhang L, Yeatman TJ, Gery S, Sebti S, Shibata D. HPP1-mediated tumor suppression requires activation of STAT1 pathways. Int J Cancer. 2008;122:1567–72. doi: 10.1002/ijc.23202. [DOI] [PubMed] [Google Scholar]
- 25.Horie M, Mitsumoto Y, Kyushiki H, Kanemoto N, Watanabe A, Taniguchi Y, Nishino N, Okamoto T, Kondo M, Mori T, Noguchi K, Nakamura Y, Takahashi E, Tanigami A. Identification and characterization of TMEFF2, a novel survival factor for hippocampal and mesencephalic neurons. Genomics. 2000;67:146–52. doi: 10.1006/geno.2000.6228. [DOI] [PubMed] [Google Scholar]
- 26.Lin K, Taylor JR, Wu TD, Gutierrez J, Elliott JM, Vernes JM, Koeppen H, Phillips HS, de Sauvage FJ, Meng YG. TMEFF2 is a PDGF-AA binding protein with methylation-associated gene silencing in multiple cancer types including glioma. PLoS One. 2011;6:e18608. doi: 10.1371/journal.pone.0018608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quayle SN, Sadar MD. A truncated isoform of TMEFF2 encodes a secreted protein in prostate cancer cells. Genomics. 2006;87:633–7. doi: 10.1016/j.ygeno.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Park YH, Seo SY, Ha M, Ku JH, Kim HH, Kwak C. Inhibition of prostate cancer using RNA interference-directed knockdown of platelet-derived growth factor receptor. Urology. 2011;77:1509. doi: 10.1016/j.urology.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 29.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem. 2002;277:31099–106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 30.Reusch HP, Zimmermann S, Schaefer M, Paul M, Moelling K. Regulation of Raf by Akt controls growth and differentiation in vascular smooth muscle cells. J Biol Chem. 2001;276:33630–7. doi: 10.1074/jbc.M105322200. [DOI] [PubMed] [Google Scholar]
- 31.Chang C, Eggen BJ, Weinstein DC, Brivanlou AH. Regulation of nodal and BMP signaling by tomoregulin-1 (X7365) through novel mechanisms. Dev Biol. 2003;255:1–11. doi: 10.1016/s0012-1606(02)00075-1. [DOI] [PubMed] [Google Scholar]
- 32.Harms PW, Chang C. Tomoregulin-1 (TMEFF1) inhibits nodal signaling through direct binding to the nodal coreceptor Cripto. Genes Dev. 2003;17:2624–2629. doi: 10.1101/gad.1127703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boswell CA, Mundo EE, Zhang C, Stainton SL, Yu SF, Lacap JA, Mao W, Kozak KR, Fourie A, Polakis P, Khawli LA, Lin K. Differential effects of predosing on tumor and tissue uptake of an 111In-labeled anti-TENB2 antibody-drug conjugate. J Nucl Med. 2012;53:1454–61. doi: 10.2967/jnumed.112.103168. [DOI] [PubMed] [Google Scholar]
- 34.Zhao XY, Schneider D, Biroc SL, Parry R, Alicke B, Toy P, Xuan JA, Sakamoto C, Wada K, Schulze M, Muller-Tiemann B, Parry G, Dinter H. Targeting tomoregulin for radioimmunotherapy of prostate cancer. Cancer Res. 2005;65:2846–2853. doi: 10.1158/0008-5472.CAN-04-4019. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–4. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 36.Er EE, Mendoza MC, Mackey AM, Rameh LE, Blenis J. AKT Facilitates EGFR Trafficking and Degradation by Phosphorylating and Activating PIKfyve. Sci Signal. 2013;6:ra45. doi: 10.1126/scisignal.2004015. doi: 10.1126/scisignal.2004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chow LM, Baker SJ. PTEN function in normal and neoplastic growth. Cancer Lett. 2006;241:184–96. doi: 10.1016/j.canlet.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 38.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, Kreisberg JI. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–71. [PubMed] [Google Scholar]
- 39.Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S, Ghosh PM. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;64:5232–6. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 40.Graff JR, Konicek BW, McNulty AM, Wang Z, Houck K, Allen S, Paul JD, Hbaiu A, Goode RG, Sandusky GE, Vessella RL, Neubauer BL. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol Chem. 2000;275:24500–5. doi: 10.1074/jbc.M003145200. [DOI] [PubMed] [Google Scholar]
- 41.Deschênes-Simard X, Gaumont-Leclerc MF, Bourdeau V, Lessard F, Moiseeva O, Forest V, Igelmann S, Mallette FA, Saba-El-Leil MK, Meloche S, Saad F, Mes-Masson AM, Ferbeyre G. Tumor suppressor activity of the ERK/MAPK pathway by promoting selective protein degradation. Genes Dev. 2013;27:900–15. doi: 10.1101/gad.203984.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labeur M, Wölfel B, Panhuysen M, Stalla J, Stalla GK, Paez-Pereda M. TMEFF2: a new endogenous modulator of the CRH signaling in corticotroph cells. Exp Clin Endocrinol Diabetes. 2010:118–P13. DOI: 10.1055/s-0030-1267015. [Google Scholar]
- 43.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 44.Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitam Horm. 2008;79:1–44. doi: 10.1016/S0083-6729(08)00401-9. [DOI] [PubMed] [Google Scholar]
- 45.Lee SJ, Lee YS, Seo KW, Bae JU, Kim GH, Park SY, Kim CD. Homocysteine enhances MMP-9 production in murine macrophages via ERK and Akt signaling pathways. Toxicol Appl Pharmacol. 2012;260:89–94. doi: 10.1016/j.taap.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 46.Xin L, Teitell MA, Lawson DA, Kwon A, Mellinghoff IK, Witte ON. Progression of prostate cancer by synergy of AKT with genotropic and nongenotropic actions of the androgen receptor. Proc Natl Acad Sci U S A. 2006;103:7789–94. doi: 10.1073/pnas.0602567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin HK, Yeh S, Kang HY, Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc Natl Acad Sci USA. 2001;98:7200–5. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overcash R, Chappell V, Green T, Geyer C, Asch A, Ruiz-Echevarría M. Androgen signaling promotes translation of TMEFF2 in prostate cancer cells via phosphorylation of the alpha subunit of the translation initiation factor 2 (eIF2α) PLoS One. 2013:e55257. doi: 10.1371/journal.pone.0055257. [DOI] [PMC free article] [PubMed] [Google Scholar]


