Abstract
Ongoing ovarian cancer screening trials are investigating the efficacy of a two-step screening strategy using currently available blood and imaging tests (CA125 and transvaginal sonography [TVS]). Concurrently, efforts to develop new biomarkers and imaging tests seek to improve screening performance beyond its current lim its. This study estimates the mortality reduction, years of life saved and cost-effectiveness achievable by annual multimodal screening using rising CA125 to select women for TVS, and predicts improvements achievable by replacing currently available screening tests with hypothetical counter parts with better performance characteristics. An existing stochastic micro-simulation model is refined and used to screen a virtual cohort of 1 million women from age 45 to 85. Each woman is assigned a detailed disease course and screening results timeline. The pre-clinical behavior of CA125 and TVS is simulated using empirical data derived from clinical trials. Simulations in which the disease incidence and performance characteristics of the screening tests are independently varied are performed in order to evaluate the impact of these factors on overall screening performance and costs. Our results demonstrate that when applied to women at average risk, annual screening using rising CA125 to select women for TVS achieves modest mortality reduction (~13%) and falls with in currently accepted cost-effectiveness guidelines. Screening outcomes are relatively insensitive to second-line test performance and costs. Identification of a first line test that perform s substantially better than CA125 and has similar costs is required in order for screening to reduce ovarian mortality by at least 25% and be reasonably cost-effective.
Introduction
The impact of epithelial ovarian cancer (EOC) screening using these rum tumor marker CA125 and transvaginal sonography (TVS) is being evaluated in two large efficacy trials. The Prostate Lung Colon and Ovary (PLCO) trial in the US failed to demonstrate a reduction in EOC specific mortality with annual screening using CA125 interpreted using a single threshold rule and TVS concurrently [1]. The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) compares two screening approaches to no screening [2]. In its single-modality imaging arm, transvaginal sonography (TVS) alone is used annually; in its multimodal arm, rising CA125 is used as a 1st line screen to select women for a 2nd line screen with TVS. The multimodal arm uses the longitudinal Risk of Ovarian Cancer Algorithm (ROCA) [3], relying on call-backs for repeat CA125 measurement to confirm exponential rise. Women with both rising CA125 and abnormal imaging results are referred for surgical consult. Early results of the trial suggest that the CA125-driven multimodal strategy outperforms the single-modality strategy using TVS. The sensitivity, specificity, and positive predictive value (PPV) for all primary invasive EOCs identified at the prevalence screen in the UKCTOCS were 89.5%, 99.8%, and 35.1% respectively for the multimodal strategy, and 75.0%, 98.2%, and 2.8% respectively for TVS alone [ 2]. Promising results using this same multimodal strategy were also recently report by Lu et al [4]. While promising, it is not yet known whether the sequential multimodal screening strategy will reduce mortality cost-effectively.
Efforts are underway to identify biomarkers and imaging strategies that perform better than currently available tests. Clinical studies have evaluated the potential of several serum marker candidates for EOC screening [5, 6], and while some have demonstrated a detection lead-time of about a year in retrospective validation studies [7, 8], none appear to perform better than CA125 either alone or as a panel. Motivated by advances in molecular biology, nanotechnology and imaging technology molecular imaging strategies are rapidly approaching the resolution necessary for EOC early detection [9]. Molecularly targeted microbubble ultrasound contrast agents are a particularly appealing approach for EOC screening because they can utilize conventional ultrasound technology, a widely available and relatively in expensive ovarian imaging method. Small animal imaging experiments demonstrate that microbubbles targeted to VEGFR2 expressed by activated endothelial cell substantially improve ultrasound signal intensity and resolution [9–11]. VEGFR2 targeted microbubbles are currently undergoing pilot testing in patients.
Knowledge about the relative effectiveness and cost-effectiveness of both conventional and new screening tools will help guide strategies for technology development and deployment toward large population EOC screening interventions, which are costly and resource intensive. Simulation modeling can be an effective and efficient method for evaluating alternative screening strategies, allowing comparison of the performance of many screening strategies under various assumptions about disease behavior and screening test performance. Modeling has been used to predict mortality reduction and cost-effectiveness of multimodal EOC screening using CA125 and TVS as first and second line tests respectively [12, 13].
It is unknown what performance characteristics and cost para meters will be required of new EOC screening tests in order to have a significant impact on EOC mortality and be cost-effective. Here we refine and extend a previously developed [14] and validated [13] EOC screening microsimulation model and use the model to address these important questions. Our results confirm earlier observations that currently available screening tests are likely to have only a modest impact on EOC mortality but are reasonably cost-effective. We further demonstrate that the performance of a multimodal EOC screening strategy is largely dependent on the characteristics of the first line test. Identification of a first line screening test that performs substantially better than CA125 and costs no more than $95 will be required in order to have a major impact on EOC mortality and be reasonably cost-effective when performed annually in average-risk post-menopausal women.
Methods
Overview
A stochastic microsimulation is used to estimate the mortality reduction, years of life saved (YLS) and cost-effectiveness of EOC screening protocols in a hypothetical cohort of 1 million women beginning at age 45 and continuing through age 85. Four distinct components comprise the model to represent 1) natural history for women with and without EOC (Natural History Component), 2) EOC screening protocols and test results (Screening Component), 3) EOC survival adjusted for age, stage, and histology at diagnosis (Survival Component), and 4) costs associated with EOC screening, diagnosis and treatment including costs incurred as a result of false positive screens (Cost Component). The model generates disease related outcomes and costs for women in the absence of screening, then superimposes a screening strategy on the cohort and calculates the impact of screening on survival and costs. Mortality reduction is measured as the decrease in deaths due to EOC divided by the EOC deaths that would have occurred in the absence of screening among women with disease present during the screening period. YLS are reported as difference in age of death with and without screening. All costs including screening, diagnostic and treatment costs are reported in 2010 US dollars, and all future costs and benefits are discounted back to 2010 using a 3% rate of return. The model calculates YLS and costs for each woman and reports both cumulatively for the entire screening cohort (additional information Supplemental Methods Sec 1).
Empirical data obtained or generated from experimental analysis, public use files or published literature provides input parameters for the model (Table 1). Where empirical data are sparse or unavailable (e.g. novel and hypothetical screening modalities) we apply our best estimates and test their robustness using sensitivity analyses. The model is stochastic in that it includes a component of randomness to represent “luck” for an individual woman. Below we highlight key features and input data for each component comprising the model.
Table 1.
Model Inputs
| Input Parameter | Disease Group | Baseline Assumption | Source |
|---|---|---|---|
| Natural History Component | |||
| Age at death from competing cause | All | N/A | Vital Statistics of the US [16] |
| Incidence and age at clinical diagnosis for cases | Cases Latent | N/A | SEER [15] |
| Incidence and age at clinical diagnosis for benign disease | Benign | N/A | Katsube [17] |
| Tumor characteristics (stage, histology and grade at clinical diagnosis) | Cases Latent | N/A | SEER [15] |
| Malignant disease duration and stage lengths | Cases Latent | See Table 2 | |
| Benign Disease duration | Benign | 9 years | PLCO [26] |
|
| |||
| Screening Component | |||
| Screening frequency | All | Annual (Age 45–85) | N/A |
| CA125 sensitivity | Cases Benign | See Figure 2 | CARET [7] |
| CA125 specificity | Healthy | 95% | Defined by the screening algorithm |
| Hypothetical Marker sensitivity | Cases Benign | 2X sensitivity of CA125 | |
| Hypothetical Marker specificity | Healthy | 95% | |
| TVS sensitivity | Cases | 63% | PLCO [26] |
| TVS specificity | Healthy | 97% | PLCO [26] |
| Hypothetical Imaging sensitivity | Cases | 90% | |
| Hypothetical Imaging specificity | Healthy | 97% | |
|
| |||
| Survival Component | |||
| EOC survival contingent on age and tumor stage, histology and grade at diagnosis | Cases | See Figure S4 | SEER [19] |
|
| |||
| Cost Component* | |||
| CA125 test cost | All | $31 | Havrilesky [12] |
| Hypothetical Marker test cost | All | $210 | |
| TVS test cost | All | $111 | Havrilesky [12] |
| Hypothetical Imaging test cost | All | $750 | |
| Laparoscopy with BSO | Benign Healthy | $4206 | Havrilesky [12] |
| EOC Treatment Costs | Cases | Yabroff [21] | |
| Initial year | Stage I | $36,671.66 | |
| Stage II | $50,718.96 | ||
| Stages III/IV | $70,452.02 | ||
| Continuing Care | All stages | $4,712.30 | |
| Last year of life** | Stage I | $27,523.12 | |
| Stage II | $46,437.70 | ||
| Stages III/IV | $69,313.90 | ||
All costs were adjusted to 2010 US dollars.
The treatment cost differential for the last year of life between early and late stage diagnoses arises by the way Yabroff et al. allocated treatment costs for patients surviving less than 24 months past diagnosis. Costs for the last 12 months of this period were allocated as last year of life costs, and the remainder considered initial year treatment costs. Our model treats cost allocation for such patients in a similar fashion. Allocation of death related costs may increase initial year treatment costs for women who die within 12 months of diagnosis.
Natural History Component
The Natural History component generates a cohort of women with ages at death and incidence of EOC, using competing risks of developing EOC or dying cancer-free that are derived from SEER [15] and the US Vital Statistics Report [16]. The generated cohort is divided into 4 groups: women with symptomatic EOC (Cases), women with non-malignant ovarian tumors (Benigns), women without ovarian disease (Healthies) and women who develop an occult EOC but die from competing causes prior to clinical diagnosis (Latents). For EOC cases and latents, tumor characteristics including age at clinical diagnosis, stage, histology and grade are derived to be consistent with US nationwide incidence rates reported by SEER [15]. The date of cancer inception, defined as the earliest time when the tumor is pathologically malignant, is calculated backwards from the date of clinical diagnosis using estimates of disease duration and stage lengths obtained by surveying gynecological oncologists (Table 2).
Table 2.
Mean disease duration and stage lengths in years for EOC cases by histology and grade*
| Low Grade | |||||
|---|---|---|---|---|---|
| Stage Length | Serous | Mucinous | Endometrioid | Adenocarcinoma NOS |
|
|
| |||||
| Stage 1 | 1.8 | 2.2 | 1.7 | 2.3 | |
| Stage 2 | 0.9 | 0.2 | 0.9 | 0.2 | |
| Stage 3 | 1.4 | 1.4 | 1.4 | 1.5 | |
| Stage 4 | 0.5 | 0.5 | 0.5 | 0.5 | |
| Total Disease | |||||
| Duration** | 4.6 | 4.3 | 4.5 | 4.5 | |
|
| |||||
| High Grade | |||||
| Stage Length | Serous | Mucinous | Endometrioid | Adenocarcinoma NOS |
Clear Cell |
|
| |||||
| Stage 1 | 1.0 | 0.6 | 1.0 | 1.5 | 0.9 |
| Stage 2 | 0.4 | 0.6 | 0.6 | 0.2 | 0.3 |
| Stage 3 | 0.8 | 0.8 | 0.8 | 0.9 | 0.8 |
| Stage 4 | 0.2 | 0.3 | 0.3 | 0.2 | 0.2 |
| Total Disease | |||||
| Duration ** | 2.5 | 2.3 | 2.6 | 2.8 | 2.2 |
|
| |||||
Calculated using physician’s estimates of EOC progression rates in the absence of treatment (detailed in Supplementary Methods Sec 2.d.)
Total disease duration was modeled using the lognormal distribution with 0.5 year standard deviation, and then divided into stages using the Dirichlet distribution to yield stages with the above average durations.
Benign disease distributions are derived from Katsube et al. [17], who report the incidence for primary ovarian tumors (all histologies) identified among women in the Denver Standard Metropolitan Statistical Area over a 10 year period. Similar to EOC cases, age at benign disease inception is back -calculated from age at clinical diagnosis assuming mean disease duration of 9 years, an estimate calculated from prevalence and incidence rates of benign ovarian tumors identified among participants in the PLCO Trial (see Supplemental Methods Sec 2.f).
Screening Component
The screening component generates for each woman a complete timeline of screening test results, superimposes an EOC screening protocol onto the cohort, and calculates shifts in disease detection from late to early stage attributable to screening. Two types of screening tests are modeled: serum biomarkers and imaging. As a base case scenario we employ a two-step screening strategy using CA125 to select women for TVS (CA125+TVS). Women are screened annually from age 45 through ages 85. CA125 test results are interpreted using the Parametric Empirical Bayes (PEB) rule, a longitudinal algorithm previously described [18]. The PEB uses parametric empirical Bayes statistical theory to generate person-specific positivity thresholds that depend on the screening history of each individual while holding the false positive (FP) rate constant across all of the screened population. Any predefined specificity level can be used. We chose the 95% specificity level for our analysis, meaning that at each screen 5% of healthy women are expected to receive a positive test result.
For EOC cases and latents, CA125 test results are assigned based on a sensitivity function that provides an estimate for the probability of a positive PEB CA125 test result at selected time intervals prior to clinical diagnosis (Figure 1). The sensitivity function is derived from analysis of CA125 levels in pre-clinical samples collected from ovarian cases identified among CARET trial participants (see Supplementary Methods Sec 3.b). Sensitivity increases with proximity to diagnosis; the baseline 5% positive test threshold (corresponding to 95% specificity) is applied at times remote from diagnosis when the function falls below this threshold. A sensitivity function for benign ovarian tumors is derived by scaling the EOC case function by a factor of 0.15, an adjustment that closely approximates the incidence of positive PEB CA125 tests attributable to benign tumors identified among 328 participants in a local EOC screening protocol (data not shown). The imaging component of the model assumes that TVS is equally sensitive throughout the disease duration once CA125 values have elevated above the positivity threshold. Because TVS is used as a second line screen, no assumptions on the performance of TVS are made when CA125 is not elevated. We chose 63% and 97% as baseline input parameters for TVS sensitivity and specificity respectively based on data from the PLCO trial as this represents the current best estimate for the performance of community-based sonography; we also perform sensitivity analysis across a range of assumptions regarding the sensitivity of TVS (Table S1). We assume that all women with both elevated markers and abnormal imaging undergo immediate diagnostic surgery. Requiring positivity for both tests yields a probability of surgical referral among healthy women of 1.5/1000 at each screen.
Figure 1. Ovarian cancer case sensitivity function for PEB CA125 and HM by time prior to clinical diagnosis*.

*The CA125 sensitivity function is derived by applying the PEB algorithm to serial CA125 levels measured in pre-clinical samples obtained from Carotene and Retinol Efficacy Trial Participants (see Supplemental Methods Sec 3). The baseline 5% positive test result corresponding to 95% specificity is applied at times remote from diagnosis when the function falls below this threshold. The sensitivity function for HM was derived by doubling both the sensitivity and lead-time of CA125. The 95% CI for each piece of the CA125 sensitivity function is estimated using the exact binomial method.
A laparoscopic bilateral salpingo-oophorectomy (BSO) is perform ed in women referred for surgery with benign ovarian tumors or normal ovaries at the time of the screen (FP screen); risk of developing EOC following BSO is assumed to be zero. EOC cases are assumed to undergo appropriate EOC surgery followed by other clinically indicated therapy. The date at screen diagnosis is defined as time of the positive screen after disease inception. Whenever the date of screen detection precedes the date at clinical diagnosis, the tumor stage at screen diagnosis is calculated by applying tumor specific estimates of stage durations (T able 2) to the date of tum or inception. A stage shift is assumed to occur whenever a tum or destined to be diagnosed clinically in late stage (III or IV) is detected in early stage (I or II) by screening. For each screen-detected case we calculate the lead-time as the time interval between the first true positive screen and the date when clinical diagnosis would have occurred in the absence of screening. The average lead-time among screen-detected cancers is reported as an output from the model.
Performance characteristics of hypothetical screening modalities
To estimate the impact of potential improvements in EOC screening tools we simulated a hypothetical biomarker (HM) with 2-fold greater sensitivity and lead-time than that of CA125 (Figure 1). These performance characteristics are arbitrary and intended to represent an optimistic appraisal of what is potentially achievable over the next decade with new biomarker discovery efforts. A sensitivity function of the HM for benign disease is derived by scaling the HM sensitivity function for EOC cases by 0.15. We also model the performance of a hypothetical imaging test (HI) that achieves a 50% improvement in sensitivity relative to TVS (from roughly 60% to 90%) while maintaining high specificity (97%). The 90% sensitivity for HI is assigned uniformly at all times following disease inception, representing a significant improvement in imaging resolution especially when used as a confirmatory test following a HM with 2X longer lead time and sensitivity compared to CA125.
Survival Component
The Survival component generates a date of death for all EOC cases detected clinically or by screening taking into account disease-specific survival and competing cause mortality. EOC survival curves extending out to 15 years post diagnosis specific to age at diagnosis (in 10 year intervals), stage (I, II, and stag es III and IV combined) and grade (low vs. high) were estimated from SEER [19] and are used to generate survival after EOC diagnosis (Figure S4). Age at death is set to the earliest of competing risk and disease specific mortalities. Cases experiencing a FP screening result and BSO prior to tumor inception are assumed to be protected from an EOC diagnosis. Women alive 15 years after EOC diagnosis are assumed to be cured with age of death determined by competing risks. We include a 0.1% risk of death associated with surgical investigation of a FP screening result [20]. For established malignancies, surgical deaths are accounted for in the SEER survival curves [19]. We assume all women die by age 110. The overall impact of screening on EOC-specific survival is reported as the total number of YLS and mortality reduction (the percentage of women saved from dying of EOC) across the entire cohort.
Cost Component
The Cost component calculates the cost of the screening program and interventions associated with EOC diagnosis and treat ment under both screening and non-screening scenarios. Three categories of costs are considered: 1) cost associated with the screening tests themselves, 2) costs associated with surgical evaluation of positive screens and 3) cancer treatment costs. Medicare reimbursement rates taken from prior reports and based on Medicare claims data are used for input cost parameters wherever possible (Table 1).
EOC treatment costs are based on Yabroff et al. [21] who estimate net EOC treatment costs by stage and phase of disease using 1999–2003 Medicare Part A and B claims data on 1,647 EOC cases identified from SEER and matched controls. Treatment costs are divided into three components: 1) costs incurred in the first year of diagnosis (including primary surgery costs), 2) costs incurred in the final year of life prior to death and 3) continuing care costs for all years between diagnosis and death. The higher initial and terminal treatment costs for cases diagnosed at stage III/IV reflect the additional expenses associated with treating more advanced disease. We assume that a woman surviving 15 years post diagnosis is cured and incurs no additional costs. All costs are reported in 2010 dollars and future costs and YLS are discounted to 2010. The cost-effectiveness of the screening program is calculated as the net cost of screening (screening costs –saving in treatment costs due to early dis ease detection) divided by the YLS attributable to screening.
The characteristics and costs of hypothetical screening tests capable of achieving the defined performance parameters are largely unknown. For our base case analysis we assume the HM and HI cost roughly 7X their current counterparts (CA125 and TVS) yielding a cost of $210 for HM and $750 for HI. The estimated cost for HM is roughly equivalent to current charges for HE4, a new FDA-approved EOC serum marker. The cost for HI is consistent with authors’ estimated lowest possible charge for targeted microbubble contrast agents when used in conjunction with a TVS screen. We also report the overall cost effectiveness of implementing these tests across a range of assumptions of screening test costs.
Results
Mortality Reduction
Mortality reduction was estimated for four different annual multimodality screening strategies under base case assumptions (Table 3). Screening using rising CA125 followed by TVS achieves a mortality reduction of 13%. Substituting HI for TVS as the 2nd line screen improves mortality reduction only modestly to 15%. Greater mortality reduction (25%) is achieved by using HM in place of CA125 as the 1st line screen prior to TVS. Separately implementing the sensitivity and the lead-time improvement associated with HM leads to a mortality reduction of 19% and 17% respectively (data not shown). Screening using both HM and HI achieves the greatest mortality reduction at 30%.
Table 3.
Mortality reduction, years of life saved and cost-effectiveness of EOC screening strategies.
| 1st line screen | None | CA125 | CA125 | HM | HM | |
|---|---|---|---|---|---|---|
| 2nd line screen | None | TVS | HI | TVS | HI | |
| Effectiveness | ||||||
| Stage at Dx: |
I | 18% | 34% | 39% | 57% 67% | |
| II | 11% | 13% | 13% | 14% 12% | ||
| III | 32% | 30% | 28% | 21% 16% | ||
| IV | 39% | 23% | 19% | 8% | 4% | |
| Stage shifts (late to early)* | N/A | 17% | 22% | 40% 48% | ||
| Total Mortality Reduction | N/A | 13% | 15% | 25% | 30% | |
| – Due to early detection of malignancy in cases | N/A | 9% | 11% | 21% 26% | ||
| – Due to BSO in cases prior to the onset of malignancy | N/A | 4% | 4% | 4% 4% | ||
| Lead time (per screen detected case) | N/A | 0.97 | 0.97 | 1.48 1.60 | ||
| YLS (per screen detected case) | N/A | 1.68 | 1.61 | 2.09 2.21 | ||
|
| ||||||
| Cost | ||||||
| Cost-effectiveness | N/A | $88,993 | $124,376 | $205,248$ | 191,441 | |
| Total cost (millions) | $865 | $1,741 | $2,397 | $5,401 | $6,068 | |
| Screening test cost (total) | $0 | $742 | $1,396 | $4,373 | $5,039 | |
| Laparoscopy cost: | ||||||
| – FP: healthy women | $0 | $128 | $128 | $128 | $128 | |
| – FP: benign disease | $81 | $84 | $83 | $86 | $83 | |
| Cancer treatment cost (TP) | $784 | $787 | $791 | $814 | $819 | |
Minor inconsistencies between the proportion of women experiencing a screening related stage shift and changes in stage distribution relative to the ne screen control group are attributable to ovarian cancer prevention in a small number of screened women who undergo BSO prior to tumor development as a consequence of a false positive screen. This prevention reduces the number of ovarian cancer cases overall and the denominator used in the stage shift and distribution calculations.
Because we assume that a positive screen necessitates BSO even in the absence of EOC, surgical evaluation of FP screens can potentially prevent EOC in women destined to develop EOC later. Consequently we separately evaluated the proportion of the overall mortality reduction attributable to early detection of established disease versus disease prevention from FP surgeries. The proportion of overall mortality reduction attributable to early detection of established disease increases from roughly 70% for CA125+ TVS to 86% for the best performing screening strategy of HI + HM. Absolute mortality reduction attributable to disease prevention is 4% across all four screening scenarios as the overall specificity (and hence FP rate) of the screening programs are identical.
Cost-Effectiveness
Annual multimodal screening using CA125+TVS yields a cost of $88,993 per YLS (Table 3). Implementing HI in place of TVS at a cost of $750 per test saves more lives but is less efficient costing $124,376 per YLS. Screening using HM at a cost of $210 has a major impact on mortality reduction but substantially increases cost regardless of the second line test. Overall cost-effectiveness of the HM+TVS and HM+HI strategy is $205,248 and $191,441 respectively. HI is more cost-effective than TVS because cost savings from the increase in YLS exceed the additional costs associated with the test.
Our assumptions about the costs of the hypothetical screening tests affect the overall cost-effectiveness of the screening strategies (Figure 2, Figure S4). The overall cost-effectiveness of replacing TVS by HI was $71,772 for a HI test with a cost equivalent to that of TVS ($111), increasing by $9,128 YLS for each fold increase in test cost. Replacing CA125 by HM results in cost per YLS of $40,926 for a HM with a cost equivalent to that of CA125 ($31), increasing by $28,458 for every fold increase in the test cost. The cost per YLS of HM+TVS remains below the generally acceptable threshold of $100,000 provided the cost of HM is $95 or less. The cost per YLS estimated for CA125+HI strategy remains below the $100,000 YLS threshold provided the cost of HI is no greater $454 per test.
Figure 2. Cost-effectiveness of screening using hypothetical screening tests by screening test cost*.
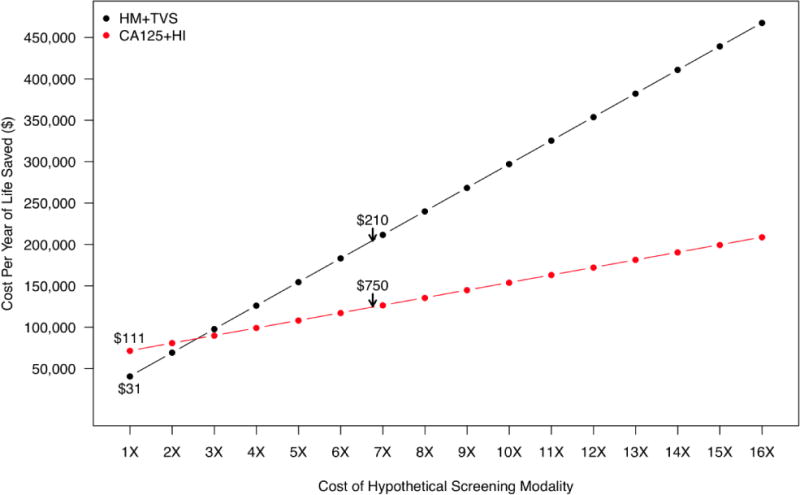
*Hypothetical screening test costs are presented as fold cost relative to the cost of CA125 ($31) and TVS ($111). $210 and $750 correspond to the base-case assumptions for the cost associated with HM and HI respectively.
Cost-effectiveness is improved by screening populations at increased risk. We evaluated the cost-effectiveness of annual screening using each strategy when applied to populations with EOC incidence rates of 2X, 4X, and 8X relative to the general population (Figure 3, Table S2). As expected, the cost-effectiveness of all the screening programs improves in proportion to the increase in disease incidence because, as more women are screen -detected in elevated risk populations (increased pre-test likelihood), the increase in number of YLS exceeds the increase in screening costs. The mortality reduction for a given screening program is unaffected by disease incidence because the performance characteristics of the screening tests do not change.
Figure 3. Cost-effectiveness of ovarian cancer screening strategies in populations at increased risk*.
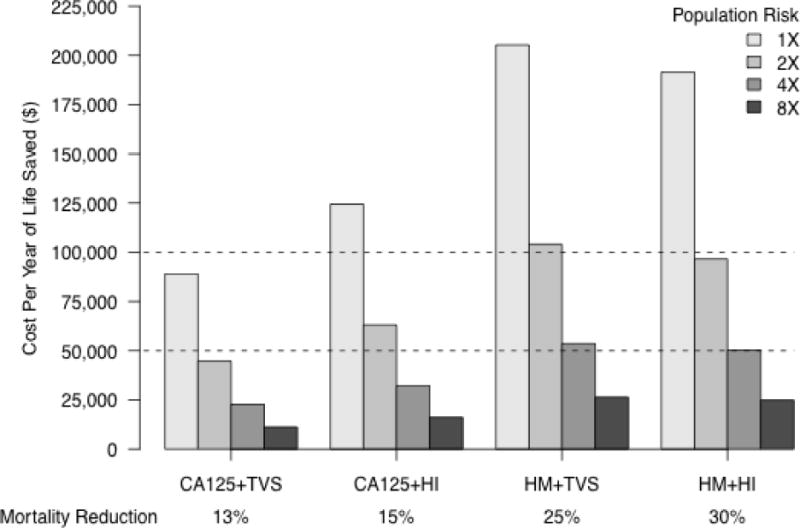
*We evaluated the cost-effectiveness of all four bi-modal screening strategies when applied to populations with increased ovarian cancer incidence rates of 2X, 4X, and 8X relative to the general population.
Screening Frequency
Screening frequency affects both mortality reduction and cost effectiveness. We modeled the interaction between screening frequency and costs for each of the four screening strategies when applied to an average risk population and compared the results across strategies (Figure 4, Table S3). As expected, mortality reduction and cost per YLS both increase for all screening strategies as screening intervals are shortened. CA125-based screening strategies do not achieve a mortality reduction of 30% even when performed semi-annually. Screening using HM+HI at least annually or HM+TVS semi-annually can achieve this target. However, HM-based screening strategies are relatively efficient due to cost of the HM which is applied at every screen.
Figure 4. Impact of screening frequency on mortality reduction and cost-effectiveness of ovarian cancer screening strategies*.

*Mortality reduction and cost-effectiveness for screening intervals of 6, 18, and 24 months are shown in addition to the base case assumption of 12 months, for each of the four screening scenarios. All other parameters were held constant.
Model Variability and Reliability
We evaluated variability in model output by conducting 100 model runs of the CA125 + TVS screening strategy using independent random number streams while holding all model parameters constant. In this analysis, the average [min, max] percent mortality reduction, YLS per screen detected case, and cost per YLS was 12.5% [11.9%, 13.3%], 0.64 [0.58, 0.70], and $87,664 [$80,072, $96,417] respectively (Table S4). This variability is attributable to the stochastic (i.e., random) generation of women’s natural history using empirically based random distributions. When different screening strategies are compared the stochastic element of the model is held constant, so that each strategy is evaluated using an identical cohort of women in order to make a level comparison.
We also validated the model for consistency. External consistency validation was conducted using boundary conditions of the various input parameters for which model outcomes could be directly calculated external to the model. Internal validations were conducted by verifying that relationships between model outputs (such as “cumulative treatment costs = cumulative survival * survival dependent treatment costs”) met expected relations (Table S5 and Supplemental Methods Sec 11).
Sensitivity Analyses
Sensitivity analysis was performed to evaluate robustness of our results to halving and doubling input parameters used in the base case (Table 4), particularly for those with limited available empirical data such as disease progression which is not directly observable in humans so that our estimates of EOC stage lengths and disease duration cannot be directly verified.
Table 4.
Impact of varying input parameter on mortality reduction and cost-effectiveness of annual screening using CA125+TVS
| Disease duration (Stage I malignant) |
.5X | 1X | 2X |
|---|---|---|---|
| Mortality reduction | 11% | 13% | 13% |
| Cost-effectiveness | $98,018 | $88,993 | $85,556 |
|
| |||
| TVS sensitivity | 31.5% | 63% | 99.9% |
| Mortality reduction | 8% | 13% | 16% |
| Cost-effectiveness | $139,779 | $88,993 | $67,605 |
|
| |||
| FP Rate (TVS specificity) | 1.5% (98.5) | 3% (97%) | 6% (94%) |
| Mortality reduction | 11% | 13% | 15% |
| Cost-effectiveness | $91,763 | $88,993 | $83,725 |
Doubling our assumptions about the duration of stage I disease does not change estimated mortality reduction and has only a minor impact on cost-effectiveness for screening using CA125+TVS. This is attributable to the fact that the empirically derived CA125 sensitivity function is conditional on time prior to clinical diagnosis and not on imputed stage at the time of the screening test. Because CA125 levels begin to rise appreciably only between one to two years prior to clinical diagnosis (Figure 2) they are relatively insensitive to the duration of early staged disease. Stage I disease duration has a more significant impact on HM driven screening strategies because of the longer lead-time attributable to HM. For example for the HM+TVS screening strategy doubling Stage I disease duration increases mortality reduction from 25% to 28% and improves cost per YLS from $205,248 to $189,532.
Although empirically derived our base-case assumptions about the performance CA125 prior to clinical diagnosis are imprecise and may either under or over-estimate the true sensitivity of CA125. Mortality reduction of 8% and 20% is achieved when the lower and upper bounds of the 95% confidence intervals for the CA125 sensitivity function are used as input parameters for CA125 performance respectively (see Table S1 Supplemental Materials)
Changes in our assumptions about the performance of TVS considerably impact both mortality reduction and cost-effectiveness. Assigning TVS near perfect sensitivity of 99.9% increases mortality reduction to 16% and reduces cost per YLS to $67,605. Importantly the 16% mortality reduction associated with 99.9% sensitivity analysis for TVS also defines the upper bounds of what is potentially achievable for a HI test when used as follow-up to an elevated CA125. Lowering the specificity of TVS to 94% (doubling the FP rate to 6%) increases mortality reduction from 13% to 15%, and improves cost-effectiveness because it lead s to more diagnostic surgeries in women destined to develop cancer later on.
Discussion
We used an updated microsimulation model to estimate the potential benefits of EOC screening. Simulation of annual screening using rising CA125 to select women for TVS predicts mortality reduction of approximately 13% at a cost of $89,000 per YLS. Semi-annual screening increases the mortality reduction to roughly 20% but is less cost-effective at $117,350 per YLS. The mortality reduction we identified is consistent with Havrilesky et al. who, using a Markov transition state model, estimated a mortality reduction of 14.7% for an annual sequential screening strategy when applied to a post-menopausal population. Mortality reduction estimated by that model fell to 10.9% when the authors accounted for variability in EOC aggressivene ss. Combined these data suggest the potential effectiveness of EOC screening using currently available tools is likely to be modest.
The updated model incorporates new functionality and is more empirically driven than that used in earlier work. The natural history component has been expanded to account for heterogeneity in EOC behavior by incorporating representations of disease duration and survival broken down by tum or histology and grade. We now also use empiric data obtained from analysis of CA125 levels in pre-clinical blood samples rather than a simple exponential model to characterize the trajectory of CA125 prior to diagnosis. These data demonstrate initial elevation in CA125 levels between 1–2 years prior to clinical diagnosis and provide a more realistic approximation of the potential benefit of screening using CA125. In our earlier report we estimated an average lead time for annual CA125-based screening of roughly 28 months which is too optimistic and likely explains the more significant mortality reduction (31%) reported earlier [14].
Our assumptions regarding EOC disease duration are based on gynecologic and medical oncologists’ estimates of disease progression in the absence of therapy. Because they cannot be validated we evaluated their impact on our results using sensitivity analysis. We focused on stage I disease duration as this is likely to have the greatest impact on screening performance. In a sensitivity analysis that included halving and doubling the estimate of stage I duration, mortality reduction for annual screening using CA125 and TVS varied by only 2%. Recently Brown et al. estimated that high grade serous cancers spend on average 4 years as in-situ, Stage I and II cancers based on an analysis of pathologic findings in risk-reducing salpingo-oophorectomy specimens in patients with BRCA1 mutations [22]. Substituting these more empirically driven estimates of disease duration and limiting analysis to serous EOC did not significantly impact our predictions of screening performance (See Table S6).
Our results demonstrate that the effectiveness of a multimodal EOC screening strategy is largely dependent on the performance of the first line test. Substituting the better performing HM for CA125 increased mortality reduction by 12% (fro m 13% to 25%). Mortality reduction increased to 28% in a sensitivity analysis where the duration of Stage I disease was doubled. The impact of improvements in imaging test performance was not as dramatic. In screening strategies where CA125 is used as the first line test mortality reduction increases only 2–3% when TVS is replaced by better performing HI and in a sensitivity analysis where TVS is assumed to have near perfect sensitivity. When combined with HM a s the first line screen, HI increased mortality reduction by 5% compared to imaging using TVS (26% vs. 21%). We did not evaluate screening strategies that use imaging as a 1st line screen because of the reported lower sensitivity of TVS compared to CA125 in early results from both the UKCTOCS and PLCO trials and because HI as a first line test would likely be prohibitively expensive when used annually. A screening strategy that employs a biomarker as the 2nd line screen is potentially appealing due to increased sensitivity [23] and low cost. However, we did not model such a strategy out of concern that physicians would be reluctant to proceed to surgery without a confirmatory imaging test.
It is unclear how best to identify biomarkers that perform twice as well as C A125. Despite tremendous effort, no newly discovered EOC biomarkers performed better than CA125 in the critical pre-clinical phase of the disease [8, 24]. Circulating auto-antibodies are appealing because of the inherent tendency of the immune system to amplify in response to minute amounts of antigen. Markers that are EOC specific and not present in unaffected women are also appealing as they lack background signal. This class of markers includes gene fusions and/or translocations that may be discovered using newer deep-sequencing technologies. A candidate gene fusion expressed in roughly 15% of the lethal serous EOC subtype has been recently identified [25]. In theory, an im aging test with appropriate performance characteristics could be used as a 1st line test. However cost is a critical issue as the test would be applied to everyone. Our analysis of the impact of cost of the first line test on the cost-effectiveness of screening suggests that screening using HI as a first line test at a cost of $750 per test might be feasible if significant mortality reduction could be achieved with screening once every few years. Brown et al. estimate that with a screening interval of 24 months it will be necessary to reliably detect tumors no larger than a few millimeters in size in order to identify 50% of high grade serous EOC in Stage II or earlier. This level of resolution is potentially within reach of newer imaging strategies.
Supplementary Material
Acknowledgments
Funding Source: This work was supported by the Pacific EOC Research Consortium, Award Number P50 CA083636 from the National Cancer Institute. Support from the NCI U01 CA152637 (to NU and CWD, Chris Li as primary investigator) and the Canary Foundation (to SH) is also gratefully acknowledged.
Footnotes
Conflict of Interest
None of the authors listed above (Charles Drescher, Sarah Hawley, Jason Thorpe, Simone Marticke, Sanjiv Gambhir, Martin McIntosh, and Nicole Urban) has declared any conflict of interest with the above manuscript.
References
- 1.Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011 Jun 8;305(22):2295–303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 2.Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol. 2009 Apr;10(4):327–40. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
- 3.Skates S, Troiano R, Knapp RC. Longitudinal CA125 detection of sporadic papillary serous carcinoma of the peritoneum. Int J Gynecol Cancer. 2003 Sep-Oct;13(5):693–6. doi: 10.1046/j.1525-1438.2003.t01-1-13050.x. [DOI] [PubMed] [Google Scholar]
- 4.Lu KH, Skates S, Bevers TB, Newland W, Moore RG, Leeds L, et al. A prospective U.S. ovarian cancer screening study using the risk of ovarian cancer algorithm (ROCA) 2010 ASCO Annual Meeting J Clin Oncol. 2010 p. (suppl; abstr 5003) [Google Scholar]
- 5.Havrilesky LJ, Whitehead CM, Rubatt JM, Cheek RL, Groelke J, He Q, et al. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol Oncol. 2008 Sep;110(3):374–82. doi: 10.1016/j.ygyno.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 6.Palmer C, Duan X, Hawley S, Scholler N, Thorpe JD, Sahota RA, et al. Systematic evaluation of candidate blood markers for detecting ovarian cancer. PLoS One. 2008;3(7):e2633. doi: 10.1371/journal.pone.0002633. PMC2440813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson GL, McIntosh M, Wu L, Barnett M, Goodman G, Thorpe JD, et al. Assessing lead time of selected ovarian cancer biomarkers: a nested case-control study. J Natl Cancer Inst. 2010 Jan 6;102(1):26–38. doi: 10.1093/jnci/djp438. PMC2802285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramer DW, Bast RC, Jr, Berg CD, Diamandis EP, Godwin AK, Hartge P, et al. Ovarian cancer biomarker performance in prostate, lung, colorectal, and ovariancancer screening trial specimens. Cancer Prev Res (Phila) 2011 Mar;4(3):365–74. doi: 10.1158/1940-6207.CAPR-10-0195. PMC3085251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutz AM, Willmann JK, Drescher CW, Ray P, Cochran FV, Urban N, et al. Early diagnosis of ovarian carcinoma: is a solution in sight? Radiology. 2011 May;259(2):329–45. doi: 10.1148/radiol.11090563. [DOI] [PubMed] [Google Scholar]
- 10.Willmann JK, Kimura RH, Deshpande N, Lutz AM, Cochran JR, Gambhir SS. Targeted contrast-enhanced ultrasound imaging of tumor angiogenesis with contrast microbubbles conjugated to integrin-binding knottin peptides. J Nucl Med. 2010 Mar;51(3):433–40. doi: 10.2967/jnumed.109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willmann JK, Cheng Z, Davis C, Lutz AM, Schipper ML, Nielsen CH, et al. Targeted microbubbles for imaging tumor angiogenesis: assessment of whole-body biodistribution with dynamic micro-PET in mice. Radiology. 2008 Oct;249(1):212–9. doi: 10.1148/radiol.2491072050. PMC2657857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havrilesky LJ, Sanders GD, Kulasingam S, Chino JP, Berchuck A, Marks JR, et al. Development of an ovarian cancer screening decision model that incorporates disease heterogeneity: implications for potential mortality reduction. Cancer. 2011 Feb 1;117(3):545–53. doi: 10.1002/cncr.25624. [DOI] [PubMed] [Google Scholar]
- 13.Urban N, McIntosh M, Clarke L, Jacobs I, Karlan B, Anderson G, Drescher D. Ovarian Cancer. 2. Vol. 6. New York: Oxford University Press; 2002. Socio-economics of ovarian cancer screening; pp. 199–208. [Google Scholar]
- 14.Urban N, Drescher C, Etzioni R, Colby C. Use of a stochastic simulation model to identify an efficient protocol for ovarian cancer screening. Control Clin Trials. 1997 Jun;18(3):251–70. doi: 10.1016/s0197-2456(96)00233-4. [DOI] [PubMed] [Google Scholar]
- 15.SEER Cancer Statistics Review. 1973–1991. National Cancer Institute; 1994. NIH Publication No. 94-2789. [Google Scholar]
- 16.U.S. Department of Health and Human Services. PHS Centers for Disease Control. NCHS. VitaI Statistics of the U.S. 1988 Vol. II Mortality, Part A, Table 14. Sect. 1. General Mortality. 1991;7 [Google Scholar]
- 17.Katsube Y, Berg JW, Silverberg SG. Epidemiologic pathology of ovarian tumors: a histopathologic review of primary ovarian neoplasms diagnosed in the Denver Standard Metropolitan Statistical Area, 1 July-31 December 1969 and 1 July-31 December 1979. Int J Gynecol Pathol. 1982;1(1):3–16. doi: 10.1097/00004347-198201000-00003. [DOI] [PubMed] [Google Scholar]
- 18.McIntosh MW, Urban N, Karlan B. Generating longitudinal screening algorithms using novel biomarkers for disease. Cancer Epidemiol Biomarkers Prev. 2002 Feb;11(2):159–66. [PubMed] [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results (SEER) Program. ( http://seer.cancer.gov/) SEER*Stat Database: Incidence – SEER 9 Regs Limited-Use, Nov 2009 Sub (1973–2007) – Linked To County Attributes – Total U.S., 1969–2007 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2010, based on the November 2009 submission.
- 20.Appendix: 2003 Statistics on All-listed Procedures in US Hosptials. Available from: http://stats.bls.gov/cpi/home.htm#data.
- 21.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008 May 7;100(9):630–41. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 22.Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med. 2009 Jul;6(7):e1000114. doi: 10.1371/journal.pmed.1000114. PMC2711307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urban N, Thorpe JD, Bergan LA, Forrest RM, Kampani AV, Scholler N, et al. Potential role of HE4 in multimodal screening for epithelial ovarian cancer. J Natl Cancer Inst. 2011 Nov 2;103(21):1630–4. doi: 10.1093/jnci/djr359. PMC3206037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu CS, Pinsky PF, Cramer DW, Ransohoff DF, Hartge P, Pfeiffer RM, et al. A framework for evaluating biomarkers for early detection: validation of biomarker panels for ovarian cancer. Cancer Prev Res (Phila) 2011 Mar;4(3):375–83. doi: 10.1158/1940-6207.CAPR-10-0193. 3057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salzman J, Marinelli RJ, Wang PL, Green AE, Nielsen JS, Nelson BH, et al. ESRRA-C11orf20 Is a Recurrent Gene Fusion in Serous Ovarian Carcinoma. PLoS Biology. 2011 Sep;9(9):e1001156. doi: 10.1371/journal.pbio.1001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Partridge E, Kreimer AR, Greenlee RT, Williams C, Xu JL, Church TR, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009 Apr;113(4):775–82. 2728067. doi: 10.1097/AOG.0b013e31819cda77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


