Abstract
Previous research has identified cognitive impairment in children with sickle cell anemia (SCA, Hemoglobin SS) compared with controls, partly accounted for by overt neuropathology after clinical stroke, “covert” (“silent”) infarction, and severity of anemia. However, cognitive deficits have also been identified in children with SCA with no history of stroke and a normal T2-weighted magnetic resonance imaging (MRI) scan. Our aim was to investigate whether nocturnal hemoglobin oxygen desaturation and sleep fragmentation could be associated with cognitive impairment in children with SCA. We assessed 10 children with SCA (9 with normal MRI) using neuropsychological measures of executive function. Cognitive assessment was immediately followed by overnight polysomnography to record nocturnal hemoglobin oxygen saturation and sleep arousals. Decreases in hemoglobin oxygen saturation and/or increased sleep arousals were associated with reduced performance on cognitive assessment. Nocturnal hemoglobin oxygen desaturation and sleep fragmentation may be a contributing factor to executive dysfunction in SCA.
Keywords: Anemia, Sickle cell, Intelligence, Neuropsychology, Oximetry, Sleep arousal disorders, Polysomnography
INTRODUCTION
Homozygous sickle cell anemia (SCA; HbSS) is a disorder in which a gene mutation leads to the formation of a sickle hemoglobin molecule containing an abnormal globin protein. Although retaining the ability to carry oxygen, the abnormality causes the cell to polymerize after deoxygenating, forming the rigid sickle shape which has pathological consequences (Schatz & McClellan, 2006). The incidence of stroke is high and the pathology typically involves the large arteries supplying anterior–middle cerebral boundary zones (Rothman, Fulling, & Nelson, 1986).
Mechanisms of Neuropsychological Impairment in SCA
Some children with SCA and neuropsychological deficit have “covert” or “silent” infarction, which refers to infarctions detectable on T2-weighted MRI at 1.5 Tesla (T) but without a focal neurological deficit lasting >24 hr and typically smaller than those associated with overt stroke. Even in the absence of overt infarction, IQ is significantly lower in children with SCA than age matched controls (85.6 vs. 91.2; Schatz, Brown, Pascual, Hsu, & DeBaun, 2001; Schatz, Finke, Kellett & Kramer, 2002). The cause of cognitive impairment in the absence of obvious infarction on T2-weighted MRI is unclear, but in part may be related to hypoxia related to anemia, a hallmark of the disease (Gold, Johnson, Treadwell, Hans, & Vichinsky, 2008; Steen et al., 2005).
SCA and Executive Functions
In addition to a significant reduction in intellectual functioning children with SCA are at risk for deficits in many specific cognitive domains, including executive functioning. One study reported that 53% of HbSS children with silent infarction had deficits in executive function, compared to 13% of HbSS children with no infarctions and 0% of control siblings without SCA (Schatz et al., 2001).
Effects of Nocturnal hemoglobin desaturation and disrupted sleep
One mechanism which may account for the presence of executive dysfunction in the absence of obvious neuropathology is low hemoglobin oxygen saturation associated with sleep-disordered breathing (SDB) and the resulting sleep fragmentation caused by increased sleep arousals. SDB is relatively common in children with SCA, with one group reporting 40% affected (Needleman et al., 1999). Sleep disruption has been shown to have a negative impact on attention and executive function in adults (Waters & Bucks, 2011). Children with SDB exhibit disrupted sleep and oxygen desaturation linked to a degree of executive dysfunction not accounted for by daytime sleepiness (Beebe and Gozal, 2002; Bourke et al., 2011).
The aim of this study was to investigate any association of nocturnal oxygen saturation and sleep quality with neuropsychological measures of executive functioning. There was a particular focus on neurologically normal children with SCA who had a normal T2-weighted MRI to provide insight into potential mechanisms related to cognitive problems in the absence of infarction.
METHODS
Participants
This study was reviewed and approved by the Brent National Health Service research ethics committee. Participants with homozygous SCA were recruited in London during April to September 2009 from a sample enrolled with the Sleep Asthma Cohort study, a three-site 5-year (2005–2010) observational study (Johnson et al., 2010), which included 236 children. On arrival, consent/assent from the parent/guardian and child were confirmed and the neuropsychological assessment was administered, taking approximately 50 min. Following a short break, the child was prepared for and underwent overnight polysomnography (Johnson et al., 2010). The following morning, participants underwent a transcranial Doppler (TCD).
Materials
Tests of executive function
Delis-Kaplan Executive Function Systems (D-KEFS)
Executive functioning was assessed using two subtests from the D-KEFS assessment battery (Delis, Kaplan, & Kramer, 2001). The D-KEFS Tower test measures spatial planning, rule learning, and inhibition of impulsive responding. The overall achievement score is based upon the number of correct towers and how many moves taken to complete the tower. The D-KEFS Sorting test measures problem solving, concept formation and conceptual reasoning. Scores for the D-KEFS sorting test are given for the accuracy of sorts and description of sorts.
Behaviour Rating Inventory of Executive Functions (BRIEF) – Parent Form
The BRIEF parent form is a parental rating questionnaire containing 86 questions relating to eight clinical scales belonging to one of two domains, behavioral regulation index (BRI) and the meta-cognition index (MCI), which are combined to give the general executive composite (GEC) score. The scale provides age and gender based t scores with a higher score indicating poorer executive functions (Mean = 50; SD = 10) (Gioia, Isquith, Guy, & Kenworthy, 2000).
Tests of intelligence
Wechsler Abbreviated Scale of Intelligence (WASI)
The WASI was selected as a reliable but brief measure of intelligence; due to time constraints, only the two item version was administered in 15 min. This form contains only the vocabulary and matrix reasoning items but provides an estimate for full-scale IQ (FSIQ; Wechsler, 1999).
Polysomnography
Standard sleep acquisition systems (Embla N-4000, Broomfield, CO), sensors, and data collection procedures were used and followed current professional society standards for overnight collection of sleep and breathing data in children except that carbon dioxide was not measured (Johnson et al., 2010). Cardiorespiratory sensors in the SAC protocol included: chest and abdominal wall motion by inductive plethysmography (Embla Xact-Trace™ bands, Broomfield, CO), airflow by both nasal pressure cannula and by oronasal by thermocouple (Pro-Tech Services, Woodinville, WA), and hemoglobin oxygen saturation by pulse oximetry (SpO2) (Masimo™ Rad-8 oximeter, Irvine, CA).
Full-channel overnight polysomnography started at the child’s usual bedtime and ended at the child’s spontaneous waking or as late as 7:00 a.m. Studies were centrally scored by research assistants at the Case Sleep Reading Center (Cleveland, OH) who were blinded to clinical correlates (Johnson et al., 2010).
Arousals and respiratory events were scored using recommended pediatric criteria, except that arousals alone were not used to define hypopneas. Respiratory variables included the obstructive apnea hypopnea index [hypopnea corroborated by at least a 3% desaturation (OAHI), mean and minimum SpO2, and percent of total sleep time with saturation values below 95%].
Data Analysis
Key variables were not normally distributed so non-parametric statistics were used. Data analysis, first, consisted of an exploratory analysis of the participants’ performance on neuropsychological measures of executive and intellectual functioning compared to normative scaled scores using one-sample Wilcoxon Signed Rank Tests. The second analysis consisted of correlations, using Spearman’s rank (Spearman’s rho) correlation coefficients aiming to identify any associations between nocturnal SpO2, sleep fragmentation, and neuropsychological measures.
RESULTS
This analysis included 10 participants (4 male and 6 female), mean age 12 years (range, 8–16 years). For three participants, the head of the household had a Master’s degree, for five, he or she had some college education but no degree, while one parent had completed high school only and one refused to answer. Three families were single parent. There were 2, 3, and 4 children in the household aged <16 years for 6, 2, and 2 participants, respectively. Nine participants had previously undergone a screening MRI as part of the Silent Infarct Transfusion trial (Casella et al., 2010) and the other had an MRI after a transient episode of visual loss. On T2-weighted MRI, the symptomatic participant had a small lesion in the left temporal lobe; the remaining MRIs were normal. Cerebral blood flow velocity on TCD was in the normal range in all participants. There were no differences in frequency of snoring (5/10 recruited sample) or any of the sleep variables or in demographics (marital status, education of head of household) between these patients and the total of 226 or the subgroup of 152 patients aged ≥8 years not recruited. Average nocturnal SpO2 (mean = 96.6%; median = 98.4%; range, 89.3–99.6) fell within the normal range typically observed in a pediatric population although the minimum SpO2 (mean = 88.9%; median = 90%; range, 76.9–96%) was considerably lower than the minimum SpO2 value of 94.6% reported in the normal population (Uliel, Tauman, Greenfeld, & Sivan, 2004).
Mean Arousal Index (AI; mean = 9.8; median = 10.2; range, 5.9–12.5) was marginally higher than the previously published mean arousals from the normal population (mean = 8.8; SD = 3.8; Traeger et al., 2005). The mean OAHI value was .98 (median = .19; range, 0.00–5.08). Three of our participants had an OAHI of 1.0 or above which could be considered outside the normal range (Traeger et al., 2005). Mean scores on the neuropsychological tests are presented in Table 1.
Table 1.
Mean scale scores from the neuropsychological assessment
| Mean | Median | Range | SD | |
|---|---|---|---|---|
| Full-scale IQ WASI | 85.7 | 89.5 | 57–105 | 15.67 |
| (87)a | (91) | (57–105) | (16.12) | |
| D-KEFS | 5.4 | 5.5 | 2–10 | 2.95 |
| Correct Sorts | (5.78) | (6) | (2–9) | (2.86) |
| D-KEFS | 5.5 | 5.0 | 2–10 | 2.59 |
| Descriptions Score | (5.78) | (5) | (2–9) | (2.58) |
| D-KEFS Tower | 8.4 | 9.0 | 1–12 | 3.37 |
| Achievement Score | (8.67) | (10) | (1–12) | (3.46) |
| BRIEFb | 64 | 66.5 | 39–94 | 16.89 |
| BRI (t-score) | (67.33) | (67) | (39–94) | (15.59) |
| BRIEFb | 56 | 57 | 41–79 | 10.99 |
| MCI (t-score) | (58.11) | (59) | (41–79) | (10.65) |
| BRIEFb | 60.3 | 57 | 39–88 | 14.11 |
| GEC (t-score) | (62.2) | (63) | (39–88) | (13.51) |
The values after excluding the participant with a lesion are shown in brackets.
BRIEF t-score: Higher t-score refers to poorer performance (M = 50, SD = 10), WAIS FSIQ (M = 100, SD = 15), D-KEFS scaled score (M = 10, SD = 3).
WASI = Wechsler Abbreviated Scale of Intelligence; FSIQ = full-scale intelligence quotient; D-KEFS = Delis-Kaplan Executive Function Systems; BRIEF = Behaviour Rating Inventory of Executive Functions; BRI = behavioral regulation index; MCI = Metacognition Index; GEC = general executive composite.
Executive Function and IQ
A one sample Wilcoxon Signed Rank Test was carried out to compare the D-KEFS subtest scores with the normative mean of 10. Both the Sorting accuracy score (Z = −2.8; p <.01) and the Sorting description score (Z = −2.8; p <.01) were significantly below the normative mean but the Tower total achievement score was not (Z = −1.4; p = .17). IQ was also significantly lower than the population mean of 100 (Z = −2.5; p = .013). Executive function scores were not correlated with IQ scores and sorting scores did not correlate with Tower scores. The two Sorting scores were highly correlated (rho = .98; p <.001) so only the correct sorts score were used for analysis. The BRIEF GEC t score (mean = 60.3; median = 61.0; range, 39–88) indicated mildly elevated problems in executive functioning but within the normal range.
Relation of Executive Function and IQ to Sleep Variables
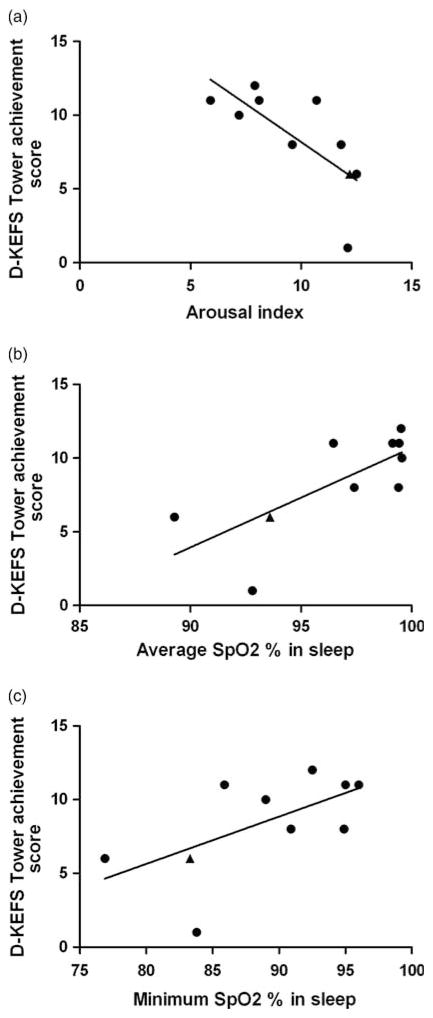
A summary of the associations is shown in Table 2 and Figure 1. The Tower task total achievement score demonstrated significant correlations with both mean (rho = .71; p = .021) and minimum SpO2 (rho = .68; p = .031). Similarly an inverse correlation was identified between percentage of the study with SpO2 <95% and Tower performance (rho = −.63; p = .05). The arousal index was also inversely correlated with the Tower achievement score (rho = −.78; p = .008) but OAHI was not (Table 2).
Table 2.
Spearman’s rho correlations between Arousal Index, Nocturnal Hemoglobin Oxygen saturation (SpO2), Obstructive Apnea/Hypopnea Index, and neuropsychological measurements
| Arousal Index (AI) | Average SpO2% in sleep | % Sleep time SpO2 < 95% | Minimum SpO2% in sleep | Obstructive Apnea/Hypopnea Index (OAHI) | |
|---|---|---|---|---|---|
| Full-scale IQ WASI | −.552† | .539 | −.767** | .576† | .081 |
| (−.550) | (.517) | (−.780*) | (.567) | .075 | |
| D-KEFS | −.357 | .135 | −.492 | .560† | −.383 |
| Correct Sorts | (−.227) | (−.067) | (−.393) | (.471) | −556 |
| D-KEFS Tower | −.778** | .710* | −.631 † | .679* | −.048 |
| Achievement Score | (−732*) | (.613†) | (−.502) | (596†) | −.091 |
Note. Values in brackets represent analysis excluding participant with lesion.
p ≤ .05.
p ≤ .01.
p ≤ 0.1.
WASI: Wechsler Abbreviated Scale of Intelligence; D-KEFS :Delis-Kaplan Executive Function Systems.
Fig. 1.
Scatterplots showing the relationship between the Delis-Kaplan Tower achievement scaled score and (a) Arousal Index, (b) Average overnight hemoglobin oxygen saturation (SpO2) and (c) Minimum overnight hemoglobin oxygen saturation. Patients with normal MRI are represented by closed circles and the patient with a small lesion is represented by a closed triangle.
Correlations between the Card Sort task and sleep variables revealed a single trend for association between the number of correct sorts and mean SpO2 (rho = .56; p = .092). IQ was significantly correlated with percentage of the study with SpO2 <95% (rho = −.77; p = .01) and was additionally associated with minimum SpO2 (rho = .58; p = .08) and AI (rho = .55; p = .098). When the data were re-analyzed excluding the participant with the MRI lesion, similar associations were identified, although with a reduced effect size (see Table 2). Average daytime saturation was not significantly related to neuropsychological measures.
DISCUSSION
Neuropsychological Functioning
The previous literature suggests that children with sickle cell disease are at risk of general and specific cognitive problems, including executive functioning. In our sample the mean IQ was almost 15 points below the population average (1 SD), that is, in the low average range. This level of impairment is similar to that reported in previous studies of those without cerebral infarction (Schatz et al., 2002). However the scores on the BRIEF were worse than previously reported in a group with none of the standard predictors of pathology such as an abnormal TCD (Kral et al., 2003) or evidence of infarction on a standard T2-weighted MRI, which must be considered when interpreting our results. Although in terms of physiological parameters and demographics, our sample is comparable to the remainder of our SCA cohort, their neuropsychological profile may be more severe, perhaps because families were self-selecting in that families with children experiencing problems at school were more likely to participate.
In line with the previous literature, we found evidence of impairment in executive functioning. In our sample, performance on the Sorting test was impaired to a greater degree than Tower task performance. Group mean scores on the Tower test were within 1 SD, and did not significantly differ from the normative mean. However, the means for both Sorting task measures in these 10 children were more than one standard deviation below the normative mean, and 4/10 performed below two standard deviations. This, along with the high BRIEF scores, supports previous suggestions that children with SCA show some impairment in executive functioning.
Impact of Oxygen Desaturation and Sleep fragmentation on Executive Functions
Given the high prevalence of nocturnal oxygen desaturation in children with SCA (Needleman et al., 1999), we hypothesized that this may be one potential mechanism leading to deficits in executive functioning. Our results suggested an association between measures of nocturnal oxygen saturation and the Tower task. Mean and minimum overnight SpO2 were significantly correlated with performance on the Tower task and there was a trend for an association with sleep-time spent with SpO2 <95% saturation, providing evidence that desaturation is linked to poorer executive function. In contrast, the Sorting task demonstrated no association with oxygen saturation variables. It is possible that our study lacked the required power to detect such differences, a problem exacerbated because the majority of our sample performed poorly on this task.
We hypothesized that increased sleep arousals would have an impact on executive functioning. The results indicate that there was a significant association between the AI and the Tower task, supporting previous literature suggesting that sleep fragmentation may impact on executive functioning (Waters & Bucks, 2011). Prefrontal cortical areas vital for executive functioning may be particularly vulnerable to disruptions in sleep as REM-sleep periods appear vital for recuperation of these areas overnight (Muzur, Pace-Schott, & Hobson, 2002).
Intellectual Functioning
Our analyses also revealed associations between sleep variables and general intellectual functioning. In particular, greater sleep-time spent with SpO2 <95% was associated with lower IQ. This suggests a possible role for disordered sleep in both general functioning and specific executive domains. Although scores on tests of IQ and executive functioning did not correlate, a larger sample size would have allowed us to investigate the interplay of these two constructs. This should be an important consideration in future research.
Association Between Reduced Nocturnal Oxygen Saturation and Sleep Arousals
Our results revealed that sleep measurements of oxygen saturation and arousal index score are highly correlated with each other. Specifically, decreases in oxygen saturation over the sleep period were highly correlated to arousals. This is not surprising as it is well known that arousal from sleep is an important mechanism of defense against night time hypoxic events. Significant dips in oxygen saturation may result in sleep fragmentation, potentially related to neuropsychological problems (Johnston, Grant, Wilkinson, & Walker, 1998). Of interest, daytime saturation did not show the same association with neuropsychological scores, although previous research has shown that daytime saturation is highly correlated with overnight saturation (Johnson et al., 2010).
Limitations
This study was carried out as a novel preliminary investigation into associations between nocturnal variables and temporally related neuropsychological assessments. Due to its exploratory nature this research suffers from several limitations. The primary limitation is the small sample size which has limited the power of our analyses and also limited us to use of non-parametric statistics. In addition our group appears to contain those with more severe executive problems than would be predicted by the previous literature. Future replication of these results will require a larger and more representative sample. Despite identifying potentially important associations between physiological variables and cognitive performance we are, at this stage, unable to tease apart the relative importance of each. Further studies will be needed to examine the relative importance of nocturnal dips in saturation and low baseline saturation.
In summary, data has been presented supporting previous literature which suggests deficits in IQ and executive functions in SCA and presents an initial argument for the importance of nocturnal systemic oxygen saturation and sleep fragmentation as mechanisms for such deficits in the absence of clear neuropathology. The identification of such mechanisms for neuropsychological problems in SCA may be particularly important given that treatment for anemia and nocturnal desaturation are options in the SCA population.
Acknowledgments
We thank the children and their families who took part in the study and Dr. Carol Rosen for advice about the interpretation of the polysomnography. The study was supported by the National Heart, Lung, and Blood Institute (5-RO1-HL079937).
Footnotes
To the best of our knowledge no conflicts of interest exist.
References
- Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. Journal of Sleep Research. 2002;11(1):1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- Bourke RS, Anderson V, Yang JS, Jackman AR, Killedar A, Nixon GM, Horne RS. Neurobehavioral function is impaired in children with all severities of sleep disordered breathing. Sleep Medicine. 2011;12(3):222–229. doi: 10.1016/j.sleep.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Casella JF, King AA, Barton B, White DA, Noetzel MJ, Ichord RN, DeBaun MR. Design of the silent cerebral infarct transfusion (SIT) trial. Pediatric Hematology/Oncology. 2010;27(2):69–89. doi: 10.3109/08880010903360367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Examiner’s manual for the Delis Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Gioia GA, Isquith PK, Guy S, Kenworthy L. BRIEF: Behaviour Rating Inventory of Executive Functions manual. Lutz, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- Gold JI, Johnson CB, Treadwell MJ, Hans N, Vichinsky E. Detection and assessment of stroke in patients with sickle cell disease: Neuropsychological functioning and magnetic resonance imaging. Pediatric Hematology and Oncology. 2008;25:409–421. doi: 10.1080/08880010802107497. [DOI] [PubMed] [Google Scholar]
- Johnson MC, Kirkham FJ, Redline S, Rosen CL, Yan Y, Roberts I, DeBaun MR. Left ventricular hypertrophy and diastolic dysfunction in children with sickle cell disease are related to asleep and waking oxygen desaturation. Blood. 2010;116(1):16–21. doi: 10.1182/blood-2009-06-227447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RV, Grant DA, Wilkinson MH, Walker AM. Repetitive hypoxia rapidly depresses arousal from active sleep in newborn lambs. Journal of Physiology. 1998;510(2):651–659. doi: 10.1111/j.1469-7793.1998.651bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral MC, Brown RT, Nietert PJ, Abboud MR, Jackson SM, Hynd GW. Transcranial doppler ultrasonography and neurocognitive functioning in children with sickle cell disease. Pediatrics. 2003;12(2):324–331. doi: 10.1542/peds.112.2.324. [DOI] [PubMed] [Google Scholar]
- Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends in Cognitive Science. 2002;6:475–481. doi: 10.1016/s1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- Needleman JP, Franco ME, Varlotta L, Reber-Brodecki D, Bauer N, Dampier C, Allen JL. Mechanisms of nocturnal oxyhemoglobin desaturation in children and adolescents with sickle cell disease. Pediatric Pulmonology. 1999;28(6):418–422. doi: 10.1002/(sici)1099-0496(199912)28:6<418::aid-ppul6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Fulling KH, Nelson JS. Sickle cell anemia and central nervous system infarction: A neuropathological study. Annals of Neurology. 1986;20:684–690. doi: 10.1002/ana.410200606. [DOI] [PubMed] [Google Scholar]
- Schatz JR, Brown RT, Pascual JM, Hsu L, DeBaun MR. Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology. 2001;56(8):1109–1111. doi: 10.1212/wnl.56.8.1109. [DOI] [PubMed] [Google Scholar]
- Schatz J, Finke RL, Kellett JM, Kramer JH. Cognitive functioning in children with sickle cell disease: A meta-analysis. Journal of Pediatric Psychology. 2002;27(8):739–748. doi: 10.1093/jpepsy/27.8.739. [DOI] [PubMed] [Google Scholar]
- Schatz J, McClellan CB. Sickle cell disease as a neurodevelopmental disorder. Mental Retardation & Developmental Disabilities Research Review. 2006;12:200–207. doi: 10.1002/mrdd.20115. [DOI] [PubMed] [Google Scholar]
- Steen RG, Fineberg-Buchner C, Hankins G, Weiss L, Prifitera A, Mulhern RK. Cognitive deficits in children with sickle cell disease. Journal of Child Neurology. 2005;20:102–107. doi: 10.1177/08830738050200020301. [DOI] [PubMed] [Google Scholar]
- Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2–9 years old: Additional data and review of the literature. Pediatric Pulmonology. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–878. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- Waters F, Bucks RS. Neuropsychological effects of sleep loss: Implication for neuropsychologists. Journal of the International Neuropsychological Society. 2011;17:571–586. doi: 10.1017/S1355617711000610. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler scale of intelligence manual (trans) San Antonio, TX: Harcourt Brace & Company; 1999. [Google Scholar]