Summary
Asthma is associated with increases in sickle cell disease (SCD)-related morbidity and mortality. A thorough evaluation for asthma in children with SCD is important and may involve methacholine challenge (MCh). In this report, we present a 14-year-old male with SCD who was admitted for an acute painful episode following MCh. Pain events after MCh have not been previously reported in children with SCD. The risk–benefit ratio should be strongly considered prior to performance of MCh in this patient population, and all possible complications, including an acute painful episode, should be openly discussed with the parents and pediatric patient.
Keywords: methacholine challenge, sickle cell disease, pain, asthma
INTRODUCTION
A physician diagnosis of asthma is associated with increased rates of painful episodes,1 acute chest syndrome (ACS),1–4 and overall mortality5 in individuals with sickle cell disease (SCD). While the biological basis for this relationship is not completely clear, it is possible that inflammation associated with asthma augments the proinflammatory state of SCD,6–9 resulting in increased morbidity and mortality. Accurate diagnosis of asthma in children and adults with SCD may require additional tests for asthma risk factors, including bronchodilator responsiveness, allergy skin testing, and methacholine challenge (MCh). In children with known mild-to-moderate asthma, MCh, as a measure of airway hyper-responsiveness, is a safe test10 that is relatively specific for a diagnosis of asthma.11,12 Although the clinical utility of MCh for diagnosing asthma in individuals with SCD is not well defined, no serious adverse events have been documented to date.13–15 In this report, we describe an adolescent with SCD who underwent a MCh as part of a clinical research study and was subsequently hospitalized for an acute painful episode.
CASE REPORT
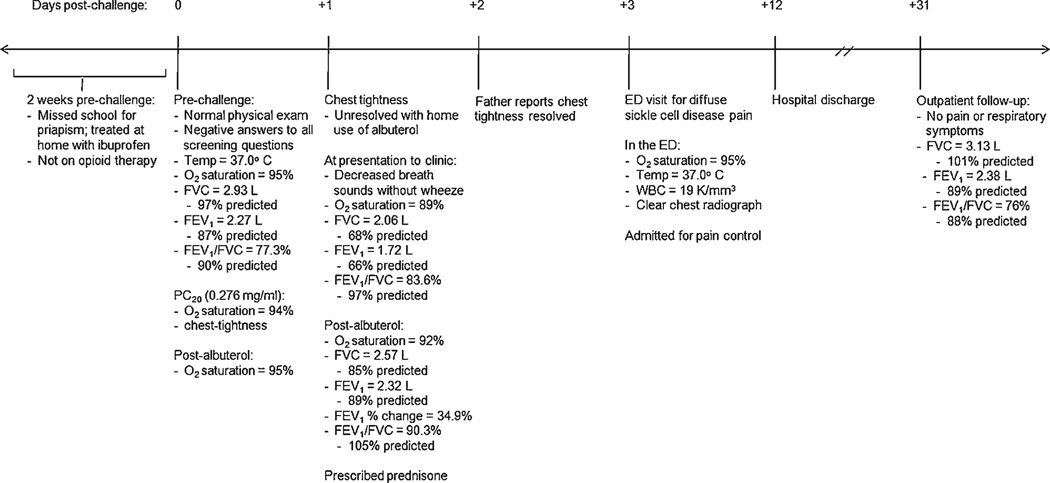
As part of the multi-center, NIH-funded Sleep and Asthma Cohort (SAC) Study, a 14-year-old male with hemoglobin SS (HbSS) was administered a MCh at a participating site in the United States. The study was approved by all three Institutional Review Boards in St. Louis, Cleveland, and London, UK, and appropriate informed assent from the subject of this report and consent from the parent were obtained. His past medical history was significant for four hospital admissions, one each for pain, priapism, upper respiratory infection, and cholestectomy; however, none were within the previous 4 months. He had no past history of respiratory symptoms or asthma. His recent medical history was significant for episodes of priapism in the 2 weeks prior to testing (Fig. 1). To optimize participant safety, a rigorous screening process per SAC protocol occurred prior to performing the MCh. This included a physical examination, spirometry, and questions about any new or worsened respiratory symptoms within the past 24 hours (hr), use of bronchodilator (past 12 hr) or extended-release theophylline (past 24 hr), use of an opioid or increase in daily opioid therapy within the last 48 hr, as well as any respiratory infections, hospitalizations for ACS or respiratory illness, or use of oral corticosteroids within the past month.
Fig. 1.
Adverse event timeline.
Based on the screening results (Fig. 1), the patient fully qualified for the MCh which was then performed according to ATS guidelines16 and as previously described in children with SCD.15 The PC20 was 0.276 mg/ml, at which point, the patient’s oxygen saturation (SpO2) decreased to 94% and he noted chest tightness. After albuterol administration, his SpO2 returned to baseline (95%) and the chest tightness resolved. Because of the positive challenge, he was instructed to take albuterol every 4 hr for the next 24 hr as delineated by protocol.
On day 1 post-challenge, the patient’s chest tightness returned on awakening, and albuterol use at home failed to yield significant improvement. He was referred to the SCD clinic where physical examination and spirometry were repeated (Fig. 1). Albuterol was administered, and his SpO2 and lung function improved, although not all measurements returned to baseline (Fig. 1). As a result, oral prednisone, 2 mg/kg for a 5-day course, was initiated.
The patient’s chest tightness resolved the following day; however, on day 3 post-challenge, he presented to the emergency department complaining of diffuse body pain (Fig. 1). The patient was admitted for pain control, a continuous drip of morphine at 5 mg/hr was started, and the course of corticosteroids was continued and then tapered over 7 days. The hospital course was uncomplicated, with transition to oral pain medication prior to discharge on day 12 post-challenge. At the outpatient follow-up 19 days after discharge, lung function had returned to baseline and the patient denied any pain or respiratory symptoms (Fig. 1).
DISCUSSION
This case report documents the first pain event following a MCh in an adolescent with SCD. Although we cannot definitively determine if the MCh caused the acute painful episode, the tight temporal relationship between the challenge and subsequent pain episode provides evidence of an association. After review of this event, we recommend implementation of the following precautionary measures for the post-challenge period: (1) inform patients to contact clinic personnel if chest heaviness, tightness, or wheezing occurs within 24 hr post-test and (2) provide a peak flow meter for patients to record and phone in values at 3 and 6 hr post-test. Although the adolescent was not taking opioids for the priapism episodes in the 2 weeks prior to testing, these episodes raise the possibility of an ongoing vaso-occlusive process. Therefore, we advise a detailed review of a patient’s recent SCD-related events prior to MCh, and exclusion of individuals with any events in the 30 days prior to testing. Based on this case, MCh should only be administered to a child with SCD after careful consideration of the potential risks and benefits of the test, and an open discussion of these risks, including an acute painful episode, with the parents and pediatric patient.
ACKNOWLEDGMENTS
This work was supported in part by the National Heart, Lung, and Blood Institute, K12 HL08710 (J.J.F.) and RO1 HL079937 (M.R.B., R.C.S., J.K.), and the Doris Duke Charitable Foundation, 2004061 (J.K.P.).
REFERENCES
- 1.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108:2923–2927. doi: 10.1182/blood-2006-01-011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd JH, Moinuddin A, Strunk RC, DeBaun MR. Asthma and acute chest in sickle-cell disease. Pediatr Pulmonol. 2004;38:229–232. doi: 10.1002/ppul.20066. [DOI] [PubMed] [Google Scholar]
- 3.Duckworth L, Hsu L, Feng H, Wang J, Sylvester JE, Kissoon N, Sandler E, Lima JJ. Physician-diagnosed asthma and acute chest syndrome: associations with NOS polymorphisms. Pediatr Pulmonol. 2007;42:332–338. doi: 10.1002/ppul.20582. [DOI] [PubMed] [Google Scholar]
- 4.Knight-Madden JM, Forrester TS, Lewis NA, Greenough A. Asthma in children with sickle cell disease and its association with acute chest syndrome. Thorax. 2005;60:206–210. doi: 10.1136/thx.2004.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with increased mortality in individuals with sickle cell anemia. Haematologica. 2007;92:1115–1118. doi: 10.3324/haematol.11213. [DOI] [PubMed] [Google Scholar]
- 6.West MS, Wethers D, Smith J, Steinberg M. Laboratory profile of sickle cell disease: a cross-sectional analysis. The Cooperative Study of Sickle Cell Disease. J Clin Epidemiol. 1992;45:893–909. doi: 10.1016/0895-4356(92)90073-v. [DOI] [PubMed] [Google Scholar]
- 7.Singhal A, Doherty JF, Raynes JG, McAdam KP, Thomas PW, Serjeant BE, Serjeant GR. Is there an acute-phase response in steady-state sickle cell disease? Lancet. 1993;341:651–653. doi: 10.1016/0140-6736(93)90418-g. [DOI] [PubMed] [Google Scholar]
- 8.Pathare A, Kindi SA, Daar S, Dennison D. Cytokines in sickle cell disease. Hematology. 2003;8:329–337. doi: 10.1080/10245330310001604719. [DOI] [PubMed] [Google Scholar]
- 9.Kato G, Martyr S, Blackwelder WC, Nichols JS, Coles WA, Hunter LA, Brennan ML, Hazen SL, Gladwin MT. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br J Haematol. 2005;130:943–953. doi: 10.1111/j.1365-2141.2005.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covar RA, Colvin R, Shapiro G, Strunk R. Safety of methacholine challenges in a multicenter pediatric asthma study. J Allergy Clin Immunol. 2006;117:709–711. doi: 10.1016/j.jaci.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Liem JJ, Kozyrskyj AL, Cockroft DW, Becker AB. Diagnosing asthma in children: what is the role for methacholine broncho-provocation testing? Pediatr Pulmonol. 2008;43:481–489. doi: 10.1002/ppul.20801. [DOI] [PubMed] [Google Scholar]
- 12.Birnbaum S, Barreiro TJ. Methacholine challenge testing: identifying its diagnostic role, testing, coding, and reimbursement. Chest. 2007;131:1932–1935. doi: 10.1378/chest.06-1385. [DOI] [PubMed] [Google Scholar]
- 13.Vendramini EC, Vianna EO, De Lucena Angulo I, De Castro FB, Martinez JA, Terra-Filho J. Lung function and airway hyperresponsiveness in adult patients with sickle cell disease. Am J Med Sci. 2006;332:68–72. doi: 10.1097/00000441-200608000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Ozbek OY, Malbora B, Sen N, Yazici AC, Ozyurek E, Ozbek N. Airway hyperreactivity detected by methacholine challenge in children with sickle cell disease. Pediatr Pulmonol. 2007;42:1187–1192. doi: 10.1002/ppul.20716. [DOI] [PubMed] [Google Scholar]
- 15.Strunk RC, Brown MS, Boyd JH, Bates P, Field JJ, DeBaun MR. Methacholine challenge in children with sickle cell disease: a case series. Pediatr Pulmonol. 2008;43:924–929. doi: 10.1002/ppul.20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing—1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]